Abstract
Cardiovascular risk factors develop in childhood and adolescence. This enumerative review addresses whether sleep characteristics, including sleep duration, continuity, quality, and daytime sleepiness, are associated with cardiovascular risk factors in young people. Thirty-nine studies were identified that examined the following risk factors: metabolic syndrome, glucose and insulin, lipids, blood pressure, and cardiovascular responses to psychological stressors. Due to the availability of other reviews, 16 longitudinal studies of obesity published in 2011 and later were also included in this report. Excluded from the review were studies of participants with suspected or diagnosed sleep disorders and reports from sleep deprivation experiments. Combining studies, evidence was strongest for obesity, followed by glucose, insulin, blood pressure (especially ambulatory blood pressure) and parasympathetic responses to psychological stressors. There was little evidence for metabolic syndrome cluster, lipids, and blood pressure responses to psychological stressors. The more positive associations were obtained for studies that incorporated objective measures of sleep and included adolescents. The foundational evidence is almost entirely cross-sectional, except for work on obesity. In summary, available evidence suggests that the associations between sleep characteristics and cardiovascular risk vary by risk factor. It is time to conduct studies to determine antecedent and consequent relationships and to expand risk factors to include markers of inflammation.
Keywords: sleep, children, cardiovascular risk factors, lipids, blood pressure, obesity, glucose, insulin
1. Introduction
Elevated risk factors for cardiovascular diseases are apparent in children and adolescents and relate to subclinical cardiovascular disease (CVD) later in life. For example, 6% of female adolescents and 20% of male adolescents had high fasting blood glucose (≥ 100 mg/dl) in the National Health Administration Examination Survey (NHANES) study.1 Up to 9.8% of children and adolescents had systolic hypertension, and up to 7.1% had diastolic hypertension in an analysis of 58,698 children and adolescents enrolled in 11 studies.2 Blood pressure (BP) at age 13 predicted adulthood BP at age 24, in addition to elevated lipids and glucose.3 Autopsy studies of young adults who died from traumas reported a linear relationship between number of cardiovascular risk factors and intima surface covered with fatty streaks in the coronary arteries: 0, 1, 2, and 3/4 risk factors had, respectively, 1.3%, 2.5%, 7.9%, and 11.0%; the extent of fibrous-plaque lesions in the coronary arteries was 12 times as great in persons with 3 or 4 risk factors, compared to those with none.4 The greater the number of risk factors (cigarette smoking, elevated lipids, BP, and body mass index (BMI)) in adolescence the greater carotid intima medial thickness in both men and women in adulthood.5 A combination of risk factors among children was associated with reduced carotid artery elasticity and increased stiffness.6;7 The metabolic syndrome, a combination of elevated BP, triglycerides, waist circumference, glucose, and low high density lipoprotein levels (HDL-C), in childhood and adolescence predicted CVD in adulthood.8
A burgeoning epidemiological literature suggests that sleep patterns are related to CVD morbidity and mortality in adulthood.9–13 In particular, either short or very long sleep duration, fragmented sleep, and insomnia-like symptoms have been connected to risk for CVD. Supporting these epidemiological data are a series of experiments depriving healthy participants of sleep for varying lengths of time and observing acute changes in cardiovascular risk factors, including BP, heart rate, glucose and insulin metabolic indices, and inflammation.14–16 In recognition of the early origins of CVD, the extent to which sleep patterns are related to cardiovascular risk factors in children and adolescents has recently been a focus of investigation. The primary purpose of the present paper is to synthesize evidence on the association between sleep characteristics of young people and their cardiovascular risk factors, in particular, metabolic syndrome, glucose and insulin, lipids, BP, and heart rate and BP responses to stressful tasks, e.g., mental arithmetic or giving a speech. Stress-induced cardiovascular responses are included because of their association with risk with incident hypertension and CVD.17
Another purpose of the paper is to update prior reviews on the relationship between obesity and sleep characteristics. A 2008 meta-analysis of 17 studies found that the risk of being overweight or obese decreased by 9% for each additional hour of sleep, with effects stronger in boys than in girls.18 Two literature reviews of sleep and obesity have been published since that time. Magee and Hale reported that all 7 longitudinal studies they reviewed observed a relationship between short sleep and increased weight and/or adiposity,19 while Guidolin and Gradisar20 reported that neither of the 2 longitudinal studies they identified observed a relationship. In the current review, we summarize the longitudinal studies that were published after January 2011 and were not included in the two previous reviews.
We chose to synthesize available evidence based on an enumerative or descriptive review, as opposed to using meta-analytic techniques, for several reasons. First, we did not want to combine all cardiovascular risk factors into one quantitative analysis because associations may vary by risk factor and by sleep characteristic. Even within one type of cardiovascular risk factor or sleep characteristic, there can be quite different approaches to assessment. Although the study findings could be pooled initially to test for heterogeneity across studies, our review is aimed at identifying which risk factors seem to be linked. A compelling reason for meta-analysis is the increased power that results in combining individual studies with relatively small sample sizes. In this review multiple studies have large sample sizes and should have sufficient power to be considered in an enumerative review.
The sleep characteristics we reviewed are those considered to be the major dimensions of sleep health, i.e., duration, continuity, perceived quality, and daytime sleepiness.21 We also included studies on sleep architecture when available and did not include sleep disordered breathing (SDB). Another major dimension of sleep health, timing, was not reviewed because of few studies in children and adolescence. Our general hypothesis is that short sleep, less continuous sleep, poorer quality, and more daytime sleepiness would be associated with elevated levels of cardiovascular risk factors. Because long sleep has also been associated with elevated CV risk in adulthood, we also identified studies that tested for a curvilinear relationship with risk factors. After summarizing the evidence for each risk factor, the review identifies subgroups that have stronger associations with sleep characteristics. The paper also highlights methodological issues and identifies directions for future research.
2. Methods
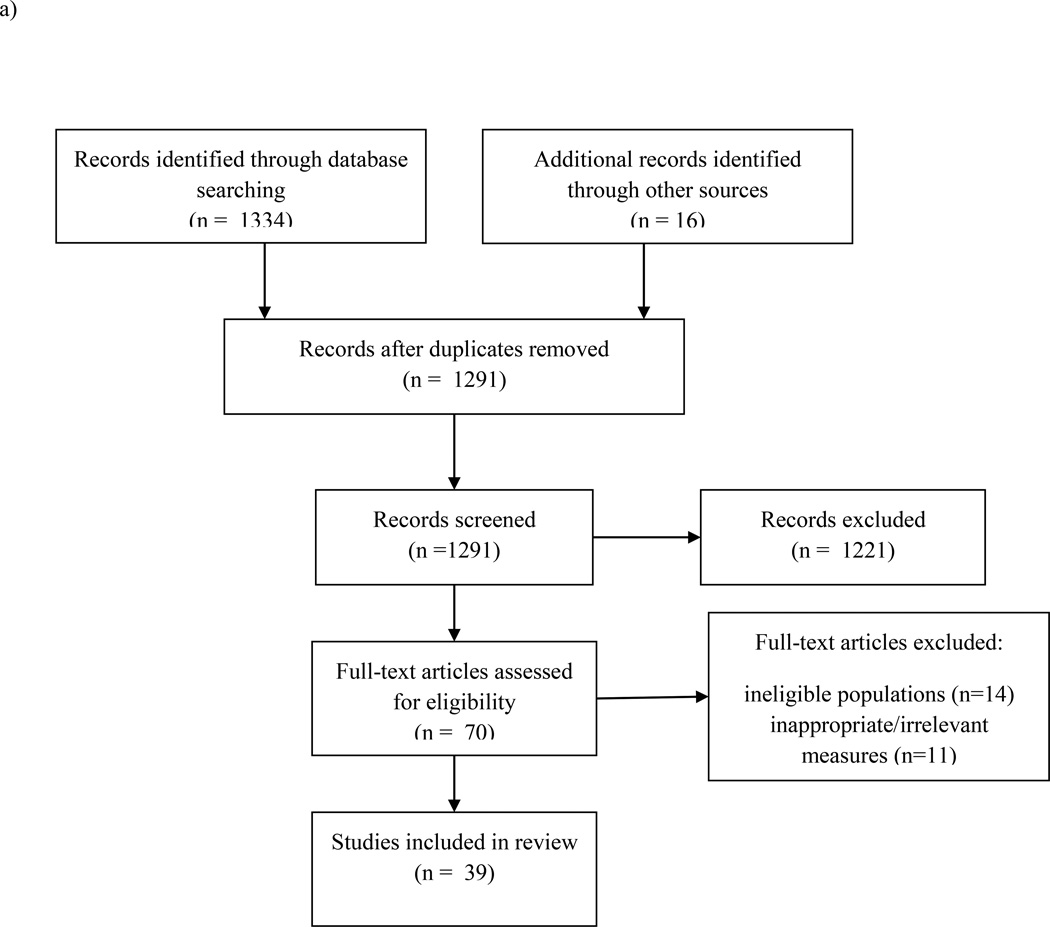
We used PubMed and PsycInfo to search for articles. We first searched for articles examining sleep and cardiovascular risk factors, excluding obesity, using the following combination of search terms: (“sleep” OR “actigraphy”) joined by “AND” with a cardiovascular risk factor (“metabolic syndrome,” OR “lipids,” OR “cholesterol,” OR “blood pressure,” OR “insulin,” OR “glucose,” OR “heart rate variability,” OR “cardiovascular”). “NOT” qualifiers included “apnea” and “breathing.” Age limiters (0 years – 29 yrs) were used to refine the search. Reference lists were used to identify additional articles. We included studies that a) had a mean sample age of 24 or younger, in accordance with the Centers for Disease Control and Prevention’s22 definition of youth, and b) investigated sleep duration, continuity, quality, or sleepiness in relation to one or more of the cardiometabolic risk factors identified above. We also included studies on sleep architecture when available. Sleep continuity refers to the consolidation of one’s sleep throughout the night (e.g., sleep latency, sleep efficiency, wake after sleep onset), and sleep quality refers to the subjective assessment of how good or poor one’s sleep is.21 Excluded from review were total or partial sleep deprivation experiments and studies that focused exclusively on clinical samples (e.g., psychiatric) or participants with sleep disorders. Figure 1a displays the number of records identified, screened, and excluded. Thirty-nine studies met criteria for inclusion. These studies are listed in Table 1 according to category of risk factors, with those reporting multiple risk factors listed first. Within risk factor category, studies are listed by year of publication.
Figure 1.
a. Flow diagram of study selection for sleep and cardiovascular risk factors.
Note: Included studies had a) had a mean sample age of 24 or younger, and b) investigated sleep duration, continuity, quality, timing, or sleepiness in relation to one or more of the cardiometabolic risk factors (metabolic syndrome, glucose/insulin, lipids, blood pressure, cardiovascular stress responses). We also included studies on sleep architecture when available. Excluded from review were total or partial sleep deprivation experiments and studies that focused exclusively on clinical samples or participants with sleep disorders.
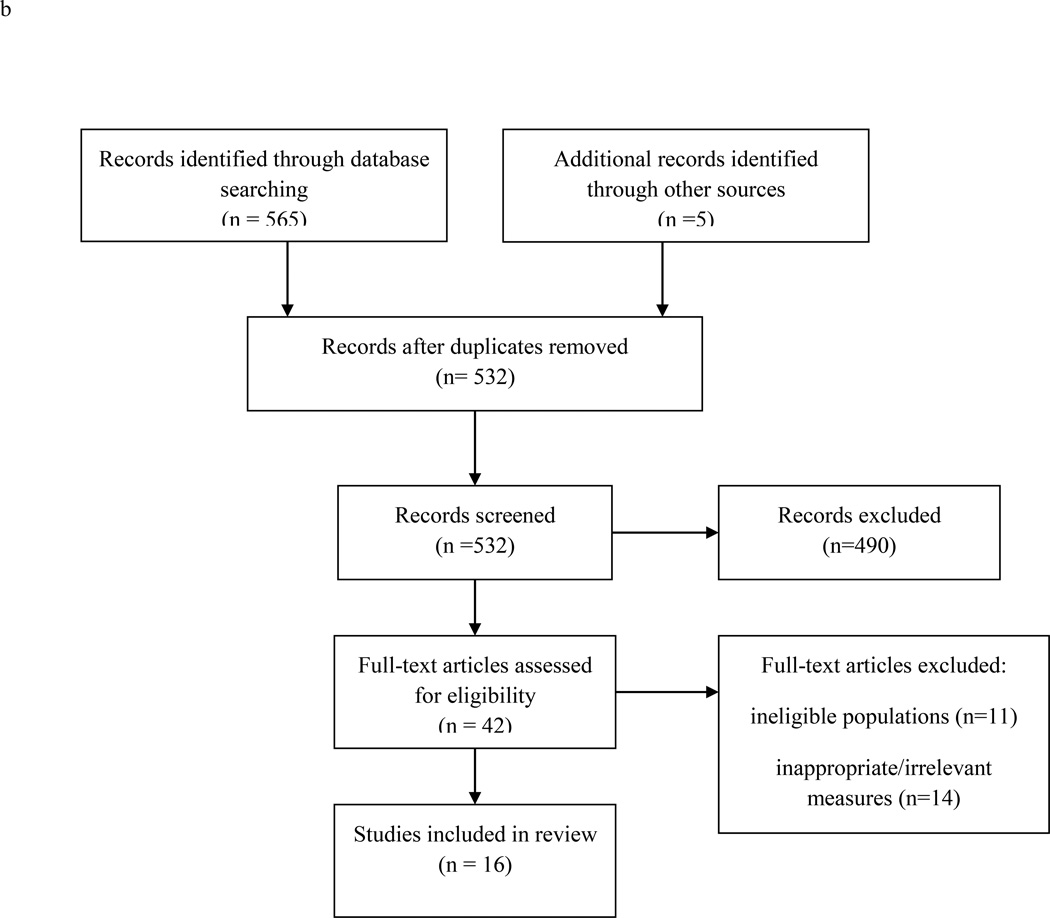
b. Flow diagram of longitudinal study selection for sleep and obesity.
Note: Included studies a) had a mean sample age of 24 or younger, b) were published in 2011 or later c) were not included in recent reviews of sleep and obesity in youth, and d) used a longitudinal design to examine sleep as a predictor of body mass index or adiposity. Excluded from review were total or partial sleep deprivation experiments and studies that focused exclusively on clinical samples or participants with sleep disorders.
Table 1.
Sleep and Cardiovascular Risk Factors organized by Category of Risk
| First Author | Sample | Study Design |
Sleep Measures | CV Risk Factors | Covariates | Results |
|---|---|---|---|---|---|---|
| Metabolic Syndrome and Multiple Risk Factors | ||||||
| IglayReger23 | 37 obese U.S. adolescents, 54.1% female, ages 11–17; M =14.0 ± 0 yrs |
cross- sectional |
≥ 5 nights actigraphy- assessed sleep duration |
MetS composite risk score |
BMI, physical activity duration and intensity |
↓sleep duration ↑ MetS composite risk score |
| Lee 26 | 1187 Korean adolescents, 46.9% female, ages 12–18; M =15.0 ± .1 yrs |
cross- sectional |
self-reported sleep duration |
MetS and MetS components |
age, sex, household income, caloric intake, physical activity |
↓sleep duration ↑BP, ↑BMI, ↑waist circumference ↓ triglycerides NS glucose, HDL-C, MetS |
| Azadbakht24 | 5528 Iranian children ages 10–18; M =14.7 yrs |
cross- sectional |
parental report of sleep duration |
BP, lipids, glucose, BMI %, physical activity |
SES, family history, physical activity, BMI, age |
all NS for multivariate analyses |
| Berentzen36 | 1481 Dutch children ages 11–12; M =12.7 ± .4 yrs |
cross- sectional |
self-report time in bed on school day, sleep pattern, nighttime awakenings, trouble falling asleep, daytime sleepiness |
cholesterol, HbA1c, BP |
age, height, maternal education, puberty, screen time |
↓ time in bed ↑ BMI, waist circumference ↑daytime sleepiness ↓HDL-C, ↑TC/HDL-C in girls only. NS boys no effects for BP, HbA1c |
| Rey-Lopez37 | 699 European adolescents, 51.6% female, ages 12.5- 17.5; M =14.8 yrs |
cross- sectional |
self-reported sleep duration |
HOMA-IR, triglycerides, TC, HDL-C, systolic BP |
age, sex, SES, physical activity |
all NS |
| Countryman27 | 367 U.S. adolescents 27% female, 45.8% Hispanic, 30.8% Black, ages 15–17; M =16.1 ± .7 yrs |
cross- sectional |
latent sleep factor (composed of self-reported sleep duration over past 7 days, fatigue, & sleep quality) |
latent MetS factor (composed of obesity, insulin resistance, lipids, and BP) |
gender, parent education |
In structural equation model, sleep was indirectly associated with increased risk of MetS via decreased aerobic fitness |
| Narang39 | 4104 Canadian adolescents;51% male, M =14.6 ± .5 yrs |
cross- sectional |
self-reported sleep quality self-reported sleep duration |
TC, HDL-C, non- HDL-C, prehypertension (≥90 - <95th % based on age, sex, height) or hypertension (≥99th %) |
sex, family history of CVD, adiposity, nutrition, physical activity, screen time |
↓sleep quality ↑ non-HDL-C ↑ hypertension NS TC NS HDL-C ↓self-report duration all NS |
| Kong25 | 2053 Hong Kong children ages 6–18 years, M = 13.3 yrs |
cross- sectional |
self-reported sleep duration in full sample, actigraphy for 24 hrs in 138 children selected on obesity |
lipids, metabolic syndrome |
age, gender, BMI, pubertal stage |
↓ self-reported duration ↑ TC and LDL-C in multivariate analyses in secondary students, NS for primary students; no report for actigraphy sleep, BP, glucose |
| Sung38 | 133 obese U.S. adolescents in tertiary care weight management clinic, 66% female; ages 10–16 yrs. M=13.2 ± 1.8 yrs |
cross- sectional |
self-reported sleep duration, parent-reported sleep duration, 7 nights actigraphy- assessed sleep duration |
MetS, MetS components (waist circumference, triglycerides, BP, HDL-c, glucose), HOMA-IR |
age, gender, race, SES, BMI, obstructive apnea |
↓self-report duration ↓ triglycerides NS for all others ↓parent-report duration ↓ HDL cholesterol NS for all others ↓actigraphy duration ↓ triglycerides NS for all others |
| Hitze35 | 414 German children ages 6–20; M =13.0 ± 3.4 yrs |
cross- sectional |
self-report after 11, parent report before 11, cutoffs based on age |
manual BP, lipids, glucose, leptin, adiponectin, HOMA-IR |
age |
↓sleep duration ↑insulin, HOMA-IR, leptin in girls. NS after adjustment for waist circumference; NS in boys |
| Gangwisch40 | 14257 U.S. adolescents in ADD Health, 51% female, grades 7–12 at baseline |
longitud- inal |
self-report sleep duration at 2 times averaged |
self-report of doctor diagnosing high cholesterol 6–7 years later |
physical activity, emotional distress, BMI groups, age, sex, race, alcohol and smoking |
↓sleep duration ↑cholesterol in females; NS in males. Test for sex interaction NS |
| Glucose and Insulin Metabolism Studies | ||||||
| Androutsos29 | 2026 Greek children, 50.1% female, ages 9–13 |
cross- sectional |
parental report of sleep duration |
HOMA-IR | gender, Tanner stage, waist circumference, parent BMI, SES, birth weight |
children with an unhealthy “lifestyle pattern,” consisting of ↓ sleep duration, ↑ screen time, and ↑sugary drink consumption, had ↑HOMA-IR |
| Zhu34 | 118 healthy Chinese children and adolescents; 55.1% female, moderate-to-severe OSA excluded M=13.1 ± 3.3 yrs |
cross- sectional |
1 night PSG -TST -sleep efficiency |
2-hr oral glucose tolerance test, insulin sensitivity (Matsuda index) |
age, gender, BMI, pubertal status, AHI |
↓TST ↑ glucose levels ↓ insulin sensitivity ↓sleep efficiency ↑ glucose levels ↓ insulin sensitivity ↓% stage 3 ↑glucose ↓insulin sensitivity |
| Matthews 32 | 245 healthy U.S. adolescents, 56% African American, 53% female; ages 14–19 M = 15.7±1.3 yrs |
cross- sectional |
diary & actigraphy sleep duration for 1 week, fragmentation |
HOMA-IR, glucose | age, race, gender, BMI, waist circumference |
↓ nocturnal sleep ↑HOMA-IR stronger in males, effect due to weekday sleep ↑fragmentation ↑glucose |
| Javaheri 30 | 471 U.S. adolescents in Cleveland Children’s Sleep and Health study; 50.7% female, 42.7% minority race; ages 13–19 M =15.7 ± 2.2 yrs |
cross- sectional |
actigraphy- assessed sleep duration |
HOMA-IR | Model 2: age, sex, race, physical activity, preterm history Model 3: above + waist circumference |
Model 2: curvilinear association of sleep duration with ↑HOMA-IR Model 3: only long sleep duration related to ↑HOMA-IR |
| Koren 31 | 62 obese U.S. adolescents, 54.8% African American, 37.1% White, 55% female; ages 8–17.5 M = 14.4 ± 2.1 yrs |
cross- sectional |
in clinic PSG TST, sleep stages |
HOMA-IR, OGTT, IGI, WBISI |
extent of obesity, OSA |
curvilinear association of TST with ↑glucose and HbA1c; NS with HOMA-IR, WBISI, IGI; ↑N3 ↑IGI and AIRg (i.e., beta cell function) |
| Tian33 | 619 obese & 617 nonobese Chinese children, matched by age; ages 3–6 M = 5.3 ± .9 yrs |
cross- sectional |
parent-reported sleep duration |
fasting glucose, hyperglycemia (fasting glucose ≥ 100 mg/dL) |
BMI, age, sex, birth weight, gestational age, systolic BP, parents’ education and BMI, breast- feeding, timing of food introduction, disease in past month, diet, sweetened beverage consumption, TV viewing, physical activity |
↓ sleep duration ↑ glucose ↑ hyperglycemia in obese only Glucose NS after adjusting for waist circumference |
| Flint28 | 40 obese U.S. children from weight clinic (32 with SDB), ages 3.5–18.5 M =12.3 ± 4.2 yrs |
cross- sectional |
in clinic PSG for sleep duration, efficiency, AHI, % stages |
OGTT insulin and glucose, HOMA-IR, WBISI |
None |
<6 hr sleep ↑fasting and peak insulin, HOMA-IR, WBISI, and ↓ % REM in univariate analyses; did not report sleep efficiency |
| Blood Pressure Studies | ||||||
| Kuciene 44 | 6940 Lithuanian children ages 12–15; M =13.4 yrs |
cross- sectional |
self-report TST | SBP, DBP (oscillometric) ≥90th, ≥95th %ile based on age, sex, height |
BMI groups, physical activity, smoking, age, sex |
Compared to ≥8hr, ↓ sleep ↑ risk for ≥90th, ≥95th % BP |
| Meininger45 | 366 U.S. adolescents 53.6% female, 37% Black, 31% Hispanic, 29% White, ages 11–16 |
cross- sectional |
24-hr actigraphy- assessed sleep (nighttime and daytime sleep duration) |
24-hr ambulatory SBP and DBP on a school day |
age, sex, racial/ethnic group, mother’s education, BMI, sexual maturation, physical activity, position and location during BP reading |
↓nighttime sleep duration ↑ ambulatory SBP NS ambulatory DBP ↓daytime sleep duration ↑ ambulatory SBP ↑ ambulatory DBP |
| Paciência52 | 1771 Portuguese 13 year-olds, 53.5% female |
cross- sectional |
self-reported sleep duration |
prehypertension (BP > 90th %ile for sex, age, and height) |
females: BMI, caffeine intake, depression males: BMI, caffeine intake, sports |
↑sleep duration females: ↑ BP males: NS |
| Archbold41 | 334 U.S. Hispanic & white children, 6–11 years; M =9.03 ± 1.63 |
longitud- inal for 5 years |
in-home PSG- based SDB, TST |
Obesity, BP ? | sex, ethnicity, age, change in obesity |
↑obesity and ↓TST related to ? SBP; NS DBP |
| Mezick46 | 246 healthy U.S. adolescents; 53.3% female, 56.5% Black; ages 14–19, M =15.7 ± 1.3 yrs |
cross- sectional |
7 nights actigraphy- assessed sleep duration; efficiency |
24 hr ambulatory BP, nighttime ambulatory BP, daytime ambulatory BP |
age, sex, race, BMI |
↓sleep duration ↑ 24-hr SBP ↑ 24-hr DBP ↑ nighttime SBP ↑ nighttime DBP NS daytime SBP, DBP efficiency not related to BP |
| Guo54 | 4902 Chinese children, ages 5–18, M = 10.9 ± 2.7 yrs |
cross- sectional |
parental report of TST |
BP (mercury column) ≥ 90% for age, sex, height, or ≥ 120/80, hypertensive ≥ 95% |
age, BMI, waist circumference, physical activity |
↓TST ↑ SBP, DBP levels among boys 11–14 ↑ DBP among girls 11–14 ↓ DBP among boys 5–10 years. NS in other age groups |
| Bayer49 | 7701 German children, 49% female, ages 3–10 |
cross- sectional |
parental report of sleep duration standardized within age group |
MAP (oscillometric) |
BMI (age-, sex- specific), parental report of physical activity |
↓ sleep duration ↑MAP, NS in multivariate analysis |
| Javaheri 42 | 238 U.S. adolescents without clinical sleep apnea in Cleveland Children’s Sleep and Health study; 48.3% female, 45% White; M = 13.7± .7 yrs |
cross- sectional |
5–7 nights of actigraphy -sleep duration -sleep efficiency |
prehypertension defined as BP ≥ 90th percentile for sex, age, and height, continuous resting SBP and DBP |
age, BMI, socio- economic status, models examining continuous BP outcomes also adjusted for sex, race, term status |
↓sleep duration prehypertension, SBP, DBP all NS ↓ sleep efficiency ↑ prehypertension ↑ SBP ↑DBP associations replicated using PSG |
| Wells47 | 4452 Brazilian adolescents, ages 10–12 |
cross- sectional |
self-report bedtime & wake up time during week |
oscillometric BP ≥120/80 |
physical activity, BMI groups, sex, SES, birth weight, length, maternal health habits |
↓ sleep duration ↑SBP NS DBP |
| Sampei53 | 117 Japanese children, ages 5–6 |
cross- sectional |
parental report of sleep + teacher report of naps |
mercury column BP | age, sex, BMI, school |
↓TST ↓ SBP |
| Au43 | 143 normal weight Chinese children and adolescents, 42% female, AHI ≥ 5 excluded, ages 10- 17.9; M = 14.3 ± 1.8 yrs |
cross- sectional |
1-night PSG -sleep time -sleep efficiency 7-day sleep diary duration |
24-hr ambulatory BP -prePSG BP -in-bed BP -postPSG BP |
age, gender, BMI, AHI, parental hypertension |
↓PSG sleep time ↑ postPSG SBP NS other BP outcomes ↓PSG sleep efficiency ↑ in-bed SBP ↑ in-bed DBP ↑ postPSG DBP NS other BP outcomes ↓sleep diary duration ↑ prePSG SBP ↑ prePSG ↑ in-bed SBP ↑ in-bed DBP ↑ postPSG SBP NS postPSG DBP |
| Hannon48 | 49 obese, nondiabetic U.S. adolescents, 48.1% female, 57.1% White, 40.7% Black, ages 12–18; M = 14.4 yrs |
cross- sectional |
1-night PSG -REM % -SWS % -TST -sleep latency -time to REM |
resting oscillometric BP assessed morning after PSG. |
age or pubertal stage, sex, race, BMI, AHI |
↓REM % ↑ SBP ↑ DBP ↓SWS % ↑ SBP ↑ DBP NS for other sleep parameters |
| Reactivity/HRV Studies | ||||||
| Mezick56 | 79 healthy normal weight U.S. college students, 100% male, ages 18–29, M = 19 ± 2.0 yrs |
cross- sectional |
7 nights actigraphy- assessed sleep duration |
HR reactivity, BP reactivity, HRV reactivity, HR recovery, BP recovery, HRV recovery |
age, race, BMI, daily caffeine, daily nicotine, stress task appraisals, naps |
↓ duration NS HR reactivity NS SBP reactivity NS DBP reactivity ↑HRV-HF reactivity ↑ HR recovery NS SBP recovery ↑ DBP recovery NS HRV-HF recovery |
| Martikainen55 | 241–274 Finnish 8- year-olds (number varied by outcome) |
cross- sectional |
sleep disturbance, Scales: 6 subscale scores (yes/no at least 1 sleep problem 2- 3x per week) |
Cardiovascular reactivity, Ambulatory BP 24 hours |
sex, age, height, BMI, start time, education, maternal licorice pregnancy yes/no |
excessive somnolence ↑HF-HRV at rest SDB ↑ CO + HR reactivity |
| Williams57 | 98 U.S. college students; 50% female, 74% White, 7% Latino/a, M = 23 ± 5.8 yrs |
cross- sectional |
prior month sleep quality, prior night sleep quality & TST |
HR reactivity, SBP reactivity, DBP reactivity |
baseline CV parameters |
↓prior month quality NS HR reactivity NS SBP reactivity ↓ DBP reactivity ↓prior night quality all NS ↓prior night TST all NS (marginal effect on SBP reactivity) |
| Michels58 | 334 Belgian children, 47% female, ages 5–11, actigraphy in subsample of N = 165 |
cross- sectional and longitudinal |
parent-reported sleep duration, actigraphy- assessed latency, TST, efficiency |
resting HRV, (HF- HRV and HF/LF ratio) |
age, sex, physical activity, parental education, stress |
↓parent-reported sleep duration NS HF-HRV NS LF/HF ratio ↑actigraphy latency ↓HF-HRV ↑LF/HF ratio ↓actigraphy efficiency NS HF-HRV ↑ LF/HF ratio ↓actigraphy TST NS HF-HRV ↑ LF/HF ratio longitudinal results replicated cross- sectional results |
| El-Sheikh59 | 224 U.S. children, 64% European American, 36% African American, 46% female, ages 8–10 |
cross- sectional |
7 nights actigraphy- TST, sleep activity, wake after sleep onset |
resting RSA, vagal withdrawal during stress |
age, sex, race, BMI, asthma |
↑ Wake after sleep onset ↑ vagal withdrawal during stress all other main effects NS ↓ resting RSA and ↑ vagal withdrawal during stress interacted to predict ↑ wake after sleep onset, ↑ sleep activity TST NS |
| Elmore- Staton60 |
29 U.S. preschoolers; 31% female, 64% European American, ages 3–5, M=3.99 ± .69 yrs |
cross- sectional |
actigraphy- assessed TST, efficiency, sleep activity |
resting RSA | age, sex, ethnicity |
↓ sleep efficiency & ↑ sleep activity ↓ RSA trend for ↓ TST, ↓ RSA |
| Martikainen50 | 231–265 Finnish 8 year olds (number varied by outcome) |
cross- sectional |
actigraphy- TST, efficiency, fragmentation |
CV reactivity, ambulatory BP |
sex, age, height, BMI, maternal licorice during pregnancy, parental education |
all NS |
| Shaikh51 | 489 Gujarti Indian adolescents; 42% female, ages 16–19 |
cross- sectional |
self-reported sleep duration (≥ 7 vs. < 7 hrs) |
resting BP, DBP reactivity |
none reported | those sleeping <7 hrs had higher DBP reactivity vs. those sleeping ≥ 7 hrs; resting SBP and DBP were NS |
| El-Sheikh61 | 41 U.S. children, 44% female, ages 6–12; M = 10.06 ± 1.74 yrs |
cross- sectional |
4 nights actigraphy- TST, sleep efficiency, self-reported sleep-wake problems and sleepiness |
resting RSA, vagal withdrawal during stress |
gender, age, puberty status |
↓TST ↓ vagal withdrawal during stress ↑ sleep-wake problems ↑ resting RSA, ↓ vagal withdrawal during stress sleep efficiency NS |
We then searched PubMed and PsycInfo for longitudinal studies on sleep and obesity, using the following combination of search terms: [(“sleep” OR “actigraphy”) AND (“body mass index” OR “overweight” OR “obesity” OR “waist circumference”) AND (“prospective” OR “longitudinal”)]. “NOT” qualifiers included “apnea” and “breathing.” Age limiters (0 years – 29 yrs) were used to refine the search. Reference lists were used to identify additional articles. We included only those studies that a) had a mean sample age of 24 or younger, b) were published in 2011 or later, c) were not included in recent reviews of sleep and obesity in youth19;20 and d) used a longitudinal design to examine sleep as a predictor of BMI or adiposity. Excluded from review were total or partial sleep deprivation experiments and studies that focused exclusively on clinical or sleep-disordered samples. Figure 1b displays the number of records identified, screened, and excluded. Sixteen studies met criteria for inclusion. These studies are listed in Table 2 by year of publication.
Table 2.
Longitudinal Studies of Sleep and Obesity
| First Author | Sample | Study Design | Sleep Measures | Obesity Measures |
Covariates | Results |
|---|---|---|---|---|---|---|
| El-Sheikh63 | 273 children, mean age of 9.4 yrs at Time 1 |
longitudinal, Time 1: 2009/2010, Time 2: 2010/2011, Tim 3: 2011–2012 |
sleep-wake problems (School Sleep Habits Survey), actigraphy- assessed sleep duration |
BMI | sex, ethnicity, puberty, income-to- need ratio, asthma, medication use |
↑ sleep problems at Time 1 = ↑ BMI at Time 3 in girls. ↓ sleep duration at Time 1 = ↑ BMI at Time 3 in boys and girls, and ↑increase in BMI in girls. |
| Scharf66 | 10,700 4–5 yr olds in Early Childhood Longitudinal Study-Birth Cohort (N=7000 at age 5) |
longitudinal, measures assessed at ages 4 and 5 |
parent-reported weeknight sleep duration, based on bedtime and waketime |
BMI z-score | sex, race/ethnicity, SES, TV viewing |
↓ sleep duration at age 4 =↑ BMI increase by age 5. Later bedtime at age 4 = ↑ BMI increase by age 5. |
| Taveras69 | 1046 children in Project Viva, 6 months old at baseline |
longitudinal, yearly assessments from infancy until 7 years |
sleep duration score, based on parent reports at each yearly assessment vs. published norms for sleep duration |
BMI z-score, total fat mass index, trunk fat mass index, skinfold thickness, waist and hip circumference at age 7 |
age, gender, race/ethnicity, maternal age, maternal BMI, maternal education, maternal parity, household income, TV viewing time |
lowest sleep duration group = ↑ BMI, total and trunk fat mass index, skinfold thickness, waist and hip circumference at age 7 vs. reference group sleep duration after age 2 = NS with BMI at age 7 |
| Chang73 | 6220 children in 5th grade at baseline, in the Early Childhood Longitudinal Study- Kindergarten Cohort |
longitudinal, children followed from 5th through 8th grade |
sleep duration derived from parent-reported bedtime and official school start time |
3 BMI groups: healthy weight (<85th % for age, gender), overweight (≥ 85th% - <95th %) or obese (≥95th %) |
gender, age, parental health, child health, race/ethnicity, parent education, family structure, poverty level |
“obese” in 5th grade ↑ sleep duration predicted moving into “overweight” or “healthy” category by 8th grade (vs. staying in obese category) “healthy weight” in 5th grade ↑ sleep duration predicted moving into “overweight” or “obese” category by 8th grade (vs. staying in healthy category) |
| Magee75 | 1079 children 4–5 yrs at Wave 1 in the Longitudinal Study of Australian Children |
longitudinal, 4 waves of data across 6 yrs |
parent-reported diary duration for first 3 waves; self-reported duration at Wave 4 |
3 BMI groups based on weight at 4 waves: healthy weight (↓ IOTF overweight at all waves), early onset obesity (↑ IOTF at all waves), later onset obesity (↓ IOTF at first wave but ↑ at later waves) |
mother and father BMI, mother education, child birth weight |
healthy weight trajectory: mixed associations between sleep duration and change in BMI later onset obesity: longitudinal associations NS early onset obesity: ↓ sleep duration at age 6–7 =↑ BMI at age 8–9; ↓ sleep duration at age 8–9 predicted ↑ BMI at age 10–11 |
| Mitchell64 | 1390 adolescents in 9th grade (Philadelphia suburban high school) at baseline |
longitudinal, assessments every 6 months thru 12th grade |
self-reported sleep duration (weighted average for school and weekend night) |
self-reported BMI |
study wave, gender, race, self-reported physical activity, maternal education, screen time |
↓ sleep duration = ↑ increases in BMI from age 14 to 18 |
| Lytle77 | 723 adolescents age 14.7 yrs at baseline |
longitudinal, first cohort: baseline, 12 mos, 24 mos second cohort: baseline, 24 mos |
self-reported sleep duration averaged across each assessment |
BMI, % body fat measured by bioelectrical impedance |
grade, race, parent education, school lunch, 24 hr energy intake, depression, pubertal status, physical activity, screen time/sedentary behavior |
all longitudinal relationships NS in boys and girls |
| Araujo70 | 1171 Portuguese adolescents, 13 yrs old at Time 1 |
longitudinal, 4-year follow-up |
self-reported weeknight sleep duration |
BMI z-score and body fat % measured by bioelectrical impedance |
parental education, Mediterranean Diet Quality Index |
boys: ↓ sleep duration at age 13 = ↑ BMI, ↑ body fat % at age 17. girls: ↑ sleep duration at age 13 = ↑ BMI change by age 17 All NS after adjusting for baseline adiposity |
| O’Dea65 | 939 children in New South Wales, ages 7–12 at baseline |
longitudinal, 4 annual assessments starting in 2007 |
self-reported weeknight sleep duration |
BMI | gender, school SES (physical activity not related to BMI and therefore not included as covariate) |
univariate analysis: upper tertile of sleep at year 1 = ↓ weight gain and ↓ increase in BMI between years 1 and 4 vs. lower tertile of sleep. multivariate analysis: consistently ↑ sleep = ↓ BMI |
| Storfer-Isser71 | 313 children ages 8–11 at baseline in the Cleveland Children’s Sleep and Health Study |
longitudinal, 3 assessments approx 4 yrs apart |
parent-reported sleep duration at Times 1 and 2; self-reported sleep duration at Time 3 (weighted mean for weekday/weekend) |
BMI z-score | age, race, birth weight, SES |
boys: ↓ sleep duration at age 8–11 predicted ↑ BMI at ages 12–15 and 16–19; NS after adjusting for baseline BMI. girls: all NS |
| Tatone- Takuda72 |
>1000 Canadian children, approx 2.5 yrs at baseline |
longitudinal, annual assessments across 4–5 yrs |
parent-reported sleep duration, assessed annually, used to create 3 groups from 2.5 to 6 yrs: 1) short- persistent/increasing 2) 10-hr persistent, 3) 11-hr persistent |
BMI at ages 6 and 7 |
child overweight or obese at 2.5 yrs, mother’s immigrant status, mother overweight or obese, household income, vegetable/fruit consumption |
boys: ↑ sleep group, ↓ BMI at age 6 (shortest 2 groups had ↑ BMI than longest group) and at age 7 (shortest group had ↑ BMI than longest group) girls: sleep, BMI NS |
| Carter62 | 244 children in New Zealand, age 3 at baseline |
longitudinal, assessments every 6 mos from age 3 to age 7 |
actigraphy-assessed sleep duration |
BMI z-score, various adiposity measures from bioelectrical impedence |
age, sex, maternal education and income, maternal BMI, birth weight, smoking in pregnancy, ethnicity, behavioral variables assessed at ages 3–5 (diet, activity, TV) |
↑ sleep duration at ages 3–5 = 1) ↓ BMI at age 7, 2) ↓ increase in BMI from age 3 to 7, and 3) ↓ risk of overweight at age 7. each hr of sleep = 61% reduction in risk of overweight or obese at age 7 |
| Diethelm74 | 481 German children in the DONALD study |
longitudinal, multiple assessments in first 2 yrs after birth; annual assessments from ages 2–7 |
parent-reported sleep duration at age 1.5 and 2 yrs used to create 3 groups: consistently long, consistently short, inconsistent |
BMI, fat mass index, fat free mass index from ages 2 to 7 yrs (mass indices based on skinfold thickness) |
sex, birth year, birth weight, rapid weight gain |
Inconsistent & consistently short sleepers at age 1.5–2 yrs = ↑ odds of excess body fat at age 7; consistently short sleepers showed progressively higher fat mass index levels until age 7 vs consistently long sleepers. BMI and fat free mass NS between sleep groups |
| Hiscock76 | 3857 infants (3–18 months) and 3844 preschoolers (4.3– 5.6 yrs) at Wave 1 in the Longitudinal Study of Australian Children |
longitudinal, Wave 1: 2004, Wave 2: 2006 |
parent-reported diary sleep duration |
infants: weight-for-age, adjusted for birth length preschoolers: BMI z-score |
sex, Wave 1 weight | NS; Sleep duration at Wave 1 did not predict BMI z-score at Wave 2 for either cohort |
| Seegers67 | 1916 children in Quebec Longitudinal Study of Kindergarten Children, 10 yrs at baseline |
longitudinal, annual assessments across 3 yrs |
parent-reported weekday sleep duration, assessed annually, used to calculate 3 trajectories: 1) short sleepers, 2) 10.5 hr sleepers, 3) 11 hr sleepers |
BMI, based on annual parent reports of height and weight, used to create 3 groups: 1) normal BMI 2) overweight 3) obese |
sex, immigrant status, family income, birth weight, parent education, pubertal status (ages 11–13), television and physical activity (age 13) |
short sleepers and 10-hr sleepers had ↑ risk of overweight and obese at age 13 versus 11 hr sleepers ↑ sleep duration at age 10 predicted ↓ BMI at age 13 |
| Silva68 | 304 children in the Tucson Children’s Assessment of Sleep Apnea study, 6–12 yrs at baseline |
longitudinal, baseline and 5- year follow-up |
PSG-assessed TST at baseline and follow- up, used to create 3 groups: 1) ≤ 7.5 hr/night 2) > 7.5 - < 9 hr/night 3) ≥ 9 hr/night daytime sleepiness |
BMI z-score at baseline and follow-up |
ethnicity, sleep disordered breathing at baseline and follow-up, age |
≤ 7.5 hr/night vs. ≥ 9 hr/night at baseline = ↑ odds of obesity at 5-yr follow-up. ≤ 7.5 hr/night vs. ≥ 9 hr/night at baseline = ↑ increase in BMI over 5 yrs daytime sleepiness NS |
We first describe the overall pattern of results within risk factor category below and discuss whether the pattern of results varies according to the sleep construct (duration, quality, continuity, and architecture; see Table 3). Then we discuss whether the findings vary by study characteristics: sleep measure (polysomnography (PSG), actigraphy, parent/self report), samples from United States vs. other, obesity within the sample, and age of participants. We considered the study as positive if any of the sleep characteristics in a given report were related to the risk factor in the expected direction and noted when the findings were in subgroups only. For example, if short sleep duration but not sleep continuity was related to BP, we considered it as a positive study; or if short sleep duration was related to BP in boys, but not girls, we considered it positive in subgroups. For the summaries where we characterized findings by study characteristics, e.g., comparing studies of children vs. adolescents, we considered the study positive if a sleep measure and multiple cardiovascular risk factors within a report were related as expected, and mixed, if it was less than a majority of the risk factors but at least one relationship in the expected direction. Thus, for example, if a report concerned BP, glucose, and total cholesterol in relation to sleep duration in elementary school aged children, and found expected associations for 2 of the 3, it would be considered positive; and mixed if there was one association, and 0 null. These judgments relied on the multivariate analyses where available. About one-third of the studies (14/38) that examined sleep duration in relation to cardiovascular risk markers reported that curvilinear or other statistical tests were used to investigate the potential association between long sleep and risk factors.
Table 3.
Results by Sleep Characteristic
| Risk Factor | Sleep Duration | Sleep Quality/Sleepiness | Sleep Continuity | Sleep Architecture |
|---|---|---|---|---|
| Metabolic Syndrome | 1 positive | 1 null/nonsignificant | -- | -- |
| 1 partial | ||||
| 3 null/nonsignificant | ||||
| Glucose/Insulin | 8 positive (6 short sleep, 2 curvilinear)1 |
1 null/nonsignificant | 2 positive | 2 positive |
| 5 null/nonsignificant | ||||
| Total Cholesterol, LDL, | 3 positive2 | 3 positive3 | -- | -- |
| HDL | 5 null/nonsignificant | |||
| Triglycerides | 2 opposite (↓ sleep, ↓ triglycerides) |
-- | -- | -- |
| 2 null/nonsignificant | ||||
| Blood Pressure | 8 positive | 1 positive | 2 positive | 1 positive |
| 2 opposite (↓sleep, ↓BP) |
2 null/nonsignificant | 2 null/nonsignificant | ||
| 1 partial/mixed | ||||
| 11 null/nonsignificant | ||||
| Cardiovascular | 2 positive | 1 null/nonsignificant | 1 null/nonsignificant | -- |
| Reactivity | 2 null/nonsignificant | 1 opposite | ||
| Heart Rate Variability | 2 positive | 2 opposite | 3 positive | -- |
| 2 null/nonsignificant | 1 null/nonsignificant | |||
| 1 opposite | ||||
2 of the 8 positive findings in subgroups only
3 of the 3 positive findings in subgroups only
2 of the 3 positive findings in subgroups only
3. Results
3.1 Multiple risk factors
There are 10 studies that reported multiple risk factors in relation to sleep, with 5 specifically reporting results for the combined index comprising the metabolic syndrome. The individual components of the metabolic syndrome are considered in the sections below. Of the 5 on the metabolic syndrome, samples ranged from 37 to 1187. Participant ages ranged from 6 to 18 years. One reported that shorter sleep duration was associated with higher risk for the metabolic syndrome among 37 obese adolescents, 23 whereas three studies reported null effects for sleep duration.24–26 The fifth study combined multiple characteristics of sleep into a latent factor and reported no direct effect of sleep on metabolic syndrome;27 however, there was an indirect effect of overall sleep characteristics being associated with lower aerobic fitness, which was, in turn, related to the metabolic syndrome. In sum, evidence is weak that sleep characteristics are related to the metabolic syndrome.
3.2 Glucose and insulin metabolism
There are 13 studies that reported associations between sleep characteristics and measures reflecting glucose and insulin metabolism. These measures included a variety of outcomes: fasting glucose, homeostasis model assessment of insulin resistance (HOMA-IR), 2-hour glucose tolerance test (OGTT), hemoglobin A1c (HbA1c; a measure of glycated hemoglobin), Matsuda index of insulin sensitivity, acute insulin response test (AIRg), insulinogenic index of insulin secretion (IGI), and whole body insulin sensitivity index (WBISI). Sample sizes ranged from 133 to 2053. Participant age ranged from 3 to 26 years. All studies were cross-sectional. Of the 13, 8 reported the hypothesized associations with sleep characteristics: with short duration,28–35 with decreased continuity,28;32;33 and with sleep stages.28;31 Of the 8 positive studies, 2 reported a curvilinear association with sleep duration: adjustment for waist circumference resulted in only long duration being related to higher HOMA-IR,30 whereas adjustment for obesity resulted in the curvilinear association of short sleep time with glucose and HbA1c remaining.31 One of the 8 studies tested the association of a combined index of shorter sleep duration, more screen time, and higher sugary drink consumption with HOMA-IR, but did not perform a separate analysis of sleep duration. This study did adjust for a number of important covariates, including waist circumference, gender, parental BMI, and birth weight.29 Two studies reported hypothesized associations in subgroups. Short sleep duration was associated with insulin, HOMA-IR, and leptin in girls only but these associations became nonsignificant with adjustment for waist circumference.35 The other found that short sleep was related to high fasting glucose in obese but not nonobese children.33 Five studies reported no associations;24;26;36–38 all of these were studies of the metabolic syndrome and 1 was from a sample in a weight management clinic. In sum, the majority of the evidence suggests that sleep characteristics are related to indices of glucose and insulin metabolism.
3.3 Lipids
There were 7 studies that reported associations between lipids and sleep characteristics. These measures included total cholesterol, low density lipoprotein cholesterol (LDL-C) or non-high density lipoprotein cholesterol (HDL-C), HDL-C, and triglycerides; sample sizes ranged from 1198 to 4104. Participant age ranged from 10 to 26 years. For high triglycerides, there were 2 null studies24;37 and 2 studies opposite to prediction.26;38 For high LDL-C or non HDL-C, there was a positive association with poor sleep quality but not with short sleep duration,39 another with short sleep duration in secondary students but not in primary students.25 For low HDL-C, there was one positive study with high daytime sleepiness in girls but not boys,36 and another with short parent-reported sleep duration but not with actigraphy measured sleep duration,38 and 3 null studies.26;37;39 One study with no direct measures of lipids found that female adolescents who reported short sleep duration on several occasions indicated 6–7 years later that they had been diagnosed by a physician as having high cholesterol.40 The same relationship was not apparent in males. In sum, the evidence does not support sleep characteristics being associated with lipids.
3.4 Blood pressure
There were 21 studies that examined sleep characteristics and BP. Sample sizes ranged from 49 to 6940, participant ages ranged from 3 to 19, and studies varied widely in terms of using cutoffs for prehypertension/hypertension, continuous BP readings, or a combination of both. Only one of the 21 studies used a longitudinal design. Archbold et al.41 performed in-home PSG assessment of children ages 6–11 and reported that shorter total sleep time (TST) at baseline predicted an increase in resting systolic (S) BP over 5 years, after adjusting for age, sex, ethnicity, SDB, and change in obesity. There was also a marginal effect of SDB on increase in SBP at follow-up.
Of the cross-sectional studies, 7 reported a relationship between decreased sleep duration or continuity and higher BP.26;42–47 One study reported that poorer sleep quality was associated with hypertension.39 In addition, 1 study that examined sleep stages reported that percentage of REM and slow wave sleep were each inversely associated with BP measurements taken the next morning.48 Eight studies observed no relationship between sleep and BP,24;35–38;49–51 and 2 studies reported that longer sleep was associated with higher BP.52;53 One study reported mixed results, such that shorter parent- reported sleep was associated with increased BP in 11–14 year-old boys and girls, but with decreased diastolic (D) BP in younger boys.54 In sum, the evidence linking sleep characteristics to BP in children and adolescents is mixed.
Of the 20 cross-sectional studies listed above, 4 used ambulatory BP monitoring over a period of 24 hours or longer. Three of these reported that decreased sleep duration or decreased sleep continuity was associated with elevated BP for a portion or all of the ambulatory monitoring period.43;45;46 In contrast, Martikainen et al. reported that neither self-reported sleep disturbance nor actigraphy-assessed sleep characteristics were associated with ambulatory BP in a sample of Finnish 8-year-olds.50;55 Thus, it is possible that multiple measurements of BP collected over an extended time period may reveal more robust associations with sleep than a limited number of clinic assessments.
3.5 Cardiovascular responses to stress and heart rate variability
Four studies examined sleep in relation to heart rate or BP responses to stress. Sample sizes ranged from 79 to 489, and participant age ranged from 8 to 29 years. Each study used a different stress task to measure cardiovascular responses. Results across the studies were mixed. Two studies reported that shorter sleep was associated with elevated or prolonged DBP response to stress;51;56 however, one of these failed to control for a number of important covariates.51 In a third study, poor sleep quality was associated with blunted DBP reactivity during a semi-structured stress interview in college students, and no other associations between sleep and reactivity were observed.57 The fourth study reported no association between actigraphy-measured sleep or self-reported sleep disturbances and heart rate or BP responses to stress in young children.50;55
Six studies examined sleep in relation to high-frequency heart rate variability (HF-HRV) or respiratory sinus arrhythmia (RSA), which are markers of parasympathetic nervous system activity. The studies varied in whether they investigated parasympathetic activity during rest and/or during psychological stress tasks. Sample sizes ranged from 29 to 334. Participant ages ranged from 3 to 29 years. The one prospective study reported that both decreased actigraphy- assessed sleep time and decreased sleep continuity, but not parent reports of sleep, predicted a higher resting ratio of low-frequency (sympathetic) to high-frequency (parasympathetic) power at 1-year follow-up in 165 children.58 Of the five cross-sectional studies, 3 reported positive results, such that shorter or less continuous sleep was linked to lower HF-HRV/RSA either at rest or during stress,56;59;60 while 2 studies reported null effects or effects in the opposite direction.50;55;61 In sum, 4 of 6 studies supported a relationship between decreased sleep duration or continuity and decreased parasympathetic activity.
3.6 Comparisons of studies by sleep measure, country of origin, obesity as a covariate, and age
3.6.1 Sleep characteristics
Sleep duration or TST was examined in the majority of studies. Across cardiovascular risk factors, 25 findings were reported showing a relationship between shorter sleep and elevated risk in the whole sample or in sub-groups. Thirty findings were null. There were 7 reported associations between longer sleep and elevated risk. Of the 11 reports of sleep continuity (including wake after sleep onset, sleep efficiency, sleep latency, and/or fragmentation), there were 7 instances of decreased continuity being associated with elevated risk and 4 null reports. Of the 12 reports of sleep quality, disturbance, or daytime sleepiness; 4 were positive, 5 were null, and 3 indicated that more sleepiness or worse quality was associated with elevated risk. In sum, there were no clear differences in cardiometabolic risk by type of sleep characteristic.
3.6.2 Subjective vs. objective report of duration or continuity
Eighteen studies assessed sleep duration or continuity using PSG or actigraphy, while 20 studies used a self-report measure of sleep duration or continuity. Regardless of cardiovascular risk factor, results tended to be more positive for studies that used an objective sleep assessment, with 11 positive, 4 mixed, 2 null, and 1 reporting longer sleep and elevated risk. Studies that used self-reports of duration or continuity were more likely to report null results, with 3 positive, 8 mixed, and 7 null, and 2 reporting longer sleep and elevated risk.
3.6.3 Country of origin
Eighteen papers reported on data collected in the United States and 21 papers reported on data collected in other countries, including Belgium, Brazil, Canada, China, Finland, Germany, Greece, Hong Kong, India, Iran, Japan, Korea, Lithuania, Netherlands, and Portugal. Regardless of cardiovascular risk factor, reports were similar in terms of those supporting the hypothesized direction of effect: for studies originating in the U.S., 10 were positive, 1 null, 6 mixed, and 1 opposite to hypotheses, vs. elsewhere 7 were positive, 4 null, 8 mixed, and 2 opposite to hypothesis.
3.6.4 Obesity
Adiposity measured as BMI or waist circumference served as a covariate in 27 studies; 11 studies did not adjust for measures of adiposity. In general the studies that adjusted for adiposity did not differ in the extent of support for associations with risk factors from those studies that did not adjust for adiposity. Four studies only recruited obese adolescents and children; of these 2 were positive and 2 were mixed. One study reported in subanalyses that shorter sleep was related to fasting glucose > 100 mg/dl only in obese children.
3.6.5 Age
Age of participants ranged from preschool to young adulthood (early 20’s). Nineteen studies reported a mean age of 14 years or older or conducted age-stratified analyses in older adolescents. Nine of these studies were positive, 7 were mixed, and 3 were null. Ten studies reported a mean sample age of 10 or younger or conducted age-stratified analyses in younger adolescents. Of these, 3 reported positive results, 1 reported mixed results, 5 reported null results, and 1 was negative. Thus, full or partial support for relationships between sleep and cardiovascular risk was more likely in older versus younger youth.
3.7 Longitudinal Studies of Obesity
We identified 16 longitudinal studies of sleep and obesity published since 2011 and not included in previous reviews.19;20 Note that the cross-sectional studies outlined in Table 1 that included measures of weight are not included in this review. All studies focused on sleep duration, with only 1 study also including a measure of sleep disturbance. In terms of sleep duration, 8 studies reported that shorter sleep predicted higher BMI, greater increases in BMI, or greater risk of overweight/obesity over time compared to longer sleep duration, in either the full sample or in both boys and girls.62–69 Three studies reported that shorter sleep was predictive of higher BMI or greater weight increases in boys but not girls.70–72 Three studies reported that associations between sleep duration and anthropometric outcomes varied by other sub-groups, such as age or outcome of interest.73–75 Two studies reported that sleep duration was not a significant predictor of BMI over time.76;77
4. Discussion
Our enumerative review of the association of cardiovascular risk factors and sleep characteristics in children and adolescents revealed several findings. First, evidence for associations with sleep characteristics is most consistent for obesity, then glucose and insulin metabolism, followed by BP (especially 24 hour ambulatory BP), and parasympathetic responses to psychological stressors . The evidence suggesting that short sleep leads to increased risk for obesity is particularly striking, especially given the longitudinal designs of the studies, and that obesity increases risk for other cardiovascular risk factors and tracks over time in young people.
On the other hand, evidence suggests null or weak associations for metabolic syndrome cluster, lipids, and BP responses to psychological stressors. These conclusions should not be considered definitive in light of many more reports regarding obesity, glucose and insulin metabolism, and BP than for metabolic syndrome, lipids, and BP responses to stress. Further examination of these parameters may reveal a different pattern of results using longitudinal designs and more thorough assessment of sleep characteristics. In that regard, it is noteworthy that the only longitudinal study other than those related to obesity did find that short sleep predicted increases in blood pressure across five years; in contrast to the number of cross-sectional studies of blood pressure that found no associations.41
Second, given the large number of studies with a variety of participants from many developed countries, it is not surprising that the strength of associations varied by several key covariates or descriptors of the study. It appears that the associations with cardiovascular risk factors are somewhat more consistent in older than younger children and in studies that used “objective” measures of sleep as opposed to self- or parent-report. The finding that objective measures may reveal stronger associations than subjective measures indicates that future studies should preferentially use objective measures. It may be that child- or parent-report measures of sleep are less accurate for children because they usually do not sleep in the room with their parents and because of the challenges of obtaining reliable self-report data regarding any characteristics from younger children. Objective measures, e.g., actigraphy or in home polysomnography, usually accompanied by sleep diaries, are feasible in large scale studies but do require compliant participants and are more expensive and labor intensive than self- or parent-report measures.
Surprisingly the results did not vary substantially by the specific sleep characteristic (i.e., duration, continuity, quality) or by whether obesity was introduced as a covariate. The latter may be due to the number of studies with only obese participants, as well as the substantial number of studies in countries that do not have epidemics of obesity as is occurring in the United States.
Third, with the exception of studies of obesity, almost all studies reviewed herein were cross-sectional in nature. Given the foundation of research summarized in this review, it is now time to examine antecedent and consequent relationships among sleep characteristics and cardiometabolic factors. All the risk factors considered are impacted by weight gain so longitudinal studies must examine how weight change affects the relationships between sleep and change in cardiovascular risk factors. Otherwise, relationships that may be attributed to the risk factors may be secondary to weight gain. Finally, it also time to conduct randomized trials to address whether improving sleep can lead to improving cardiovascular risk factors.
Fourth, only two studies included indices of mental health as covariates. A very large literature suggests that depression and anxiety are consistently related to future cardiovascular morbidity, mortality, and subclinical CVD.78 Poor sleep is also intertwined with depression and anxiety,79 and the mechanisms accounting for their associations may be similar to the mechanisms accounting for associations between depression and anxiety and cardiovascular risk.80 It is important to include indices of depression and anxiety in future studies to examine whether poor sleep leads to mental health problems, which, in turn, lead to cardiometabolic risk later in life, or whether mental health problems lead to poor sleep, which then leads to cardiovascular risk.
Fifth, although timing of sleep is a key characteristic of sleep health, we only found one relevant study. Scharf and DeBoer66 noted that a later bedtime in 4-year-olds predicted an increase in BMI by age 5. Previous studies not included in this review also suggest an association between variability in sleep timing and risk factors, including higher BMI,81 inflammatory activity, 82 and sympatho-adrenal- medullary activity. 83 The sleep-wake cycle, as well as many of the physiological systems implicated in cardiometabolic disease risk, including glucose metabolism, adipocyte function, and vascular function, are closely tied to and may be influenced by circadian rhythms84;85 or circadian preference.86 Thus, it is possible that that circadian dysregulation is an underlying cause of both disrupted sleep and variations in CVD risk markers.
We did not include inflammatory risk factors because of the few studies available, other than those concerning SDB. However, elevated levels of a generic marker of inflammation, C-reactive protein (CRP), were related to shorter sleep duration in several cross-sectional studies of adolescents.87–89 Inflammation is an important arena for investigation, especially given substantial literature showing that CRP and interleukin 6 predict the early development of CVD.90 As investigations move forward in this arena, it is important to measure SDB, especially in populations of overweight children and adolescents.
Finally, the review does not address whether effects are stronger in lower SES or minority children and adolescence because rarely did papers report moderation effects by SES or race. More generally, it is worthwhile to consider that poor sleep may inform the relationship between low SES and minority status with cardiovascular disease risk. Supporting this notion is ample evidence that low SES in childhood is related to elevated cardiovascular morbidity and mortality in adulthood,91;92 although low SES is not consistently related to cardiovascular risk factors in childhood.93 Black children and adolescents have shorter sleep than their white counterparts but they report fewer insomnia-like symptoms.81;94–96 This pattern of results by race is consistent with meta-analyses documenting similar associations in adults.97;98 Evidence regarding the influence of SES on sleep of children and adolescence is less clear but it is reasonable to hypothesize adverse effects of low SES because environmental factors, such as inadequate heating and cooling, noise, irregular routines, and stress, do covary with SES and impact sleep.
The present review has a number of strengths and weaknesses. The strengths are clear specification of inclusion criteria for studies summarized in the review; detailed description of sleep measures and cardiovascular risk factors; and identification of types of studies and participants that show more positive vs. null associations. The weaknesses of the review are primarily a consequence of the current status of the literature: absence of a sufficient number of longitudinal studies that would permit conclusions regarding antecedent and consequent relationships; the heterogeneity of the studies making meta-analysis less attractive; key information lacking in some papers, e.g., adjustments for obesity, age stratification that is not comparable across studies; few studies examining the impact of long sleep duration; and few studies of inflammatory markers. Furthermore, little is known about variability in sleep patterns or sleep timing. Addressing these weaknesses in future research, however, provides guidelines for additional investigation.
Given the state of the field, it may be premature to recommend health policy and service delivery changes at this time. The exception is obesity, with the caveat that no clinical trials are yet available. Should short, discontinuous, and low quality sleep be related to prevention of obesity, there are many ways that sleep can be improved. For example, school start times can be delayed to permit more opportunity to sleep. Health care providers can provide materials on improving sleep hygiene and parents and students educated regarding the negative effects of poor sleep. Sleep interventions could target obese children.
5. Conclusions
Cardiovascular risk factors emerge in childhood and adolescence and impact long-term cardiovascular health. The extent to which sleep characteristics play a role in understanding cardiovascular risk in young people is an area of active international investigation. While an important topic, substantial challenges exist in addressing the roles of sleep characteristics. Sleep patterns change dramatically during childhood and adolescence. Rates of maturation vary across boys and girls as well as within gender such that age adjustments and grouping by age are not sufficient proxies. Demands of school and home superimpose constraints that entrain circadian and sleep patterns that vary by culture. Although general standards for optimal sleep exist according to developmental stages, they do not take into account the sleep “needs” of the individual based on their diet, activity pattern, environment, and genetic make-up. Thus far, cross-sectional evidence provides the bulk of the relevant data we have reviewed and no clinical trials are available. Yet hypothesized relationships may be obtained in longitudinal data even when they are not obtained in cross-sectional data. Although the current state of evidence varies by risk factor, there are enough positive findings, particularly in studies employing the more objective measures of sleep and including adolescent samples, to provide support for substantial future efforts to understand the links between sleep and cardiovascular risk in young people.
Supplementary Material
Highlights.
We review 55 studies of cardiovascular risk factors, obesity, and sleep in youth.
There are 39 studies of sleep and risk factors, most of which are cross-sectional.
In cross-sectional studies, the most consistent evidence links sleep to glucose/insulin.
Data from 16 longitudinal studies suggest that short sleep predicts obesity.
More longitudinal studies that use objective sleep measures are needed.
Acknowledgements
This work was supported by the National Institutes of Health grant HL025767.
Abbreviations
- M
mean
- NS
nonsignificant
- MetS
metabolic syndrome
- BMI
body mass index
- BP
blood pressure, S systolic, D diastolic
- MAP
mean arterial blood pressure
- HR
heart rate
- CO
cardiac output
- HF-HRV
high frequency heart rate variability
- LF-HRV
low frequency heart rate variability
- RSA
respiratory sinus arrhythmia
- HbA1c
hemoglobin A1c
- HDL-C
high density lipoprotein cholesterol
- TC
total cholesterol
- LDL-C
low density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- IGI
insulinogenic index of insulin secretion
- WBISI
whole body insulin sensitivity index
- AIRg
acute insulin response to glucose
- OGTT
oral glucose tolerance test
- SDB
sleep disordered breathing
- TST
total sleep time
- REM
rapid eye movement
- N3
sleep stage #3
- SWS
show wave sleep
- PSG
polysomnography
- IOTF
International Obesity Task Force
- CVD
cardiovascular disease
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Li C, Ford ES, Zhao G, Mokdad AH. Associations of health risk factors and chronic illnesses with life dissatisfaction among U.S. adults: the Behavioral Risk Factor Surveillance System, 2006. Prev Med. 2009;49:253–259. doi: 10.1016/j.ypmed.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502–508. doi: 10.1161/HYPERTENSIONAHA.109.134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rademacher ER, Jacobs DR, Jr, Moran A, Steinberger J, Prineas RJ, Sinaiko A. Relation of blood pressure and body mass index during childhood to cardiovascular risk factor levels in young adults. J Hypertens. 2009;27:1766–1774. doi: 10.1097/HJH.0b013e32832e8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children young adults The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 5.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 6.Iannuzzi A, Licenziati MR, Acampora C, et al. Carotid artery stiffness in obese children with the metabolic syndrome. Am J Cardiol. 2006;97:528–531. doi: 10.1016/j.amjcard.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- 8.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 9.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 10.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews KA, Strollo PJ, Jr, Hall M, et al. Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle-aged men and women: Pittsburgh SleepSCORE study. Sleep. 2011;34:711–716. doi: 10.5665/SLEEP.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sands MR, Lauderdale DS, Liu K, et al. Short sleep duration is associated with carotid intima-media thickness among men in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke. 2012;43:2858–2864. doi: 10.1161/STROKEAHA.112.660332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21:57–64. doi: 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- 14.Broussard J, Brady MJ. The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract Res Clin Endocrinol Metab. 2010;24:763–773. doi: 10.1016/j.beem.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Beydoun MA, Wang Y. Obesity. Vol. 16. Silver Spring; 2008. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis; pp. 265–274. [DOI] [PubMed] [Google Scholar]
- 19.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev. 2012;16:231–241. doi: 10.1016/j.smrv.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidolin M, Gradisar M. Is shortened sleep duration a risk factor for overweight and obesity during adolescence? A review of the empirical literature. Sleep Med. 2012;13:779–786. doi: 10.1016/j.sleep.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention NCfCDPaHPDoAaSH, Health Resources and Services Administration MaCHBOoAH, National Adolescent Health Information Center UoCSF. Improving the Health of Adolescents & Young Adults: A Guide for States and Communities. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 23.Iglayreger HB, Peterson MD, Liu D, et al. Sleep duration predicts cardiometabolic risk in obese adolescents. J Pediatr. 2014;164:1085–1090. doi: 10.1016/j.jpeds.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azadbakht L, Kelishadi R, Khodarahmi M, et al. The association of sleep duration and cardiometabolic risk factors in a national sample of children and adolescents: the CASPIAN III study. Nutrition. 2013;29:1133–1141. doi: 10.1016/j.nut.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Kong AP, Wing YK, Choi KC, et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep Med. 2011;12:659–665. doi: 10.1016/j.sleep.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Lee JA, Park HS. Relation between sleep duration, overweight, and metabolic syndrome in Korean adolescents. Nutr Metab Cardiovasc Dis. 2014;24:65–71. doi: 10.1016/j.numecd.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Countryman AJ, Saab PG, LlAbre MM, Penedo FJ, McCalla JR, Schneiderman N. Cardiometabolic risk in adolescents: associations with physical activity, fitness, and sleep. Ann Behav Med. 2013;45:121–131. doi: 10.1007/s12160-012-9428-8. [DOI] [PubMed] [Google Scholar]
- 28.Flint J, Kothare SV, Zihlif M, et al. Association between inadequate sleep and insulin resistance in obese children. J Pediatr. 2007;150:364–369. doi: 10.1016/j.jpeds.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 29.Androutsos O, Moschonis G, Mavrogianni C, et al. Identification of lifestyle patterns, including sleep deprivation, associated with insulin resistance in children: the Healthy Growth Study. Eur J Clin Nutr. 2014;68:344–349. doi: 10.1038/ejcn.2013.280. [DOI] [PubMed] [Google Scholar]
- 30.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158:617–623. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koren D, Levitt Katz LE, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34:2442–2447. doi: 10.2337/dc11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35:1353–1358. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Z, Ye T, Zhang X, et al. Sleep duration and hyperglycemia among obese and nonobese children aged 3 to 6 years. Arch Pediatr Adolesc Med. 2010;164:46–52. doi: 10.1001/archpediatrics.2009.233. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Li AM, Au CT, et al. Association between sleep architecture and glucose tolerance in children and adolescents. Diabetes. doi: 10.1111/1753-0407.12138. In press. [DOI] [PubMed] [Google Scholar]
- 35.Hitze B, Bosy-Westphal A, Bielfeldt F, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr. 2009;63:739–746. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 36.Berentzen NE, Smit HA, Bekkers MB, et al. Time in bed, sleep quality and associations with cardiometabolic markers in children: the Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Sleep Res. 2014;23:3–12. doi: 10.1111/jsr.12087. [DOI] [PubMed] [Google Scholar]
- 37.Rey-Lopez JP, de Carvalho HB, de Moraes AC, et al. Sleep time and cardiovascular risk factors in adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Sleep Med. 2014;15:104–110. doi: 10.1016/j.sleep.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Sung V, Beebe DW, Vandyke R, et al. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. Sleep. 2011;34:891–898. doi: 10.5665/SLEEP.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narang I, Manlhiot C, Davies-Shaw J, et al. Sleep disturbance and cardiovascular risk in adolescents. CMAJ. 2012;184:E913–E920. doi: 10.1503/cmaj.111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gangwisch JE, Malaspina D, Babiss LA, et al. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33:956–961. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children's Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26–30. doi: 10.1016/j.jpeds.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Au CT, Ho CK, Wing YK, Lam HS, Li AM. Acute and chronic effects of sleep duration on blood pressure. Pediatrics. 2014;133:e64–e72. doi: 10.1542/peds.2013-1379. [DOI] [PubMed] [Google Scholar]
- 44.Kuciene R, Dulskiene V. Associations of short sleep duration with prehypertension and hypertension among Lithuanian children and adolescents: a cross-sectional study. BMC Public Health. 2014;14:255. doi: 10.1186/1471-2458-14-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meininger JC, Gallagher MR, Eissa MA, Nguyen TQ, Chan W. Sleep duration and its association with ambulatory blood pressure in a school-based, diverse sample of adolescents. Am J Hypertens. 2014;27:948–955. doi: 10.1093/ajh/hpt297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59:747–752. doi: 10.1161/HYPERTENSIONAHA.111.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells JC, Hallal PC, Reichert FF, Menezes AM, Araujo CL, Victora CG. Sleep patterns and television viewing in relation to obesity and blood pressure: evidence from an adolescent Brazilian birth cohort. Int J Obes (Lond) 2008;32:1042–1049. doi: 10.1038/ijo.2008.37. [DOI] [PubMed] [Google Scholar]
- 48.Hannon TS, Tu W, Watson SE, Jalou H, Chakravorty S, Arslanian SA. Morning blood pressure is associated with sleep quality in obese adolescents. J Pediatr. 2014;164:313–317. doi: 10.1016/j.jpeds.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bayer O, Neuhauser H, von KR. Sleep duration and blood pressure in children: a cross-sectional study. J Hypertens. 2009;27:1789–1793. doi: 10.1097/HJH.0b013e32832e49ef. [DOI] [PubMed] [Google Scholar]
- 50.Martikainen S, Pesonen AK, Feldt K, et al. Poor sleep and cardiovascular function in children. Hypertension. 2011;58:16–21. doi: 10.1161/HYPERTENSIONAHA.111.172395. [DOI] [PubMed] [Google Scholar]
- 51.Shaikh WA, Patel M, Singh S. Association of sleep duration with arterial blood pressure profile of gujarati Indian adolescents. Indian J Community Med. 2010;35:125–129. doi: 10.4103/0970-0218.62571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paciencia I, Barros H, Araujo J, Ramos E. Association between sleep duration and blood pressure in adolescents. Hypertens Res. 2013;36:747–752. doi: 10.1038/hr.2013.36. [DOI] [PubMed] [Google Scholar]
- 53.Sampei M, Dakeishi M, Wood DC, Murata K. Impact of total sleep duration on blood pressure in preschool children. Biomed Res. 2006;27:111–115. doi: 10.2220/biomedres.27.111. [DOI] [PubMed] [Google Scholar]
- 54.Guo X, Zheng L, Li Y, et al. Association between sleep duration and hypertension among Chinese children and adolescents. Clin Cardiol. 2011;34:774–781. doi: 10.1002/clc.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martikainen S, Pesonen AK, Jones A, et al. Sleep problems and cardiovascular function in children. Psychosom Med. 2013;75:682–690. doi: 10.1097/PSY.0b013e31829cc915. [DOI] [PubMed] [Google Scholar]
- 56.Mezick EJ, Matthews KA, Hall MH, Richard JJ, Kamarck TW. Sleep duration and cardiovascular responses to stress in undergraduate men. Psychophysiology. 2014;51:88–96. doi: 10.1111/psyp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams PG, Cribbet MR, Rau HK, Gunn HE, Czajkowski LA. The effects of poor sleep on cognitive, affective, and physiological responses to a laboratory stressor. Ann Behav Med. 2013;46:40–51. doi: 10.1007/s12160-013-9482-x. [DOI] [PubMed] [Google Scholar]
- 58.Michels N, Clays E, De BM, Vanaelst B, De HS, Sioen I. Children's sleep and autonomic function: low sleep quality has an impact on heart rate variability. Sleep. 2013;36:1939–1946. doi: 10.5665/sleep.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Sheikh M, Erath SA, Bagley EJ. Parasympathetic nervous system activity and children's sleep. J Sleep Res. 2013;22:282–288. doi: 10.1111/jsr.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elmore-Staton L, El-Sheikh M, Vaughn B, Arsiwalla DD. Preschoolers' daytime respiratory sinus arrhythmia and nighttime sleep. Physiol Behav. 2012;107:414–417. doi: 10.1016/j.physbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 61.El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children's sleep problems. Dev Psychobiol. 2005;46:307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- 62.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Sheikh M, Bagley EJ, Keiley MK, Erath SA. Growth in Body Mass Index From Childhood Into Adolescence: The Role of Sleep Duration and Quality. The Journal of Early Adolescence. 2014 [Google Scholar]
- 64.Mitchell JA, Rodriguez D, Schmitz KH, Audrain-McGovern J. Sleep duration and adolescent obesity. Pediatrics. 2013;131:e1428–e1434. doi: 10.1542/peds.2012-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Dea JA, Dibley MJ, Rankin NM. Low sleep and low socioeconomic status predict high body mass index: a 4-year longitudinal study of Australian schoolchildren. Pediatr Obes. 2012;7:295–303. doi: 10.1111/j.2047-6310.2012.00054.x. [DOI] [PubMed] [Google Scholar]
- 66.Scharf RJ, DeBoer MD. Sleep timing and longitudinal weight gain in 4- and 5-year-old children. Pediatr Obes. 2014 doi: 10.1111/ijpo.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seegers V, Petit D, Falissard B, et al. Short sleep duration and body mass index: a prospective longitudinal study in preadolescence. Am J Epidemiol. 2011;173:621–629. doi: 10.1093/aje/kwq389. [DOI] [PubMed] [Google Scholar]
- 68.Silva GE, Goodwin JL, Parthasarathy S, et al. Longitudinal association between short sleep, body weight, and emotional and learning problems in Hispanic and Caucasian children. Sleep. 2011;34:1197–1205. doi: 10.5665/SLEEP.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taveras EM, Gillman MW, Pena MM, Redline S, Rifas-Shiman SL. Chronic sleep curtailment and adiposity. Pediatrics. 2014;133:1013–1022. doi: 10.1542/peds.2013-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Araujo J, Severo M, Ramos E. Sleep duration and adiposity during adolescence. Pediatrics. 2012;130:e1146–e1154. doi: 10.1542/peds.2011-1116. [DOI] [PubMed] [Google Scholar]
- 71.Storfer-Isser A, Patel SR, Babineau DC, Redline S. Relation between sleep duration and BMI varies by age and sex in youth age 8–19. Pediatr Obes. 2012;7:53–64. doi: 10.1111/j.2047-6310.2011.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatone-Tokuda F, Dubois L, Ramsay T, et al. Sex differences in the association between sleep duration, diet and body mass index: a birth cohort study. J Sleep Res. 2012;21:448–460. doi: 10.1111/j.1365-2869.2011.00989.x. [DOI] [PubMed] [Google Scholar]
- 73.Chang Y, Gable S. Predicting weight status stability and change from fifth grade to eighth grade: the significant role of adolescents' social-emotional well-being. J Adolesc Health. 2013;52:448–455. doi: 10.1016/j.jadohealth.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Diethelm K, Bolzenius K, Cheng G, Remer T, Buyken AE. Longitudinal associations between reported sleep duration in early childhood and the development of body mass index, fat mass index and fat free mass index until age 7. Int J Pediatr Obes. 2011;6:e114–e123. doi: 10.3109/17477166.2011.566338. [DOI] [PubMed] [Google Scholar]
- 75.Magee CA, Caputi P, Iverson DC. The longitudinal relationship between sleep duration and body mass index in children: a growth mixture modeling approach. J Dev Behav Pediatr. 2013;34:165–173. doi: 10.1097/DBP.0b013e318289aa51.. [DOI] [PubMed] [Google Scholar]
- 76.Hiscock H, Scalzo K, Canterford L, Wake M. Sleep duration and body mass index in 0–7-year olds. Arch Dis Child. 2011;96:735–739. doi: 10.1136/adc.2010.204925. [DOI] [PubMed] [Google Scholar]
- 77.Lytle LA, Murray DM, Laska MN, Pasch KE, Anderson SE, Farbakhsh K. Examining the longitudinal relationship between change in sleep and obesity risk in adolescents. Health Educ Behav. 2013;40:362–370. doi: 10.1177/1090198112451446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 79.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9(Suppl 1):S3–S9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
- 80.Mezick EJ, Hall M, Matthews KA. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep Med Rev. 2011;15:51–63. doi: 10.1016/j.smrv.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. 2011;12:239–245. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okun ML, Reynolds CF, III, Buysse DJ, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mezick EJ, Matthews KA, Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnston JD. Physiological links between circadian rhythms, metabolism and nutrition. Exp Physiol. 2014;99:1133–1137. doi: 10.1113/expphysiol.2014.078295. [DOI] [PubMed] [Google Scholar]
- 85.Prasai MJ, George JT, Scott EM. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res. 2008;5:89–95. doi: 10.3132/dvdr.2008.015. [DOI] [PubMed] [Google Scholar]
- 86.Roeser K, Obergfell F, Meule A, Vogele C, Schlarb AA, Kubler A. Of larks and hearts--morningness/eveningness, heart rate variability and cardiovascular stress response at different times of day. Physiol Behav. 2012;106:151–157. doi: 10.1016/j.physbeh.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 87.Hall MH, Lee L, Matthews KA. Sleep duration during the school week is associated with C-reactive protein risk groups in healthy adolescents. Sleep Med. 2014 doi: 10.1016/j.sleep.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, et al. Sleep duration and emerging cardiometabolic risk markers in adolescents The AFINOS study. Sleep Med. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79:1544–1551. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 91.Galobardes B, Lynch JW, Davey SG. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 92.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 93.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: how and why do these relationships change with age? Psychol Bull. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 94.Adam EK, Snell EK, Pendry P. Sleep timing and quantity in ecological and family context: a nationally representative time-diary study. J Fam Psychol. 2007;21:4–19. doi: 10.1037/0893-3200.21.1.4. [DOI] [PubMed] [Google Scholar]
- 95.Matthews KA, Hall M, Dahl RE. Sleep in healthy black and white adolescents. Pediatrics. 2014;133:1189–1196. doi: 10.1542/peds.2013-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts RE, Roberts C, Chan W. Ethnic differences in symptoms of insomnia among adolescents. Sleep. 2006;29:359–365. doi: 10.1093/sleep/29.3.359. [DOI] [PubMed] [Google Scholar]
- 97.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–259. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 98.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12:209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.