Abstract
The delivery of postoperative combined modality adjuvant therapy for completely resected pancreatic adenocarcinoma was initially shown to be beneficial based on a prospective, randomized trial published 30 years ago. Since then, oncologists have debated whether chemotherapy alone, chemoradiation, or both are optimal adjuvant therapies following pancreatectomy for pancreatic ductal adenocarcinomas (PDAC). No global consensus has emerged, and there is no one superior modality despite randomized trials in part, to poor trial design, poor patient selection, and poor therapy options itself. We need to have a disciplined approach to the selection of patients for pancreatectomy, pathologic assessment of surgical resection margins, and postoperative (pre-treatment) imaging. In the era of the multidetector CT optimized for pancreatic imaging, tumors of “borderline resectability” have emerged as a distinct subset of PDAC. The attempt to standardize the definition of borderline resectable is a work in progress and modified with time. This distinction (between resectable and borderline resectable) is essential to minimize potentially confounding results of clinical trials. Additionally, preoperative therapy is not only preferred but mandatory in a large population of borderline resectable patients. Ultimately, as we develop more effective systemic therapies for PDAC, proceeding with surgery after a period of induction therapy will be even more compelling especially if there is a clear positive impact on overall survival.
Keywords: Pancreatic adenocarcinoma, Borderline resectable pancreatic adenocarcinoma, Preoperative treatment, Neoadjuvant treatment
Introduction
Surgery plays an undisputed central role in the curative management of localized pancreatic adenocarcinoma (PDAC). Unfortunately, the majority of patients with radiographic evidence of localized disease will recur with metastases. Even with excellent perioperative supportive care and low mortality in high-volume centers, ∼80 % of resected patients will develop metastases and die of their disease within 5 years [1, 2]. This is largely due to the presence of micro-metastatic disease at the time of attempted resection [3].
As a result, multimodality therapy involving systemic and radiation therapy has become integral to the preoperative and adjuvant settings. While earlier research focused on treatment strategies in the adjuvant setting, those results have informed more recent work in the preoperative setting. Additionally, improved imaging technologies with rigorous diagnostic criteria allow for identification of borderline resectable pancreatic adenocarcinoma (BRPC) patients, a group which may particularly benefit from preoperative therapy.
Strong Evidence in Support of Adjuvant Therapy for Pancreatic Cancer
5-Fluorouracil-Based Adjuvant Therapy
Standard treatment for unresectable pancreatic cancer in the 1980s involved fluorouracil-based (5-FU) regimen with limited trials investigating the role of adjuvant chemotherapy [4]. The earliest randomized prospective trial for operable PDAC was published in 1985 by the Gastrointestinal Tumor Study Group (GITSG) [5]. Forty-three patients were randomized to observation or 5-FU with radiation after surgery. Survival was 20 vs. 11 months in treatment vs. observation arms, respectively. The European Organization of Research and Treatment of Cancer (EORTC) conducted a study comparing 5-FU-based chemoradiation vs. observation in patients with resected pancreatic head or periampullary cancers (defined as tumors of the distal common bile duct, papilla of Vater, or duodenum). Median overall survival (OS) in the treatment group was 24.5 vs. 19 months in the observation group; however, this difference did not achieve significance (p = 0.208) [6]. Pancreatic head adenocarcinoma patients appeared to derive larger relative benefit than those with periampullary cancers; unfortunately, the study was under powered to analyze this subgroup.
The European Study Group for Pancreatic Cancer (ESPAC) launched a more robust effort to determine the contributions of adjuvant chemotherapy and chemoradiation for resected PDAC [7, 8]. Two hundred eighty-nine patients were randomized to one of four arms: observation; chemotherapy with bolus 5-FU and leucovorin both given daily for 5 days every 28 days for 6 months; chemoradiation with bolus 5-FU, given during the first 3 days of split-course external beam radiotherapy (EBRT); or chemoradiation followed by 6 months of chemotherapy with bolus 5-FU and leucovorin. The study analyzed the survival outcomes using a 2 × 2 factorial design, pooling survival data based on randomization to chemotherapy (yes or no), or chemoradiation (yes or no). When overall survival (OS) durations of the four arms were compared, there was no survival difference among them. However, using the 2 × 2 factorial design, patients who received chemoradiation did worse (median OS of 15.9 months) than those not receiving chemoradiation (median OS of 17.9 months, p = 0.05). Conversely, patients who received chemotherapy had a median OS of 20.6 vs. 15.5 months for those who did not receive chemotherapy (p = 0.009). The investigators concluded that adjuvant chemotherapy improved survival, and that chemoradiation not only failed to benefit patients, but was detrimental.
Gemcitabine-Based Adjuvant Therapy
Because gemcitabine is modestly superior to bolus 5-FU for the treatment of advanced PDAC, its integration into adjuvant therapy trials was a logical next step, as discussed in three large studies. In the CONKO-001 (Charité Onkologie 001) trial, resected patients received adjuvant gemcitabine vs. observation for 6 months with reported improvement in both disease-free survival (DFS) and OS with adjuvant chemotherapy [9]. The long-term outcomes data confirms the benefit of adjuvant gemcitabine. The treatment group had significant improvement in DFS at 13.4 months vs. 6.7, and median OS for adjuvant gemcitabine vs. observation was 22.8 vs. 20.2, respectively (HR = 0.76; p = 0.01) [10].
The Radiation Therapy Oncology Group (RTOG) prospective randomized trial (RTOG 9704) compared gemcitabine with infusional 5-FU as the systemic component of therapy with all patients also receiving 5-FU-based chemoradiation. Five hundred thirty-eight patients were enrolled in the study with the majority of patients having pancreatic head tumors. There was no difference in the OS for the two groups. For patients with pancreatic head tumors (n = 388), the addition of gemcitabine to adjuvant 5-FU-based chemoradiation was associated with a survival benefit (20.5 vs. 16.9 months, p = 0.05).
The ESPAC-3 trial randomized 1088 patients following pancreatectomy and adequate recovery to receive gemcitabine or bolus 5-FU and leucovorin for 6 months. There was no difference in overall survival, although gemcitabine was better tolerated [11].
As with earlier single-agent studies, ongoing multiple agent chemotherapy trials in advanced disease will offer insights applicable to adjuvant treatment. The Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) trial demonstrated a survival benefit of combination nab-paclitaxel and gemcitabine vs. gemcitabine alone in metastatic PDAC [12]. The ongoing APACT study compares the same chemotherapy agents in the adjuvant setting.
Adjuvant Trials in Periampullary Cancers
The role of adjuvant treatment in periampullary disease is less well characterized. As discussed above, the EORTC trial showed no significant benefit with chemoradiation. The ESPAC-3 periampullary cancer trial specifically addressed adjuvant chemotherapy in this population, comparing 5-FU, gemcitabine, and observation [13]. The investigators found a trend towards survival benefit with adjuvant therapy that was significant on multivariable analysis. Despite being one of the largest periampullary trials to date, subgroup analysis was underpowered to determine survival benefit between regimens.
Analysis of Large Randomized Adjuvant Trials
ESPAC-1, CONKO-001, and RTOG 9704 all appear to reflect some problems with quality control—the inability to determine the presence or absence of a gross complete resection at the time of surgery and R0/R1 on final pathology, and no central review of imaging or operative reports.
The available data suggests that some form of adjuvant therapy is probably better than no therapy, particularly since many of the study patients were likely receiving therapy for persistent local disease or early metastatic disease which was not detected due to the absence of high-quality postoperative/pre-treatment imaging or the accurate interpretation of such studies if performed. Moreover, if the results are reviewed objectively, no single postoperative approach can claim conclusive superiority to others.
At the University of Texas, M.D. Anderson Cancer Center, (MDACC), we treat PDAC patients who have undergone upfront surgery, with up to a total of 6 months of gemcitabine-based chemotherapy. The current role of radiation remains controversial. At MDACC, most patients are candidates for adjuvant chemotherapy alone, partially because of our preoperative approach that utilizes radiation before surgery (discussed below). When we encounter a patient with a high risk of local relapse (R1 resection), we consider adjuvant chemoradiation to address potential residual disease based on the overall risk of distant vs. isolated locoregional recurrence.
Emerging Support for Preoperative Systemic Therapy
Preoperative therapy has a sound rationale to include initiation of therapy shortly after diagnosis rather than weeks after surgery, treatment of a relatively well-perfused tumor bed, and provision of a time interval to assess for onset of overt metastatic disease prior to surgical intervention. Despite the benefit of adjuvant treatment, it is delayed or denied in nearly 25 % of patients [6] due to postoperative complications, comorbidities, or prolonged recovery. Preoperative therapy is generally well tolerated and has a high completion rate. Preoperative treatment provides a several-months window where patients with microscopic metastatic disease may “declare” themselves and surgical morbidity is avoided. In situations that require medical optimization to improve performance status prior to surgery—such as biliary decompression or treatment of pulmonary embolus—systemic therapy can be used to minimize or prevent disease progression. Thus far, preoperative strategies for pancreatic cancer have not been widely adopted despite growing evidence of the potential benefits of this approach for other gastrointestinal tumors including rectal cancer, gastric cancer, and esophageal cancer. A common reason for rejecting preoperative therapy centers on the potential for local tumor progression, which may preclude surgical intervention. However, published to date suggests that this risk is not substantial.
Potential benefit was first shown with preoperative radiation therapy in the early 1980s [14]. Since then, studies of preoperative therapy have utilized both chemotherapy and radiation, either in combination or sequentially. The majority of the studies from the 1990s employed 5-FU-based regimens, for radiosensitization and EBRT [15–17]. As would be expected, the patients who underwent preoperative therapy and resection had significantly better survival than those who did not. However, there are several limitations to interpretation of these studies, mainly the lack of strict resectability criteria.
Two large studies reported by MDACC studied preoperative therapy using gemcitabine-based chemoradiation (Gem-XRT) and systemic therapy followed by chemoradiation (Gem-Cis followed by Gem-XRT). The Gem-XRT study enrolled 86 resectable patients who received seven weekly doses of gemcitabine and 30 Gy of radiation over 2 weeks, followed by restaging 4–6 weeks after completing treatment [18]. Of those enrolled, 64 (74 %) underwent pancreaticoduodenectomy (PD) and 57 (89 % of resected) achieved R0 resection. Median OS for the resected patients was 34 vs. 7 months for the unresected cohort. The second trial with Gem-Cis followed by Gem-XRT enrolled 79 patients who successfully completed treatment with gemcitabine/cisplatin for 4 cycles followed by gemcitabine-based chemoradiation with 30 Gy in 10 fractions [19]. Of those who completed treatment, 52 (66 %) were treated operatively and 50 (96 %) achieved an R0 resection. OS was 31 months for resected patients and 10.5 months for those not resected. Additionally, isolated locoregional progression precluding surgery was low in both studies.
There are few trials investigating preoperative chemotherapy in the resectable population without the addition of radiation. O’Reilly and colleagues [20] prospectively investigated the effect of preoperative gemcitabine and oxaliplatin in 38 patients. Thirty-five patients completed treatment with 4 cycles of gemcitabine and oxaliplatin, 27 (77 %) underwent PD and among those, 20 (74 %) achieved R0 resection. The median OS was 27.2 months for resected patients. While small, this study suggests that preoperative chemotherapy alone may improve patient selection and disease control in patients with clearly resectable PDAC.
Given the lack of randomized data, the role of preoperative therapy in the PDAC population is less clear than adjuvant therapy. There is now reasonable evidence that preoperative therapy will select patients most likely to benefit from surgical resection. A meta-analysis of existing trials identified benefit in unresectable cases at presentation (largely BRPC) over resectable PDAC [21].
At MDACC, we give resectable patients an opportunity for preoperative therapy, ideally through clinical trials when possible. As the adjuvant and metastatic therapies for PDAC become more effective, the preoperative logic will be more sound; these therapies can then be adopted in the preoperative setting, and allow the most aggressive and definitive part of treatment, i.e., surgery, to come last.
Borderline Resectable Pancreatic Cancer: Criteria and Management
The best evidence for preoperative multimodality therapy has been established for the highest risk patients with localized disease. In the meta-analysis by Gillen et al. [21], patients with initially unresectable disease treated with preoperative therapy were likely to respond to therapy—4.8 % of patients achieved a complete response and 30.2 % achieved a partial response. These rates were higher with combination chemotherapy compared with monotherapy. Significantly, 33.2 % of patients underwent surgical exploration with resection (79 % R0 resection rate). In the era of the multidetector CT optimized for pancreatic imaging, tumors of “borderline resectability” have emerged as a distinct subset of PDAC. Patients with BRPC are poor candidates for upfront surgery because they are at a high risk for margin-positive resection with initial surgery. Multiple studies have reported that patients with margin-positive resection do poorly with a life expectancy between 8–12 months, which is no different from patients with locally advanced PDAC [22, 23]. The rationale for preoperative treatment for BRPC is similar to potentially resectable PDAC although with a greater emphasis on maximizing R0 resection [24].
Detailed imaging studies have found, with a threshold of 180° of vessel involvement, unresectability could be predicted with very high specificity and high sensitivity [25, 26]. Accurate imaging with pancreatic-phase thin-section helical CT plays an essential role, and criteria for BRPC has been modified with time through the National Comprehensive Cancer Network (NCCN), initial descriptions from MDACC, and consensus conferences, the first being sponsored by the Americas Hepato-Pancreato-Biliary Association (AHPBA)/Society for Surgery of the Alimentary Tract (SSAT)/Society of Surgical Oncology (SSO). Briefly, it allows superior mesenteric vein (SMV)-portal vein (PV) abutment, encasement, or occlusion; superior mesenteric artery (SMA) abutment; abutment or short-segment encasement of the common hepatic artery; and no involvement of the celiac trunk. Table 1 describes the various groups’ criteria, and it remains a work in progress.
Table 1.
Primary radiologic criteria for borderline resectable pancreatic cancer
| AHPBA-SSAT-SSO | Intergroup (alliance A021101) | MDACC | NCCN | |
|---|---|---|---|---|
| SMV-PV | Abutment, encasement, or occlusion | At least 180° interface between tumor and vessel OR reconstructable occlusion | Occlusion | Abutment with impingement or narrowing |
| SMA | Abutment | Less than 180° interface between tumor and vessel | Abutment | Abutment |
| CHA | Abutment or short-segment encasement | Reconstructable, short-segment interface between tumor and vessel | Abutment or short-segment encasement | Abutment or short-segment encasement |
| Celiac trunk | No abutment or encasement | Less than 180° interface between tumor and vessel | Abutment | No abutment or encasement |
AHPBA Americas Hepatopancreatobiliary Association, SSAT Society for Surgery of the Alimentary Tract, SSO Society of Surgical Oncology, SMV superior mesenteric vein, PV portal vein, SMA superior mesenteric artery, CHA common hepatic artery
MDACC published one of the largest retrospective studies of preoperative treatment of BRPC to date [27]. One hundred twenty-nine patients were identified as BRPC by either the MDACC or AHPBA/SSO/SSAT criteria; 70 met both sets of criteria. Patients were primarily treated with either sequential gemcitabine-based chemotherapy followed by chemoradiation or chemoradiation alone. The average survival in the surgical group was 32 months while in the unresected population it was only 12 months.
Several prospective trials for BRPC have been conducted to identify effective regimens, and several trials are ongoing. A combination of 5-FU, irinotecan, and oxaliplatin (FOLFIRINOX) is being actively studied in BRPC. Paniccia [28] reported on a small retrospective cohort of patients who received FOLFIRINOX. Approximately half received only chemotherapy while the rest received chemotherapy followed by chemoradiation. In spite of expected toxicities, nearly 90 % of patients completed chemotherapy. Eighty-five percent underwent resection, and all those patients achieved R0 resection. A separate study looking at a modified FOLFIRINOX regimen with reduced doses found similar rates of resection and high R0 resection rates with less toxicities [29]. The Alliance A021101 intergroup trial for BRPC (22 patients) evaluated preoperative FOLFIRINOX followed by capecitabine-based chemoradiation, and early data shows a 68 % resection rate in this population.
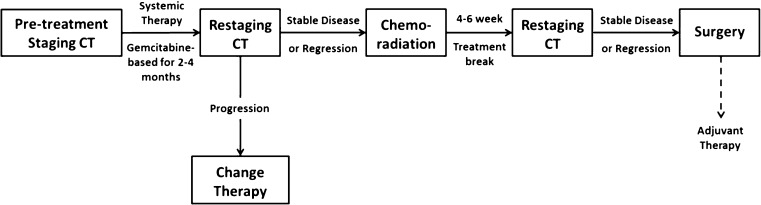
At our institution, we treat most BRPC patients with a combination of chemotherapy and chemoradiation. Typically, we use gemcitabine-containing multi-agent regimens or FOLFIRINOX in sequence with chemoradiation therapy, ideally in a protocol setting. Figure 1 outlines our approach to management of borderline resectable disease.
Fig. 1.
Management algorithm for borderline resectable pancreatic cancer. Adjuvant therapy should be considered based on length of preoperative therapy and pathology. Preoperative FOLFIRINOX and other novel combinations are preferred in protocol setting
Barriers to Preoperative Therapy
Patients with localized PDAC are frequently treated for biliary obstruction with endoscopic stent placement. For resectable cancers, stent placement is not necessary when upfront surgical intervention will definitively address obstruction. However, in patients where a prolonged course of preoperative therapy is planned, metal stents should be used as they are more likely to remain patent; stent occlusion is likely to interrupt therapy and can result in life-threatening infection [30]. Prehabilitation is a newer concept that refers to the enhancing a patient’s functional capacity prior to medical or surgical intervention. PDAC patients with poor functional status are more likely to have poor outcomes, and multidimensional programs to address debilitation, improve nutrition, and optimize comorbid conditions allow better completion of preoperative treatment and improve surgical outcomes. Additionally, directly addressing patient expectations for surgery through preoperative therapy is essential. Multimodality treatment courses are long and both physically and emotionally taxing—if a patient is unprepared, they are less likely to complete therapy. Finally, preoperative therapy by nature is a multidisciplinary approach to optimize chance of curative tumor resection. Tumor boards and early involvement of all practitioners in treatment planning minimizes delays and facilitates management based on individual and institutional expertise.
Summary and Future Trends
A more disciplined approach needs to be taken to define tumor resectability preoperatively, and to ensure that patients who undergo surgery have a significantly higher chance of achieving a complete resection of all gross disease. This may decrease the number of patients eligible for upfront surgery and subsequent adjuvant therapy. However, if preoperative therapy is carefully designed and delivered, especially to patients with tumors objectively classified as borderline for immediate resection, more patients may ultimately benefit from surgery and enjoy long-term survival.
At this time, we lack predictive biomarkers to help assess candidates for surgery or a particular therapy. Unfortunately, Human Equilabrative Nucleoside Transporter 1 (hENT1) expression nor Secreted Protein Acidic and Rich in Cysteine (SPARC) were useful to predict response to neither gemcitabine nor nab-paclitaxel, respectively. There is an urgent need to develop relevant prognostic and predictive markers of response. While many therapies have failed over the past decade, there is presently an explosion of trials targeting the molecular features of this disease. Beside PDAC tumor genomic profiling, there is a significant interest in exploiting a “liquid biopsy” platform using exosomes, circulating tumor cells, and cell-free DNA from blood to study genomic and proteomic alterations that can then inform therapy.
Acknowledgments
Conflict of Interest
Dr. Reilley, Dr. Shroff, and Dr. Varadhachary have no conflicts to disclose.
References
- 1.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225(5):621–633. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 3.Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246(5):734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 4.Wright JC. Update in cancer chemotherapy: gastrointestinal cancer, cancer of the pancreas. J Natl Med Assoc. 1986;78(6):519–527. [PMC free article] [PubMed] [Google Scholar]
- 5.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 6.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–1585. doi: 10.1016/S0140-6736(01)06651-X. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 14.Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer. 1980;46(9):1945–1949. doi: 10.1002/1097-0142(19801101)46:9<1945::AID-CNCR2820460908>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Kamthan AG, Morris JC, Dalton J, et al. Combined modality therapy for stage II and stage III pancreatic carcinoma. J Clin Oncol. 1997;15(8):2920–2927. doi: 10.1200/JCO.1997.15.8.2920. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16(1):317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 17.Pisters PW, Abbruzzese JL, Janjan NA, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. 1998;16(12):3843–3850. doi: 10.1200/JCO.1998.16.12.3843. [DOI] [PubMed] [Google Scholar]
- 18.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 19.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260(1):142–148. doi: 10.1097/SLA.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234(6):758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Tamm EP, Loyer EM, Faria S, et al. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging. 2006;31(5):568–574. doi: 10.1007/s00261-005-0194-y. [DOI] [PubMed] [Google Scholar]
- 26.Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg. 2014;18(2):269–278. doi: 10.1007/s11605-013-2374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 28.Paniccia A, Edil BH, Schulick RD, et al. Neoadjuvant FOLFIRINOX application in borderline resectable pancreatic adenocarcinoma: a retrospective cohort study. Medicine (Baltimore) 2014;93(27):e198. doi: 10.1097/MD.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22(4):1153–1159. doi: 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulay BR, Parepally M. Managing malignant biliary obstruction in pancreas cancer: choosing the appropriate strategy. World J Gastroenterol. 2014;20(28):9345–9353. doi: 10.3748/wjg.v20.i28.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]