Abstract
In a pure volume overloaded (VO) heart, interstitial collagen loss is degraded by matrix metalloproteinases (MMPs) that leads to left ventricular (LV) dilatation and heart failure. Cardiac fibroblasts are the primary source of extracellular matrix proteins that connect cardiomyocytes. The goal of this study was to determine how VO affects intracellular procollagen in cardiac fibroblasts. Using the aortocaval fistula (ACF) model in Sprague-Dawley rats, we demonstrate that cardiac fibroblasts isolated from 4 and 12 wk ACF animals have decreased intracellular procollagen I compared to the fibroblasts from age-matched shams. The reduction of procollagen I is associated with increased autophagy as demonstrated by increased autophagic vacuoles and LC3-II expression. To test the relationship between autophagy and procollagen degradation, we treated adult cardiac fibroblasts with either an autophagy inducer, rapamycin, or an inhibitor, wortmannin, and found that procollagen I protein levels were decreased in fibroblasts treated with rapamycin and elevated in wortmannin-treated cells. In addition, we demonstrated that VO induces oxidative stresses in cardiac fibroblasts from 4- and 12 wk ACF rats. Treatment of cultured cardiac fibroblasts with an oxidative stress-inducing agent (DMNQ) induces autophagy and intracellular procollagen I and fibronectin degradation, which is reversed by wortmannin but not by the global MMP inhibitor (PD166793). Mechanical stretch of cardiac fibroblasts also induces oxidative stress and autophagic degradation of procollagen I and fibronectin. Our results suggest that in addition to the well-known effects of MMPs on extracellular collagen degradation in VO, there is a concurrent degradation of intracellular procollagen and fibronectin mediated by oxidative stress-induced autophagy in cardiac fibroblasts.
Keywords: Volume overload, cardiac fibroblast; oxidative stress; autophagy; intracellular procollagen; matrix metalloproteinase
1. Introduction
A pure cardiac volume overload (VO) caused by aortocaval fistula (ACF) or primary mitral regurgitation (MR) is initially marked by inflammation and oxidative stress that eventually progresses to left ventricular (LV) dilatation, LV wall thinning, cardiomyocyte elongation and thinning, and heart failure [1-7]. This adverse eccentric LV remodeling is associated with a loss of interstitial collagen matrix that connects cardiomyocytes to each other and leads to spherical LV dilatation and heart failure. A large body of work from multiple laboratories has documented an upregulation of matrix metalloproteinases (MMPs) in mediating this extracellular collagen breakdown in the LV remodeling of a pure VO [2, 7-10]. MMP inhibitors have been shown to attenuate extracellular collagen loss and LV dilatation in the ACF rats [11]. Pretreatment with MMP inhibition decreases oxidative stress and improves LV diastolic and systolic dysfunction in vivo at the 4 week time point in the ACF mouse [12]. Taken together, these studies suggest that increased inflammation and oxidative stress in a pure VO leads to MMP activation, interstitial collagen loss, LV dilatation and heart failure.
The fibroblast is the most plentiful cell type in the heart and plays a critical role in the maintenance of extracellular matrix (ECM) homeostasis [13, 14]. Cardiac fibroblasts are the primary source of ECM proteins as well as proteins involved in ECM degradation that include MMPs and tissue inhibitors of MMPs (TIMPs) [14]. The ECM proteins include fibrillar collagen types I and fibronectin, as well as less abundant type III, IV, V and VI-collagen, laminin, elastin, and proteoglycans. The synthesis of ECM proteins can be regulated by various growth factors such as platelet-derived growth factor, basic fibroblast growth factor and TGF-β in development or disease conditions [14]. In vitro treatment of rat cardiac fibroblasts with interleukin-1β, TNF-α or oxidative stress decreases collagen synthesis and increases MMP activities [15, 16]. However, how collagen synthesis and degradation are balanced within fibroblasts is largely unknown in VO.
Autophagy is a major cellular pathway that degrades misfolded/aggregated proteins and damaged organelles. Autophagy involves the formation of double membrane vesicles called autophagosomes that enclose macromolecular aggregates and cellular organelles for degradation by the lysosomes [17]. It can be upregulated by both external and intracellular stress signals that include amino acid starvation, endoplasmic reticulum (ER) stress, hypoxia and oxidative stress. Besides MMPs that degrade proteins in the extracellular space, emerging evidence suggests that autophagy is involved in procollagen degradation within the fibroblasts. In mesangial cells from mouse kidney, autophagy promotes intracellular degradation of type I procollagen in response to TGF-β1 treatment [18]. In rat cardiac fibroblasts, β2-adrenergic stimulation triggers autophagy and procollagen degradation [19].
In current study, we investigate the role of cardiac fibroblasts in ECM degradation in response to pure VO. We report a marked increase of autophagy associated intracellular procollagen degradation in cardiac fibroblasts isolated from 4 and 12 wk ACF rats, for which we have identified as compensated and decompensated stages of ACF VO in the rat [7]. In vitro studies with isolated cardiac fibroblasts demonstrate that the autophagic degradation of procollagen I and fibronectin is increased when the fibroblast cells are treated with either oxidative stress or mechanical stretch. Thus in a pure VO, this process exacerbates the ECM homeostatic imbalance by decreasing ECM production in the face of interstitial ECM loss through MMP activation.
2. Materials and methods
2.1. Animal studies
Adult male Sprague-Dawley rats (200-250g) at 12 weeks of age were subjected to either sham or ACF surgery, as described previously in our laboratory [1-5, 7, 20]. Briefly, rats were anesthetized with isoflurane (2% at 2L/min oxygen) in a well-ventilated aseptic area 10 min prior to the surgery. After the surgery, the skin was closed using metallic wound clips. Rats were administered with buprenorphine (0.05 mg/Kg; IP) preoperatively and after surgery. Every effort was made to minimize any discomfort to the animals used in these studies and the animals were euthanized following anesthesia with isofluorane and exsanguination after rapid removal of the heart. This method is consistent with the recommendations of the Panel of Euthanasia of the American Veterinary Medical Association. All procedures were approved and performed according to the guidelines of the Institutional Animal Care and Use Committees of the University of Alabama at Birmingham (Animal Resource Program, Protocol 140909251) and followed the National Institute of Health's ‘Guide for the Care and Use of Laboratory Animals’.
2.2. Echocardiographic measurement
Echocardiography was performed prior to sacrifice using the Visualsonics imaging system (Vivo 2100, Toronto, Canada) with the rats under isoflurane anesthesia as previously described [2, 5, 7]. Echocardiography dimensions (wall thickness and chamber diameter) were obtained using software included in the Visualsonics system.
2.3. Isolation of cardiac fibroblasts
Adult rat cardiac fibroblasts were isolated by recirculating perfusion buffer supplemented with 1 mg/mL type II collagenase (Invitrogen, CA) as previously described [1]. Briefly, the heart was perfused with perfusion buffer (120 mmol/L NaCl, 15 mmol/L KCl, 0.5 mmol/L KH2PO4, 5 mmol/L NaHCO3, 10 mmol/L HEPES, and 5 mmol/L glucose, pH 7.0) for 5 min and digested with perfusion buffer containing 1 mg/mL collagenase for 30 min at 37°C. The right ventricle and atria were removed before the perfused-heart was minced. The cell suspension was then mixed with stop buffer (perfusion buffer containing 10 mg/ml bovine serum albumin) to prevent further digestion. The cell suspension was added to a mesh cell collector and the flow-through was centrifuged at 80 g for 3 min to remove most of the cardiomyocytes. The supernatant containing mainly cardiac fibroblasts was centrifuged at 400 g for 8 min and washed by PBS twice then resuspended in DMEM supplemented with antibiotics (penicillin/streptomycin, 1%), L-glutamine and 10% FBS. Cells were subjected to differential plating on uncoated cell culture dishes (10 cm diameter) for 120 min. Non-adherent cells (mostly cardiomyocytes, endothelial and smooth muscle cells) were removed. We previously demonstrated that the purity of the preparation is greater than 95% with very little myofibroblast differentiation [2]. Cultured cells by this protocol were vimentin-positive after immunostaining with anti-vimentin antibody (Millipore #AB5733; 1:500) and negative for smooth muscle alpha-actin or fibronectin (1:100 dilutions, Sigma-Aldrich, MO). Adult rat cardiac fibroblasts were used from passages 2 to 3 in all of the described studies.
2.4. Monitoring autophagy by immunoblotting and fluorescence microscopy
The increase in autophagy was monitored by immunoblotting with an LC3-specific antibody. Briefly, cardiac fibroblasts were lysed in RIPA buffer containing protease and phosphatase cocktail inhibitors (Thermo Fisher Scientific, IL). Lysates (10–40 μg) were separated on a 4–20% gradient Bis/Tris gel (Invitrogen, CA) by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes probed with primary antibodies overnight (4°C), and then with appropriate horse-radish-peroxidase-conjugated secondary antibodies (1:5000). Bands were visualized by enhanced chemiluminescence (Thermo Fisher Scientific, IL), scanned and analyzed by image J software (NIH, Bethesda, MD). A rabbit polyclonal antibody against LC3 was purchased from Sigma-Aldrich (Cat. # L8918) and used at 1:500 dilution. Immunoblotting of GAPDH (GeneTax, Cat. #GT239) was used as a loading control. The normal control fibroblasts were treated with the autophagy enhancer (rapamycin) or inhibitor (wortmannin) for 24 h with 0.5 μM and 50 nM concentrations, respectively. 2,3-Dimethoxy-1, 4-naphthoquinone (DMNQ), an inducer of intracellular superoxide anion formation, was purchased from Sigma-Aldrich (Cat. #5439) and used at 5 μM for 16 h to treat the cultured fibroblast cells. PD166793, a broad spectrum MMP inhibitor, was purchased from Tocris Bioscience and used at 100 μM for 16 h.
2.5. Monitoring of procollagen I and fibronectin protein expression in cardiac fibroblasts
The expression of procollagen in cardiac fibroblast cells was determined by immunoblotting as described above. A rabbit polyclonal antibody against collagen I was purchased from Abcam (Cat. #ab34710) and used at 1:500 dilution. A mouse monoclonal antibody against fibronectin was also purchased from Abcam (Cat. #ab6328) and used at 1:1000 dilution.
2.6. Extrinsic mechanical stretch and transmission electron microscopy of cardiac fibroblasts
Cardiac fibroblasts (50,000 cells/cm2) were cultured on BioFlex culture plates without any coating (Flexcell International Corp., Hillsborough, NC, USA) in DMEM medium containing 10% FBS, 2 mM glutamine, 10 U/mL penicillin, and 100 mg/mL streptomycin as previously described [2, 21]. The medium was changed 24 h before initiation of the experiment and cells were subjected to cyclic strain on the Flexcell Strain system (model FX-5000) at a level of distension sufficient to promote an increment of approximately 20% in surface area at the point of maximal distension on the culture surface. Cyclic stretch was performed for 24 h at 1 Hz without interruptions. After stretch, cardiac fibroblasts were fixed overnight in 2.5% glutaraldehyde in Sorensen's phosphate buffer (Electron Microscopy Sciences, PA, #15980). Transmission electron microscopy (TEM) was performed by EMLabs, Inc, Birmingham, AL. After post-fixation with 1% osmium tetroxide in 0.1 M cacodylate buffer, fibroblasts were dehydrated with a graded series of ethanol and embedded in Epon resin. Semi-thin (0.5 μm) and ultra-thin (90 nm) sections were cut, mounted on copper grids, and post-stained with uranyl acetate and lead citrate. Sections were viewed at 60 kv with a Philips 201 transmission electron microscope (FEI Co., OR) for qualitative changes in the ultrastructure. At least 3 samples of each condition were examined and representative images were shown in the figures.
2.7. Measurement of interstitial collagen (Collagen Volume Percent) in rat LV tissue
Interstitial collagen in LV tissue of 4 and 12 wk sham and ACF rat was analyzed as previously described [2]. All measurements were performed in a blinded manner. Results are presented as the mean ± SEM values computed from the average of individual measurements obtained from each heart.
2.8. Assessment of oxidative stress in cardiac fibroblasts isolated from sham and ACF rats
The production of reactive oxygen species (ROS) was monitored with 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein (CM-DCF, Life Technologies, Cat. #C6827), a ROS-sensitive fluorescent indicator, as previously described by our laboratory [1]. Briefly, cardiac fibroblasts isolated from sham and ACF rats were cultured for 36 h in a 4-chamber slides (BD Bioscience) and incubated with 4 μM CM-DCF dye solution for 20 min in a dark humidity chamber at RT. Cells were washed 3 times with 1X serum free medium at RT, chambers were removed, and slides were coverslipped with 1X serum free medium, CM-DCF images were captured using a Leica DM6000 Epifluorescence microscope at 488 nm excitation. Mean fluorescence intensity (a.u.) and background were measured using SimplePCI software. Data was expressed as net intensity.
2.9. Measurement of MMP-2 activity by gel zymography
The activity of matrix metalloproteinases (MMP) in isolated cardiac fibroblasts were measured as described previously [2]. Cell lysates (5 μg) were separated by running a 10% SDS-PAGE in a gelatin-containing gel followed by zymography according to the manufacturer's instructions (Novex, Invitrogen). The bands corresponding to the MMP-2 activity were scanned and quantified with Image J. Data were expressed as fold changes relative to the sham control.
2.10. Quantitation of mRNA levels
Total RNA was extracted from cardiac fibroblasts from 4 and 12 wk sham and ACF rats, and from isolated normal fibroblasts after 24 h of 20% cyclical stretch using RNeasy Mini Kit (catalog no. 74106; Qiagen) according to the manufacturer's protocol as previously described in our lab [22]. Total collagen I was evaluated using Assay-On-Demand primer mix (Collα1, collagen type I, alpha 1, assay ID: Rn01463848_m1). Ribosomal protein, large, P0 (Rplp0; assay ID: Rn00821065_g1) was studied in parallel as internal controls. TaqMan One-Step RT-PCR reactions were performed in 25 μl final volumes containing 5 μl (10× dilution of stock) of RNA sample, AmpErase UNG (2×, 10 μl), MultiScribe reverse transcriptase and RNase inhibitor (40×, 1 μl), primers and probe (20×, 1 μl), and RNase-free water (3 μl). Five 1-log serial dilution reactions were conducted in duplicate. The input amount of target mRNA was normalized to Rplp0 mRNA as an endogenous control. Results are plotted as fold changes relative to sham or normal control.
2.11. Statistical analysis
All data were presented as means ± SE. An unpaired Student's t-test was used to compare age-matched shams versus ACF groups and to compare control versus stretch groups. One-way ANOVA with Student-Newman-Keuls post hoc test was used in Figures 3 and 5 to determine the effects of rapamycin, wortmannin, DMNQ and PD166793 on cardiac fibroblasts. A P-value of less than 0.05 was used for statistical significance.
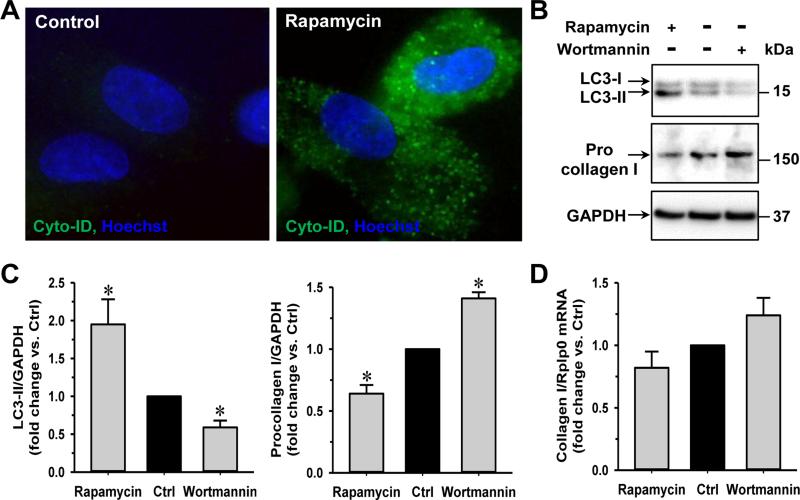
Fig. 3. Procollagen I is decreased by autophagy enhancer (rapamycin) and increased by autophagy inhibitor (wortmannin).
Cardiac fibroblasts isolated from adult rats are cultured in 6-well plates before 0.5 μM rapamycin or 50 nM wortmannin treatments for 24 h. (A) Rapamycin increases the formation of autophagic vacuoles as labeled by Cyto-ID (green). (B) Representative western blot images showing rapamycin treatment increases the LC3-II expression and decreases procollagen I protein in fibroblasts while wortmannin treatment has opposite effects. (C) Quantification of LC3-II, procollagen I protein and (D) collagen I mRNA after rapamycin or wortmannin treatments. n = 5, *P<0.05 vs. control (Ctrl).
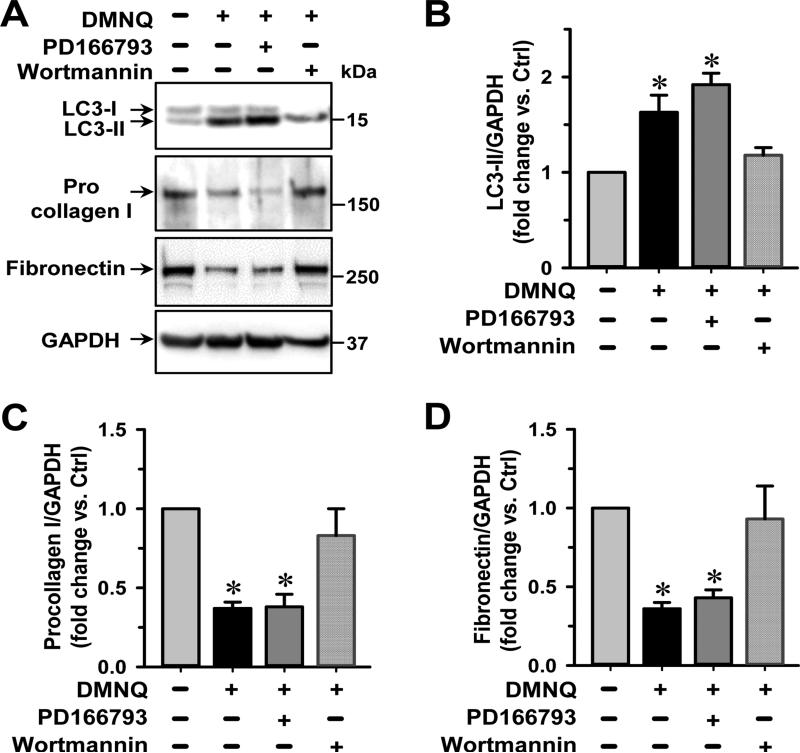
Fig 5. Autophagic degradation of procollagen is induced by DMNQ.
Cardiac fibroblasts are isolated from adult normal rat and cultured in 6-well tissue culture plates followed by treatments with DMNQ (5 μM) and an MMP inhibitor (PD166793, 100 μM) or an autophagy inhibitor (wortmannin, 50 nM) for 16 h. (A) Representative images of immunoblotting show an increased expression of LC3-II and decreased level of intracellular procollagen I and fibronectin by DMNQ that are reversed by autophagy inhibition not MMP inhibition. (B-D). Quantitative results of LC3-II, procollagen I and fibronectin protein expression, respectively. n = 5. *P<0.05 vs. untreated control (Ctrl).
3. RESULTS
3.1. LV remodeling in 4 and 12 wk ACF rats
Previously, we reported a differential regulation of genes related to inflammation and ECM homeostasis during different stages of ACF [7]. In our analysis, we found that the 4-5 wk ACF is the compensated stage and 12-15 wk ACF is the decompensated stage. In the current study, echocardiography was performed to determine the level of LV remodeling in 4- and 12-wk ACF rats and age-matched sham animals. Table 1 shows significant increases of LV end-diastolic dimension (LVEDD), LV end-systolic dimension (LVESD) and LV end-diastolic dimension/posterior wall thickness ratio (LVEDD/PW) in both 4 and 12 wk ACF rats compared to the sham controls. These results indicate an eccentric remodeling of the heart in chronic ACF rats as we have shown previously [2, 4, 7]. In the 12 wk ACF rats, there is a significant decrease in LV fractional shortening (LVFS) and velocity of circumferential shortening (VCFr), while the LVFS and VCFr in 4 wk ACF rats does not differ from age-matched shams, indicating decreased systolic function and decompensation in 12 weeks of ACF.
Table 1.
Body weight, heart rate and LV functional parameters at 4 and 12 wk rats.
| 4wk Sham (n=6) |
4wk ACF (n=6) |
12wk Sham (n=6) |
12wk ACF (n=6) |
|
|---|---|---|---|---|
| BODY WT (gm) | 343±6 | 359±5 | 472±22 | 485±26 |
| HR (beats/min) | 348±5 | 321±5* | 335±18 | 322±8 |
| LVEDD (mm) | 7.3±0.2 | 10.3±0.2* | 8.2±0.2 | 11.3±0.2* |
| LVESD (mm) | 4.7±0.2 | 6.6±0.2* | 5.1±0.1 | 7.7±0.2* |
| LVEDD/PW | 4.3±0.3 | 6.5±0.5* | 4.8±0.2 | 6.4±0.6* |
| LVFS (%) | 40±3 | 36±3 | 42±2 | 30±1* |
| VCFr | 7.4±0.4 | 6.7±0.3 | 7.4±0.3 | 4.8±0.1* |
Values are expressed as mean ± SEM. In each group, values in parentheses represent n.
P<0.05 vs. age-matched shams. ACF: aortocaval fistula; HR: heart rate; LVEDD: Left ventricular (LV) end-diastolic dimension; LVESD: LV end-systolic dimension; LVEDD/PW: LV end-diastolic dimension to posterior wall thickness ratio; LV FS: LV fractional shortening; VCFr: velocity of circumferential shortening.
3.2. Increased autophagy and procollagen degradation in cardiac fibroblasts of ACF rats
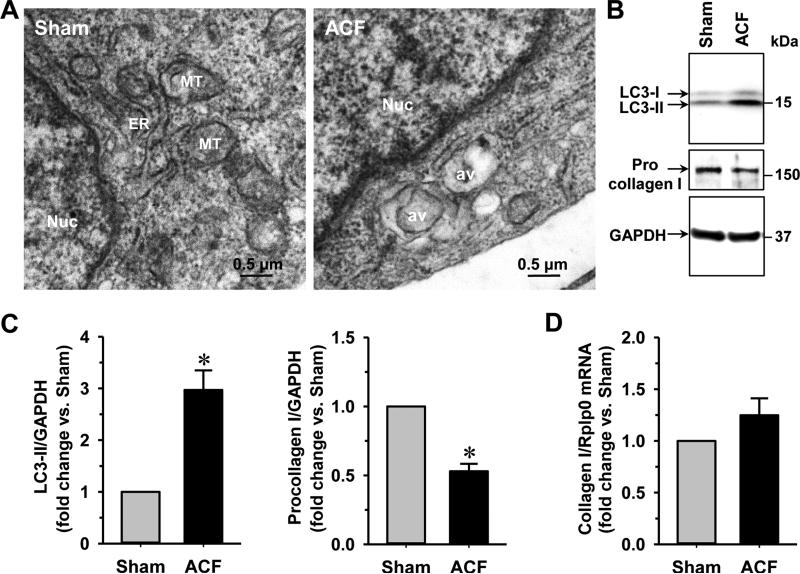
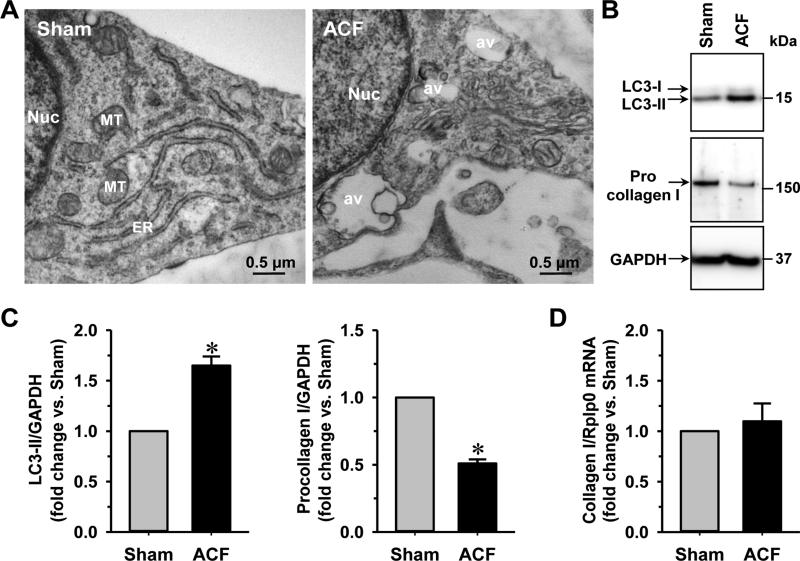
Cardiac fibroblasts have been shown to play a critical role in LV remodeling due to their function in the generation of extracellular matrix [19, 23, 24]. We first analyzed the ultrastructure of cardiac fibroblasts isolated from 4 wk ACF rats in comparison to the sham controls. The formation of autophagic vacuoles was evident in cardiac fibroblasts isolated from the ACF animals as shown by transmission electron microscopy (TEM) (Fig. 1A). Since the type-II microtubule-associated protein 1 light chain 3 (LC3-II) is widely used as a marker of autophagy induction, we measured the level of LC3-II production in cardiac fibroblasts from the sham and ACF rats by immunoblotting. As shown in Figs. 1B & 1C, the level of LC3-II increased ~3 fold in cardiac fibroblasts isolated from 4 wk ACF rats compared to the age-matched shams, indicating that ACF induces autophagic activity in cardiac fibroblasts. Protein level of procollagen I in cardiac fibroblasts isolated from ACF rats was decreased ~50% (Figs. 1B & 1C). The mRNA levels of collagen I, however, were the same in cardiac fibroblasts of the sham and ACF animals (Fig. 1D), suggesting that the change of procollagen I after 4 wk ACF was a posttranscriptional effect. At 12 weeks of ACF, the LC3-II levels were increased ~1.5 fold and procollagen I levels were reduced ~50% when compared to the age-matched shams (Fig. 2), indicating that the loss of procollagen production was a long-term effect. In addition, examination of isolated fibroblasts from 12 wk ACF hearts by TEM also demonstrated large autophagic vacuoles as the 4 wk ACF fibroblasts.
Fig. 1. Cardiac fibroblasts isolated from 4 wk ACF rats have an increased level of autophagy and decreased procollagen I.
(A) Cardiac fibroblasts are isolated from 4 wk sham and ACF rats. TEM analysis demonstrates an increase of autophagic vacuoles. (B) Cardiac fibroblast expression of procollagen I and an autophagic marker (LC3-II) in 4 wk sham and ACF rats are shown by immunoblotting with GAPDH as loading control. (C) Quantification of procollagen I and LC3-II in sham and ACF rats. (D) Fold changes of fibroblast collagen I mRNA level from 4 wk sham and ACF rats. n = 6. *P<0.05 vs. age-matched shams. Nuc: nucleus; av: autophagic vacuoles.
Fig. 2. Cardiac fibroblasts isolated from 12 wk ACF rats have an increased level of autophagy and decreased procollagen I.
(A) TEM of cardiac fibroblasts isolated from 12 wk sham and ACF rats demonstrates a marked increase of autophagic vacuoles. (B) Representative Western blot images of procollagen I and LC3. (C) Quantification of procollagen I and the LC3-II in 12 wk sham and ACF rats. (D) Fold changes of fibroblast collagen I mRNA level from sham and ACF rats. n = 6. *P<0.05 vs. age-matched shams. Nuc: nucleus; av: autophagic vacuoles.
3.3. Degradation of procollagen I is mediated through autophagy in cardiac fibroblasts
Because we saw a corresponding induction of autophagy and loss of procollagen I upon ACF, we tested whether autophagy induction promoted procollagen I degradation. We compared the effects of the autophagy inducer (rapamycin) with the autophagy inhibitor (wortmannin) on the procollagen I levels in cardiac fibroblasts isolated from normal adult rats and cultured with either 0.5 μM rapamycin or 50 nM wortmannin for 24 h. Rapamycin treatment induced an increase of autophagic vacuoles as indicated by increased labeling of Cyto-ID (Fig. 3A) as well as LC3-II production as shown by immunoblotting (Figs. 3B & 3C). Correspondingly, the level of procollagen I was reduced after rapamycin treatment. In contrast, inhibition of autophagy by wortmannin, a PI3-kinase inhibitor, increased the level of procollagen I. There were no significant changes in the collagen 1 mRNA transcripts after rapamycin or wortmannin treatment (Fig. 3D). These results suggest that autophagy is directly involved in the degradation of procollagen I.
3.4. Oxidative stress induces autophagic degradation of procollagen in cardiac fibroblasts
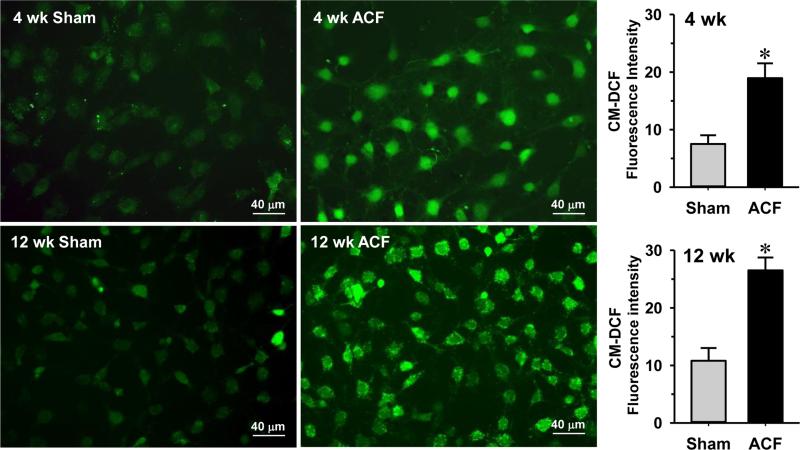
We previously reported that there is an increased oxidative stress in cardiomyocytes from 24 hr and 8 wk ACF rats [1, 3, 21]. Here we tested whether oxidative stress also occurred in cardiac fibroblasts isolated from ACF rats. Cardiac fibroblasts were cultured and loaded with CM-DCF, a fluorescent indicator for ROS. Fig. 4 shows that cardiac fibroblasts from 4 and 12 wk ACF rats have a ~2 fold increase in DCF fluorescence compared to the sham controls, indicating that the fibroblasts isolated from the ACF rats were undergoing oxidative stress.
Fig. 4. Oxidative stress is increased in isolated cardiac fibroblasts from 4 and 12 wk ACF rats.
Analysis of ROS by 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein (CM-DCF), a fluorescent marker of oxidative stress, in isolated fibroblasts from 4 and 12 wk ACF rat hearts indicates a significant increase in ROS in ACF rats. n = 80 cells/group. *P<0.05 vs. age-matched shams.
Next, we tested if the oxidative stress promoted the intracellular procollagen degradation by inducing autophagy. Cardiac fibroblasts isolated from normal adult rats were cultured with or without DMNQ, a cell-permeable redox cycling quinone that generates superoxide production. In Fig. 5, we show that DMNQ treatment increases LC3-II production ~2 fold, confirming that oxidative stress induces autophagy. Furthermore, the increased autophagy parallels a 50% decrease of procollagen I levels. Fibronectin levels were also decreased by DMNQ, indicating that oxidative stress induced autophagy affects the breakdown of ECM proteins in general. To further support the connection between the oxidative stress-induced autophagy and the degradation of ECM proteins, we demonstrated that DMNQ effects on procollagen and fibronectin levels were reversed by autophagy inhibition (wortmannin treatment), suggesting that autophagy was responsible for the loss of ECM proteins. In contrast, the broad-spectrum MMP inhibitor (PD166793) did not reverse the effects of DMNQ on procollagen and fibronectin degradation, suggesting MMP activation does not degrade intracellular procollagen and fibronectin before they are secreted to the extracellular environment.
3.5 Mechanical stretch induces autophagy-mediated procollagen/fibronectin loss and oxidative stress in cardiac fibroblasts
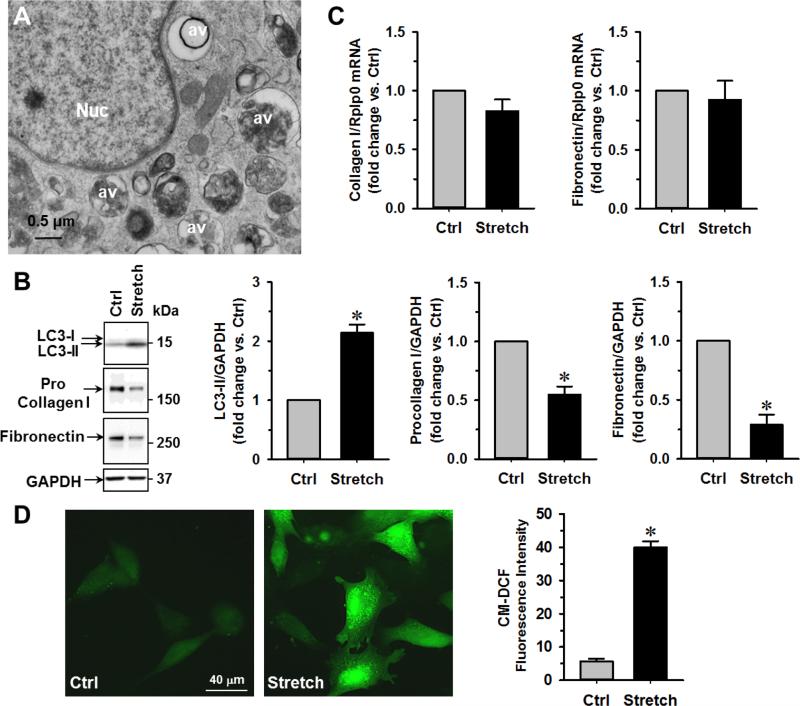
The results above suggest that chronic VO in vivo in ACF rats reduces intracellular collagen production through the activation of autophagy. Next, we used normal adult cultured cardiac fibroblasts undergoing cyclic mechanical stretch to simulate VO in vitro. TEM analyses of stretched fibroblasts demonstrated the presence of multiple perinuclear late-stage autophagic vacuoles defined by the presence of electron dense debris (Fig. 6A), along with a 2-fold increase in the LC3-II and significant loss of intracellular procollagen I and fibronectin protein (Fig. 6B) without significant changes on their mRNA levels (Fig. 6C). To test if these changes are due to the increased oxidative stress, we measured the production of reactive oxygen species (ROS) after cyclic stretch of fibroblasts for 24 h and found a 5-fold increase in CM-DCF fluorescence in stretched fibroblasts (Fig. 6D). These in vitro experiments of cyclic stretch suggest that like the conditions in VO, mechanical stretch-induced oxidative stress may be an important stimulus to autophagy-mediated ECM loss in isolated cardiac fibroblasts.
Fig. 6. Mechanical stretch results in increased autophagy and decreased procollagen I and fibronectin in isolated cardiac fibroblasts.
Normal rat cardiac fibroblasts are cultured in BioFlex culture plates and then subjected to 24 h, 20% cyclic stretch (1 Hz). (A) TEM demonstrates the formation of perinuclear autophagic vacuoles in isolated fibroblasts after stretch. Nuc: nucleus, av: autophgic vacuoles. (B) Western blot demonstrates an increase in LC3-II and decrease in procollagen I and fibronectin in stretched versus unstretched control cells. n = 5, *P<0.05 vs. control (Ctrl). (C) Quantitative RT-PCR shows no significant changes in collagen I and fibronectin mRNA after stretch. (D) There is a 5-fold increase in ROS production after stretch as demonstrated by CM-DCF staining.
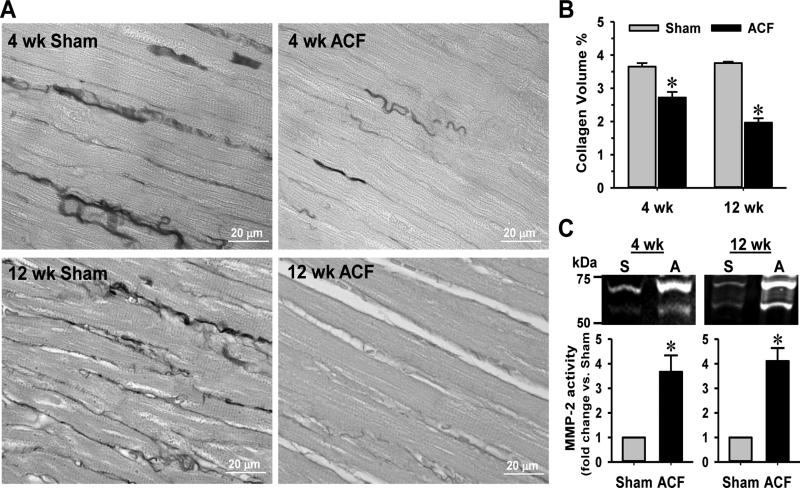
3.6. Interstitial collagen loss is associated with MMP-2 activation in ACF rats
The interstitial collagen in LV heart tissues from 4 and 12 wk ACF rats was reduced ~30% compared to the sham controls (Figs 7A & 7B). It is widely accepted that the loss of extracellular matrix proteins such as collagen is attributed to the increased activity of MMPs [10]. Indeed, the MMP-2 level was increased ~3.5 fold and ~4 fold in 4 and 12 wk ACF cardiac fibroblasts, respectively, compared to age-matched shams (Fig. 7C).
Fig. 7. ACF induces interstitial collagen loss and MMP-2 activation in cardiac fibroblasts.
(A) Interstitial collagen in LV of 4 and 12 wk sham and ACF rats are stained with PASR and (B) the collagen volume percentages quantified as described in the Materials and methods. (C) MMP-2 activity in isolated cardiac fibroblasts is analyzed by gel zymography. n = 6. *P<0.05 vs. age-matched shams
4. Discussion
The pathophysiologic mechanisms of VO are related to not just an ECM breakdown, but also to a failure to replace ECM, in spite of increased cardiac renin, angiotension-converting enzyme (ACE), chymase activity, and Ang II levels [2, 25]. Here we demonstrate that during the course of a compensated (4 wk) and decompensated (12 wk) stage in VO, cardiac fibroblasts produce a strong autophagic response that leads to increase intracellular degradation of procollagen and fibronectin. The results of the current investigation demonstrate a heretofore-unrecognized role of autophagy that contributes to the excessive loss of ECM in the process of LV dilatation.
The fibroblast is the most plentiful cell type in the rat heart and is the source of ECM proteins, MMPs and tissue inhibitors of MMPs (TIMPs) [13, 27]. Much has been written about the pathophysiologic importance of upregulation and balance of MMPs and TIMPs for LV remodeling in models of coronary artery occlusion [26], later stages of pressure overload [24] and pure VO [8-10]. It is of interest that Schaper and coworkers demonstrated that LV dilatation and a decrease in LV ejection fraction that occurs in the later stages of pressure overload in human aortic stenosis is associated with an increased MMP/TIMP ratio [24]. It has been suggested that this imbalance is an important mechanism of LV dilatation that occurs early and throughout the course of a pure VO [7]. In VO, there is a strong correlation between MMP-2 activation and the ECM collagen degradation as we reported in ACF rats [1, 2, 9, 10].
One of the major factors in MMP activation is increased oxidative stress that occurs during cardiac injury [27]. Indeed, we have demonstrated increased cardiomyocyte oxidative stress with cyclical stretch in vitro and in both early and later 8 wk ACF cardiomyocytes [1, 3, 21]. Here we demonstrate a similar finding of increased oxidative stress in fibroblasts stretched in vitro and in fibroblasts taken from 4 and 12 wk ACF left ventricles (Fig. 3 and Fig. 6). We found that cardiac fibroblasts isolated from 4 and 12 wk ACF rats also have elevated MMP-2 activity compared to sham controls (Fig. 7C). Increased oxidative stress can activate MMP-2 and thus mediate collagen degradation in the extracellular space. Consistent with this notion, we demonstrated that the global MMP inhibitor PD166793 prevents interstitial collagen loss in LV tissue of 24 hours VO heart [1]. However, PD166793 has no effect on oxidative stress-induced procollagen degradation (Fig. 5); indicating MMP-2 is not involved in intracellular procollagen degradation.
Previous studies from our lab demonstrate increased LV MMP-2 activity at an early 2 wk stage and late 12 wk ACF but not at 4 wk ACF rats [7]. In the current investigation, we provide evidence of increased autophagy in cardiac fibroblasts taken from both 4 and 12 wk ACF left ventricles. The induction of autophagy is demonstrated by the elevated LC3-II expression and increase in autophagic vacuoles by TEM. Thus, under all conditions of ACF in vivo, and DMNQ treatment and cyclical stretch in vitro, there is oxidative stress induction in fibroblasts that is associated with the decrease in procollagen I. A further connection between autophagic induction and intracellular procollagen breakdown is demonstrated in Fig. 3 where pharmacologic autophagy enhancement or inhibition in isolated fibroblasts has directionally opposite effects on the procollagen I protein but no influence on its mRNA.
Previous studies have also demonstrated a similar finding of autophagic digestion of procollagen under stimuli other than oxidative stress. Stimulation of fibroblast autophagic procollagen digestion has also been demonstrated by beta-adrenergic stimulation of adult rat cardiac fibroblasts [19] and in TGF-β stimulated mouse kidney mesangial cells [18]. In both studies, the investigators speculated that the autophagic procollagen breakdown is a compensatory mechanism that reduces fibrosis in the presence of excessive adrenergic stimulation that occurs in heart failure and in TGF-β-mediated kidney fibrosis, respectively. Aging dermal fibroblasts also have decreased collagen production [28, 29] and increased autophagic procollagen digestion, which has also been implicated in the matrix loss and decreased tensile strength in the aging skin [30]. Taken together, these collective studies in fibroblasts support our findings that autophagy plays a role in ECM homeostasis through intracellular degradation of procollagen and fibronectin in the VO heart.
Important to the net collagen loss in a pure VO, our studies demonstrate a dual function of cardiac fibroblasts on ECM loss by decreasing collagen production through autophagy and secreting MMPs to degrade interstitial collagen. While the balance of MMP/TIMP activity in different stages of LV remodeling may fluctuate [7-8, 31], the induction of autophagy and procollagen decrease at both a compensated stage of 4 wk ACF and decompensated stage of 12 wk ACF demonstrate a continual loss of collagen via autophagy. Many extracellular and intracellular signals might stimulate autophagy under VO; however, we posit that the combined stimulus of adrenergic drive in a pure VO [32] and oxidative stress may represent the chronic stimuli that turn on this autophagic response as well as a secretory MMP activity in fibroblasts. The combination of both events sets up a multi-mechanistic and unremitting collagen loss between cardiomyocytes and results in adverse LV eccentric remodeling.
In summary, we provide evidence that cardiac fibroblasts produce less collagen in the VO heart. This finding is consistent with the mounting evidence for the wide range of dynamic functional and phenotypic alterations of fibroblasts including cytokine production and transformation in response to various cardiac stresses [23, 33, 34]. The fact that fibroblasts make up approximately two-thirds of the cell population in the heart combined with their strategic location in the cardiac interstitium further underscore the importance of fibroblasts in adverse eccentric LV remodeling in VO.
5. Conclusion
The current study shows that in the ACF model of VO, cardiac fibroblasts have an increased autophagic degradation of newly synthesized intracellular procollagen I and fibronectin. This further disturbs the ECM homeostatic balance in the heart by decreasing fibroblast procollagen I production in the face of increased interstitial ECM degradation by MMPs and results in net ECM loss and adverse LV remodeling in a pure VO.
Highlights.
Volume overload (VO) induces oxidative stress and autophagy in cardiac fibroblasts from 4 and 12 wk aortocaval fistula (ACF) rats.
ACF rats have decreased intracellular procollagen in cardiac fibroblasts.
The intracellular procollagen and fibronectin degradation is mediated through autophagy and is induced by oxidative and mechanical stresses.
Both intracellular procollagen degradation by autophagy and interstitial collagen breakdown by matrix metalloproteinases contribute to the extracellular matrix loss and LV remodeling in VO.
Acknowledgments
Funding:
This work was supported by grants from Department of Veteran Affairs for Merit Review (Grant 1BX001050-01 to C.C.W. and Grant 1CX000993-01 to L.J.D.) and NIH (Grant P01 HL051952 to L.J.D.).
Abbreviations
- VO
volume overload
- MMP
matrix metalloproteinase
- ACF
aortocaval fistula
- DMNQ
2,3-Dimethoxy-1, 4-naphthoquinone
- MR
mitral regurgitation
- ECM
extracellular matrix
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- ER
endoplasmic reticulum
- TEM
transmission electron microscopy
- ROS
reactive oxygen species
- CM-DCF
5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein
- LV
left ventricular
- LVEDD
LV enddiastolic dimension
- LVESD
LV end-systolic dimension
- LVEDD/PW
LV end-diastolic dimension/posterior wall thickness ratio
- FS
fractional shortening
- VCFr
velocity of circumferential shortening
- LC3-I and II
type-I and II microtubule-associated protein 1 light chain 3
- TIMP
tissue inhibitor of MMP
- PO
pressure overload
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None declared.
References
- 1.Ulasova E, Gladden JD, Chen Y, Zheng J, Pat B, Bradley W, et al. Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. J Mol Cell Cardiol. 2011;50:147–56. doi: 10.1016/j.yjmcc.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei CC, Chen Y, Powell LC, Zheng J, Shi K, Bradley WE, et al. Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss. PLoS One. 2012;7:e40110. doi: 10.1371/journal.pone.0040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancey DM, Guichard JL, Ahmed MI, Zhou L, Murphy MP, Johnson MS, et al. Cardiomyocyte mitochondrial oxidative stress and cytoskeletal breakdown in the heart with a primary volume overload. Am J Physiol Heart Circ Physiol. 2015;308:H651–63. doi: 10.1152/ajpheart.00638.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan TD, Rothstein EC, Aban I, Tallaj JA, Husain A, Lucchesi PA, et al. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol. 2007;49:811–21. doi: 10.1016/j.jacc.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 5.Gladden JD, Ahmed MI, Litovsky SH, Schiros CG, Lloyd SG, Gupta H, et al. Oxidative stress and myocardial remodeling in chronic mitral regurgitation. Am J Med Sci. 2011;342:114–9. doi: 10.1097/MAJ.0b013e318224ab93. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, et al. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J Am Coll Cardiol. 2010;55:671–9. doi: 10.1016/j.jacc.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YW, Pat B, Gladden JD, Zheng J, Powell P, Wei CC, et al. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol. 2011;300:H2251–60. doi: 10.1152/ajpheart.01104.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson KR, Guggilam A, Cismowski MJ, Galantowicz ML, West TA, Stewart JA, Jr., et al. Temporal pattern of left ventricular structural and functional remodeling following reversal of volume overload heart failure. J Appl Physiol (1985) 2011;111:1778–88. doi: 10.1152/japplphysiol.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol. 2002;283:H518–25. doi: 10.1152/ajpheart.00218.2000. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JA, Jr., Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–9. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 11.Chancey AL, Brower GL, Peterson JT, Janicki JS. Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circulation. 2002;105:1983–8. doi: 10.1161/01.cir.0000014686.73212.da. [DOI] [PubMed] [Google Scholar]
- 12.Cox MJ, Hawkins UA, Hoit BD, Tyagi SC. Attenuation of oxidative stress and remodeling by cardiac inhibitor of metalloproteinase protein transfer. Circulation. 2004;109:2123–8. doi: 10.1161/01.CIR.0000127429.53391.78. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–91. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 14.Butt RP, Laurent GJ, Bishop JE. Collagen production and replication by cardiac fibroblasts is enhanced in response to diverse classes of growth factors. Eur J Cell Biol. 1995;68:330–5. [PubMed] [Google Scholar]
- 15.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–65. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 16.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 17.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem. 2012;287:11677–88. doi: 10.1074/jbc.M111.308460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aranguiz-Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, et al. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta. 2011;1812:23–31. doi: 10.1016/j.bbadis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Wei CC, Lucchesi PA, Tallaj J, Bradley WE, Powell PC, Dell'Italia LJ. Cardiac interstitial bradykinin and mast cells modulate pattern of LV remodeling in volume overload in rats. Am J Physiol Heart Circ Physiol. 2003;285:H784–92. doi: 10.1152/ajpheart.00793.2001. [DOI] [PubMed] [Google Scholar]
- 21.Gladden JD, Zelickson BR, Wei CC, Ulasova E, Zheng J, Ahmed MI, et al. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radic Biol Med. 2011;51:1975–84. doi: 10.1016/j.freeradbiomed.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rab A, Bartoszewski R, Jurkuvenaite A, Wakefield J, Collawn JF, Bebok Z. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol. 2007;292:C756–66. doi: 10.1152/ajpcell.00391.2006. [DOI] [PubMed] [Google Scholar]
- 23.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polyakova V, Hein S, Kostin S, Ziegelhoeffer T, Schaper J. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol. 2004;44:1609–18. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Ruzicka M, Keeley FW, Leenen FH. The renin-angiotensin system and volume overload-induced changes in cardiac collagen and elastin. Circulation. 1994;90:1989–96. doi: 10.1161/01.cir.90.4.1989. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Z, Ou L, Liu Y, Liu X, Li F, Sun B, et al. Granulocyte colony-stimulating factor exacerbates cardiac fibrosis after myocardial infarction in a rat model of permanent occlusion. Cardiovasc Res. 2008;80:425–34. doi: 10.1093/cvr/cvn202. [DOI] [PubMed] [Google Scholar]
- 27.DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial matrix metalloproteinase-2: inside out and upside down. J Mol Cell Cardiol. 2014;77:64–72. doi: 10.1016/j.yjmcc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117:1218–24. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 29.Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–8. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tashiro K, Shishido M, Fujimoto K, Hirota Y, Yo K, Gomi T, et al. Age-related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem Biophys Res Commun. 2014;443:167–72. doi: 10.1016/j.bbrc.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 31.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, et al. Beta1-adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol. 2006;291:H147–51. doi: 10.1152/ajpheart.00951.2005. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–53. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110:5588–93. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]