Abstract
Background
Electroencephalography (EEG) and related measures have a long and productive history in child psychopathology research and are currently experiencing a renaissance in interest, particularly for use as putative biomarkers.
Method and Scope
First, the recent history leading to the use of EEG measures as endophenotypes and biomarkers for disease and treatment response are reviewed. Two key controversies within the area of non-invasive human electrophysiology research are discussed, and problems that currently either function as barriers or provide gateways to progress. First, the differences between the main types of EEG measurements (event related potentials, quantitative EEG, and time-frequency measures) and how they can contribute collectively to better understanding of cortical dynamics underlying cognition and behavior are highlighted. Second, we focus on the ongoing shift in analytic focus to specific cortical sources and source networks whose dynamics are relevant to the clinical and experimental focus of the study, and the effective increase in source signal-to-noise ratio (SNR) that may be obtained in the process.
Conclusions
Understanding of these issues informs any discussion of current trends in EEG research. We highlight possible ways to evolve our understanding of brain dynamics beyond the apparent contradictions in understanding and modeling EEG activity highlighted by these controversies. Finally, we summarize some promising future directions of EEG biomarker research in child psychopathology.
Introduction
Electroencephalography (EEG) has a long history in child psychopathology research and was the first methodology used for examining human cortical brain activity among children. EEG recordings from the human scalp were first successfully recorded by Hans Berger (1929), whose observations regarding prominent brain oscillations in the 8-12 Hertz (Hz) “alpha” band range still hold true today. Soon, thereafter, Jasper (1938) reported increased slow-wave activity among children who were institutionalized for problem behaviors, many of whom might today qualify for child psychiatric diagnoses. Since those first qualitative observations, EEG and metrics derived from it, including trial-averaged event-related potentials (ERPs), have been widely used in child psychopathology studies. This is likely because of the many advantages of using EEG for functional brain imaging -- including its flexibility and portability, its non-invasive nature and wide subject acceptance, and its excellent temporal resolution and relatively low cost.
In recent decades, new mathematical, technological, and neuroscientific advances have allowed EEG measures to become an important data source for a new quantitative science of macroscopic or ‘mesoscopic’ (Freeman, 2000) cortical brain dynamics. Over time, scientific interest in EEG, particularly as applied to child psychopathology research and practice, has waxed or waned depending on experimental questions of the day. Currently, EEG is experiencing a renaissance, as suggested by the number of Pubmed citations with keywords ‘EEG, ‘ERP’, and ‘brain oscillation,’ for which Figure 1 shows some interesting trends: The early importance of EEG brain imaging before the development of fMRI, the emergence of ERP studies since the introduction of this term and measure in the early 1960s, leading to the predominance of the ‘ERP’ keyword over the keyword ‘EEG’ itself in published reports during a nearly 30-year period (1980 to 2007), the difference peaking about 1989. Also, note the continuing increase over the last 40 years in studies using the keywords ‘brain oscillations,’ which may reflect the growing recognition that brain dynamic phenomena are incompletely captured by ERP and static spectral “quantitative EEG” (qEEG) measures (Makeig, 1993; Pfurtscheller, Aranibar, & Maresch, 1979). Ongoing applications of new EEG measures are revealing information about cortical brain dynamics in EEG data that go well beyond traditional ERP and qEEG measures.
Figure 1.
Relative percent of PubMed citations retrieved by ‘All Fields’ search terms: ‘EEG’ + ‘ERP’ + ‘Brain Oscillations’. The percent of citations for each search term relative to the total number of citations returned by a search for any of the three terms is plotted relative to the other two search terms. For visual clarity, ‘Brain Oscillations’ citations are graphed with a dotted line according to the Y-axis labels on the right; ‘EEG’ with a solid line and ‘ERP’ with a dashed line according to the Y-axis labels on the left.
Focus of the current review
Currently, many applications of EEG research within child psychopathology are being proposed and interpreted within the context of endophenotypes and biomarkers for disease and treatment response. Biomarkers are defined as, “a characteristic that is objectively measured and evaluated as an indication of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definition Working Group, 2001). Because child psychiatric disorders involve significant clinical heterogeneity in etiology, symptomatic presentation, developmental course, and treatment response, the identification of biomarkers to detect early risk factors, facilitate diagnosis, and potentially predict treatment outcome would be of great value to the field. Accordingly, this topic will be the primary focus of the review. First, we review the recent history leading up to this development. Then, we discuss two key controversies within the area of non-invasive human electrophysiology research that currently function either as gateways or barriers to progress. Understanding of these issues informs any discussion of current trends in EEG research. We then highlight possible ways to evolve our understanding of brain dynamics beyond the apparent contradictions in understanding and modeling EEG oscillatory activity as highlighted by these controversies. Finally, we summarize some promising future directions of EEG biomarker research in child psychopathology.
Use of EEG endophenotypes for gene discovery
In the past 10 to 15 years, our understanding of psychopathology (in both child and adult forms) has evolved from a focus on unique factors (genetic, environmental, neurobiologic, and cognitive) underlying specific psychiatric disorders to one aimed at examining shared factors across different forms of psychopathology. This paradigmatic shift started in large part due to advances made by the Human Genome Project and subsequent focus on identifying genetic factors underlying complex traits. Because of the significant heterogeneity that is present across nearly all Diagnostic and Statistical Manual (DSM) (APA, 2013) psychiatric disorders, the need arose for greater use of intermediate phenotypes or ‘endophenotypes’ between the diagnostic phenotypes and the underlying causal (i.e., genetic and environmental) factors (Gottesman & Gould, 2003). Theoretically, endophenotypes were meant to be genetically ‘simpler’ phenotypes that lay along the same etiologic pathway but were closer to the underlying gene action. It was commonly believed that endophenoytpes would confer more power to detect gene variants signifying increased risk for disorders, thereby reducing etiologic heterogeneity and allowing delineation of more homogeneous subgroups within a diagnosis.
EEG and ERP measures were considered excellent candidate endophenotypes given they are quantitative neurobiological measures that can be much less expensive to collect than magnetic resonance imaging (MRI) measures (McLoughlin, Makeig, & Tsuang, 2014) and have high heritability estimates: ~0.80 for spectral power and ~0.50 for (some) ERP peak amplitudes within healthy populations (Smit, Posthuma, Boomsma, & Geus, 2005). Heritability of EEG measures is similarly high within psychiatric populations including ADHD (Loo et al., 2010; McLoughlin, Palmer, Rijsdijk, & Makeig, 2014), Bipolar Disorder (Bestelmeyer, Phillips, Crombie, Benson, & St Clair, 2009), and Schizophrenia (Hall et al., 2011), particularly when recorded and contrasted during cognitive processing versus resting conditions and in combination with cognitive test performance (Loo et al., 2010).
Many early endophenotype studies were based on overly simplistic models of gene action and ignored the influence of environmental variables on gene expression (Valdar et al., 2006). For example, early genetic investigations of psychiatric disorders were designed to detect the effects of a relatively small set of putative risk genes with moderate to large effects. In addition, it was assumed that most cognitive and neurobiologic endophenotypes had a simpler genetic architecture than psychiatric diagnoses, which now appears to not be the case (Flint & Munafo, 2007). Finally, the majority of genetic variance was thought to be captured by common genetic variants (or polymorphisms) rather than rare variants or copy number variations. In current models of psychiatric genetics, however, complex traits (and associated endophenotypes) are thought to be the result of hundreds, perhaps thousands of genetic variations, each of small effect. As a result, the endophenotype approach for identifying genetic loci for psychiatric disorders has failed to deliver the previously hoped for successes, with few notable exceptions.
With the growing feasibility, cost-effectiveness, and use of whole-genome sequencing, however, a more complete account of genetic variation underlying complex traits and psychiatric conditions will soon be available. With this will come a renewed need for endophenotypes to assist with functional interpretation of the genetic sequence variants (de Geus, 2010). This ‘reverse endophenotype’ approach is a strong justification for increased use of EEG measures in genetic investigations. Decoupled from examining the genetic pathways associated with disease and neurobiological functions, EEG measures shown to be useful for disease prediction, diagnosis, characterization, and treatment monitoring are increasingly referred to as biological markers or ‘biomarkers’ rather than endophenotypes.
Shift to shared genetic and mechanistic factors across disorders
As genetic studies failed to pinpoint the expected risk genes responsible for psychiatric disorders, the concept of disorder-specific genetic risk factors and mechanisms started to shift to shared etiologic and neurobiologic factors that may be operant across disorders. Early studies in this area demonstrated that genetic linkage findings across several child psychiatric disorders including autism spectrum disorder (ASD), attention-deficit/ hyperactivity disorder (ADHD), and dyslexia overlapped at a rate significantly greater than chance (Smalley, Loo, Yang, & Cantor, 2005). Theories regarding common genetic architecture for a broad band of internalizing and externalizing disorders have been proposed, with a small proportion of disorder unique genes and environmental experience factors determining the specific manifestation and diagnosis (Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011). Recent genetic findings from the Psychiatric Genetics Consortium across five psychiatric disorders: ADHD, ASD, Bipolar Disorder, Schizophrenia, and Major Depressive Disorder validate the idea that shared heritability and genetic loci underlie multiple psychiatric disorders (Cross-Disorder Group of the Psychiatric Genomics et al., 2013).
Simultaneous with the shift to common genetic factors came a convergence of ideas across neuroscience and clinical science leading to the examination of common or shared neurobiologic mechanisms that underlie different psychiatric disorders. Although this multidimensional view of psychopathology has always had proponents, the concept currently has much wider and growing acceptance in the scientific and clinical communities. Within the clinical community, greater recognition of psychopathology dimensions have been incorporated into the DSM diagnostic criteria as dimensional specifiers, which have replaced some diagnostic subtypes, as well as elimination of exclusion criteria prohibiting the diagnosis of co-morbid disorders such as ADHD and ASD.
Within the research community, organizations including the U.S. National Institute of Mental Health (NIMH) have proposed a move away from characterizing psychopathology based on psychiatric diagnoses but rather according to translational dimensions of functioning such as cognitive processes, positive and negative valence, arousal and self-regulation (Research Domain Criteria; RDOC). Each of these dimensions can be characterized by a translational, multi-level pathway (i.e., genes, neural circuitry, brain dynamics, and behavior) that can inform searches for both underlying neural mechanisms and potential treatments. Consistent with this approach, NIMH has more recently issued guidance for pre-clinical and intervention trials (e.g., NIH FAST-Fail trials) that makes central the need for measurement of biomarkers in addition to behavioral symptoms in gauging treatment response.
Collectively, this broader focus on etiologic and symptomatic heterogeneity within psychiatric disorders and new focus on examination of domains of function across disorders has implications for efforts to develop more robust, highly cost effective EEG-based biomarkers for psychiatric disorders. It seems unlikely that a single biomarker measure of any type can capture all of the variance within a diagnostic category (Lenartowicz & Loo, 2014). Instead, finding biomarkers for neural circuits associated with domains of functioning that are abnormal across several disorders seems a potentially more fruitful approach.
Current controversies
Interest in EEG research is again on the rise in large part because refined EEG measures can now increase the amount of relevant information about brain function that can be derived from the collected data. Below we discuss two controversies in EEG analytic approaches that may serve as barriers to progress in using EEG in child psychopathology. First, we discuss differences between the most common types of EEG measures (ERP, qEEG, and event-related time-frequency measures) and how their combination may contribute to better understanding of cortical dynamics underlying cognition and behavior. Second, we highlight the ongoing shift in analytic focus to specific cortical sources and source networks whose dynamics are relevant to the clinical and experimental focus of the study, and the effective increase in biomarker signal-to-noise ratio (SNR) that may be obtained using source-resolved EEG measures.
CONTROVERSY I: Use of averaged event-related potentials (ERPs) vs. power spectra (qEEG) vs. time-frequency measures
Of the many possible outcome variables that can be extracted from EEG-recorded scalp data, the two that have most frequently been applied in psychiatric research are mean EEG power spectra during some specified time period(s) (often called ‘quantitative EEG’ or qEEG measures), and peak amplitudes and latencies of ERPs extracted from EEG data by averaging across data epochs identically time-locked to some set of experiment events. Although the same hardware, software, and scalp signals are used to compute these different measures, there has historically been a deep divide (with little cross-over) between researchers who use ERP or qEEG measures. The principal difference between these measures is the signal processing methods used to derive them. Below, we briefly describe these and then discuss potential paths to bridge the divide in future research.
Event-related potential
An ERP is computed from EEG activity within a number of data epochs selected as being time-locked to the occurrence of some class of events of interest, such as particular stimulus onsets or subject responses. The time-locked data epochs (or ‘trials’) are averaged, producing a single trial-average waveform consisting of a series of positive and negative peak deflections. The brief (~1-sec) ERP time series are typically quantified by measuring the latencies and amplitudes of the major peaks, and tested for statistical robustness using standard means (T-tests, ANOVAs, or non-parametric bootstrap methods). After averaging many trial epochs, only event-related EEG deflections that are both time-locked (occur at fixed latencies relative to the time-locking events) and phase-locked (i.e., at least somewhat consistently positive- or negative-going across trials) at some set of latencies following (or sometimes preceding) the time-locking events remain in the trial-average ERP waveform. Similarly, only those ERP data features that arise more or less consistently across subjects are reflected in the group ‘grand average’ ERP. If oscillatory EEG complexes following the time-locking events do not occur at a consistent latency or with a constituent (e.g., positive or negative) phase, nothing of these features will remain in the ERP after averaging across many trials. Thus, analyzing only average ERPs extracted from the recorded EEG signals typically leaves many of its features effectively unexamined.
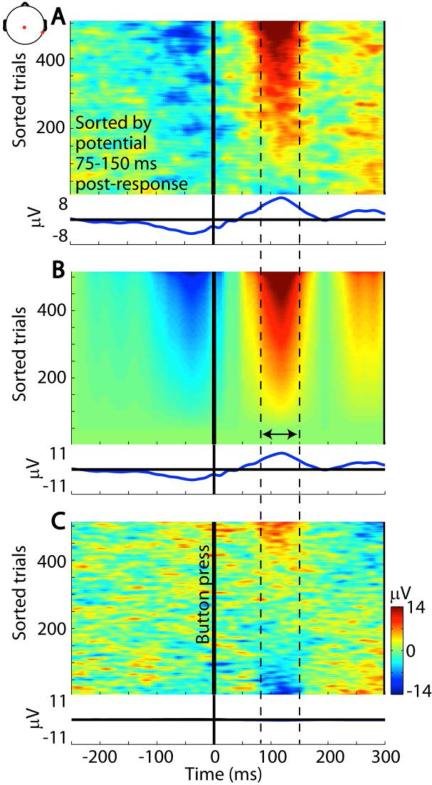
Importantly, ERPs do not reveal how many or which of the trials contain deflections with the same phase (positive or negative) as the trial-average ERP at that latency. For most ERP features, this figure is quite a bit less than 100%, as can be ascertained by plotting the single trials sorted by potential at a latency of interest. Figure 2A, from Makeig & Onton (2009), shows an ‘ERP-image’ view of such a collection of trials whose ERP average contains a prominent positivity peaking about 110 ms after subject button presses. However, sorting trials by potential in this latency window shows that this positivity only occurs in about 75% of the trials and not in the other 25%. Thus, activity sequences appearing in ERPs typically do not occur in all trials, and indeed might even not occur in entirety in any single trial. Nonetheless, the amplitudes and latencies of several ERP peaks (e.g., error-related negativity, P3, slow cortical potential, etc.) are typically examined in ERP studies without any attention to their (possibly systematic) trial-to-trial variability – or to accompanying EEG phenomena that do not appear in the ERPs (for example, periods of alpha power ‘blocking’ or ‘flooding’ time-locked but not phase-locked to the same events).
Figure 2.
Insufficiency of the ERP model. (A) Color-indicated single trials time locked to participant button presses in a cognitive task paradigm (channel: Cz to average reference). The trials are sorted (bottom to top) in increasing order of mean potential in a (dashed-line indicated) postresponse latency window and lightly smoothed with a 20-trial moving average. The trial-average ERP is shown below the ERP-image panel. The prominent postresponse positivity in the ERP actually occurs in less than 80% of the single trials. (B) The trial-average ERP regressed on each trial activity, plotted in the same trial order. The model trial average, shown below, matches the actual trial ERP. (C) The difference between the data in panels A and B shows that activity in the postresponse latency window, for a subset of most positive-going and negative-going (top and bottom) trials, is not proportional to the expression of the whole ERP in those trials. (Reprinted from Makeig & Onton, 2012, which also shows many other phenomena in these data not evident in this figure)
Preliminary efforts to study single-trial ERP variability have yielded a more refined understanding of ERP features and their association with underlying neural and cognitive mechanisms. Several studies have examined the associations of reaction time variability (RTV) and of trial-by-trial variability in the P300 component (a positive voltage deflection near 300 ms associated with stimulus evaluation and response selection) in ADHD. Analyses of P3b and lateralized readiness potential (LRP) latency distributions to infrequent or unexpected (‘oddball’) stimuli suggest that reaction time variability in ADHD may be in large part due to processing differences related to response emission rather than to stimulus interpretation (Saville et al., 2014). Furthermore, the heterogeneity in the spatial distribution and low amplitude of the P3 were the strongest predictors of RTV (Bender et al., 2015).
Examination of trial-by-trial ERP variability may also lead to a clearer understanding of differences between diagnostic subgroups. Trial-by-trial ERP analysis revealed that the distribution of P3 peak amplitudes differed significantly between children with ADHD and controls. However, further differences dependent on ADHD subtype emerged (Heinrich et al., 2014). Specifically, children with ADHD Combined Type had a larger proportion of low-amplitude P3 peaks in single trials, indicating a sub-optimal neural state before stimulus presentation, whereas those with ADHD Inattentive Type had a larger proportion of relatively high-amplitude P3 responses, suggesting reduced capacity of resource allocation. Because clinical heterogeneity within and among child psychiatric disorders is a significant barrier to progress in identifying etiologic factors and choosing treatments, measures that can account for ERP and EEG variability as well as mean expression may constitute more sensitive and specific biomarkers.
Use of ERP features as biomarkers of diagnosis and treatment outcome has been moderately successful. For example, the error-related negativity (ERN), a heavily studied fronto-central negative ERP peak following (~50 msec) incorrect button press responses in speeded choice response tasks is thought to index performance monitoring. ERN amplitude is enhanced in anxiety and related disorders such as obsessive-compulsive disorder (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Olvet & Hajcak, 2008), but is relatively stable across development (Meyer, Weinberg, Klein, & Hajcak, 2012). Furthermore, the size of the ERN can predict the development of anxiety disorders in the subsequent three years (Meyer, Proudfit, & Klein, 2014). These facts suggest it can be used as a biomarker of risk and may be a potential target for early interventions in childhood anxiety.
Similarly, two ERP components – the mismatch negativity (MMN), a frontocentral negative wave between 100-200 ms elicited by infrequent deviant auditory stimuli, and P3a have been validated as potentially useful biomarkers for schizophrenia. Specifically, significantly reduced MMN and P3a amplitudes occur among patients with schizophrenia, including first episode and high-risk individuals (Atkinson, Michie, & Schall, 2012), are associated with some demographic and clinical characteristics (Tarasenko, Swerdlow, Makeig, Braff, & Light, 2014), and predate the onset of psychosis (Shaikh et al., 2015). Thus, several abnormalities in some ERP peak amplitudes have been reliably associated with specific diagnostic groups. However, to be useful as biomarkers, measurements of their sensitivity and specificity in individual subjects are required.
Within-ADHD, subject classification success using single ERP features has been modest, hovering in the range of 60-80% (Johnstone, Barry, & Clarke, 2013; Liechti et al., 2013). Recent studies have applied machine learning methods to combined ERP measures, with more success. Both Mueller et al. (Mueller, Candrian, Kropotov, Ponomarev, & Baschera, 2010) and Nazvahani et al. (Nazhvani, Boostani, Afrasiabi, & Sadatnezhad, 2013) used machine learning algorithms and multiple ERP measures to achieve ADHD versus control classification accuracy in excess of 90%. Mueller et al. (2010) reported sensitivity and specificity of 91% in predicting diagnosis in a sample of 150 adults (75 with ADHD), exploiting a combination of five brain source-resolved ERP features associated with response-inhibition identified using independent component analysis (ICA). In a smaller sample (n = 36), Nazhvani et al. (2013) maximized the accuracy of group discrimination using a combination of ERP amplitudes in response to visual evoked responses to flashes of light. This approach achieved an accuracy of 94.6% for discriminating adults with ADHD from controls and an accuracy of 92.9% for distinguishing adults with ADHD from those with bipolar mood disorder. Also, Heinrich et al., (2014) used a combination of ERP (Cue-P3) and transcranial magnetic stimulation (short interval cortical inhibition) measures to discriminate between ADHD and control subjects, with a 90% success rate. It is possible that more complex models utilizing multiple measures may likely yield biomarkers with increased discriminative validity.
Strengths of average ERP and other ‘time domain’ EEG measures can exploit the inherent, precise time resolution of EEG recording. Unlike hemodynamic imaging, EEG can give information about brain activity with better than 1-millisecond (ms) time resolution (though ±2-4 ms resolution is typically used in most EEG recordings), making it well suited to record and model the moment-to-moment fluctuations in distributed macro- and meso-scopic scale cortical dynamics involved in nearly all sensory, cognitive, and motor processes. Based on the exact timing of features in the recorded EEG signals, fine temporal distinctions can be made between early perceptual, bottom up, and top down modes of information processing. This makes EEG (including ERP) measures particularly valuable when assessing phenomena that occur or evolve at 0.1-sec and finer time scale, such as perception, cognition, event evaluation, action planning, and motor action itself. However, the misconception that data features appearing in the EEG data not reflected in an average ERP are ‘task-irrelevant EEG noise,’ can be a conceptual barrier to achieving a more complete understanding of the meso- and macro-scopic brain dynamic processes by making use of more of the information contained in the recorded EEG data.
Quantitative EEG (qEEG)
EEG spectral power is estimated by separating the EEG recordings using mathematical methods including the Fast Fourier Transform (FFT) and its variants into a sum of activities within narrow frequency bands. Power spectra represent the magnitude or magnitude-squared power of complex activity patterns as a function of EEG frequency (measured in cycles per second, Hertz [Hz]). Because EEG signals themselves occupy a wide spectrum of frequencies (from 0.1 Hz and below to 250 Hz and above), power spectral averages can capture information about parallel EEG processes occurring in distinct frequency ranges that contribute to different aspects of cognitive, sensory, or motor processing. While definitions vary, mean amplitude or power within well-known frequency bands, including delta (2- 4 Hz), theta (4-8 Hz), alpha (9-12 Hz), beta (13-30 Hz), and gamma (>30 Hz), are often reported. Estimation of the mean power spectrum at each scalp channel or of average power across several electrodes has been most frequently performed across the whole duration of a continuous recording period. This has sometimes been referred to as qEEG measurement (to contrast with still earlier non-quantitative analysis methods based solely on visual inspection, still widely used in neurology). Mean power spectral estimation is most appropriate for conditions in which EEG signals might be expected to be relatively stationary; the extent to which this is the case deserves detailed examination.
The power spectrum at rest is often modeled as a marker of trait-like brain function indexing variables such as developmental level, baseline level of affect reactivity, or arousal. The best known example of using EEG spectral power as a biomarker for diagnosis is in ADHD, where high values of the theta to beta ratio, recorded from the vertex or top of the head, have been claimed to be strongly associated with diagnosis of attention-deficit/hyperactivity disorder (ADHD). Early studies (Monastra, Lubar, & Linden, 2001; Snyder et al., 2008) suggested high accuracy in identifying people with ADHD; a meta-analysis reported a large effect size of 3.08 (Snyder & Hall, 2006). However, recent empiric and meta-analytic studies (Arns, Conners, & Kraemer, 2013; Buyck & Wiersema, 2014; Liechti et al., 2013; Loo et al., 2013; Ogrim, Kropotov, & Hestad, 2012; Williams et al., 2010) have not supported the association between ADHD and the theta/beta ratio measure, a conceivable suggested reason being a general increase in this ratio among normal control populations in recent years (Arns et al., 2013). While this theta/beta ratio measure may be elevated in a subgroup of individuals with ADHD, neither the characteristics of this subgroup nor what brain state is indexed by the theta/beta ratio have been determined.
In contrast, EEG resting spectral power has been shown to be a robust marker of neurodevelopment. Age-related maturation of the EEG power spectrum has long been noted, with attenuated slower wave activity (delta & theta bands) and increased faster wave activity (alpha & beta bands) with increasing age (Gasser, Jennen-Steinmetz, Sroka, Verleger, & Mocks, 1988; John et al., 1980; Luchinger, Michels, Martin, & Brandeis, 2012). In a recent study, Buyck and Wiersma (2014) reported using several EEG measures for predicting age classification (i.e., children, adults) with high accuracy (theta/beta ratio, 97%; absolute and relative theta power, 91-94%; relative beta power, 90%). These developmental shifts have been compared with MRI measures to help interpret the EEG findings. Combining concurrent resting-state fMRI and EEG measures, Luchinger and colleagues (2012) suggested that EEG changes during development may reflect age-related changes in emphasis and integration of local and long-range networks, as inferred from spatial coherency in BOLD signals during instructed rest periods.
While the use of the mean qEEG power spectrum as a primary dependent variable does not capitalize on the temporal resolution of EEG, it does make use of its other advantages including its noninvasiveness, relatively low cost, and flexibility of use with a wide range of subject and patient populations. New signal processing methods and widely available computing power (see below) make it more feasible to extract information from analyses extending across large datasets, making large-scale studies computationally tractable. And, like average ERP measures, mean power spectral measures greatly reduce the dimensionality of the recorded EEG data, facilitating analysis including comparisons with other measures of interest such as fMRI BOLD changes or behavioral performance. While reducing the (temporal and spatial) dimensionality of the data is often necessary to achieve a coherent result, both of these approaches result in a vast reduction in information content about EEG signals generated by the many cortical processes contributing to the recorded EEG during the experiment.
qEEG and ERP measures share relatively little redundant information, since variations in the amplitude and latency of an ERP peak (representing only a tiny fraction of the power in the whole EEG) may have little correlation with variations in the EEG power spectrum during the same recording period. qEEG power spectral measures may therefore be used fruitfully in combination with ERP measures although, as noted above, these measures have historically been studied in isolation from one other. As we will discuss below, using newer analytic methods to identify relevant measures of specific cortical source processes contributing to the scalp data may give much more robust information relevant to questions of psychiatric interest than simply collapsing the whole scalp data to a few summary measures at one or the mean of a few scalp channels (Lenartowicz et al., 2014; Mullen, Acar, Worrell, & Makeig, 2011).
Event-related time/frequency measures
Advances in computer technology and signal processing software are attracting an increasing number of researchers to performing more general time-frequency analyses that combine and extend the strengths of both ERP and qEEG spectral power measures. Time/frequency analyses extract changes in spectral power and phase across a given set of data epochs time-locked to a set of similar events of interest, allowing sub-second resolution of changes in brain oscillatory activity. The term ‘event-related spectral perturbation’ (ERSP) was suggested for the time/frequency measure summarizing mean spectral power changes at each frequency surrounding the time-locking events (Makeig, 1993); various other terms have since been offered. Event-related spectral power increases or decreases within a single frequency band (e.g., the 8-12 Hz alpha band) have been called event-related synchronization [ERS] or desynchronization [ERD], respectively, since early research with electrodes implanted in animal brains suggested loss of local spatial cortical field synchronization during periods of higher-frequency activity (Pfurtscheller & Aranibar, 1979).
ERSPs, like ERPs, can be used to compare EEG activity in different trial-latency windows as well as in different trial subsets. For example, Lenartowicz et al. (2014) used ERSP analysis to examine power spectral differences within working memory (WM) task trial-latency windows in ADHD, which suggested that WM performance deficits were specifically related to (bottom-up) visual encoding versus (top-down) WM maintenance.
In addition to the clear benefit of allowing finer event-related time resolution across the full EEG power spectrum, time-resolved measures of brain oscillatory activity features are largely conserved and associated with similar functional significance across mammalian species (Buzsaki, Logothetis, & Singer, 2013). This enhances the use of EEG to study and develop translational models of neural circuits, the importance of which is emphasized in the NIMH RDOC initiative. Animal models of brain oscillations have been well studied and can inform our understanding of the functional significance of various brain rhythms. For example, subcortical generators of theta-band activity during memory tasks are in the hippocampus, based on basic animal research (Arnolds et al., 1980; Buzsaki 2002), whereas alpha-band activity (8-12 Hz) has a known generator mechanism in radially arrayed cortico-thalamic loops (Lopes da Silva, 1977). This cross-species link differentiates EEG from other brain imaging modalities, such as fMRI, since measures of oscillatory field phenomena may be more direct measures of brain activity than, for instance, measures used to study BOLD-signal functional connectivity, which rely on statistical estimates (typically linear measures of co-variation) calculated on indirect (metabolic) indices of regional brain activity.
A way forward
A straightforward (although at the outset not simpler) way to proceed toward more specific and robust models of EEG dynamics is to combine ERP, mean qEEG spectral power, and event-related time/frequency (ERSP and other) measures of oscillatory activity rather than limiting analyses to just one measure. This allows a more comprehensive examination of the changes in EEG activity contained in the collected EEG signals and more complete tests for possible relationships between and across measures of interest.
One interesting idea illustrating this direction is the analysis of pre-event oscillatory dynamics, in particular power spectra, as indexing context-aware ‘preparatory’ state differences, and post-event ERPs and ERSPs as indexing temporally precise cortical processing of events. For example, several studies have demonstrated that pre-stimulus EEG spectral power may predict subsequent event-related cortical activation (as measured using ERPs and ERSPs) as well as subject performance on cognitive challenges including memory encoding and retrieval (Addante, Watrous, Yonelinas, Ekstrom, & Ranganath, 2011), motor inhibition, and visual processing (van Dijk, Schoffelen, Oostenveld, & Jensen, 2008). Whether pre-stimulus spectral power EEG features may index attention linked to arousal and/or engagement of specific attention networks remains to be tested empirically. However, more attention is warranted to relationships between pre-stimulus EEG dynamic differences, ensuing stimulus-driven cortical responses, and behavioral performance differences.
In summary, new analytic tools are making modeling of ongoing EEG dynamics and its relationship to performance, subject status, etc., increasingly possible and informative. For most EEG researchers, however, collaboration with others with expertise in technical areas involved (e.g., signal processing, specific clinical populations, etc.) is advisable since each by itself is becoming its own advanced field of study. Fruitful collaborations between basic and clinical researchers may bring more computationally complex analyses to clinical population data, thereby developing more precise biomarkers of developmental risk, manifestations of psychopathology, and treatment response.
CONTROVERSY II: Measures of ‘source activities’ versus scalp channel potentials
Another important and still controversial issue in EEG modeling and measurement is whether to use measures derived directly from EEG signals recorded at one or a few neighboring scalp electrode channels, or to analyze the activity of underlying cortical ‘sources’ derived by analytic methods from the recorded scalp channel data. Because EEG potentials are recorded on the scalp, far from the cortical regions (or EEG source processes) from which the constituent signals of interest originate, only potentials from areas that achieve some degree of local field synchrony have sufficient summed strength to contribute noticeably to scalp EEG signals. The fluctuating potential from each of these cortical source areas is volume-conducted through brain and head tissues, thereby spreading the net signal of the source area broadly across the scalp surface. This results in the core problem of scalp recordings: that each individual scalp EEG signal contains mixtures of information from many cortical sources whose signals are summed with each other (as well as with non-brain artifacts from eye-blinks, muscle tension, etc.) by passive volume conduction. The fundamental fact of the resulting spatial blurring of source activities in scalp EEG signals means that most measures computed on scalp channel data cannot be assigned a particular brain origin. Instead, they typically contain summed potentials from both relevant and irrelevant brain and non-brain sources, thereby limiting their effective signal-to-noise ratio (SNR) and statistical value.
Although mixing of electrical signals at the scalp is a biophysical fact upon which all investigators agree, how to best deal with the mixed scalp signals is an issue of considerable current disagreement. While scalp channel data measures may have useful information value – e.g., when their interpretation in terms of brain processes is not a goal of the analysis – they remain measures of cortical source admixtures. If, however, EEG signals are to be linked to brain function (i.e., as biomarkers indexing activity within a particular network or neural circuit) with the highest possible statistical power, it is necessary to un-mix the sources that form the individual EEG channel signals and then perform analyses on the unmixed source signals.
The same is the case when EEG measurements are to be related to any other brain imaging measures (from fMRI, anatomy, etc.). The interpretation of many ERP features (P1, N1, etc.) gained widely recognized value in psychiatry and other fields only when source modeling and parallel intracranial data became available and confirmed their brain origins. To make EEG biomarkers meaningful, their cortical sources must be known, a point implicit in the recent mission statement of the NIMH Division of Translational Research and Neuroscience and Basic Behavioral Science focusing the goals of their basic research programs directly on clarifying the brain mechanisms of psychopathology.
The un-mixing of EEG scalp data into identified cortical sources is known as the EEG ‘inverse problem.’ Modeling the locations and orientations of the EEG sources contributing to scalp data patterns can be described as an‘ill-posed’ problem because any scalp data map, is compatible with multiple source solutions. One solution can only be preferred using additional assumptions regarding the feasible number, locations, and/or orientations of the cortical source areas; in practice, many solutions can be rejected as physiologically implausible. The skill of identifying and selecting components representing plausible brain activity generally requires training and practice through teaching, training, or collaborating with experienced researchers.
Several freely available software environments and commercial applications1 have tools for estimating cortical source activities that contribute to EEG features of interest. Most of these environments have graphic user interfaces that make their operation more accessible to researchers who are not comfortable with writing data analysis scripts. To fully understand the nature of the more advanced computations made available in these signal processing environments, those who have not used such tools may want to collaborate with other, more experienced users to learn to properly implement and interpret appropriate measures and approaches.
These comparatively recent advances in signal processing include the use of independent component analysis (ICA; (Makeig, Bell, Jung, & Sejnowski, 1996; Makeig et al., 2002) to isolate individual cortical source signals originating in different brain and non-brain processes that contribute to the EEG signals. ICA has the same (linear un-mixing) format as other types of latent variable modeling (principal component analysis, factor analysis, latent cluster analysis, factor mixture modeling) for which the determination of the number of factors or clusters is guided by various fit measures (eigenvalues, AIC, BIC) and by the amount of variance accounted for in the data. Similar measures exist in ICA approaches; the amount of data variance accounted for by each source and the degree of measured independence of its time course from those of other source signals (Delorme, Palmer, Onton, Oostenveld, & Makeig, 2012) may be used to assess the physiological plausibility of the component process.
The distinguishing feature of ICA versus other linear decomposition approaches is that the scalp channel signals are assumed to sum statistically (and therefore also functionally) independent source signals with stable spatial scalp projection patterns (Makeig, Debener, Onton, & Delorme, 2004). In actual data, these include eye blinks, saccades, electrocardiographic activity, single-channel noise from a loose electrode, and individual scalp muscle activities. Other sources (unmixed by ICA from contamination by non-brain source activity) represent the projections of spatially stable cortical sources. Because the connectivity structure of cortex is highly weighted toward local neural connections, and thalamocortical connectivity is primarily radial, these brain sources are typically compatible with local field activity within a small (cm-scale) cortical source patch. The locations of each of these source patches can be estimated with either a (patch-equivalent) single dipole model or, when an individual electrical head model is available, with a cortical patch model. Note, however, that all approaches to ICA decomposition do not perform equally well, and the success of the approach depends on its application to a sufficient amount of well-collected and adequately pre-processed data.
A way forward
Many EEG researchers continue to assess only scalp channel data measures of their data, despite the logical impediments to their neuroscientific interpretation. Investigators in this position may find it useful to perform side-by-side comparison of scalp and source data measures to better understand the added value of using source-resolved EEG signals. Doing so may demystify the source localization process and allow deeper understanding of differences between channel and source space measures. As an example from our own work, we present further analysis of data examined in a recent report in which we used source-level EEG analysis to discover which cognitive processes (e.g., vigilance, encoding, maintenance) during spatial working memory (SWM) are deficient in ADHD (Lenartowicz et al., 2014). To demonstrate ways of interpreting and validating source solutions, we examined the scalp channel and source-resolved EEG data from two subject groups for 1) similar time/frequency patterns, 2) amount of overlapping variance accounted for in both sets of data, and 3) association with the cognitive/behavioral phenomena of interest. These comparisons illustrate the ability to compare channel and source measures and to test whether source-resolved analysis can yield more statistically robust information.
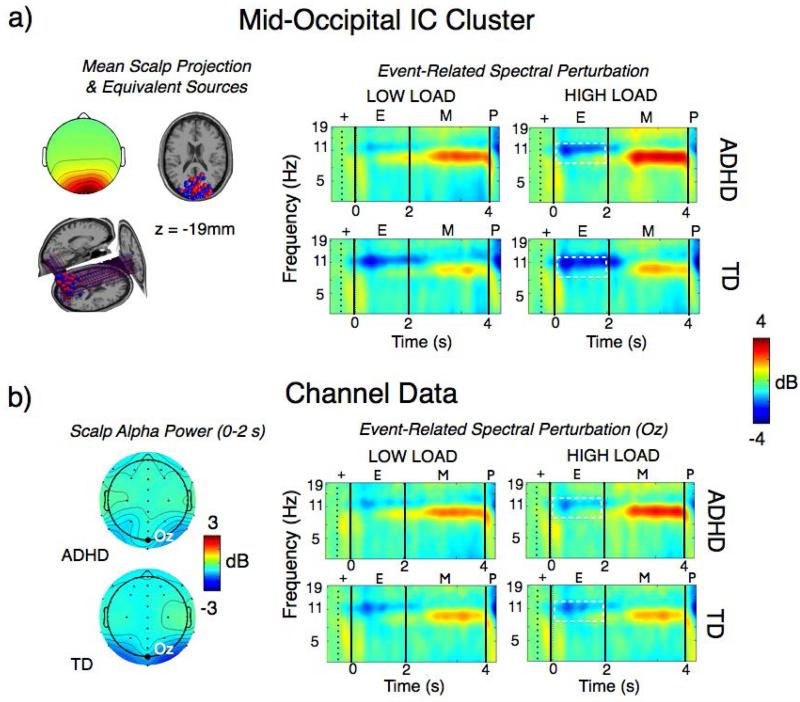
Participants were 43 ADHD and 37 typically developing (TD) controls, 7-14 years of age. ICA decomposition was used to identify 12 clusters of source-resolved independent component (IC) process activities that differed significantly across clinical groups (as validated using non-parametric bootstrap statistics with false discovery rate correction for multiple comparisons). These spatial IC clusters were localized to mid-frontal and central occipital cortical areas respectively (see Lenartowicz et al., 2014 for details). Because of space constraints, we present here only the results for an occipital source cluster centered at or near the occipital pole (x= 8 mm, y= −89 mm, z= −19 mm). The scalp projection of these occipital cluster processes was strongest at posterior occipital scalp electrodes. Thus, we first examined the ERSPs for both the mid-occipital source cluster and the overlying posterior scalp channels (e.g.., Oz; see Fig 3). By visual inspection, both the source and scalp ERSPs have similar spectral power patterns, strongly suggesting that their physiological sources are related.
Figure 3.
Comparison of source-resolved versus scalp channel data. (A) One cluster of Independent component (IC) source locations and corresponding cluster mean event-related spectral perturbation (ERSP) time/frequency measures for a spatial working memory task performed by 43 ADHD and 43 typically developing (TD) children 7–14 years of age. Results for low and high memory load conditions are shown. (B) Equivalent ERSP measures for nearest scalp electrode channel Oz. Topographical distribution of the alpha band activity (in black dashed boxes) during the Encoding (E) time window are shown on the cartoon heads
The occipital source cluster, however, shows stronger signal changes, particularly in alpha band desynchronization (blue) during the stimulus encoding (E) period (white dashed line boxes). Examination of event-related signal changes and overall variance accounted for by each suggests that the source and channel ERSP features do not index exactly the same functional source activations. The mean decrease in alpha power (8-12 Hz) from occipital sources during the Encoding time period accounted for 34% of variance (SE, 2.1%) in the ERSP for scalp channel Oz (Figure 3), and 43% of variance (SE, 2.8%) in the ERSP for the more inferior channel Iz (not shown). Thus, even though the ERSP patterns (in Fig. 3a and b) look similar, the most strongly contributing and most directly underlying independent brain sources accounted for only less than half the variance in the ERSPs for the most closely related EEG scalp channels.
We then assessed how much variance in the raw scalp channel data was accounted for by the back-projected IC source activities in the central occipital cluster. This tells us to what extent these cortical source activities were represented in the supervening scalp channel recordings. For the mid-occipital cluster, we evaluated the mean data variance accounted for across the three scalp channels showing the strongest subject group differences (Iz, O1, and O2). For the TD group, the IC source cluster on average accounted for 29.0% (SE=2.6) of scalp channel data variance; for the ADHD group this figure was 25.5% (SE=2.8). Thus, the scalp projection of this IC source cluster accounted for a low (but significant) portion of data variance in the EEG signals recorded at the nearest scalp channels. These results illustrate the expected breadth of contributions of a localized cortical IC source or source cluster across scalp channels.
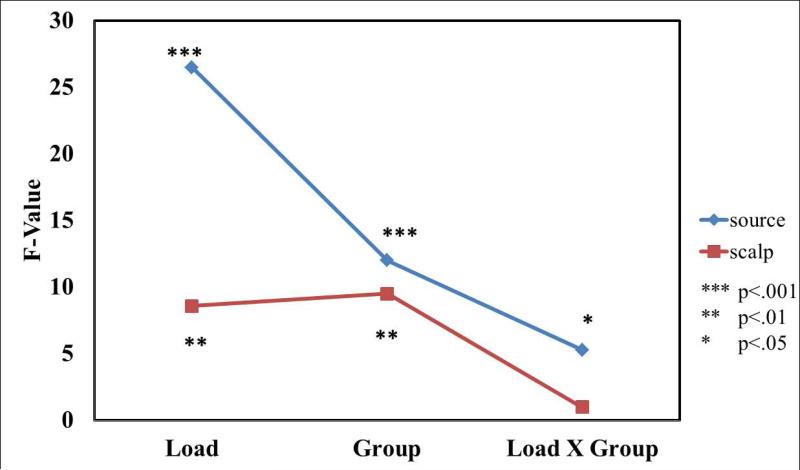
Finally, we evaluated the extents to which the central occipital IC source cluster ERSPs, versus ERSPs from the most closely related scalp channels, could account for diagnostic group differences in the spatial working task. Consistent with the alpha suppression scalp distribution pattern plotted in Figure 3B, we used an average of the supervening Iz, O1, and O2 channel ERSPs (in essence, a simpler-to-specify but less focused spatial filter) to estimate scalp channel-level dynamics. We performed separate repeated-measure ANOVAs, with diagnostic GROUP and memory LOAD as factors, first on the Encoding (E) period alpha band ERD (dotted box in Fig. 3) for the central occipital source cluster data and then for the same time window for the supervening scalp channel data. The results are presented in Fig. 4. For the effects of LOAD, GROUP and LOAD × GROUP, we observed significantly higher F values for the mid-occipital source cluster than for the most closely related channel data (FLOAD(1,69) = 26.5 vs. 8.6; FGROUP(1,69) = 12.0 vs. 9.5; FLOAD × GROUP(1,69) = 5.2 vs. 0.6 ). Thus, the unmixed EEG signals in the source-resolved central occipital cluster were more strongly associated with the clinical group differences of interest. These effects were partially masked in the scalp signals but were unmasked by ICA source separation and spatial source clustering.
Figure 4.
Independent component (IC) source activities in a midoccipital cortical source cluster are better correlated with ADHD diagnostic group and memory load differences than spectral power changes for the most closely related scalp channel signals, Iz, O1, and O2
These results demonstrate that it is possible to compare source-resolved and scalp-channel data so as to better understand their interrelationships. While the source cluster and scalp-channel alpha suppression patterns were correlated, the source-resolved IC cluster results represented a more coherent set of (cortical) processes, projected by volume conduction across many of the scalp EEG channels. ICA source measures can thus carry an SNR advantage that confers a distinct advantage for identifying EEG associations with cognitive and diagnostic effects that is not available from measures computed from only one or more of the scalp channel signals. Such testing of the added value of source-resolved versus scalp-channel data measures may be a desirable or necessary step for current and future EEG researchers considering the adoption of advanced signal processing methods who may feel reluctant to abandon their practice of basing their data analysis solely on scalp channel measures.
Future directions using advanced signal processing techniques for EEG and ERP analysis
We believe that adoption of a more comprehensive analysis approach combining time and frequency domain measures, analyzed with advanced signal processing techniques to separate and locate physiological sources of EEG signal differences have great potential for advancing child psychiatry research. Use of these techniques can allow researchers to more precisely understand the brain origins and mechanisms represented in collected scalp EEG data. Decomposition of scalp signals into separate non-brain artifact and cortical sources can facilitate identification of the most pertinent information, thus enabling researchers to develop more physiologically precise biomarkers of cortical dysfunction. Identification of more effective biomarkers can in turn improve diagnostic discrimination, increase understanding of pathophysiology, and potentially aide in monitoring and prediction of treatment response.
Increased use of advanced signal processing tools will allow several methodological advances in future studies of child psychopathology and treatment. Because child psychiatric disorders involve significant clinical heterogeneity in etiology, neurobiology, symptomatic presentation, developmental course, and treatment response, the methods and measures we use need to increase in complexity. To capture the heterogeneity of the underlying sources, an increased focus on multivariate classifiers is needed. The need to move beyond use of single measures of any type -- genetic, neurologic, cognitive or behavioral -- as biomarkers is clearly evident in the current child psychopathology literature. This makes our suggestion to combine of various EEG-derived measures (ERP, spectral power but also phase, coherence and others) increasingly relevant, not only for more comprehensive understanding of the data available in the signal but also to more accurately represent the heterogeneity within the samples and populations we seek to study. Overall, recent studies that have utilized multivariate classifiers have had higher classification rates than studies using single measures, regardless of type the measure used (candidate genes, cognitive tests, EEG/ERP, MRI). The need for multivariate classifiers makes sense given the diagnostic and population heterogeneity evident among child samples and the complexity of the dysfunctional brain processes that underlie behavioral differences.
To model complex relationships in the data, advanced statistical methods including machine learning, graph theory, discriminant functions, and logistic regression are also increasingly required. Many current studies focus on characterizing group mean differences only, however, this is insufficient to determine the usefulness of a candidate biomarker. Analyses that examine how well individual subjects can be typified, and the sensitivity, specificity, positive/negative predictive power, and receiver operating characteristics (ROC) of the single or combined measure must be used to evaluate the discriminative validity and utility of a candidate biomarker.
We anticipate that the use of advanced signal processing and statistical methods to identify multivariate classifiers will result in the identification of multidimensional landscapes of interpersonal differences defined by brain network dynamics, cognitive processes, and behavioral symptoms. This will be more likely to map onto translational domains of function than the current few diagnostic categories, and will define a richer landscape within which individual and group differences can be quantified. Such ‘landscapes’ can be thought of as multidimensional extensions of the ‘spectrum’ concept of individual differences currently used in autism research. Ideally, such multidimensional approaches will open a richer space of physiologically defined differences in brain dynamics in which patient heterogeneity is captured and quantified rather than ignored. Better understanding of heterogeneity will allow more accurate understanding of each individual relative to others with respect to development, diagnosis, treatment response, and cognitive function.
These methodological directions will not only allow identification of better, more effective biomarkers and a reconceptualization of the diagnostic and treatment target from being mediated by a single clinical category label to being defined by an individual's position in a varied but coherent landscape of individual differences. These new methods also increase the feasibility of a wider range of EEG studies. EEG study paradigms have been limited by traditional methods of EEG data analyses sensitive to differences across EEG systems and sites (limiting multisite EEG studies) and to movement-related artifacts (limiting mobile EEG studies). Refined electrical head models based on structural MR images (Akalin Acar & Makeig, 2010) will be more commonly available, allowing ever more accurate localization of effective EEG sources within and then across individuals. This will facilitate multimodal brain imaging studies and the ability to integrate the excellent temporal resolution of source-resolved EEG with information derived from other imaging modalities such as fMRI, diffusion tensor imaging (DTI), and MR spectroscopy. Finally, the currently scattered resource of existing, carefully collected EEG data sets (each analyzed until now only for the amplitudes and latencies of a few ERP peaks or power in frequency bands) can be collected and subjected to collective data mining efforts that make use of combined sample sizes in the thousands or more.
Conclusions
EEG recording and analysis is currently experiencing a renaissance in interest and in both range and depth of applications, particularly in the search for endophenotypes and biomarkers for etiologic factors, individual diagnosis, risk for disorder, and treatment response. The field of child psychopathology research would benefit from increased integration of the various EEG methods previously used in isolation to measure and model EEG signals. Improved characterization of EEG signals in terms of underlying cortical source processes, and incorporation of their time/frequency characteristics will advance EEG as a sensitive and physiologically specific functional brain imaging modality for studying cortical activation patterns underlying child psychopathology. Use of these methods will likely lead to the identification of multivariate classifiers using advanced statistical analytic techniques that will result in multidimensional landscapes of inter-individual differences defined by brain network dynamics, cognitive processes, and behavioral symptoms. Similarly, identifying network/circuit level models underlying functional domains, necessary for RDOC models, will require comparison of source-resolved EEG with other measures and models of brain function. These and other rapidly evolving advances in data collection, signal processing and statistical analysis will allow the next generation of EEG studies (multisite, multimodal imaging, mobile EEG, brain-computer interface, meta-analytic, etc.) to flourish and to extend our insights into both normal and pathological brain processes and development.
Key points.
There has been a shift to common genetic and mechanistic factors across psychiatric disorders; electroencephalography (EEG) measures are well suited to be used as putative biomarkers within this approach.
In order to be more effective biomarkers of neural circuit activity, EEG research must successfully contend with controversies within the field regarding: 1. the use of different EEG measurements (event-related potentials, quantitative EEG, and time frequency measures); and 2. analysis of cortical sources rather than scalp signals.
Advances in signal processing software are making it increasingly feasible for researchers to successfully address both controversies within their own research.
Future directions using advanced signal processing and statistical methods will result in identification of better, more effective biomarkers and a reconceptualization of a diagnostic or treatment target to a map of translational domains that will define a richer landscape within which individual and group differences can be quantified.
Acknowledgments
This work was supported by National Institutes of Health grants: NS80160 (SKL) MH101282 (SKL), NS47293 (SM) and The Swartz Foundation (SM).
Footnotes
An annotated list of programs can be obtained from the first author.
The authors have declared that they have no competing and potential conflicts of interest.
References
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proc Natl Acad Sci U S A. 2011;108(26):10702–10707. doi: 10.1073/pnas.1014528108. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalin Acar Z, Makeig S. Neuroelectromagnetic forward head modeling toolbox. Journal of Neuroscience Methods. 2010;190(2):258–270. doi: 10.1016/j.jneumeth.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition Author; Arlington, VA: 2013. [Google Scholar]
- Arns M, Conners CK, Kraemer HC. A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. J Atten Disord. 2013;17(5):374–383. doi: 10.1177/1087054712460087. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71(2):98–104. doi: 10.1016/j.biopsych.2011.08.023. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Bender S, Banaschewski T, Roessner V, Klein C, Rietschel M, Feige B, Laucht M. Variability of single trial brain activation predicts fluctuations in reaction time. Biol Psychol. 2015;106:50–60. doi: 10.1016/j.biopsycho.2015.01.013. doi: 10.1016/j.biopsycho.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Berger H. Uber das elecktrenkephalogramm des meschen. Archiv Fur Psychiatrie Und Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry Res. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definition Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Buyck I, Wiersema JR. Resting electroencephalogram in attention deficit hyperactivity disorder: developmental course and diagnostic value. Psychiatry Res. 2014;216(3):391–397. doi: 10.1016/j.psychres.2013.12.055. doi: 10.1016/j.psychres.2013.12.055. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80(3):751–764. doi: 10.1016/j.neuron.2013.10.002. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics, C. Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Wray NR. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. doi: 10.1038/ng.2711. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJ. From genotype to EEG endophenotype: a route for post-genomic understanding of complex psychiatric disease? Genome Med. 2010;2(9):63. doi: 10.1186/gm184. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Palmer JA, Onton J, Oostenveld R, Makeig S. Independent EEG sources are dipolar. PLoS One. 2012;i7(2):e30135. doi: 10.1371/journal.pone.0030135. doi: 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ. Neurodynamics: An exploration in mesoscopic brain dynamics. Springer; Berlin: 2000. [Google Scholar]
- Gasser T, Jennen-Steinmetz C, Sroka L, Verleger R, Mocks J. Development of the EEG of school-age children and adolescents. II. Topography. Electroencephalogr Clin Neurophysiol. 1988;69(2):100–109. doi: 10.1016/0013-4694(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, Rijsdijk F. The genetic and environmental influences of event-related gamma oscillations on bipolar disorder. Bipolar Disord. 2011;13(3):260–271. doi: 10.1111/j.1399-5618.2011.00925.x. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich H, Busch K, Studer P, Erbe K, Moll GH, Kratz O. Refining the picture of reduced alerting responses in ADHD - a single-trial analysis of event-related potentials. Neurosci Lett. 2014;582:49–53. doi: 10.1016/j.neulet.2014.08.050. doi: 10.1016/j.neulet.2014.08.050. [DOI] [PubMed] [Google Scholar]
- Jasper H, Solomon P, Bradley C. Electroencephalographic analyses of behavior problem children. Am J Psychiatry. 1938;95(3):641. [Google Scholar]
- John ER, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210(4475):1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Clarke AR. Ten years on: a follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2013;124(4):644–657. doi: 10.1016/j.clinph.2012.09.006. doi: 10.1016/j.clinph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J Child Psychol Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry. 2011;68(2):181–189. doi: 10.1001/archgenpsychiatry.2010.192. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Delorme A, Walshaw PD, Cho AL, Bilder RM, McGough JJ, Loo SK. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J Neurosci. 2014;34(4):1171–1182. doi: 10.1523/JNEUROSCI.1765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Loo SK. Use of EEG to diagnose ADHD. Curr Psychiatry Rep. 2014;16(11):498. doi: 10.1007/s11920-014-0498-0. doi: 10.1007/s11920-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti MD, Valko L, Muller UC, Dohnert M, Drechsler R, Steinhausen HC, Brandeis D. Diagnostic value of resting electroencephalogram in attention-deficit/hyperactivity disorder across the lifespan. Brain Topogr. 2013;26(1):135–151. doi: 10.1007/s10548-012-0258-6. doi: 10.1007/s10548-012-0258-6. [DOI] [PubMed] [Google Scholar]
- Loo SK, Cho A, Hale TS, McGough J, McCracken J, Smalley SL. Characterization of the theta to beta ratio in ADHD: identifying potential sources of heterogeneity. J Atten Disord. 2013;17(5):384–392. doi: 10.1177/1087054712468050. doi: 10.1177/1087054712468050. [DOI] [PubMed] [Google Scholar]
- Loo SK, Hale TS, Hanada G, Macion J, Shrestha A, McGough JJ, Smalley SL. Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;29(4):368–377. [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH. The cortical source of the alpha rhythm. Neuroscience Letters. 1977;6:237–241. doi: 10.1016/0304-3940(77)90024-6. [DOI] [PubMed] [Google Scholar]
- Luchinger R, Michels L, Martin E, Brandeis D. Brain state regulation during normal development: Intrinsic activity fluctuations in simultaneous EEG-fMRI. Neuroimage. 2012;60(2):1426–1439. doi: 10.1016/j.neuroimage.2012.01.031. doi: 10.1016/j.neuroimage.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993;86(4):283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Touretzky D, Mozer M, Hasselmo M, editors. Independent component analysis of electroencephalographic data. Advances in Neural Information Processing Systems. 1996:145–151. [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Makeig S, Onton J. ERP features and EEG dynamics: An ICA perspective. In: Luck S, Kappenman E, editors. Oxford Handbook of Event-Related Potential Components. Oxford University Press; 2009. [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295(5555):690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Makeig S, Tsuang MT. In search of biomarkers in psychiatry: EEG-based measures of brain function. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(2):111–121. doi: 10.1002/ajmg.b.32208. doi: 10.1002/ajmg.b.32208. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Palmer JA, Rijsdijk F, Makeig S. Genetic overlap between evoked frontocentral theta-band phase variability, reaction time variability, and attention-deficit/hyperactivity disorder symptoms in a twin study. Biol Psychiatry. 2014;75(3):238–247. doi: 10.1016/j.biopsych.2013.07.020. doi: 10.1016/j.biopsych.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Klein DN. Increased Ern Predicts the Onset of Anxiety Disorders in Children. Psychophysiology. 2014;51:S7–S7. [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev Cogn Neurosci. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15(1):136–144. doi: 10.1037//0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- Mueller A, Candrian G, Kropotov JD, Ponomarev VA, Baschera GM. Classification of ADHD patients on the basis of independent ERP components using a machine learning system. Nonlinear Biomed Phys. 2010;4(Suppl 1):S1. doi: 10.1186/1753-4631-4-S1-S1. doi: 10.1186/1753-4631-4-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen T, Acar ZA, Worrell G, Makeig S. Modeling cortical source dynamics and interactions during seizure. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1411–1414. doi: 10.1109/IEMBS.2011.6090332. doi: 10.1109/IEMBS.2011.6090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazhvani AD, Boostani R, Afrasiabi S, Sadatnezhad K. Classification of ADHD and BMD patients using visual evoked potential. Clin Neurol Neurosurg. 2013;115(11):2329–2335. doi: 10.1016/j.clineuro.2013.08.009. doi: 10.1016/j.clineuro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Ogrim G, Kropotov J, Hestad K. The QEEG theta/beta ratio in ADHD and normal controls: Sensitivity, specificity, and behavioral correlates. Psychiatry Res. 2012 doi: 10.1016/j.psychres.2011.12.041. doi: S0165-1781(11)00825-0 [pii] 10.1016/j.psychres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr Clin Neurophysiol. 1979;46(2):138–146. doi: 10.1016/0013-4694(79)90063-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A, Maresch H. Amplitude of evoked potentials and degree of event-related desynchronization (ERD) during photic stimulation. Electroencephalogr Clin Neurophysiol. 1979;47(1):21–30. doi: 10.1016/0013-4694(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Saville CW, Feige B, Kluckert C, Bender S, Biscaldi M, Berger A, Klein C. Increased reaction time variability in attention-deficit hyperactivity disorder as a response-related phenomenon: evidence from single-trial event-related potentials. J Child Psychol Psychiatry. 2014 doi: 10.1111/jcpp.12348. doi: 10.1111/jcpp.12348. [DOI] [PubMed] [Google Scholar]
- Shaikh M, Dutt A, Broome MR, Vozmediano AG, Ranlund S, Diez A, Bramon E. Sensory gating deficits in the attenuated psychosis syndrome. Schizophr Res. 2015;161(2-3):277–282. doi: 10.1016/j.schres.2014.12.021. doi: 10.1016/j.schres.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Smalley SL, Loo SK, Yang MH, Cantor RM. Toward localizing genes underlying cerebral asymmetry and mental health. Am J Med Genet B Neuropsychiatr Genet. 2005;135B(1):79–84. doi: 10.1002/ajmg.b.30141. doi: 10.1002/ajmg.b.30141. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42(6):691–697. doi: 10.1111/j.1469-8986.2005.00352.x. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23(5):440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Snyder SM, Quintana H, Sexson SB, Knott P, Haque AF, Reynolds DA. Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Res. 2008;159(3):346–358. doi: 10.1016/j.psychres.2007.05.006. doi: S0165-1781(07)00156-4 [pii] 10.1016/j.psychres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Tarasenko MA, Swerdlow NR, Makeig S, Braff DL, Light GA. The auditory brainstem response to complex sounds: a potential biomarker for guiding treatment of psychosis. Front Psychiatry. 2014;5:142. doi: 10.3389/fpsyt.2014.00142. doi: 10.3389/fpsyt.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdar W, Solberg LC, Gauguier D, Cookson WO, Rawlins JN, Mott R, Flint J. Genetic and environmental effects on complex traits in mice. Genetics. 2006;174(2):959–984. doi: 10.1534/genetics.106.060004. doi: 10.1534/genetics.106.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28(8):1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Hermens DF, Thein T, Clark CR, Cooper NJ, Clarke SD, Kohn MR. Using brain-based cognitive measures to support clinical decisions in ADHD. Pediatr Neurol. 2010;42(2):118–126. doi: 10.1016/j.pediatrneurol.2009.08.010. doi: 10.1016/j.pediatrneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]