Abstract
Background
With initiation of transcatheter aortic valve replacement (TAVR) programs, centers may see changes in surgical aortic valve replacement (SAVR) populations and related outcomes due to more high-risk patients undergoing TAVR rather than SAVR. Little data exists on the potential changes in the risk profiles and outcomes of SAVR patients from the pre- to post-TAVR eras. As such, this study sought to evaluate changes in the SAVR population at a tertiary referral center following TAVR program initiation.
Methods
Using a single-center valve surgery database, annual volume, patient characteristics, operative details, and predicted and observed mortality for patients undergoing isolated SAVR or SAVR + CABG from 2006 to 2013 were evaluated. Patients were divided into 3 eras: 1) Pre-TAVR (1/2006–6/2009), 2) Transition (7/2009–3/2011), and 3) TAVR (4/2011–6/2013). The primary analysis compared predicted and observed mortality in Pre-TAVR and TAVR eras.
Results
From 2006 to 2013, 1,380 SAVR patients were identified with 505 (36.6%), 330 (23.9%), and 545 (39.5%) patients from the Pre-TAVR, Transition, and TAVR eras, respectively. SAVR case volume increased from 131 to 256 cases/year (95.4% increase) pre- to post-TAVR. Predicted risk of mortality (PROM) for SAVR patients from the Pre-TAVR to TAVR eras by the STS-PROM was stable near 3.8% (p=0.82). Crude 30-day SAVR mortality trended down from 2.8% in the Pre-TAVR era to 1.5% post-TAVR (p=0.23).
Conclusion
Consistent with prior studies, initiation of a TAVR program was associated with increased SAVR volume. Risk profiles for SAVR patients in the TAVR era remained similar by STS-PROM, indicating generally stable risk among surgical patients after launching a TAVR program. These data suggest that significant changes in the risk profiles of SAVR patients should not be expected with the initiation of a TAVR program. Further research will need to re-evaluate these changes once TAVR becomes more widely available.
Keywords: aortic valve replacement, cardiac surgery, transcatheter, emerging technology
An aging population and increasing recognition of aortic valve disease have led to substantial growth in aortic valve surgery over the last 10 years, with nearly 45,000 aortic valve surgeries being performed in the United States in 2010 [1-3]. With the potential to transform the landscape of aortic valve surgery, transcatheter aortic valve replacement (TAVR) has generated significant uncertainty around the future of surgical aortic valve replacement (SAVR) [4]. Over 7,000 TAVRs have been performed in the U.S. and captured by the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry from 2011 to 2012. With the Edwards SAPIEN valve demonstrating efficacy in several patient populations [5-7], the Medtronic CoreValve recently approved, and multiple other industry studies beginning [8, 9], TAVR use in the U.S. is set to expand dramatically over the next several years.
With initiation of a TAVR program, centers may see changes in their SAVR population due to high-risk patients undergoing TAVR rather than SAVR. Alternatively, increased referral of patients for TAVR who were not previously considered surgical candidates by outside providers may lead to a larger pool of high-risk patients considered appropriate for surgery by regional aortic valve referral centers. Studies from Europe, where TAVR has a longer history, have already demonstrated the potential changes to the SAVR population; however, these studies have been inconsistent and may not apply to U.S. centers [4, 10-12].
To examine the potential changes to SAVR that can be expected with the introduction of a TAVR program, we examined our SAVR population prior to the start of our TAVR program and after TAVR program initiation and evaluated differences in patient characteristics, risk profiles, and surgical outcomes.
Patients and Methods
Institutional data
Using a prospective institutional dataset, all cases of surgical aortic valve replacement were identified from January 2006 through June 2013. Patient characteristics, procedural details, and operative outcomes were extracted from the Duke Valve Surgery Database, a clinical registry of valve surgery patients at Duke University Medical Center (Durham, NC) with data collection patterned after the STS-Adult Cardiac Surgery Database [13]. Long-term mortality status was obtained through chart review, social security death index, and DEDUCE guided query [14]. Although not the focus of this analysis, institutional TAVR data was used to provide context on TAVR patients treated at our institution. Patient selection for TAVR over SAVR relied upon interdisciplinary Heart Team evaluation who collectively determined patient risk for aortic valve surgery in accordance with prior clinical trials [15, 16]. Specifically, decisions were based on assessments beyond those captured in traditional surgical risk assessment tools such as the STS Predicted Risk of Mortality (STS-PROM) [16]. Two senior cardiac surgeons and one interventional cardiologist had to agree that the patient met risk criteria for TAVR, which included comorbidities, anatomic factors unfavorable for surgical AVR such as aortic calcification, chest wall deformity, and hostile mediastinum, frailty assessments, and disability assessments in addition to STS-PROM [16]. Due to concerns that the TAVR program would only impact risk profiles and outcomes of patients with aortic stenosis, patients with aortic insufficiency were excluded in sensitivity analysis. Institutional review board waived the need for informed consent.
Study period
Patients were divided into 3 eras based on initiation of our institutional TAVR program in April, 2011: 1) Pre-TAVR period (January, 2006–June, 2009), 2) Transition period (July, 2009–March, 2011), and 3) TAVR period (April, 2011–June, 2013). The TAVR period was defined by the start of our institutional TAVR program in April, 2011, while the Transition period was developed based on concerns that patients were being referred differently due to the anticipation of TAVR availability. Due to concerns that the Transition era would be difficult to interpret with patients potentially being held back for TAVR, the primary analysis compared the Pre-TAVR and TAVR periods.
Predicted Risk of Mortality and Outcomes
Using the STS-PROM models for isolated AVR and AVR + CABG (coronary artery bypass grafting surgery) [17, 18], we calculated a predicted operative mortality for each patient based on patient and surgical characteristics. These models estimate the risk of operative mortality, defined as death during index hospitalization or within 30 days of index procedure. Mortality at 30 days was the primary outcome of the analysis. 1-year, 3-year, and time to all-cause mortality were also included as secondary outcomes.
Statistical analysis
Across the 3 time periods, patient, operative, and outcome variables were summarized using frequency and percentages for categorical variables and median and interquartile range (IQR) for continuous variables. Categorical variables were compared using Pearson’s Chi-squared and Fisher’s exact test, as appropriate. Continuous variables were compared using ANOVA. Baseline characteristics of SAVR and TAVR patients during the TAVR era were compared with similar methods. To examine trends over time, case volume, observed mortality, and mean predicted mortality were summarized based on year of surgery.
Observed to expected (O:E) mortality ratios were calculated using the observed mortality divided by average predicted mortality from the STS-PROM. Generalized linear models evaluated the differences in O:E ratios between Pre-TAVR and TAVR eras. Kaplan-Meier methods and the log-rank test were used when estimating and comparing long-term mortality. Patients in the TAVR and Transition eras were censored at 1 and 3 years respectively due to small numbers. A p-value <0.05 was considered to represent a statistically significant difference between groups. R (v. 3.02; R Foundation for Statistical Computing, Vienna, Austria, 2008) was used when performing statistical analyses.
Results
From January 2006 through June 2013, 1,380 patients underwent isolated AVR or AVR+CABG at our institution, with 36.6% (n=505) occurring during the Pre-TAVR period, 23.9% (n=330) occurring during the Transition period, and 39.5% (n=545) performed during the TAVR period. Compared to patients in the Pre-TAVR period, patients undergoing SAVR during the TAVR period were older (median age 74 vs. 69 years; p<0.001) and had higher rates of previous sternotomy (21.1% vs. 14.7%; p=0.005) and preoperative atrial fibrillation (25.9% vs. 13.7%; p<0.001); however, they were significantly less likely to have a smoking history, peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), New York Heart Association (NYHA) Class IV heart failure, unstable angina, or hypertension (Table 1).
Table 1.
Characteristics of SAVR patients by study period
| Study Period |
|||||
|---|---|---|---|---|---|
| Overall | Pre-TAVR | Transition | TAVR | P-value | |
| N | 1,380 | 505 (36.6%) | 330 (23.9%) | 545 (39.5%) | |
| Demographics | |||||
| Age | 71 (62, 79) | 69 (60, 77) | 71 (60, 78) | 74 (65, 82) | < 0.001 |
| Female | 512 (37.1%) | 185 (36.6%) | 118 (35.8%) | 209 (38.3%) | 0.717 |
| Race/Ethnicity | 0.362 | ||||
| White | 1,200 (87%) | 431 (85.3%) | 286 (86.7%) | 483 (88.6%) | |
| Black | 149 (10.8%) | 58 (11.5%) | 37 (11.2%) | 54 (9.9%) | |
| Other | 31 (2.2%) | 16 (3.2%) | 7 (2.1%) | 8 (1.5%) | |
| BSA –m2 | 2 (1.8, 2.2) | 2 (1.8, 2.2) | 2 (1.8, 2.2) | 2 (1.8, 2.2) | 0.255 |
| Comorbidities | |||||
| EF –% | 50 (50, 55) | 55 (45, 55) | 50 (50, 55) | 50 (50, 50) | 0.103 |
| Creatinine –mg/dL | 1 (0.8, 1.2) | 1 (0.8, 1.2) | 1 (0.8, 1.2) | 1 (0.8, 1.2) | 0.529 |
| Endocarditis (< 6 months) | 68 (4.9%) | 29 (5.7%) | 19 (5.8%) | 20 (3.7%) | 0.219 |
| History of smoking | 332 (24.1%) | 174 (34.5%) | 65 (19.7%) | 93 (17.2%) | < 0.001 |
| Dialysis | 37 (2.7%) | 17 (3.4%) | 11 (3.3%) | 9 (1.7%) | 0.160 |
| CVD | 148 (10.7%) | 47 (9.3%) | 38 (11.5%) | 63 (11.6%) | 0.433 |
| PVD | 117 (8.5%) | 54 (10.7%) | 30 (9.1%) | 33 (6.1%) | 0.024 |
| COPD -mild/moderate | 91 (6.6%) | 52 (10.3%) | 9 (2.7%) | 30 (5.5%) | < 0.001 |
| COPD –severe | 35 (2.5%) | 19 (3.8%) | 9 (2.7%) | 7 (1.3%) | 0.037 |
| Diabetes -non-insulin dep. | 372 (27%) | 126 (25%) | 104 (31.5%) | 142 (26.1%) | 0.093 |
| Diabetes -insulin dep. | 57 (4.1%) | 22 (4.4%) | 12 (3.6%) | 23 (4.2%) | 0.870 |
| Cardiogenic shock | 22 (1.6%) | 6 (1.2%) | 5 (1.5%) | 11 (2%) | 0.581 |
| Unstable angina | 171 (12.4%) | 92 (18.2%) | 42 (12.7%) | 37 (6.8%) | < 0.001 |
| Left main disease | 58 (4.2%) | 20 (4%) | 7 (2.1%) | 31 (5.7%) | 0.037 |
| Hypertension | 1,155 (83.7%) | 436 (86.3%) | 280 (84.8%) | 439 (80.6%) | 0.032 |
| Pre-op atrial fibrillation | 263 (19.1%) | 69 (13.7%) | 53 (16.1%) | 141 (25.9%) | < 0.001 |
| Pre-op IABP | 5 (0.4%) | 3 (0.6%) | 1 (0.3%) | 1 (0.2%) | 0.644 |
| AS | 1,212 (88%) | 427 (84.6%) | 292 (88.5%) | 493 (90.8%) | 0.008 |
| AR (≥ moderate) | 229 (16.6%) | 83 (16.4%) | 47 (14.2%) | 99 (18.2%) | 0.317 |
| MS | 51 (3.7%) | 24 (4.8%) | 7 (2.1%) | 20 (3.7%) | 0.143 |
| MR (≥ moderate) | 38 (2.8%) | 3 (0.6%) | 4 (1.2%) | 31 (5.7%) | < 0.001 |
| TR (≥ moderate) | 19 (1.4%) | 0 (0%) | 3 (0.9%) | 16 (2.9%) | < 0.001 |
| Previous MI (<21 days) | 156 (11.3%) | 58 (11.5%) | 38 (11.5%) | 60 (11%) | 0.962 |
|
Number of diseased
vessels |
0.242 | ||||
| 0 | 756 (54.8%) | 281 (55.6%) | 193 (58.5%) | 282 (51.8%) | |
| 1 | 188 (13.6%) | 63 (12.5%) | 41 (12.4%) | 84 (15.4%) | |
| 2 | 184 (13.3%) | 70 (13.9%) | 33 (10%) | 81 (14.9%) | |
| 3 | 251 (18.2%) | 91 (18%) | 63 (19.1%) | 97 (17.8%) | |
| CHF (NYHA class I-III) | 1,177 (85.3%) | 401 (79.4%) | 273 (82.7%) | 503 (92.3%) | < 0.001 |
| CHF (NYHA Class IV) | 197 (14.3%) | 102 (20.2%) | 55 (16.7%) | 40 (7.3%) | < 0.001 |
| Redo sternotomy | 235 (17%) | 74 (14.7%) | 46 (13.9%) | 115 (21.1%) | 0.005 |
Categorical data represented as frequency (%). Continuous data represented as median (IQR –interquartile range). Abbreviations as follows: SAVR –surgical aortic valve replacement, TAVR –transcatheter aortic valve replacement, BSA –body surface area, EF –ejection fraction, CVD –cerebrovascular disease, PVD –peripheral vascular disease, COPD –chronic obstructive pulmonary disease, IABP –intra-aortic balloon pump, AS –aortic stenosis, AR –aortic regurgitation, MS –mitral stenosis, MR –mitral regurgitation, TR –tricuspid regurgitation, MI –myocardial infarction, CHF –congestive heart failure, NYHA –New York Heart Association. AS and MS were defined as any degree of stenosis.
When SAVR (n=545) and TAVR (n=141) patients from the TAVR era were compared, the TAVR patients were older (82 vs. 74 years; p<0.001) and had significantly more comorbidities, including history of smoking (28.4% vs. 17.2%; p=0.004), COPD (34.0 % vs. 6.8%; p<0.001), cerebrovascular disease (19.9% vs. 11.6%; p=0.014), and peripheral vascular disease (24.8% vs. 6.1%; p<0.001; Table 2). STS-PROM was also significantly higher in TAVR patients (9.3% vs. 3.8%; p<0.001). Reasons for not undergoing surgical AVR for patients with an STS-PROM<12% included anatomic factors unfavorable for surgical AVR, frailty, and disability assessments [16], among others (Table 2). 30-day mortality was similar for TAVR and SAVR patients (2.8% vs. 1.5%; p=0.281) during the TAVR era.
Table 2.
Baseline characteristics of SAVR and TAVR patients during the TAVR era
| Variable | SAVR | TAVR | P-value |
|---|---|---|---|
| N | 545 (79.4%) | 141 (20.6%) | |
| Demographics | |||
| Age | 74 (64, 82) | 82 (77, 86) | < 0.001 |
| Female | 209 (38.3%) | 62 (44.0%) | 0.262 |
| Race/Ethnicity | 0.248 | ||
| White | 483 (88.6%) | 132 (93.6%) | |
| Black | 54 (9.9%) | 8 (5.7%) | |
| Other | 8 (1.5%) | 1 (0.7%) | |
| BSA | 2 (1.8, 2.1) | 1.9 (1.7, 2.1) | 0.163 |
| Comorbidities | |||
| EF | 50 (50, 50) | 55 (46.2, 55) | 0.451 |
| Creatinine | 1 (0.8, 1.2) | 1.2 (1, 1.4) | 0.4 |
| History of Smoking | 93 (17.2%) | 40 (28.4%) | 0.004 |
| CVD | 63 (11.6%) | 28 (19.9%) | 0.014 |
| PVD | 33 (6.1%) | 35 (24.8%) | < 0.001 |
| COPD | 37 (6.8%) | 48 (34%) | < 0.001 |
| Diabetes | 165 (30.3%) | 60 (42.6%) | 0.008 |
| Hypertension | 439 (80.6%) | 122 (86.5%) | 0.130 |
| AS | 493 (90.8%) | 126 (98.4%) | 0.002 |
| AR (≥ moderate) | 99 (18.2%) | 15 (11.0%) | 0.062 |
| MR (≥ moderate) | 31 (5.7%) | 14 (10.3%) | 0.082 |
| CHF (NYHA class I-III) | 503 (92.3%) | 120 (85.1%) | 0.013 |
| CHF (NYHA class IV) | 40 (7.3%) | 16 (11.8%) | 0.132 |
| Previous sternotomy | 115 (21.1%) | 68 (48.9%) | < 0.001 |
| STS-PROM, mean (SD) | 3.8% (2.8%) | 9.3% (5.3%) | < 0.001 |
| STS-PROM <12% | 535 (98.2%) | 87 (79.1%) | <0.001 |
| Reason for not getting SAVR among those with STS PROM<12% | |||
| Frailty | NA | 45 (51.7%) | |
| Severe pulmonary disease | NA | 10 (11.5%) | |
| Cirrhosis | NA | 2 (2.3%) | |
| Redo TAVR | NA | 3 (3.4%) | |
| Porcelain aorta | NA | 18 (20.7%) | |
| Hostile mediastinum | NA | 9 (10.3%) | |
| Outcomes | |||
| 30-day mortality | 8 (1.5%) | 4 (2.8%) | 0.281 |
Categorical data represented as frequency (%). Continuous data represented as median (IQR –interquartile range). Abbreviations as follows: SAVR –surgical aortic valve replacement, TAVR –transcatheter aortic valve replacement, BSA –body surface area, EF –ejection fraction, CVD –cerebrovascular disease, PVD –peripheral vascular disease, COPD –chronic obstructive pulmonary disease, AS –aortic stenosis, AR –aortic regurgitation, MS –mitral stenosis, MR –mitral regurgitation, TR –tricuspid regurgitation, MI –myocardial infarction, CHF –congestive heart failure, NYHA –New York Heart Association. AS was defined as any degree of stenosis.
The annual volume of SAVR cases performed rose dramatically over the study period, increasing from 131 cases in 2006 to 256 cases (95.4% increase) in 2012 (Figure 1). Predicted risk of mortality in SAVR patients remained stable over the study period, with mean predicted risk of mortality only varying between 3.5-3.8% by STS-PROM; however, the risk profile decreased in patients undergoing TAVR during the 3 years evaluated, from 11.1% in 2011 to 7.0% in 2013. The annual crude observed SAVR mortality varied during the study period from 0.8% to 4.7%, but did not demonstrate a temporal trend.
Figure 1.
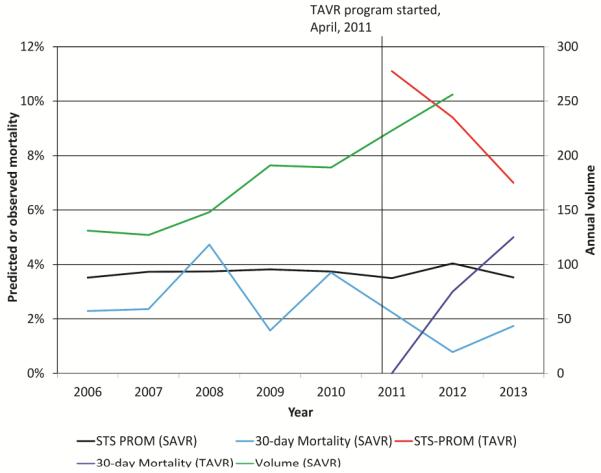
Temporal trends in SAVR and TAVR predicted and observed mortality and institutional SAVR case volume over the study period. Annual case volume is displayed on the right axis, while annual mean predicted and observed mortality rates are on the left axis.
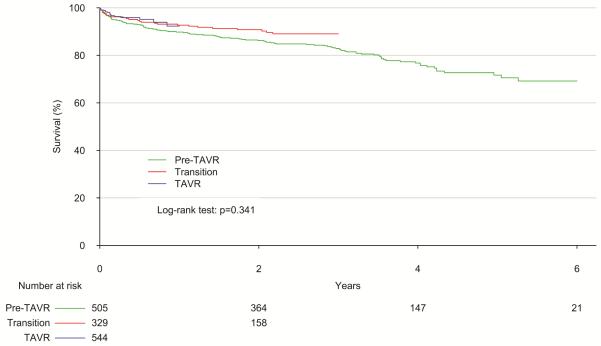
When comparing only the Pre-TAVR and TAVR periods, there was no significant difference in predicted mortality for SAVR patients by STS-PROM (3.8% vs. 3.8%; p=0.822). In addition, observed crude 30-day mortality did not differ significantly, (2.8% vs. 1.5%; p=0.230; Table 3). 1- and 3-year mortality rates were 9% and 15% in the overall cohort, and overall survival did not differ significantly by group (p=0.143; Figure 2). Observed to expected (O:E) ratios for 30-day mortality were below 1 for the STS-PROM model during each study period, although this difference was only significant in the TAVR era. There was a trend towards improved O:E mortality ratio when comparing the TAVR era (O/E: 0.39; 95% CI: 0.17-0.76) to the Pre-TAVR era (O/E: 0.74; 95% CI: 0.41-1.23); however, this improvement was not statistically significant (p=0.140). Sensitivity analysis excluding patients with aortic insufficiency demonstrated similar results.
Table 3.
Predicted and observed outcomes for SAVR by study period
| Study Period |
|||||
|---|---|---|---|---|---|
| Overall | Pre-TAVR | Transition | TAVR | P-value | |
| N | 1,380 | 505 (36.6%) | 330 (23.9%) | 545 (39.5%) | |
| Predicted Mortality | |||||
| STS-PROM (SD) | 3.7% (3.2%) | 3.8% (3.4%) | 3.6% (3.4%) | 3.8% (2.8%) | 0.822 |
| Observed mortality | |||||
| 30-day | 32 (2.3%) | 14 (2.8%) | 10 (3.0%) | 8 (1.5%) | 0.230 |
| 1-year | 89 (9%) | 50 (10%) | 21 (7.0%) | 18 (8%) | 0.341 |
| 3-year | 36 (15%) | 29 (17%) | 7 (11%) | NA | |
| Observed to expected 30-day mortality (95% confidence intervals) | |||||
| O/E | 0.62 (0.43-0.87) | 0.74 (0.41-1.23) | 0.85 (0.41-1.54) | 0.39 (0.17-0.76) | 0.140 |
P-values based on comparison of Pre-TAVR and TAVR eras only. The Transition period is displayed only for reference. For 1- and 3-year mortality, Kaplan-Meier methods were used to compare overall survival, and p-values were based on log-rank test.
Figure 2.
Overall survival after SAVR stratified by study era. Patients in TAVR and Transition eras were censored at 1 and 3 years due to low numbers. P-value by log-rank test represents comparison of Pre-TAVR and TAVR populations.
Comment
The introduction of TAVR programs in the U.S. holds the potential to dramatically change the landscape of SAVR, and it is unclear how patient populations will shift and institutional outcomes may change with TAVR initiation. Examining institutional experience beginning 5 years prior to the start of our TAVR program and continuing 2 years beyond initiation, this study found an increased SAVR case volume as the TAVR program was initiated. It also demonstrated stable SAVR STS-PROM, indicating that the overall risk profile of SAVR patients did not change dramatically with the initiation of TAVR. However, it should be noted that patients with certain comorbid conditions such as COPD, NYHA class IV heart failure, and peripheral vascular disease were significantly less likely to undergo SAVR in the TAVR era. Siphoning off these higher risk patients may have been offset by the significantly higher median age and greater frequency of redo-sternotomy among SAVR patients in the TAVR era, thereby maintaining a stable overall risk profile of the cohort. This change in patient demographics over time would suggest that some shifting of risk from SAVR to TAVR was potentially occurring, however, and further highlights the imperfections of the available preoperative risk models, as well as the continued necessity of experienced cardiac surgeon involvement in the risk assessment process.
With U.S. Food and Drug Administration approval of TAVR devices for use, and the subsequent decreased scrutiny around patient selection compared to that present during clinical trials leading to approval, concerns have been raised over the possibility of “risk creep” [4] and the extension of use into moderate-risk populations where TAVR has not been approved and may potentially be inappropriate. Evidence from Europe, where TAVR has a longer history of approved use, has demonstrated the potential of “risk creep.” The German TAVR registry indicated that 13% of TAVR patients were undergoing the procedure due to patient preference, despite a predicted mortality of less than 20% [12]. Similarly, research from the Netherlands showed that two-thirds of TAVR use had off-label criteria [11]. On the contrary, a single institution study from the United Kingdom [10] found no difference in the risk profiles of patients being referred for SAVR following the initiation of a TAVR program, showing an increase in EuroSCORE from 7.4% to 7.9% predicted risk of mortality. Similar to the current report, the UK study found a small, albeit non-significant, decrease in the observed mortality rate and a substantial increase in SAVR volume after starting a TAVR program.
Although the volume of SAVR in the U.S. has demonstrated dramatic growth over the last decade [3], the near doubling of our institutional volume over a 5-year period is likely in part due to the “halo effect” [19] of increased patient referral in response TAVR program initiation. Many of these patients may have sought out TAVR as a possible therapy despite their appropriateness for surgery, while others may not have been considered surgical candidates and were only referred because TAVR was thought to be an acceptable risk. Previous studies have demonstrated the overestimation of surgical risk and lack of referral of patients who would actually be considered appropriate surgical candidates [20, 21]; therefore, the lure of TAVR may bring surgical candidates to the attention of cardiac surgeons who would otherwise never see them. Single center studies have demonstrated this increased referral of AS for surgery [22]. A recently published national study of the STS/ACC TVT registry demonstrated an overall increase in SAVR cases in the U.S. following introduction of TAVR, with a 19% increase in SAVR volume at TAVR centers compared to only a 9% increase in centers without TAVR [19], which further supports these hypotheses.
Contrary to our expectation that the risk-profile of the SAVR patients would decrease with the arrival of TAVR, we found stable STS-PROM from the pre-TAVR to TAVR eras. However, certain patient risk factors did change significantly over this transition, with decreasing rates of smoking, COPD, NYHA class IV heart failure, PVD, and other comorbidities as mentioned above. This decrease in the comorbidity profile appears to have been offset by the increasing age and greater incidence of prior cardiac surgery among the SAVR cohort in the TAVR era. The increase in these patient characteristics is not surprising, as advanced age or prior cardiac surgery may have led providers to withhold referral prior to TAVR availability due to perceived surgical risk, and these patients only came to surgical attention because of the possibility of a less invasive procedure. This change in patient comorbidity profiles may reflect a risk shift that was simply not captured by the STS-PROM, which was developed using data from 2004-2006. Although not designed to examine this question, the aforementioned study using the STS/ACC TVT registry indicated that decreased risk profiles may already be seen in the SAVR population. Our center may have been insulated from these changes due to the draw of the TAVR program on high-risk patients, loss of some low-risk patients with STS-PROM “blind spots” to TAVR, or increased referral of high-risk patients to a high volume, tertiary referral center.
Although not statistically significant, the observed trend towards improved crude and adjusted SAVR outcomes over time in the current study may reflect this risk shift. Similar to the improved adjusted outcomes for SAVR seen in the STS/ACC TVT registry [19], our improved outcomes may be the result of the use of TAVR in the traditional high-risk cohort as well as those patients with risk features not captured by the STS-PROM. It may also simply reflect the continued improvements in surgical care over time and the lack of model calibration for these improvements.
Of note, the risk profile of our TAVR population appeared to decrease dramatically over time, going from 11.1% to 7.0% over a 3-year period (Figure 1). While this trend is concerning and may represent so-called “risk creep” [4], it may also simply indicate the increased use of this emerging technology in a patient population with risk factors not captured by the STS-PROM. In a review of reasons for selecting TAVR over SAVR in the lower risk TAVR population (STS-PROM <12%) (Table 2), the vast majority of patients had indications that are known ”blind spots” of the current STS risk model, including anatomic factors unfavorable for surgical AVR, frailty, and disability assessments. Better understanding of reasons for avoiding SAVR and comparing the outcomes of TAVR in these lower STS-PROM subgroups will be critical to improving our understanding of appropriate TAVR use.
While the current results provide important insights into the potential early changes that can be expected with the initiation of a TAVR program, ongoing research will be needed to monitor TAVR use and ensure that patients continue to be treated in accordance with evidence-based criteria utilizing a multidisciplinary “Heart Team” approach [23]. One mechanism providing this ongoing monitoring is the increased scrutiny placed on TAVR by Medicare through the STS/ACC TVT registry [24], and the corresponding robust research infrastructure to assess trends in outcomes across the U.S. [25]. As a result of this scrutiny, the post-approval U.S. TAVR experience may not match that of other devices or other countries unless future research continues to demonstrate similar short- and longer term outcomes to SAVR. However, with randomized data that only extends to 2-years [5], and good long-term results with SAVR again demonstrated in the current study with 5-year survival >70% (versus 23.7% overall 1-year mortality among patients undergoing TAVR in U.S. clinical practice) [26], caution must be applied to extending the use of TAVR beyond its approved patient population at present.
The current study has clear limitations. As a retrospective analysis, we cannot establish causality when considering case volume or patient profile changes. With treatment selection as the focus of the analysis, we examined an extensive number of potential confounders; however, the risk of unmeasured confounders and bias persists. As a single tertiary referral center with an early TAVR initiation in the context of a clinical trial [15, 16], our experience may not be generalizable to other institutions. While the STS-PROM is a robust and well-validated measure of predicted mortality, the original models were used and no calibration was made for general improvements in care and outcomes over time.
In conclusion, the initiation of an institutional TAVR program was associated with a substantial increase in SAVR volume, while predicted and observed SAVR mortality remained similar prior to and after TAVR initiation. These data would suggest that significant changes in SAVR patient risk profiles should not be anticipated with the initiation of a TAVR program, and this institutional experience may provide some guidance about expectations for other programs making similar changes. However, further research will be needed to re-evaluate the impact of TAVR on the SAVR population to ensure continued appropriate use as TAVR becomes a more accepted and widely used therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Society of Thoracic Surgeons [accessed 1/4/2015];STS Adult Cardiac Surgery Database: executive summary: 10 years 2011. Available from: http://www.sts.org/sites/default/files/documents/2011%20-%20Adult%20Cardiac%20Surgery%201stHarvestExecutiveSummary.pdf.
- 4.Desai CS, Bonow RO. Transcatheter valve replacement for aortic stenosis: balancing benefits, risks, and expectations. JAMA. 2012;308(6):573–4. doi: 10.1001/jama.2012.9427. [DOI] [PubMed] [Google Scholar]
- 5.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–95. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 6.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 8.Urena M, Doyle D, Rodes-Cabau J, et al. Initial experience of transcatheter aortic valve replacement with the St Jude Medical Portico valve inserted through the transapical approach. J Thorac Cardiovasc Surg. 2013;146(4):e24–7. doi: 10.1016/j.jtcvs.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Willson AB, Rodes-Cabau J, Wood DA, et al. Transcatheter aortic valve replacement with the St. Jude Medical Portico valve: first-in-human experience. J Am Coll Cardiol. 2012;60(7):581–6. doi: 10.1016/j.jacc.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Grant SW, Devbhandari MP, Grayson AD, et al. What is the impact of providing a transcatheter aortic valve implantation service on conventional aortic valve surgical activity: patient risk factors and outcomes in the first 2 years. Heart. 2010;96(20):1633–7. doi: 10.1136/hrt.2010.203661. [DOI] [PubMed] [Google Scholar]
- 11.Piazza N, Otten A, Schultz C, et al. Adherence to patient selection criteria in patients undergoing transcatheter aortic valve implantation with the 18F CoreValve ReValving System. Heart. 2010;96(1):19–26. doi: 10.1136/hrt.2009.172809. [DOI] [PubMed] [Google Scholar]
- 12.Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32(2):198–204. doi: 10.1093/eurheartj/ehq339. [DOI] [PubMed] [Google Scholar]
- 13.Society of Thoracic Surgeons [accessed 1/4/2015];Society of Thoracic Surgeons, Data Collection. Available from: http://www.sts.org/sts-national-database/database-managers/adult-cardiac-surgery-database/data-collection.
- 14.Horvath MM, Winfield S, Evans S, et al. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44(2):266–76. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–8. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 16.Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter Aortic Valve Replacement Using a Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis at Extreme Risk for Surgery. J Am Coll Cardiol. 2014;63(19):1972–81. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Shahian DM, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3--valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S43–62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 19.Brennan JM, Holmes DR, Sherwood MW, et al. The association of transcatheter aortic valve replacement availability and hospital aortic valve replacement volume and mortality in the United States. Ann Thorac Surg. 2014;98(6):2016–22. doi: 10.1016/j.athoracsur.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2(6):533–9. doi: 10.1161/CIRCOUTCOMES.109.848259. [DOI] [PubMed] [Google Scholar]
- 21.Charlson E, Legedza AT, Hamel MB. Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis. 2006;15(3):312–21. [PubMed] [Google Scholar]
- 22.Malaisrie SC, Tuday E, Lapin B, et al. Transcatheter aortic valve implantation decreases the rate of unoperated aortic stenosis. Eur J Cardiothorac Surg. 2011;40(1):43–8. doi: 10.1016/j.ejcts.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Tommaso CL, Bolman RM, 3rd, Feldman T, et al. Multisociety (AATS, ACCF, SCAI, and STS) expert consensus statement: operator and institutional requirements for transcatheter valve repair and replacement, part 1: transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(22):2028–42. doi: 10.1016/j.jacc.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JD, Edwards FH, Marinac-Dabic D, et al. The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62(11):1026–34. doi: 10.1016/j.jacc.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 25.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310(19):2069–77. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 26.Holmes DR, Jr., Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019–28. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]