Abstract
Posterior parietal cortex (PPC) is an extensive region of the human brain that develops relatively late in development and is proportionally large compared to that of monkeys and prosimian primates. Our ongoing comparative studies have lead to several conclusions about the evolution of this posterior parietal region. In early placental mammals, PPC likely was a small multisensory region much like PPC of extant rodents and tree shrews. In early primates, PPC likely resembled that of prosimian galagos where caudal PPC (PPCc) is visual and rostral PPC (PPCr) has eight or more multisensory domains where electrical stimulation evokes different complex motor behaviors, including reaching, hand-to-mouth, looking, protecting the face or body and grasping. These evoked behaviors depend on connections with functionally matched domains in premotor cortex (PMC) and motor cortex (M1). Domains in each region compete with each other, and a serial arrangement of domains allows different factors to successively influence motor outcomes. Similar arrangements of domains have been retained in New and Old World monkeys, and humans appear to have at least some of these domains. The great expansion and prolonged development of PPC in humans suggest the addition of functionally distinct territories. Across primates we propose that PMC and M1 domains are second and third levels in a number of parallel, interacting networks for mediating and selecting one type of action over others.
Keywords: visual cortex, motor cortex, prosimian primates, motor behavior, cortical connections
Graphical Abstract
Introduction
Posterior parietal cortex (PPC) is a region of the human brain that has been intensively investigated in non-invasive neuroimaging studies of cortical activation during sensorimotor and related cognitive processes (e.g., Sereno and Huang, 2006; Tosoni et al., 2008; Linden et al., 2010; Konen and Kastner, 2008; Filimon et al., 2009). PPC matures over a relatively long period of development in humans, and it is a relatively expanded part of the human brain in comparison to posterior parietal cortex of macaque brain (Hill et al., 2010). In addition to the wealth of information that is now available about the functional and connectional organization of PPC in humans, the functional and anatomical organization of PPC in macaque monkeys has been repeatedly investigated with anatomical and physiological approaches, including imaging studies, with direct comparisons to human PPC (e.g. Grefkes and Fink, 2005; Tsao et al., 2003; Koyama et al., 2004; Kagan et al., 2010). The general conclusion that stems from these studies is that PPC of humans and macaques consists of a number of functionally distinct subdivisions that are involved in different sensorimotor behaviors (e.g., reaching, looking, or grasping) and related cognitive functions (see Creem-Regehr, 2009; Sereno and Huang, 2013 for review). In addition, the much larger PPC of humans is thought to have additional areas or functional regions than PPC in monkeys including new regions for tool use and gestures (Frey, 2008; Peeters et al., 2009) and hemispheric asymmetries (Pouget and Driver, 2000).
Here we address the challenging issue of how the expanded and functionally complex PPC evolved from the immediate non-primate ancestors of early primates to modern humans (Kaas and Preuss, 2014). As the fossil record of skull endocasts reflects overall brain shape and size, but tells little about functional organization, our conclusions are necessarily based largely on comparative data from extant mammals. This includes results from the nearest available relatives of primates in the clade of euarchcontoglires, especially rodents, and tree shrews, and our ongoing studies of cortical organization in galagos, prosimian primates that in many ways resembles early primates in overall anatomy and relative brain size (Clark, 1959; Radinsky, 1975; Martin, 1990). Other information comes from our studies of cortical organization in small New World monkeys, and larger Old World macaque monkeys. We also briefly consider some of the data on PPC organization in humans. The accepted logic of reconstructing features of brain or other body parts from studies of contemporary mammals is that traits that are widely distributed across a clade of related species are most likely due to the widespread retention of that trait from a common ancestor (Henning, 1966; Wiley, 1981).
The antecedents of parietal-frontal networks in primates
The results of comparative studies of neocortical size and organization in extant mammals, together with estimates of neocortical size from the skull endocasts of the brains of early mammals, suggest that early mammals had little neocortex with few functionally distinct subdivisions or areas (Kaas, 2011). Distinct, separate motor areas of frontal cortex had not yet emerged, although motor functions were influenced by cortical projections to the basal ganglia, the midbrain, and brainstem. Somatosensory areas of cortex may have been especially important in motor behaviors due to a relay of information to somatosensory cortex from the basal ganglia and cerebellum via the early motor thalamus and outputs to the basal ganglia, the superior colliculus, and motor neurons of the brainstem and spinal cord. Early placental mammals already had a separate primary motor area (M1), with inputs from the motor thalamus related mainly to the cerebellum, and a premotor region with inputs from a portion of motor thalamus dominated by inputs from the basal ganglia (Young et al., 2012). The influences of prefrontal, cingulate, and other cortical areas on motor behavior likely depended less on their subcortical projections and more on their direct and indirect connections to M1 and PMC. Motor and premotor areas, free from the constraints of topographically representing somatosensory information, likely had mosaic organizations of cortical columns of movement representations, resulting in a fractured somatotopy (see Kaas, 2012), that only crudely represented the arrangement of body parts, as in present day primates, rodents and tree shrews (e.g., Gould et al., 1986; Wu et al., 2000; Remple et al., 2006; Cooke et al., 2012). For example, when near threshold current pulses in a brief series were used to stimulate M1 in squirrels, the resulting motor map was “fractured” in that the movements of given body parts were evoked from multiple different locations within the map, although a crude overall somatotopy is observed (Cooke et al., 2011). Similar results have been obtained in M1 of tree shrews (Remple et al., 2006). Such mosaic organizations are thought to be better suited for producing relevant sequences of movements (Overduin et al., 2008). This type of fractured topography is also found in somatosensory regions of the cerebellum (Shambes et al., 1978; Welker et al., 1988). Although variable across lineages, this type of modular organization in PMC and M1 evolved in mammals, and allowed “domains” especially devoted to specific, complex movement patterns to emerge. Thus, longer trains of electrical stimulation at above threshold levels of current evoke different complex movements from different locations in M1 in rats (Ramanathan et al., 2006; Bonazzi et al., 2013; Brown and Teskey, 2014), tree shrews and squirrels as well as primates. As we discuss below, M1 and PPC of primates not only have fractured somatotopies (see Sanes and Schieber, 2001, for review) but have domains for ethologically relevant complex behaviors (see Kaas et al., 2011; Graziano, 2009 for review) similar to the movement primitives (muscle synergies organized within the brain stem and spinal cord that are basic components of natural behaviors) that can be evoked from the subcortical motor structures (see Flash and Hochner, 2005; Mussa-Ivaldi and Bizzi, 2000). We suggest that the movement domains in motor cortex, access subcortical modules that organize such primitives, and directly contribute to the organization of the movements. Possibly, primitives can be combined in various ways to create “a vast repertoire of motor behaviors” (Mussa-Ivaldi and Bizzi, 2000). We also suggest that the functional benefits of a fractured somatotopy of M1 and PMC in placental mammals predisposed the emergence of functional domains, and influenced the subsequent evolution of PPC in primates. Additionally, domains in M1 and PMC likely interact with each other, to modify or combine primitives, and to allow certain complex movement patterns to emerge over others (Kaas, 2012).
In most placental mammals, PPC constitutes a small strip of cortex between the somatosensory and visual areas of cortex, extending ventrally to auditory cortex. In rodents and tree shrews, mammals closely related to primates, much of the cortex considered to be PPC unsurprisingly appears to have visual, somatosensory or auditory inputs, or some combinations of these inputs (Wallace et al., 2004; Kolb and Walkey, 1987, rats; Remple et al., 2007, tree shrews). Nevertheless, there is an overall somatotopy in the narrow band of PPC along the somatosensory cortex just caudal to S1, called the posterior medial area (PM) in rats (see Ebner and Kaas, 2015), and tree shrews (Remple et al., 2007) so that PPC is more related to the lower body medially and upper body laterally. Such a somatotopy also exists in rostral PPC of monkeys (Seelke et al., 2012) and humans (Huang et al., 2012) Thus, if the fractured somatotopy and functional domains of M1 and PMC, together with the mediolateral hindlimb to face somatotopy of PPC of the non-primate relatives of primates characterized cortex of the early ancestors of primates, these features likely influenced the subsequent evolution of PPC in primates. In particular, the position of PPC relative to somatosensory, visual and auditory areas of cortex likely favored the expansion of PPC in primates with an increased role in multisensory processing for motor guidance and control via projections to frontal cortex. Similar, expansion of PPC might occurr in other lines of mammalian evolution, including carnivores (Manger et al., 2002; Homman-Ludiye et al., 2010), but more research is needed. In addition, the existence of domains in motor cortex of the ancestors of primates may have favored the evolution of functionally matched domains in PPC, as well as the somatotopic mediolateral arrangement of these domains.
The organization of PPC and parietal-frontal sensorimotor networks in early primates
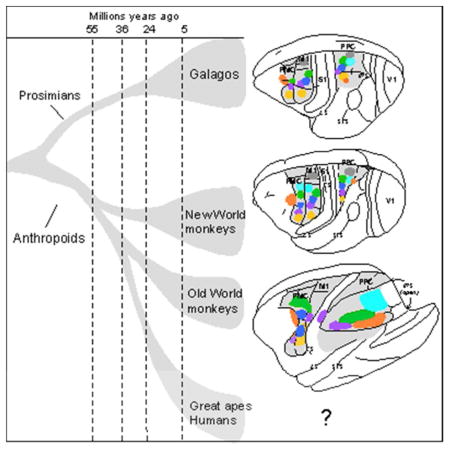
The posterior parietal region of cortex is greatly expanded and functionally divided into nodes or domains in parallel sensorimotor processing streams in all studied primates. Thus, we propose that a similar organization emerged with the evolution of early primates. Nevertheless, clear differences exist in PPC size and organization across present-day primates. Most notably, prosimian primates have absolutely and relatively less PPC than most other primates, and humans have more. Here we consider the results of our studies of PPC and frontal motor areas in galagos, a prosimian primate with a relative brain size and shape that is not much different from that of early primates (e.g., Clark, 1954; Radinsky, 1975; Bloch and Boyer, 2002). Archaic primates emerged from the common ancestors of tree shrews and flying lemurs over 80 million years ago, and the surviving branches of strepsirrhine (galagos, lemurs and lorises) and haplorhine (tarsiers, monkeys, apes and humans) primates diverged over 70 million years ago (e.g., Steiper et al., 2012). While the lemur radiation in Madagascar has impressively varied members, the more limited galago and loris radiations may have been constrained by competition with anthropoid primates. Thus, galagos may have preserved more of the ancestral features of primate brain organization than most other primates.
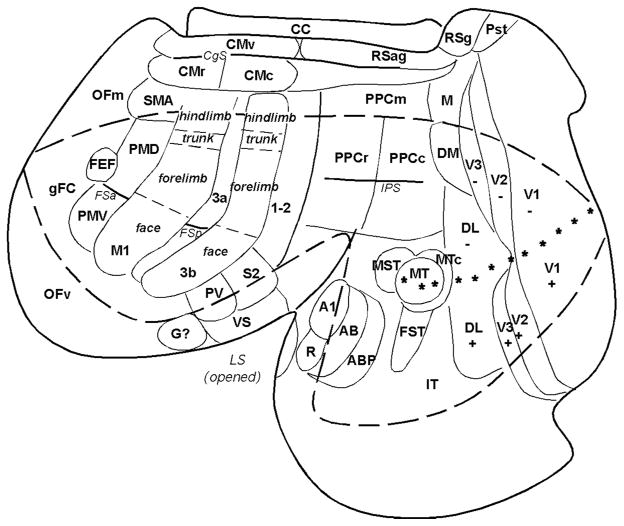
The neocortex of galagos contains a number of areas that correspond to those that are recognized in other primates, suggesting that these areas are common to all primates. Because some cortical areas are hidden in fissures in views of the intact brain, it is useful to display these areas on cortex that has been separated from the rest of the brain, and flattened as a single sheet (Fig. 1). However, some of these proposed areas of figure 1 are not accepted by all investigators, their boundaries may be somewhat uncertain, or similar areas may be termed differently. Nevertheless, an array of early visual areas can be identified in galagos and other primates, including V1, V2, V3, DL (V4), DM (V3a), MT (V5), MTC (V4t), MST, and FST. In galagos direct evidence is available for only two primary auditory areas, A1 and R (Brugge, 1982), but an auditory belt and parabelt of higher auditory processing can be presumed from studies in other primates (Kaas and Hackett, 2000). Somatosensory cortex includes a primary area, 3b or S1, 3a area, and area 1 or 1+2, PV, S2, and VS, plus a likely gustatory (G) area (Kaas, 2004). Motor cortex includes M1, the supplementary motor area, SMA, ventral and dorsal premotor areas, PMV and PMD, and the frontal eye field, FEF, of other primates (Wu et al., 2000). Subdivisions of PMV of macaque monkeys (Rizzolatti and Luppino, 2001) have not been determined in galagos. Cingulate cortex includes three of the cingulate motor areas of other primates (Wu et al., 2000), and granular and agranular retrosplenial areas have been identified (Wong and Kaas, 2010).
Fig. 1.
A surface view of the flattened neocortex of a prosimian primate (Galago garnetti). Cortex has been separated from the rest of the brain and flattened as a single sheet with proposed cortical areas imposed. This view shows cortex normally hidden in views of the intact brain. The dashed lines roughly outline the cortex that would be visible on a dorsolateral view of the cerebral hemisphere. Posterior parietal cortex contains rostral (PPCr), caudal (PPCc) and medial (PPCm) regions. Primary (V1) and secondary (V2) visual areas are common to most mammals. As in other primates galagos also have the third visual area (V3), a dorsomedial area (DM), a middle temporal area (MT), a dorsolateral area (DL or V4), fundal area of the superior temporal sulcus (FST), a MT crescent (MTc), and inferior temporal cortex (IT) of several divisions. Auditory cortex includes A1 and rostral (R) areas, plus presumed auditory belt (AB) and parabelt (APB) regions. Somatosensory cortex includes a primary area (S1 or area 3b) (note the overall somatotopy), a proprioceptive area 3a and secondary areas of 1–2, S2, parietal ventral (PV) and ventral somatosensory (VS). Motor areas include primary motor area (M1) ventral (PMV) and dorsal (PMD) premotor areas, supplementary motor area (SMA), frontal eye field (FEF) and ventral (CMv), rostral (CMr) and caudal (CMc) cingulate areas. Frontal cortex includes frontal granular cortex (gFC), medial (OFm) and ventral (OFv) orbital frontal regions. Agranular (RSag) and granular (Rsg) retrosplenial areas, area prostriata (Pst) are noted. CC= corpus callosum. Stars mark horizontal meridian. Modified from Kaas and Preuss, 2014.
In our approach, to determine the organization of PPC in galagos we adopted the long train microstimulation procedure of Graziano et al. (2002) to evoke complex movements from small regions we call domains, of PPC, PMC and M1 (Stepniewska et al., 2009, 2011). The electrical stimuli consists of 500ms trains of 0.2ms bihphasic pulses of current at 300Hz, with pulse amplitudes ranging from 20μA to 300μA. As noted by Graziano et al. (2002) these longer trains than the usually used (40–60ms), are more compatible with the time course of the natural behaviors (Stepniewska et al., 2014), and shorter trains of pulses either reveal simple movements (from M1 and PMC) or fail to evoke movements (from PPC). While there have been concerns about the “unnatural” nature of this long train stimulation (e.g. Strick, 2002), others have concluded that long train stimulation provides “the most direct evidence that the nervous system encodes motor primitives” (Overduin et al., 2012). In our experience, long train stimulation has the major advantage of reliably identifying a series of small regions of cortex where different ethologically relevant behaviors, such as reaching or grasping, are evoked. While stimulating in the grasp or reach domain consistently evokes grasping or reaching, we do not suggest that other regions of cortex, or subcortical structures (e.g. basal ganglia or thalamus), are not involved in these behaviors. Instead, we suggest that the microstimulation reveals the core cortical regions that are most directly involved in a specific behaviors, while the functional roles of regions where neurons are active during reaching or grasping, or preparing for these behaviors, are not directly apparent from microelectrode recording or functional imaging data. However, our preliminary microstimulation study on thalamus showed that complex movements similar to those evoked from cortical domains could be evoked from the “cerebellar” region of thalamus, the ventral lateral nucleus (Stepniewska et al., 2012), suggesting that “domains” involved in specific motor behaviors might be also present in thalamus. By identifying functional domains through the consequences of electrical stimulation, we can further explore their functional significance by deactivating or lesioning domains, or stimulating two or more domains at once. We can also determine the connections of such movement-defined regions. These results are complemented by studies of single neuron properties and neural activations, as these methods have been used extensively to define subregions of PPC in macaques and humans, but such complementary studies are not yet available for galagos. Yet, in both galagos and monkeys, there are studies of connections that usefully suggest the existence of similar functional relationships.
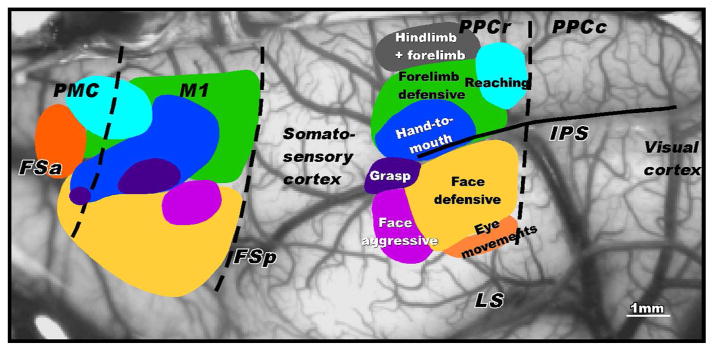
The application of long train stimulation to PPC, PMC and M1 revealed a number of interesting findings (Fig. 2; see also Stepniewska et al., 2005, 2009a, 2009b, 2011). First, long train stimulation reveled up to eight movement specific domains in each of the three regions of the cortex. Second, the movement domains were all located within the rostral half of PPC (PPCr), and no movements were evoked from the caudal half of PPC (PPCc). Third, the movement domains were arranged in a somatotopic sequence, from a hindlimb movement domains most medially to forelimb, head, and eye movement domains, in successively more lateral locations. Thus, the overall somatotopy of the domains in PPC reflected the somatotopy of more anterior somatosensory cortex. Fourth, the movement sequences for each PPC domain started within the first 50–100ms and continued for most or all of the 500 ms stimulation period, and sometimes continued briefly past the stimulation period. The movements were very stereotyped, and could be demonstrated repeatedly. All electrode penetrations within a domain produced similar but not necessarily identical movements. The movement domains were in very similar positions in different galagos. Fifth, the movement sequences seemed to reflect what Graziano et al. (2002) have called ethologically relevant behaviors. We classified the behavior by the inferred functions: 1. hindlimb and forelimb movements, possibly for running or climbing; 2. reaching away from the body; 3. defending the face and the body with a blocking movement of the forelimb, palm out; 4. a hand movement to or toward the mouth as in food retrieval; 5. grasping or manipulation movements of the hand; 6. aggressive face movements, with mouth open, lip withdrawn to expose teeth and ear rotated and sometimes flattened; 7. defensive face movements with an eye blink, grimace, and backward ear movement, sometimes with defensive forelimb movement. Sixth, the connections of the domains differed, but included widespread intrinsic connections within PPCr, indirect and direct inputs from somatosensory and extrastriate visual areas, and feedforward projections to matching domains in PMC and M1. The feedback connections from PMC and M1 to PPCr were widespread and not focused on only matching domains.
Fig. 2.
Movement domains in rostral posterior parietal cortex (PPCr), motor cortex (M1 and premotor cortex (PMC) of galagos. Long train electrical stimulation was used to evoke different complex movements from different regions (domains). In PPCr, hindlimb movements, or hindlimb with forelimb movements, were evoked from the most medial domain. The laterally adjacent domain was characterized by evoked defensive arm movements as if to fend off an attack. More caudally, stimulation evoked reaching movements of the contralateral arm. More laterally, stimulation evoked hand to mouth movements, sometimes with opening of the mouth. Face defensive movements were evoked from more lateral cortex, with eye movements evoked from the most lateral part of the responsive cortex. More rostrally, face and forelimb movements were evoked in patterns that suggested threat response (face aggressive domain). Matching domains were in M1 and PMC. The domains are illustrated on a photograph of frontoparietal cortex. FSa and FSp, anterior and posterior frontal sulci, IPS- intraparietal sulcus, LS, lateral sulcus. Modified from Stepniewska et al., 2005.
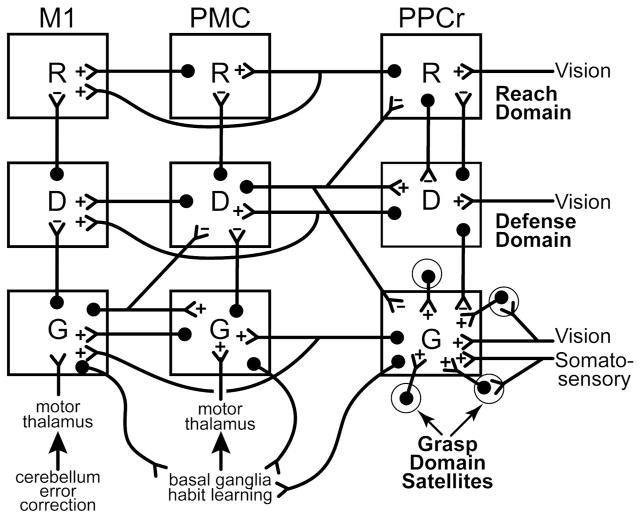
The connection patterns suggested to us that serial processing from PPCr to PMC to M1 matching domains was important. The matching domains from PPCr to PMC to M1 function as a series, with some bypassing from PPCr directly to M1. This functional relationship was supported by the results of inactivation experiments with muscimol injections (Stepniewska et al., 2014), cooling (Cooke et al., 2014), and lesions (unpublished). Inactivations of specific domains in M1 by any of the three methods abolished or greatly impaired the movements evoked by stimulation of matching domains in PMC or PPCr, while movements evoked from non-matching domains were unaffected or only somewhat altered. Inactivating PPC or PMC domains did not abolish the ability of stimulation to evoke movements from matching or non-matching domains in M1, while the inactivation of PMC domains suppressed or altered movements evoked from PPC domains. These results are all consistent with our model of domains in a series of functionally distinct, parallel parietal-frontal networks (Fig. 3).
Fig. 3.
Proposed interactions of three example classes of domains, R-reach, D-defensive and G-grasp, in rostral posterior parietal cortex (PPCr), premotor cortex (PMC) and primary motor cortex (M1) of primates. In PPC domains are activated by various combinations of visual, somatosensory, and other sensory inputs. The domains compete with each other via mutual inhibition to be most activated, and to thereby activate functionally matched domains in PMC and M1, where further competition takes place. Domains in PMC are informed by inputs from prefrontal cortex and a relay of basal ganglia inputs to the motor thalamus. Domains in M1 receive inputs from PMC and PPC, other areas of motor cortex, and a relay of cerebellar inputs to the motor thalamus. All illustrated connections are excitatory (+), but they may have inhibitory effects by selectively activating inhibitory neurons (−) that are not shown. Feedforward connections from PPCr to PMC and M1 are focused to functionally matched domains, while feedback connections are more widespread, and may result in inhibition of mismatched domains. Connections between mismatched domains largely produce inhibition while patches of cortex (satellites) around domains contribute to domain functions via dense interconnections (shown only for the PPCr domain). This model is currently under further evaluation.
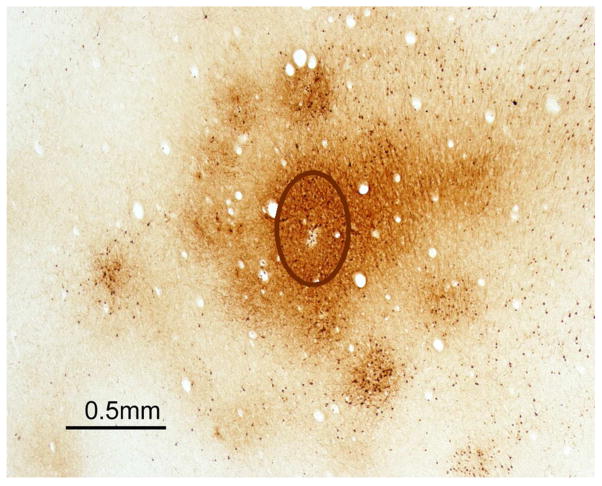
We next sought to determine how mismatched domains in each region interact. Our studies of connections indicated that domains in each region had numerous interconnections with other domains. One approach to determine how mismatched domains interact was to optically image the evoked cortical activity while stimulating domains. The results indicated that stimulating PPCr domains selectively activated matching PMC and M1 domains (Stepniewska et al., 2011). Furthermore, stimulating PPCr domains produced above threshold activation of only the stimulated domain, and immediately adjacent cortex, and not other nearby domains (abstract: Friedman et al., 2014, see Logothetis et al., 2010; Adelsberger et al., 2014 for related results). This suggested to us that the excitatory connections between domains in the same region, and likely the non-matching feedback connections, terminated mainly on inhibitory neurons, thereby reducing the activity overall in non-matching domains in the same and other regions. Most importantly, the optical imaging results indicated that the excitatory activation resulting from electrically stimulating PPCr domains does not spread widely or randomly. Instead, increases in overall activity are confined to the stimulated domain, and the immediate surround, and to matching PMC and M1 domains. The patchy activation of cortex in the immediate surround of the stimulated domain suggests that this cortex is functionally tied to the domain, and we refer to it as the satellite cortex subserving each domain. PPCr domains also have dense patches of connections around and slightly within domains that reflect the satellite concept (Fig. 4; see also Stepniewska et al., 2015).
Fig. 4.
Dense connections of a reach domain in PPCr of a galago with surrounding satellites. The photomicrograph is of a brain section cut parallel to the cortical surface after an injection of a tracer (BDA) into the reach domain identified by electrical stimulation. The injection core (outlined oval) labeled neurons and axons most densely and uniformly within the domain and in a patchy pattern along the margins of the domain, and just outside the domain. Modified from Stepniewska et al., 2015.
As the widespread connections that are intrinsic to PPCr stand in contrast to the limited zone of activations as measured with optical imaging, we suggest that the overall effect of domain interactions within a region is typically of mutual inhibition. We evaluated this hypothesis by stimulating two domains at the same time. This research is ongoing, but the initial results are supportive of the hypothesis of mutual inhibition between domains. When matching domains in PPCr and M1 or PMC were stimulated simultaneously the evoked movement was enhanced, by being faster or greater as suggested by a serial excitation model (Fig. 3). In contrast, stimulating two mismatched domains in PPCr at once typically resulted in a mutual inhibition with one movement winning out, or in an alternation of movements (one or the other movement occurred on each trial in alternation). However, different hand and arm movements were sometimes combined. As expected, the effects of stimulating two sites in a single domain were additive (also see Ethier et al., 2006). Overall, the results indicate that the mismatched domains most typically suppress each other. Such findings raise the question, what is the function of such suppressive interactions. We suggest, that each of the matching domains in the three cortical regions is involved in promoting one behavior over others. The selection or decision process starts in PPCr, which has domains dominated by sensory inputs from higher order sensory areas, and from the visually driven caudal half of PPC (PPCc) in galagos. PPCc receives inputs from visual areas V2, V3, DM, DL and MT complex, and projects to PPCr (Stepniewska et al., 2009b, 2015). Additionally, some of these visual areas project directly to parts of PPCr. Somatosensory areas 1–2, S2, PV and VS contribute somatosensory inputs, especially to the grasp domain of PPCr, which extends somewhat into area 1–2. In our view, the selection process somewhat resembles the “race model” of Logan and Cowan (1984) which held that when a go signal for a specific response is countered by a stop signal, the processing of the two signals proceed in a race to see which signal is completely processed first. This concept has been developed further in regard to signals that are processed in the frontal eye field for producing or stopping an eye movement, with stopping dependent on neural inhibition (Schall, 2005; Boucher et al., 2007). We propose that PPCr domains select one behavior over the other as sensory stimuli drive one domain sooner, or greater, than others that are then suppressed by inhibition generated by projections from the more active domain onto inhibitory neurons in other domains. The output of the most active PPC domain starts a similar process of selectively activating matching domains in PMC and M1. Domains in PMC are also activated by other inputs, including those from prefrontal cortex and thalamic inputs related to basal ganglia projections to the motor thalamus. Domains in M1 receive inputs from cingulate motor cortex, SMA, PMC, and PPCr, cerebellar inputs relayed via the motor thalamus and sensory inputs, especially proprioceptive inputs from area 3a, adding further levels of competition to the selection process. The PMC and M1 domains are involved in second and third levels of selection (Coallier et al., 2015), based on the different sources of information, as well as being involved in initiating and guiding the sequences of movements for each type of behavior. Ultimately, M1 domains have the most relevant information, and are the most critical in the selection process. The critical outputs of PMC and M1 are to brainstem and spinal motor pools of neurons, which organize the muscle synergies responsible for the motor behaviors (Bizzi et al., 2008; Roh et al., 2011).
PPC, PMC and M1 domains in New World monkeys
In addition to our studies on galagos, we have identified functional domains in two taxa of New World monkeys, owl monkeys and squirrel monkeys. Modern galagos are part of the strepsirrhine radiation, so named because they have retained the primitive feature of a rhinarium, a hairless, moist area on the nose. Modern tarsiers, monkeys, apes and humans have lost this feature, and are called haplorhines, or anthropoids, a clade that sometimes excludes tarsiers. The ancestors of extant New World monkeys, called platyrrhines, somehow rafted from Africa to South America some 35 million years ago (Schrago and Russo, 2003; Schrago, 2007) where they radiated into a number of branches, including the small marmosets and tamarins, and the more varied but larger cebids, ranging from the small owl monkey and squirrel monkey to much larger capuchins, spider monkeys, and howler monkeys. Overall, the smaller cebids appear to most closely resemble the early monkeys that made it to South America. Cortical organization has been studied extensively in both owl and squirrel monkeys, and many cortical areas have been defined, including most of those shown for galagos in figure 1. Here, we focus on the organization of PPC, PMC and M1, especially in regard to the locations of some of the functionally specialized domains that we have defined in galagos. However, our studies of such domains in squirrel monkeys and owl monkeys is ongoing and less extensive than our studies in galagos.
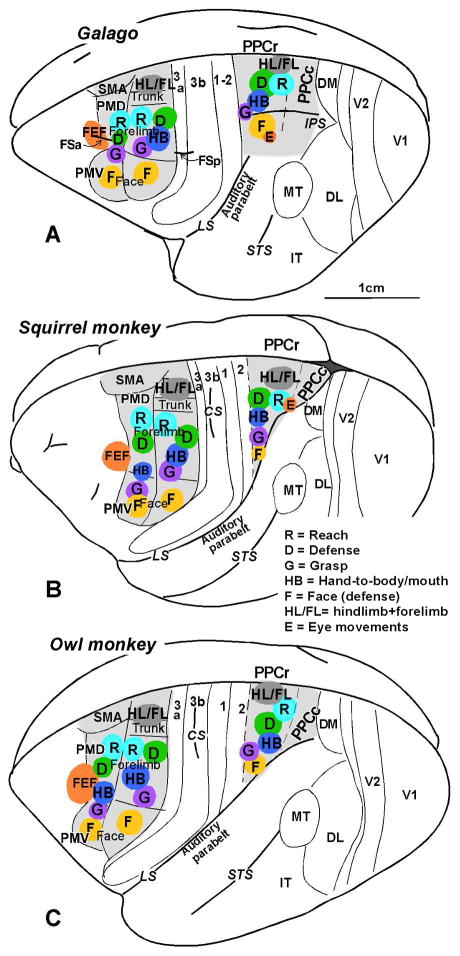
The arrangements of six or more of the functional domains in PPCr, PMC and M1 of galagos, owl monkeys, and squirrel monkeys are shown for comparison in figure 5. Note that the overall arrangement of domains in the three cortical regions is similar, and it reflects the overall hindlimb to face somatotopy, from medial to lateral, that have been described for somatosensory areas 3a, 3b, 1 and 2, and M1, in addition to ventral and dorsal premotor cortex combined. Microstimulation with 60 ms trains of current pulses at near threshold levels revealed overlapping representation in PMD and PMV, with fractured somatotopies in both areas. Hindlimb, trunk, proximal forelimb, neck and face movements were evoked in PMD and distal forelimb and orofacial movements in PMV (Preuss et al., 1996). The adjoining FEF can be considered part of premotor cortex. Thus, PMD, PMV, and FEF appear to form a larger functional unit. Most importantly, the evoked complex movements from domains in PPCr, PMC and M1 in galagos, New and Old World monkeys are very similar (Gharbawie et al., 2011a,b).
Fig. 5.
The arrangement of functional domains in (A) galagos, (B) squirrel monkeys, and (C) owl monkeys in PPCr, PMC, and M1. Matching domains are found in all three regions, where they are arranged in similar mediolateral sequences. No movement domains have been found in caudal PPC (PPCc). At least 3–4 domains (reach, grasp, defense and eye movements) are also present or likely in macaques (not shown, see text). Modified from Stepniewska et al., 2014.
Inactivation experiments with muscimol in squirrel monkeys demonstrated that the inactivation of domains in M1 abolished or greatly reduced the movements evoked from matched domains in PMC or PPC, and inactivation of PMC domains altered, reduced or abolished movements evoked from matching domains in PPCr (Stepniewska et al., 2014). Thus, as in galagos, matched domains in PPCr, PMC and M1 of New World monkeys function as a series. Domains of different types in each of the three regions were densely connected within these three regions (Gharbawie et al., 2011b). Feedforward connections favored matching domains, while feedback connections were more widespread. As in galagos, the more caudal reach domain was dominated by inputs from visual PPCc and extrastriate visual areas, while the more rostral grasp received dense inputs from somatosensory areas. As in galagos, electrical stimulation of PPC domains selectively activated M1 domains as measured with optical imaging (unpublished). In the squirrel and owl monkeys, the eye movement domain in lateral PPC was located on the rostral bank of extended lateral sulcus (Friedman et al., 2014), as there is no intraparietal sulcus in these monkeys. An early study of tracing FEF connections (Huerta et al., 1987) showed labeled neurons in the region of the eye movement domain in PPCr described here. Overall, the arrangement of the types of domains in PPCr, PMC and M1 of squirrel and owl monkeys, the evoked behaviors, the serial nature of the processing, and the cortical connections are similar to those in galagos. We conclude from these similarities that the parallel networks described here evolved in the immediate ancestors of all extant primates, and changed very little in the evolution of galagos, squirrel monkeys and owl monkeys. Comparisons of these systems in the brains of the larger New World cebus monkeys would be useful, as brains in these monkeys have changed by becoming bigger, having specializations of somatosensory cortex (Felleman et al., 1983) and possibly in other ways such as those reflecting tool use (Visalberghi et al., 2009).
There may be some differences in PPC organization between galagos and small New World monkeys, but they do not appear to be major. The PPCr region relative to the PPCc region appears to be larger in galagos, but measurements are needed. The satellites of domains, demonstrated by optical imaging in squirrel monkeys (Friedman et al., 2014) and by dense local connections in galagos (Fig. 4), have not been fully investigated, and these satellites may differ across primate taxa. Little is known about the organization of PPCc and the parts of PPCr in owl and squirrel monkeys where movements have not been evoked.
PPC, PMC and M1 domains in Old World macaque monkeys
There is a wealth of information about the organizations of PPC in macaques from the many microelectrode recording, fMRI and anatomical studies, but much of this information is difficult to relate to a framework of organization that is focused on the results of microstimulation. However, there is enough information to suggest that there are major similarities in functional organization to those described here for PPC of galagos and New World monkeys. Most notably, we have used long-train electrical stimulation to evoke grasping movements of the contralateral hand from a small zone of cortex near the tip of the intraparietal sulcus in macaque monkeys (Gharbawie et al., 2011a). The effective domain included both, the cortex of the upper bank of the caudal tip of the intraparietal sulcus and adjoining cortex of PPCr extending into area 2. The extension into area 2 was into cortex responsive to touch on the fingers. Neurons in the region of this grasp domain respond to touch of the hand and manipulation of the hand (see Seelke et al., 2012 for review). However, much of the research on response properties of neurons in PPC that is related to grasping and manipulation has been on the ventral bank of the intraparietal cortex, in a region known as the anterior intraparietal area, AIP, where neurons responses relate to the shape of the hand during grasping (Taira et al., 1990; Sakata et al., 1995; Murata et al., 2000; Gardner et al., 2007) and where cortical deactivations with muscimol produces deficits in hand preshaping for grasping (Gallese et al., 1994). While the grasping domain in macaques does not appear to be in area AIP, which is the traditionally proposed region for grasping, the grasping domain is connected to the AIP region, as well as other parts of PPC (Gharbawie et al., 2011a). Thus, it is clear that there are other regions of PPC that are involved in grasping and manipulation, but electrical stimulation may not evoke grasping from these regions, including AIP. While AIP is known to have connections with ventral premotor cortex (Luppino et al., 1999; Borra et al., 2008), the parietal grasp domain has direct connections with the grasp zone of M1 as defined by long train microstimulation (Gharbawie et al., 2011a). Other connections of the grasp domain are with part of PMV, which is also involved in grasping. Previously Graziano et al., (2002) used long train microstimulation to define functional domains (action zones) in M1 and PMC of macaques, including a manipulation zone in M1, much like our grasp domain. Thus, there is evidence for a grasping domain in PPCr with projections to the M1 grasp domain, and to a part of PMV that may have a grasp domain. Other zones defined in M1 and PMC by stimulation included a climbing region medially and reaching, hand to mouth, and defensive arm and face regions more laterally (Graziano et al., 2002, 2005). At a finer scale, somatotopies of M1 and premotor areas are fractured (eg. Qi et al., 2000). Graziano and Aflalo (2007) have suggested that the fractures in movement representations in M1 and premotor cortex, as well as the re-representations, constitute a way for the “motor repertoire” to be mapped onto the two-dimensional cortical sheet.
Long train stimulation has also been used to identify a body and head defense domain more caudally in PPC, in cortex of the fundus of the intraparietal sulcus (Cooke et al., 2003). This region was previously defined by inputs from MT as the ventral intraparietal area, VIP (Maunsell and Van Essen, 1983). Neurons in VIP respond to visual and tactile stimulation (Colby et al., 1993; Duhamel et al., 1998). Electrical stimulation of sites in VIP evoked defensive movements which included a squint or blink of the contralateral eye, a contraction of the face toward the eye, a flattening of the ear against the head, an elevation of the contralateral shoulder, and an outward movement of the arm. Very similar movements were evoked from the polysensory zone of ventral PMC (Graziano et al., 2002; Graziano and Cooke, 2006). In addition, chemically exciting the polysensory zone with bicuculline resulted in exaggerated defensive reaction to air puffs (Cooke and Graziano, 2003, 2004). Similar movements are produced by an air puff on the cheek, and they have been interpreted as defensive movements to protect the head or body from an approaching object. The connections of VIP appear to be with the polysensory zone of PMC (Luppino et al., 1999). Thus, VIP is very much like the defensive domain we have described in PPCr of galagos and New World monkeys, and the polysensory defensive zone in PMC corresponds to the defensive zone in PMC of those primates.
Another domain-like module that has been identified in macaques PPC is the lateral intraparietal area, LIP, an area originally defined by connections with the frontal eye field, FEF, and the superior colliculus, two structures involved in processing saccadic eye movements (Andersen et al., 1985). Electrical stimulation of sites in LIP produces conjugate saccades toward the contralateral hemifield (Thier and Andersen, 1998). The saccades are similar to those evoked by electrically stimulating the FEF, but the threshold currents are higher. Eye movements can be evoked by short (40ms) trains of pulses (Shibutani et al, 1984), but longer trains (200ms) are more effective (Kurylo et al., 1991). Overall, the functional properties of LIP and its anatomical connections with the FEF (Huerta et al. 1987) and the superior colliculus (Huerta et al., 1986) indicate that much of LIP directly corresponds to the eye movement domain of PPCr in galagos, owl monkeys and squirrel monkeys. The FEF has been defined by microstimulation in all these primates (Huerta et al., 1986; Preuss et al., 1996; Wu et al., 2000). Thus, matching PPCr and PMC domains for eye movements exist in prosimian primates and New and Old World monkeys. Unlike other sets of matching domains, there is no obvious eye movement domain in M1.
While long trains of electrical stimulation have not been used to define a reach domain in posterior parietal cortex of macaque monkeys, microelectrode recording results have been used to define a parietal reach region (PRR, Snyder et al., 2000) that includes medial intraparietal area MIP of Colby et al. (1988). Neurons in PRR respond during the preparation for a reaching movement with the hand and during the reach (Calton et al., 2002; Cohen and Andersen, 2002). The reaching functions of PRR, are thought to be mediated by projections to dorsal premotor cortex (Caminiti et al., 1996), the location where long train electrical stimulation evokes reaching movements in macaques (Graziano et al., 2005), and in galagos and New World monkeys. Thus, a reaching domain likely exists within PRR of macaque.
Overall, there is a good evidence for matching frontal and posterior parietal domains for grasping, defense, looking and reaching in macaques that are arranged in a spatial order that corresponds to those in galagos and New World monkeys. There obviously could be more classes of domains in PPC of macaques, and there is already good evidence for more domains in frontal cortex. The array of PPC domains is rotated in macaques so that the medial reaching domain is much more caudal in macaques compared to galagos and New World monkeys. This rotation likely reflects the greater expansion of rostromedial parts of PPC in macaques. It is also clear that much of the cortex around the grasp domain in macaques is also involved in grasping, but not so directly that electrical stimulation evokes a grasp, and this could be the case for other domains as well.
Domains in human PPC
Movement specific domains have not been demonstrated by long train stimulation in PPC of human brain. This is not surprising given the few circumstances that would justify collecting such data, and the recent use of this procedure in other primates. Yet, there is indirect evidence for grasping, defense, and eye movement domains in PPC of humans, and the domains are associated within larger regions of cortex where the response properties of neurons are compatible with such behaviors. Thus, we can infer from activity patterns in cortex where domains are likely located, as we have done for a reaching domain in the parietal reach region, PRR, of macaques. Most notably a rostrocaudal sequence of regions successively related to grasping, eye movements, and reaching have been repeatedly revealed by fMRI experiments in humans, and the regions have been compared and often homologized with the AIP, LIP, and PRR in macaques. Thus, human AIP (hAIP) is activated during the grasping and manipulation of objects (e.g., Binkofski et al., 1998; Frey et al., 2005; Culham et al., 2006; Hinkley et al., 2009; Cavina-Pratesi et al., 2010; Konen et al, 2013), as is part of ventral premotor cortex. Thus, it is possible that electrical stimulation would define grasping domains in parts of the hAIP and PMV regions of humans. There is also evidence for a more caudal human ventral intraparietal area, hVIP that represents peripersonal space, information that could be useful in evoking and guiding defensive behaviors (Bremmer et al., 2001; Sereno and Huang, 2006).
Others have identified a presumptive homolog of macaque LIP, hLIP, or the parietal eye field, as activated during saccadic eye movements (e.g., Tosoni et al., 2008; Hinkley et al., 2009; for lesion effects see Ptak and Muri, 2013), a region that is co-activated with the frontal eye field, hFEF (e.g., Vernet et al., 2014). Finally, there is fMRI evidence for a caudal reach region, the presumptive hPRR that is co-activated with part of PMD (e.g., Prado et al., 2005; Vesia et al., 2010; Konen et al., 2013). Overall, these observations and others suggest that at least some of the action domains of PPC of galagos and monkeys likely exist in humans. These domains, if identified by electrically evoked behaviors, are likely to be smaller than the regions activated by preparing for specific movements and other related cognitive acts, but this remains to be determined. There is evidence that the presumptive homologs of macaque areas AIP, VIP, LIP, and PRR are more spatially separated in the much larger human parietal cortex, perhaps to “support human-specific functions” (Konen et al., 2013), including the use of complex tools (Frey, 2007; Peeters et al., 2009). Additionally, PPC of humans contains multiple representations of the visual hemifields that appear to be related to different functions (e.g., Silver and Kastner, 2009). Together with the imaging evidence that functional networks in human PPC have some overlapping activations, we hypothesize that only restricted regions in human PPC, the movement domains, are directly involved in mediating specific behaviors.
Finally, the activation patterns provide evidence that PPC regions in humans relate directly or indirectly to functionally matching regions in PMC and M1, as in galagos and monkeys. These motor areas of cortex appear to be somatotopically fractured so that the “hand representation”, in M1 for example, contains a mosaic of small columns related to different movements of fingers, wrist and arm (e.g., Sanes and Schieber, 2001; Branco et al., 2003; Farrell et al., 2007), as in monkeys, where functional domains contain a mixture of such columns (Kaas, 2012). Moreover, there is now direct evidence for a hand-to-mouth domain in M1 of humans that is revealed by electrical stimulation (Desmurget et al., 2014). Other supporting evidence comes from high-resolution fMRI activations during actions (Meier et al., 2008). Thus, evidence is accumulating for the existence in all primates of separate, yet interacting networks for a series of ethologically relevant complex movement patterns that are mediated by interconnected PPC, PMC and M1 functionally matched domains that access subcortical motor structures while receiving various types of sensory, motor, and cognitive information from cortical and subcortical inputs. Additional research is needed to further determine how these networks and the organization of PPC are similar and different across primate taxa.
Acknowledgments
Grant Support: NIH EY002686 to JHK and NS 055843 to IS.
We thank Leah Krubitzer for helpful comments on an earlier version of this manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ROLE OF AUTHORS
Both authors prepared this review.
LITERATURE CITED
- Adelsberger H, Zainos A, Alvarez M, Romo R, Konerth A. Local domains of motor cortical activity revelaed by fiber-optic calcium recordings in behaving non-human primates. Proc Natl Acad Sci U S A. 2014;111:463–468. doi: 10.1073/pnas.1321612111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Cowan WM. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: A study using retrogradely transported fluorescent dyes. J Comp Neurol. 1985;232:443–455. doi: 10.1002/cne.902320403. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Cheung VC, d’Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev. 2008;57:125–133. doi: 10.1016/j.brainresrev.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch JI, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- Bonazzi L, Viaro R, Lodi E, Canto R, Bonifazzi C, Franchi G. Complex movement topography and extrinsic space representation in the rat foreimb motor cortex as defined by long-duration intracortical microstimulation. J Neurosci. 2013;33:2097–2107. doi: 10.1523/JNEUROSCI.3454-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Branco DM, Coelho TM, Branco BM, Schmidt L, Calcagnotto ME, Portuguez M, Paglioli Neto E, Paglioli E, Palmini A, Lima JV, Da Costa JC. Functional variability of the human cortical motor map: electrical stimulation findings in perirolandic epilepsy surgery. J Clin Neurophysiol. 2003;20:17–25. doi: 10.1097/00004691-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Duhamel JR, Graf W, Fink GR. Space coding in primate posterior parietal cortex. Neuroimage. 2001;14:S46–51. doi: 10.1006/nimg.2001.0817. [DOI] [PubMed] [Google Scholar]
- Brown AR, Teskey GC. Motor cortex is functionally organized as a set of spatially distinct representations for complex movements. J Neurosci. 2014;34:13574–13585. doi: 10.1523/JNEUROSCI.2500-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge JF. Auditory areas in primates. In: Woolsey CN, editor. Cortical sensory organization. Vol 3, Multiple auditory areas. Human Press; Clifton: 1982. pp. 59–70. [Google Scholar]
- Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci. 2002;5:580–588. doi: 10.1038/nn0602-862. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Johnson PB. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex. 1996;6:319–328. doi: 10.1093/cercor/6.3.319. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, McAdam TD, Quinlan DJ, Goodale MA, Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J Neurosci. 2010;30:10306–10323. doi: 10.1523/JNEUROSCI.2023-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, Le Gros WE. The Antecedents of Man. Edinburgh: Edinburgh University Press; 1959. [Google Scholar]
- Coallier E, Michelet T, Kalaska JF. Dorsal premotor cortex: neural correlates of reach target decisions based on a color-location matching rule and conflicting sensory evidence. J Neurophysiol. 2001 doi: 10.1152/jn.00166.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. Topographic Organization of Cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Graziano MSA. Defensive movements evoked by air puff in monkeys. J Neurophysiol. 2003;90:3317–3329. doi: 10.1152/jn.00513.2003. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Graziano MSA. Super-flinchers and nerves of steel: Defensive movements altered by chemical manipulation of a cortical motor area. Neuron. 2004;43:585–593. doi: 10.1016/j.neuron.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CSR, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Padberg J, Zahner T, Krubitzer L. The functional organization and cortical connections of motor cortex in squirrels. Cereb Cortex. 2012;22:1959–1978. doi: 10.1093/cercor/bhr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Stepniewska I, Miller DJ, Kaas JH, Kubitzer L. Reversible deactivation of motor cortex reveals functional connectivity with posterior parietal cortex in the prosomian galago (Otolemur garnetti) Soc Neurosci Abstr 432.12 (online) 2014 doi: 10.1523/JNEUROSCI.1468-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creem-Regerh SH. Sensory-motor and cognitive functions of the human posterior parietal cortex involved in manual actions. Neurobiol Learn Mem. 2009;91:166–171. doi: 10.1016/j.nlm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina-Pratesi C, Singhal A. The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia. 2006;44:2668–2684. doi: 10.1016/j.neuropsychologia.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Richard N, Harquel S, Baraduc P, Szathmari A, Mottolese C, Sirigu A. Neural representations of ethologically relevant hand/mouth synergies in the human precentral gyrus. Proc Natl Acad Sci U S A. 2014;111:5718–5722. doi: 10.1073/pnas.1321909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: Anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Ebner FF, Kaas JK. Somatosensory system. In: Paxinos G, editor. The Rat Nervous System. 4. Elsevier; 2015. pp. 673–699. [Google Scholar]
- Ethier C, Brizzi L, Darling WG, Capaday C. Linear summation of cat motor cortex outputs. J Neurosci. 2006;26:5574–5581. doi: 10.1523/JNEUROSCI.5332-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DF, Burbank N, Lettich E, Ojemann GA. Individual variation in human motor-sensory (rolandic) cortex. J Clin Neurophysiol. 2007;24:286–293. doi: 10.1097/WNP.0b013e31803bb59a. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Nelson RJ, Sur M, Kaas JH. Representation of the body surface in areas 3b and 1 of postcentral parietal cortex of cebus monkeys. Brain Res. 1983;268:15–26. doi: 10.1016/0006-8993(83)90386-4. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Huang RS, Sereno MI. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J Neurosci. 2009;29:2961–2971. doi: 10.1523/JNEUROSCI.3211-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flash T, Hochner B. Motor primitives in vertebrates and invertebrates. Curr Opin Neurol. 2005;15:1–7. doi: 10.1016/j.conb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Frey SH. What puts the how and where? Tool use and the divided visual streams hypothesis. Cortex. 2007;43:368–375. doi: 10.1016/s0010-9452(08)70462-3. [DOI] [PubMed] [Google Scholar]
- Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:1951–1957. doi: 10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res. 2005;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Friedman RM, Stepniewska I, Roe AW, Kaas JH. Intracortical microstimulation reveals movement specific domains in awake squirrel monkeys. Soc Neurosci Abstr 735.08 (online) 2014 [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport. 1994;5:1525–1529. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Qi H, Kaas JH. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci. 2011a;31:11660–11677. doi: 10.1523/JNEUROSCI.1777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex regions in new world monkeys. Cereb Cortex. 2011b;21:1981–2002. doi: 10.1093/cercor/bhq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Graziano MSA. The intelligent movement machine. Oxford University Press; New York: 2009. [Google Scholar]
- Graziano MSA, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56:234–251. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Aflalo T, Cooke DE. Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J Neurophysiol. 2005;94:4209–4223. doi: 10.1152/jn.01303.2004. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning W. Phylogenetic Systematics. Univ Illinois Press; 1966. [Google Scholar]
- Hill J, Inder T, Neil J, Diesker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley LBN, Krubitzer LA, Padberg J, Disbrow EA. Visual-manual exploration and posterior parietal cortex in humans. J Neurophysiol. 2009;102:3433–3446. doi: 10.1152/jn.90785.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homman-Ludiye J, Manger PR, Bourne JA. Immunohistochemical parcellation of the ferret (Mustela putorius) visual cortex reveals substantial homology with the cat (Felis catus) J Comp Neurol. 2010;518:4439–4462. doi: 10.1002/cne.22465. [DOI] [PubMed] [Google Scholar]
- Huang R-S, Chen C-F, Trans AT, Holstein KL, Sereno MI. Mapping multisensory parietal face and body areas in humans. Proc Natl Acad Sci U S A. 2012;109:18114–18119. doi: 10.1073/pnas.1207946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys and macaque monkeys: I. Subcortical connections. J Comp Neurol. 1986;253:415–39. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of the large, complex sensorimotor systems of anthropoid primates. J Comp Psych. 2004;17:34–52. [Google Scholar]
- Kaas JH. Neocortex in early mammals and its subsequent variations. Ann NY Acad Sci. 2011;1225:28–36. doi: 10.1111/j.1749-6632.2011.05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Evolution of columns, modules, and domains in the neocortex of primates. Proc Natl Acad Sci U S A. 2012;109:10655–10660. doi: 10.1073/pnas.1201892109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Preuss TM. Human brain evolution. In: Squire L, et al., editors. Fundamental Neuroscience. 4. London: Elsevier; 2014. pp. 901–928. [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat. 2011;5:34. doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I, Iyer A, Lindner A, Andersen RA. Space representation for eye movements is more contralateral in monkeys than in humans. Proc Natl Acad Sci U S A. 2010;107:7933–7938. doi: 10.1073/pnas.1002825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Beh Brain Res. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Konen CS, Mruczek RE, Montoya JL, Kastner S. Functional organization of human posterior parietal cortex: grasping- and reaching-related activations relative to topographically organized cortex. J Neurophysiol. 2013;109:2897–2908. doi: 10.1152/jn.00657.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci. 2008;28:8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Skavenski AA. Eye movements elicited by electrical stimulation of area PG in the monkey. Am Physiol Soci. 1991;65:1243–1253. doi: 10.1152/jn.1991.65.6.1243. [DOI] [PubMed] [Google Scholar]
- Linden DE, Thornton K, Kuswanto CN, Johnston SJ, van de Ven V, Jackson MC. The brain’s voices: comparing nonclinical auditory hallucinations and imagery. Cereb Cortex. 2011;21:330–337. doi: 10.1093/cercor/bhq097. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Augath M, Murayama Y, Rauch A, Sultan F, Goense J, Oeltermann A, Merkle H. The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci. 2010;13:1283–1291. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Manger PR, Masiello I, Innocenti GM. Areal organization of the posterior parietal cortex of the ferret (Mustela putorius) Cereb Cortex. 2002;12:1280–1297. doi: 10.1093/cercor/12.12.1280. [DOI] [PubMed] [Google Scholar]
- Martin RD. Primate origins and evolution. Princeton: Princeton University Press; 1990. [Google Scholar]
- Maunsell JHR, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurophysiol. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: a high-resolution fMRI study. J Neurophysiol. 2008;100:1800–1812. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Bizzi E. Motor learning through the combination of primitives. Philosophical Transactions of the Royal Society: Biological Sciences. 2000;355:1755–1769. doi: 10.1098/rstb.2000.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Roh J, Bizzi E. Modulation of Muscle Synergy Recruitment in primate grasping. J Neurosci. 2008;28:880–892. doi: 10.1523/JNEUROSCI.2869-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron. 2012;76:1071–1077. doi: 10.1016/j.neuron.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Driver J. Relating unilateral neglect to the neural coding of space. Curr Opin Neurobiol. 2000;10:242–249. doi: 10.1016/s0959-4388(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron. 2005;48:849–858. doi: 10.1016/j.neuron.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: A microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ptak R, Müri RM. The parietal cortex and saccade planning: lessons from human lesion studies. Front Hum Neurosci. 2013;7:254. doi: 10.3389/fnhum.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H-X, Stepniewska I, Kaas JH. Reorganization of primary motor cortex in adult macaque with long-standing amputations. J Neurophysiol. 2000;84:2133–2147. doi: 10.1152/jn.2000.84.4.2133. [DOI] [PubMed] [Google Scholar]
- Radinsky L. Primate Brain Evolution. Am Scientist. 1975;63:656–663. [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of functions after brain injury. Proc Natl Acad Sci U S A. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Kaas JH. Organization of frontoparietal cortex in the tree shrew (Tupaia belangeri). I. Architecture, microelectrode maps, and corticospinal connections. J Comp Neurol. 2006;497:133–154. doi: 10.1002/cne.20975. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VCK, Bizzi E. Modules in the brain system and spinal cord underlying motor behaviors. J Neurophysiol. 2011;106:1363–1378. doi: 10.1152/jn.00842.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Schieber MH. Orderly somatotopy in primary motor cortex: Does it exist? NeuroImage. 2001;13:968–974. doi: 10.1006/nimg.2000.0733. [DOI] [PubMed] [Google Scholar]
- Schall JD. Decision making. Curr Biol. 2005;15:R9–R11. doi: 10.1016/j.cub.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Schrago CG. On the time scale of New World primate diversification. J Phys Anthro. 2007;132:344–354. doi: 10.1002/ajpa.20459. [DOI] [PubMed] [Google Scholar]
- Schrago CG, Russo CAM. Timing the origin of New World monkeys. Mol Biol Evol. 2003;20:1620–1625. doi: 10.1093/molbev/msg172. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Padberg JJ, Disbrow E, Purnell SM, Recanzone G, Krubitzer L. Topographic maps within Brodmann’s area 5 of macaque monkeys. Cereb Cortex. 2011;22:1834–1850. doi: 10.1093/cercor/bhr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. Multisensory maps in parietal cortex. Curr Opin Neurobiol. 2013;24:39–46. doi: 10.1016/j.conb.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15:94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Shibutani H, Sakata H, Hyvärinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intension related activity in the posterior parietal cortex: a review. Vision Rev. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Steiper ER. Evidence for a convergent slow-down in primate molecular rates and its implications for the timing of early primate evolution. Proc Natl Acad Sci U S A. 2012;104:6004–6011. doi: 10.1073/pnas.1119506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A. 2005;13:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 2009a;517:765–82. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009b;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Friedman RM, Gharbawie OA, Cerkevich CM, Roe AW, Kaas JH. Optical imaging in galagos reveals parietal-frontal circuits underlying motor behavior. Proc Natl Acad Sci U S A. 2011;108:E725–732. doi: 10.1073/pnas.1109925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Gharbawie O, Burish MJ, Kaas JH. Functional maps of the motor thalamus in prosimian primates based on electrical stimulation. FENS Abstr vol. 2012;7:08733. [Google Scholar]
- Stepniewska I, Gharbawie OA, Burish MJ, Kaas JH. Effects of muscimol inactivations of functional domains in motor, premotor, and posterior parietal cortex on complex movements evoked by electrical stimulation. J Neurophysiol. 2014;111:1100–1119. doi: 10.1152/jn.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Kaas JH. Connections of the caudal portion of posterior parietal cortex in prosimian galagos. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv132. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL. Stimulating research on motor cortex. Nat Neurosci. 2002;5:714–715. doi: 10.1038/nn0802-714. [DOI] [PubMed] [Google Scholar]
- Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res. 1990;83:29–36. doi: 10.1007/BF00232190. [DOI] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- Tosoni A, Galati G, Romani GL, Corbetta M. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat Neurosci. 2008;11:1446–1453. doi: 10.1038/nn.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabre A. Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci. 2014;8:66. doi: 10.3389/fnint.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci. 2010;30:13053–13065. doi: 10.1523/JNEUROSCI.1644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalberghi E, Addessi E, Truppa V, Spagnoletti N, Ottoni E, Izar P, Fragaszy D. Selection of effective stone tools by wild bearded capuchin monkeys. Curr Biol. 2009;19:213–217. doi: 10.1016/j.cub.2008.11.064. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proc Natl Acad Sci U S A. 2004;101:2167–2172. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker W, Blair C, Shambes GM. Somatosensory projections to cerebellar granule cell Layer of giant bushbaby, Galago crassicaudatus. Brain Behav Evol. 1988;31:150–160. doi: 10.1159/000116582. [DOI] [PubMed] [Google Scholar]
- Wiley EO. Phylogenetics: The theory and practice of phylogenetic systematics. New York: John Wiley & Sons; 1981. [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the galago (Otolemur garnetti) Anat Rec. 2010;293:1033–1069. doi: 10.1002/ar.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections from multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–717. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Young M, Stepniewska I, Kaas JH. Motor cortex. In: Watson C, Paxinos G, Pueles L, editors. The Mouse Nervous System. London: Elsevier; 2012. pp. 528–538. [Google Scholar]