Abstract
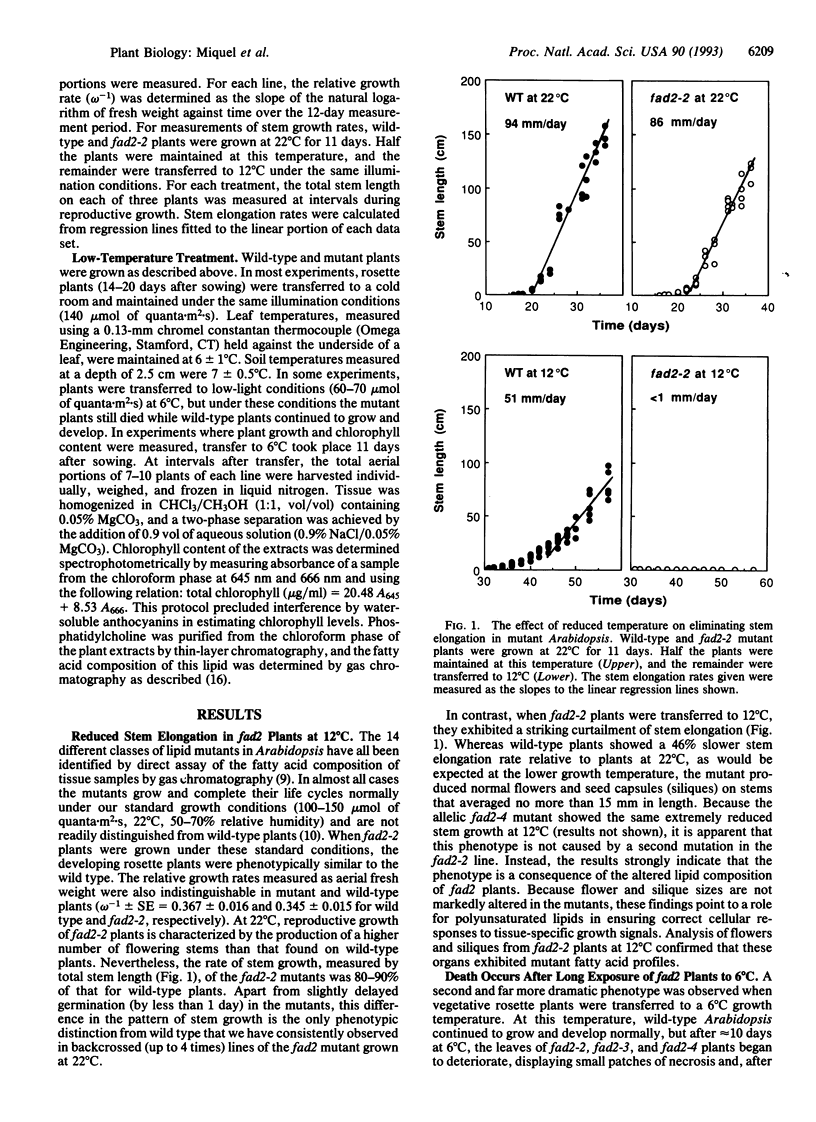
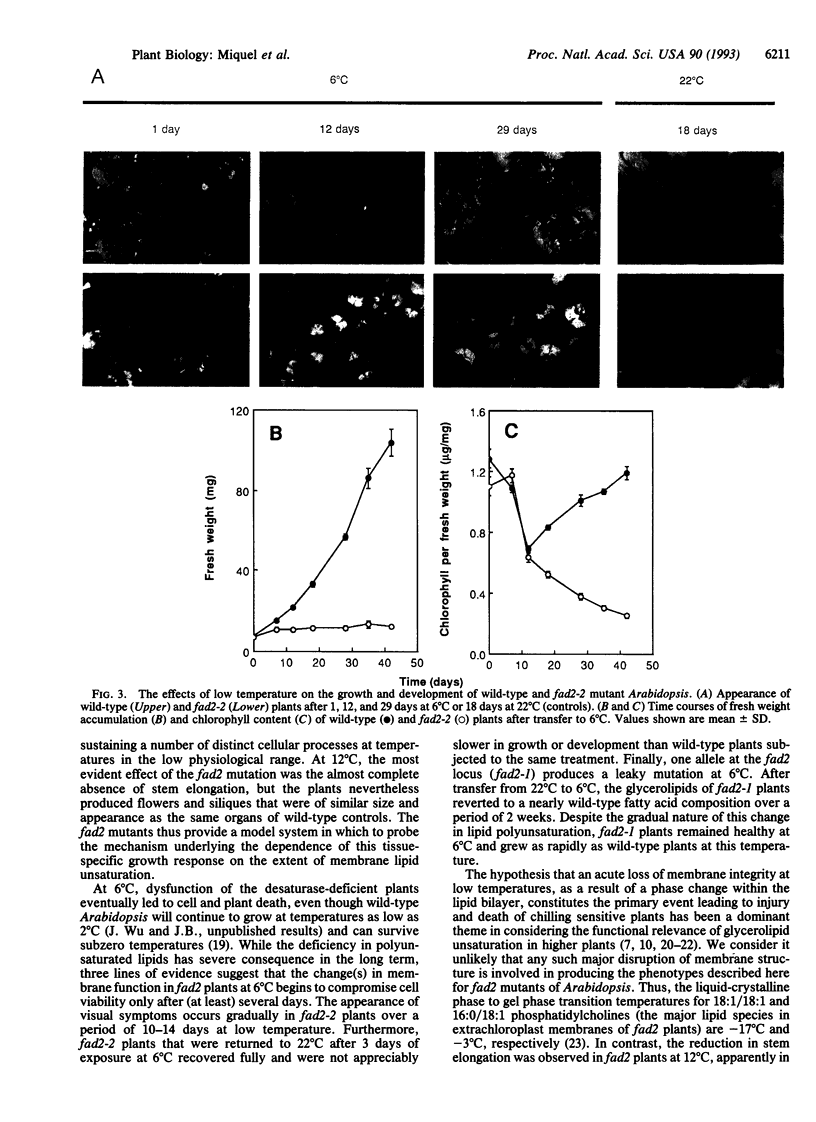
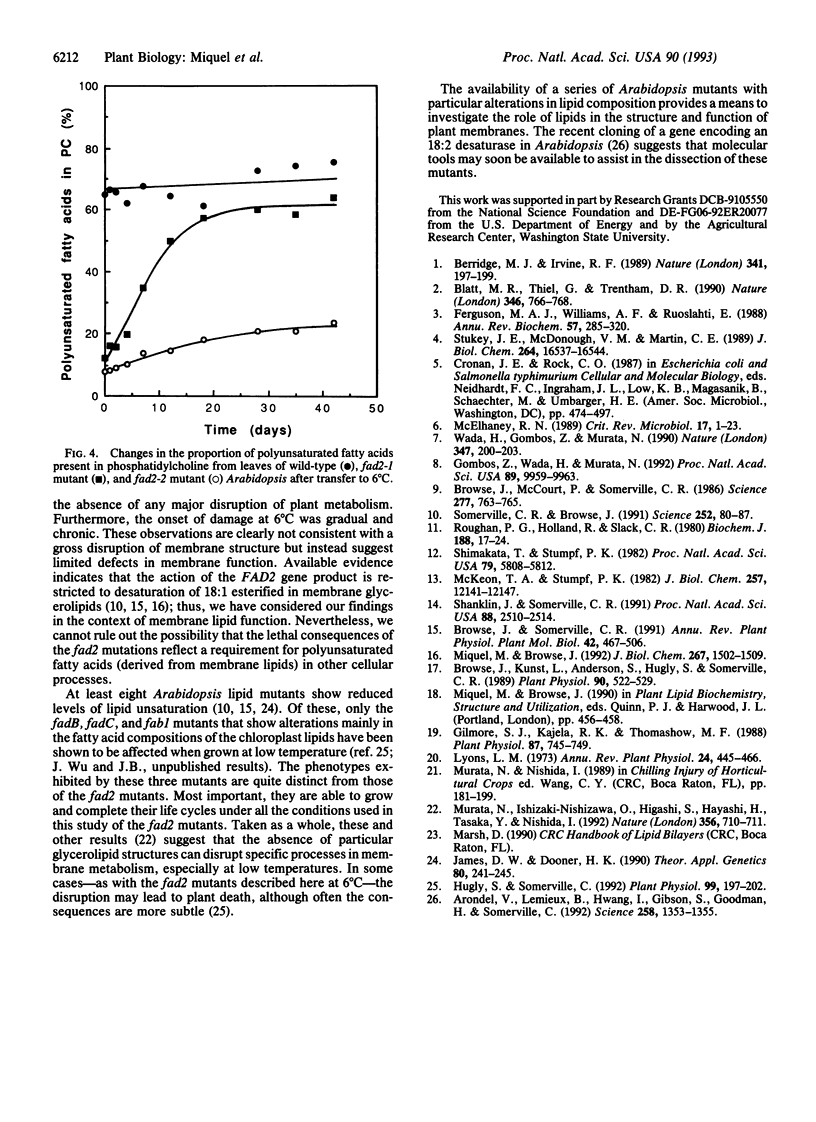
Mutants of Arabidopsis that contain reduced levels of polyunsaturated fatty acids showed growth characteristics at 22 degrees C that were very similar to wild type. By contrast, at 12 degrees C, the mutants failed to undergo stem elongation during reproductive growth although they produced normal flowers and fertile seeds. After transfer to 6 degrees C, rosette leaves of the mutants gradually died, and the plants were inviable. These different responses of the mutant plants at 12 degrees C and 6 degrees C suggest that distinct functions may be affected at these two temperatures. The gradual development of symptoms at 6 degrees C and other lines of evidence argue against a general collapse of membrane integrity as the cause of the lethal phenotype. Rather, they indicate that the decrease in polyunsaturated membrane lipids may initially have relatively limited effects in disrupting cellular function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arondel V., Lemieux B., Hwang I., Gibson S., Goodman H. M., Somerville C. R. Map-based cloning of a gene controlling omega-3 fatty acid desaturation in Arabidopsis. Science. 1992 Nov 20;258(5086):1353–1355. doi: 10.1126/science.1455229. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blatt M. R., Thiel G., Trentham D. R. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1990 Aug 23;346(6286):766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Browse J., Kunst L., Anderson S., Hugly S., Somerville C. A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol. 1989 Jun;90(2):522–529. doi: 10.1104/pp.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Gilmour S. J., Hajela R. K., Thomashow M. F. Cold Acclimation in Arabidopsis thaliana. Plant Physiol. 1988 Jul;87(3):745–750. doi: 10.1104/pp.87.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos Z., Wada H., Murata N. Unsaturation of fatty acids in membrane lipids enhances tolerance of the cyanobacterium Synechocystis PCC6803 to low-temperature photoinhibition. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9959–9963. doi: 10.1073/pnas.89.20.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S., Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 1992 May;99(1):197–202. doi: 10.1104/pp.99.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N. The influence of membrane lipid composition and physical properties of membrane structure and function in Acholeplasma laidlawii. Crit Rev Microbiol. 1989;17(1):1–32. doi: 10.3109/10408418909105720. [DOI] [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Miquel M., Browse J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem. 1992 Jan 25;267(3):1502–1509. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J., Somerville C. Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2510–2514. doi: 10.1073/pnas.88.6.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Isolation and function of spinach leaf beta-ketoacyl-[acyl-carrier-protein] synthases. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5808–5812. doi: 10.1073/pnas.79.19.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C., Browse J. Plant lipids: metabolism, mutants, and membranes. Science. 1991 Apr 5;252(5002):80–87. doi: 10.1126/science.252.5002.80. [DOI] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J Biol Chem. 1989 Oct 5;264(28):16537–16544. [PubMed] [Google Scholar]
- Wada H., Gombos Z., Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature. 1990 Sep 13;347(6289):200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]





