Abstract
Pancreatic ductal adenocarcinoma (PDA) is a challenging disease, as overall survival has not improved over the last several decades. The disease is characterized by late diagnosis, difficult major surgery in resectable patients, and a biologically chemoresistant tumor. Intense research in the field is ongoing to develop biomarkers for early detection and prognostication. Surgery is presently the crux of the management of PDA and has been standardized over the years with high-volume centers reporting <5 % operative mortality. The biggest problem is to overcome the inherent chemoresistance of the tumor that is densely fibrotic and hypoxic and has a tendency to invade surrounding neuronal plexuses. This review attempts to summarize in brief the reasons why PDA is difficult to treat, and provides a glimpse of the ongoing research in the field.
Keywords: Pancreatic ductal adenocarcinoma, Diagnosis, Surgery, Chemoresistance, Survival
Introduction
Pancreatic ductal adenocarcinoma (PDA) is a dreaded disease with a very poor 5-year survival rate of <5 % [1, 2]. Despite giant strides made in the surgical management of pancreatic cancer, the disease continues to be a harbinger of death. The operative mortality has dropped drastically to less than 5 % in high-volume centers, but the operative morbidity still remains close to 40–50 % [3, 4]. Unfortunately, nearly 80–85 % of patients present with unresectable and advanced cancer with a dismal median survival of <6 months [1, 2]. Even the best possible treatments prolong life by only ∼8–16 weeks [5].
To understand the reason behind this aggressive nature of PDA, first of all, a deep insight is imperative into the unfavorable dynamic molecular changes that occur during pancreatic carcinogenesis and metastasis.
Pancreatic Carcinogenesis
It is known that the vast majority of pancreatic cancers develop from microscopic precursors called pancreatic intraepithelial neoplasia (PanIN) that originate in small terminal (<5 mm) pancreatic ducts. These are further classified as PanIN-1, PanIN-2, and PanIN-3 on basis of increasing atypia; the associated genetic changes have been extensively studied [6]. The other precancerous lesions include intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) that are often asymptomatic and discovered incidentally [7]. However, the genetic progression model of these cystic neoplasms is less well known.
It is yet unclear if the poor survival of pancreatic cancer patients is due to early dissemination or to a delay in diagnosis. Two interesting studies bring forth both the theories. On one end of the spectrum is the study by Yaschida et al. [8] wherein they reported their results of rapid autopsies and sequencing the genomes of seven patients with end-stage pancreatic cancer to evaluate the clonal relationships among primary and metastatic cancers. They identified two categories of mutations. The commonest were the ones present in all samples from a given patient (“founder” mutations), indicating that the majority of somatically acquired mutations were present in pancreatic cancers and occurred before the development of metastatic lesions. The other category mutations were the “progressor” mutations that were present in one or more of the metastases examined, including the index metastasis, but not the parental clone. They found by Sanger sequencing that all mutations in the metastatic lesions were clonal, i.e., present in the great majority if not all neoplastic cells of the metastasis. Thus, these mutations were present in the cell that clonally expanded to become the metastasis. Further, clonal populations that give rise to distant metastases are represented within the primary carcinoma [8].
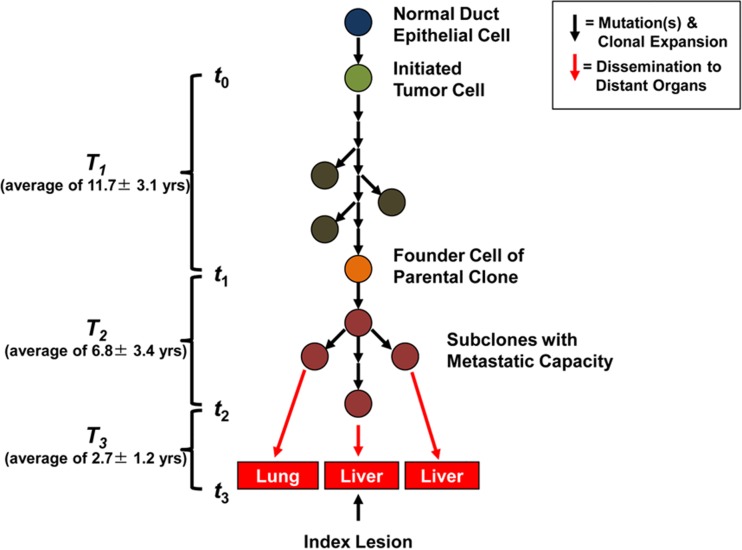
Using the mathematical model, they were further able to estimate that an average of 17 years elapses between the initiation of tumorigenesis until birth of the cell giving rise to metastasis, suggesting that a large window of opportunity for diagnosis exists while the disease is still localized (Fig. 1) [8].
Fig. 1.
Schema of the genetic evolution of pancreatic cancer. Tumorigenesis begins with an initiating mutation in a normal cell that confers a selective growth advantage. Successive waves of clonal expansion occur in association with the acquisition of additional mutations, corresponding to the progression model of pancreatic intraepithelial neoplasia (PanIN) and time T1. One founder cell within a PanIN lesion will seed the parental clone and hence initiate an infiltrating carcinoma (end of T1 and beginning of T2). Eventually, the cell that will give rise to the index lesion will appear (end of T2 and beginning of T3). Unfortunately, most patients are not diagnosed until well into time interval T3 when cells of these metastatic subclones have already escaped the pancreas and started to grow within distant organs. The average time for intervals T1, T2, and T3 for all seven patients is indicated in the parentheses at the left (reproduced with permission from [8])
On the other extreme is a study by Rhim et al. [9] in 2012. They tagged and tracked pancreatic epithelial cells in a mouse model of pancreatic cancer [9]. They found using lineage-labeled mouse models that tagged (YFP+) cells traversed the basement membrane and dissociated from any pancreatic epithelial structure (delamination) even in PanIN-2 and PanIN-3 lesions before invasive behavior could be detected by standard histology. These cells had acquired mesenchymal characteristics [epithelial-to-mesenchymal transition (EMT)]. Circulating pancreatic cells (CPCs) were also detected in the blood by flow cytometry, retained mesenchymal and stem cell characteristics, and seeded the liver. Thus, their data support a model for pancreatic cancer progression in which the seeding of distant organs occurs before, and in parallel to, tumor formation at the primary site. Additional experiments will be needed to prove that cells that enter the circulation prior to the development of frank malignancy have metastatic potential.
Extensive research thus continues in the laboratory to understand the biology of PDA, but as clinician, it is also important to understand the grim realities of the disease and difficulties in its management.
Late Diagnosis
Pancreatic cancer is a disease of the elderly and is often diagnosed at a late stage due to vague presenting symptoms that are often ignored by the patient and lack of effective early diagnostic tools (imaging/biomarkers). The presenting symptoms of this disease can be very subtle and nonspecific and may include weight loss, floating foul smelling stools, pain, dyspepsia, nausea, and depression. Painless progressive jaundice; sudden onset of adult type 2 diabetes in patients 50 years or older; unusual manifestations, such as abdominal symptoms and progressive weight loss in patients with long-standing diabetes; and in ~10 %, migratory thrombophlebitis (Trousseau’s syndrome) may be serious indicators of pancreatic cancer.
The majority of PDA is sporadic (results from somatic mutations), and only a minority of 5–10 % has a genetic component (germline mutations); these may range from high-penetrance genes (associated with high lifetime risk) to low-penetrance genes (associated with <1.5-fold increased risk) of PDA [10]. Genetic syndromes associated with pancreatic cancer include Peutz-Jeghers syndrome, familial pancreatitis, familial atypical multiple mole melanoma (FAMMM) syndrome, Lynch syndrome, hereditary breast-ovarian cancer syndrome, etc. A family history of PDA in first-degree relative increases the risk by 4.6- to 6.4-fold [11]. It is important to quantify risk as it enables screening of individuals at risk for lesions in the pancreas and, in addition, screening for extrapancreatic malignancies that may be associated with these genetic syndromes. Interestingly, knowledge regarding genetic alterations also helps predict treatment response to therapeutic agents as those with BRCA2 gene inactivation appear to be sensitive to DNA cross-linking agents [12].
However, still, no screening tool or biomarker exists that would enable the diagnosis of PDA at an early stage. The only well-researched biomarker for PDA is serum CA 19-9, a sialylated Lewis A blood group antigen, is FDA approved, but has never proved to be an effective screening tool [13]. The major drawbacks include its nonspecific expression in many benign and malignant diseases, false-negative results in Lewis-negative genotype (5–10 %), and false-positive results in the presence of biliary infection (cholangitis) and obstructive jaundice (10–60 %) [14, 15]. Serum CA 19-9 levels have a sensitivity and specificity of 79–81 % and 82–90 %, respectively, for the diagnosis of pancreatic cancer in symptomatic patients but are not useful as a screening marker because of its low positive predictive value (0.5–0.9 %) [16]. Another study revealed that elevated levels of CA 19-9 were often present in PDA relative to other pancreaticobiliary diagnoses in the analysis of 283 patients. However, 15 % of patients with PDA had normal CA 19-9 levels [17]. On the contrary, others have reported that CA 19-9 levels may be elevated in patients up to 2 years before a diagnosis of pancreatic cancer, indicating its importance as a biomarker for screening individuals at high risk [18].
Current research focuses on proteomic-based, metabolomic-based, and genomic-based biomarkers such as proteins, metabolites, microRNAs, and circulating nucleic acids in the blood, serum, urine, bile, and pancreatic juice. Using specialized tests such as microarray analyses or focused multiplex immunoassay, several PDA-associated proteins have been identified in the serum, including PGK1 (seventh step of glycolysis) [19, 20], histone H4 (chromatin component) [20], c14orf166 (modulates RNA polymerase) [21], MBL2 (innate immune response) [22], MLCK2 (skeletal muscle contraction) [22], APOC1 (lipoprotein component) [23], and APOAII (lipoprotein component) [23]. Several tests assess for metabolite profile in the urine and serum. OuYang et al. [24] reported results on metabolomic profiling of the serum using (1)H nuclear magnetic resonance (NMR). They found significantly lower levels of 3-hydroxybutyrate, 3-hydroxyisovalerate, lactate, and trimethylamine-N-oxide and significantly higher levels of isoleucine, triglyceride, leucine, and creatinine in the serum from pancreatic cancer patients versus those of healthy controls [24]. Similarly, NMR spectroscopic analysis of urinary metabolites was able to identify a complex molecular signature of PDA [25]. These preliminary results suggest that metabolomic approaches may facilitate discovery of novel pancreatic cancer biomarkers [26]. The human bile is being examined too for potential biomarkers for the cancers of the hepatobiliary tract including the pancreas as it is a rich source of metabolites linked to the pathways of tumor cell metabolism of the area [27].
MicroRNAs (miRNAs) are small ribonucleic acids involved in post-transcriptional gene regulation, and several are aberrantly expressed in pancreatic cancer and are being analyzed as potential biomarkers for PDA. Schultz et al., in their study of 409 patients with pancreatic cancer, 25 with chronic pancreatitis, and 312 healthy controls, were able to identify two diagnostic panels based on miRNA expression in the whole blood with the potential to distinguish patients with pancreatic cancer from healthy controls [28].
Jones et al. [29] published their results of sequencing of the protein-coding exons from 20,661 genes in 24 patients with advanced PDA, providing unprecedented insight into the somatic mutations in these neoplasms. They revealed that pancreatic cancers have about 63 genetic alterations, the majority being point mutations. Interestingly, 67 to 100 % of the tumors have these alterations around a core set of 12 cellular signaling pathways and processes, thereby setting a target for future therapeutic agents and diagnostic biomarkers (by detecting mutant proteins shed by the cancers) [29].
PDA most commonly carry mutations of Kirsten rat sarcoma viral oncogene (KRAS) (90 %), p16/CDKN2A (90 %), TP53 (75–90 %), and SMAD4/DPC4 (50 %) [30]. The point mutations in KRAS occur early in pancreatic neoplasia and commonly target three codons (codons 12, 13, and 61), suggesting the possibility that KRAS mutations could form the basis for gene-based tests to detect early curable pancreatic neoplasia. In fact, a study found that mutation levels were substantially higher in patients with pancreatic cancer (0.05 to 82 % of total KRAS2 molecules) versus those with chronic pancreatitis (0 to 0.7 %) using a LigAmp quantification method [31]. The greatest challenge of detection of mutant KRAS in a sample (e.g., blood) is that the detection assay should have sufficient sensitivity to detect a very small number of mutant KRAS copies, in a background of abundant non-mutated sequence coming from leucocytes.
PDA can be very aggressive, and some patients (roughly 20 %) may have an early recurrence after resection and may die of disease within a year [32]. This has implications on the management aspect as those with biologically aggressive PDAs are probably best treated initially with systemic therapy compared to those with indolent cancers who may benefit from an aggressive surgical approach. One such study by Winter et al. [32] found that, on multivariate analysis using a survival tissue microarray for PDA, MUC1 [odds ratio (OR) = 28.95, 3+ vs. negative expression, p = 0.004] and MSLN (OR = 12.47, 3+ vs. negative expression, p = 0.01) were highly predictive of early cancer-specific death. By comparison, pathologic factors (size, lymph node metastases, resection margin status, and grade) had ORs below 3 and none reached statistical significance [32].
Thus, efforts are underway to find better diagnostic, prognostic, and predictive markers that will help to individualize treatment of PDA based on DNA analysis.
Difficult Surgery
The aim of surgery for carcinoma of the pancreas is local complete resection (R0) of the carcinoma. However, surgical resection is a major abdominal undertaking and is beset with complications.
Arterial Anomalies
The pancreas is situated deep in the retroperitoneum. It is in intimate contact with major abdominal vessels such as the superior mesenteric vein (SMV)/portal vein (PV), superior mesenteric artery (SMA), common hepatic artery (CHA), hepatic artery (HA), gastroduodenal artery (GDA), celiac axis (CA), and pancreaticoduodenal arcades. It is well known that aberrations in the hepatic arterial anatomy are frequent and normal anatomy is observed in only 55 to 79 % of patients [33]. The majority of the replaced/accessory arteries originate from branches of either the CA or the SMA, and they are likely to have an abnormal course. At times, there is stenosis of the CA with blood being directed to the liver via the pancreaticoduodenal arcades and GDA. Ligation of GDA (which is a routine step during pancreaticoduodenectomy) hence can have a disastrous impact on the blood supply to the liver. Most of these arterial anomalies can usually be identified by routine preoperative high-quality pancreatic protocol computed tomography scans with or without the aid of post-processed volume-rendered images [34]. It is vital to identify these arterial anomalies to prevent their inadvertent damage during surgery, compromising the vascular supply of the liver or an oncologically safe resection [35].
Neuronal Invasion
The pancreaticoduodenal arterial arcades, veins, and nerves are situated on the fusion fascia of Treitz that also covers the pancreas, extrapancreatic nerve plexuses, SMA, and PV [36]. PDA has a special tendency to invade neuronal plexus, and its prevalence may reach up to 100 % [37, 38]. It carries a strong association with local recurrence after curative resection and poor prognosis [39]. This neuronal invasion occurs mostly by continuous growth of PDA cells along the nerves towards the extrapancreatic neural plexus. Interestingly, more than 50 % of patients may have neuronal invasion in normal pancreatic areas that are distant from the main tumor, a phenomenon that is termed “nex” and is a harbinger of poor prognosis [40].
Thus, the Japanese literature emphasizes examination by frozen section of the entire dissected end of the nerve plexus during surgery and that should be confirmed to be negative for cancer. If positive for cancer, additional resection of the nerve plexus should be performed to ensure a R0 resection [36].
Pancreaticoenteric Anastomosis
To maintain pancreatic outflow, pancreaticoenteric anastomosis is created by anastomosing the pancreatic remnant to either the jejunum [pancreaticojejunostomy (PJ)] or the stomach [pancreaticogastrostomy (PG)]. This is technically and prognostically the most important part of pancreaticoduodenectomy as failure of this anastomosis results in postoperative pancreatic fistula (POPF) that can, at times, be life-threatening. PJ has often been described as the “Achilles heel” of pancreaticoduodenectomy [41]. The PJ leak rate is so preoccupying that, to minimize leak rates, over 70 technique variations of PJ have been attempted [42]. This indicates inherent failures of each technique.
A multitude of adjunct techniques have also been tested to minimize leak rate such as use of pancreatic duct stents, glue, octreotide, etc. Despite this, the procedure is associated with significant morbidity of around 40–50 % [3, 4] and hence should be preferably carried out in high-volume centers (>16 cases/year) within the purview of multidisciplinary team so as to achieve the best outcome [43].
The Tumor Microenvironment and Chemoresistance
PDA is highly chemoresistant and is a major reason for poor prognosis [44, 45]. PDA is characterized by extensive stromal/fibrotic reaction that comprises up to 90 % of the tumor volume. Further, it is extremely hypovascular and most of the pancreatic tumor mass consists of activated (myo)fibroblasts, immune cells, and extracellular matrix components, such as collagen, desmin, fibronectin, and hyaluronic acid [46–48]. An important subtype within the stromal population is pancreatic stellate cells (PSCs), which have emerged as pancreas-specific myofibroblasts. In the normal pancreas, PSCs are located in the periacinar space and account for about 4–7 % of all pancreatic cells. In the healthy pancreas, PSCs are quiescent (non-activated) and are characterized by cytoplasmic lipid droplets containing vitamin A. Upon activation, PSCs play a central role in the formation of fibrotic extracellular matrix (desmoplasia) and can also induce axonal sprouting, increased neurite density, and perikaryonal hypertrophy of neurons under in vitro conditions [49, 50]. In addition to PSCs induced desmoplasia, cancer-associated fibroblasts suppress blood vessel formation leading to the sparse vasculature, making drug delivery through this “stromal fortress” extremely difficult [51].
PDA is, thus, a profoundly hypovascular and hypoxic tumor. Direct measurements of oxygen partial pressure in human pancreatic tumors using the Eppendorf (Hamburg, Germany) polarographic electrode revealed that pancreatic tumors are significantly hypoxic, though the adjacent normal pancreas has normal oxygenation [52]. This is also evident by imaging techniques as a hypoenhancing pancreatic mass is observed in PDA patients whenever contrast-enhanced scans are obtained. Poor perfusion and hypoxic microenvironment can have important effects on radiosensitivity and aggressive behavior of the tumor [53].
The advent of various genetically engineered mouse models (GEMMs) of pancreas cancer has marked a milestone in understanding the biological implications of the tumor stroma and provides novel opportunities for preclinical testing of new agents directed against various targets in PDA. For example, sonic hedgehog (SHH) pathway plays important roles during embryonic development and is aberrantly re-expressed in PDA promoting desmoplasia in pancreatic carcinogenesis. In the KPC mouse model, pharmacologic inhibition of SHH by the smoothened inhibitor IPI-926 resulted in stromal depletion, increased microvessel density and patency, and improved delivery of gemcitabine in the intratumoral compartment [54]. Preliminary results available from a phase Ib/II trial in patients with metastatic pancreatic cancer using IPI-926 and gemcitabine reveal that the drug is well tolerated and radiographic partial responses were seen in 31 % of patients [55].
A breakthrough was recently obtained by enhancing chemotherapeutic drug delivery into the dense fibrous stroma of PDA using albumin-bound paclitaxel that is a nanoparticle form of paclitaxel. A large, open-label, international, randomized phase III MPACT trial on 861 patients with metastatic pancreatic cancer and no prior chemotherapy revealed higher overall survival (OS) (8.7 vs. 6.6 months; P < 0.0001; HR = 0.72) in patients randomized to receive gemcitabine plus albumin-bound paclitaxel versus gemcitabine alone [56]. Updated results of the MPACT trial confirm that long-term survival is possible with gemcitabine plus albumin-bound paclitaxel [57].
One proposed mechanism of action (though contradictory) is that the albumin in albumin-bound paclitaxel (nab-paclitaxel) may bind to SPARC, an extracellular matrix protein that is upregulated in the stroma of pancreatic tumors, thereby increasing the delivery of paclitaxel to the neoplastic cells [58, 59]. However, a recent analysis in GEMM found that nab-paclitaxel alters the sensitivity of pancreatic tumors to gemcitabine through downregulation of cytidine deaminase, leading to higher concentrations of dFdCTP in tumors [60].
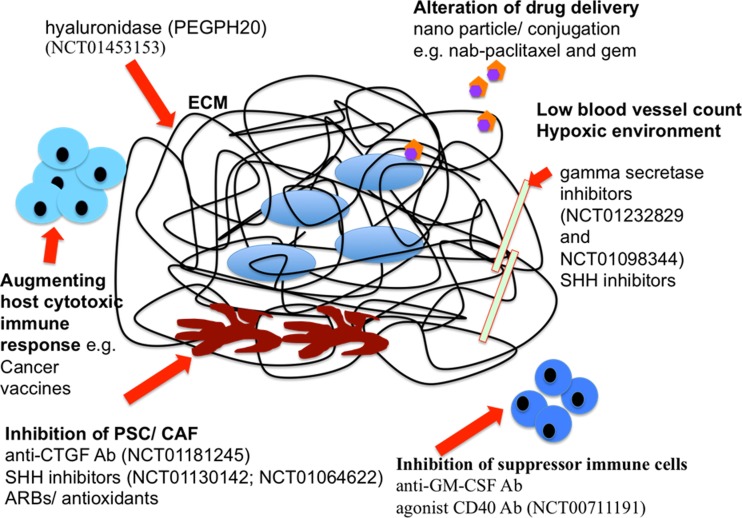
A number of new therapeutic agents targeting various tumor stroma host interactions are currently under active investigation. Discussing them in detail is out of the scope of this paper, though few are summarized in Fig. 2.
Fig. 2.
Diagrammatic representation of tumor microenvironment in PDA with new therapeutic targets. Ab antibody, CAF cancer-associated fibroblast, CTGF connective tissue growth factor, ECM extracellular matrix, GM-CSF granulocyte-macrophage colony-stimulating factor, PDA pancreatic ductal adenocarcinoma, PSC pancreatic stellate cell, SHH sonic hedgehog, nab-paclitaxel albumin-bound paclitaxel, ARB angiotensin II type 1 receptor blocker. Red arrows indicate the site of action. 1. PSC and CAF can be targeted by inhibition of SHH, CTGF, and ARBs. 2. ECM components lead to desmoplasia, creating barriers for drug delivery that can be circumvented by drug alteration and conjugation such as nab-paclitaxel and human recombinant PEGylated hyaluronidase (PEGPH20). 3. Tumor vessels are compressed by dense tumor stroma, resulting in a hypoxic environment; tumor vasculature can be targeted by SHH and gamma-secretase inhibitors. 4. Suppressor immune cells can be targeted by agonist CD40 antibodies or anti-GM-CSF antibodies. 5. Host immune response can be strengthened by cancer vaccines such as GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines [67]. 6. Selected ongoing and recently completed clinical trials are mentioned by National Clinical Trial (NCT) number, and details can be obtained online at http://clinicaltrials.gov/
Novel Imaging Techniques
In addition to standard imaging modalities, several promising techniques are under development that may improve diagnostic imaging. Advanced imaging such as diffusion-weighted magnetic resonance imaging (DW-MRI) and dynamic contrast-enhanced (DCE) MRI takes the advantage of altered perfusion of pancreatic tumor to provide functional contrast relative to normal or inflamed pancreatic tissue [61, 62]. Advanced endoscopic ultrasound techniques such as contrast-enhanced ultrasound and ultrasound elastography (which measures tissue perfusion and stiffness, respectively) have shown promise, although they are highly operator dependent [63, 64].
New targeted imaging probes are under investigation too. Plec1-targeting peptides (tPTP) have been successfully analyzed as a contrast agent for single-photon emission computed tomography (SPECT) in an orthotopic and liver metastasis murine model of PDA in vivo. Hence, it may be used to identify primary and metastatic PDA by imaging and may also detect preinvasive PanIN-3 lesions [65].
A group of researchers recently published a study evaluating polarization gating spectroscopic measurements using fiber optic probes of early increase in blood supply that detects variables, such as deoxyhemoglobin concentration (DHb) and mean blood vessel radius (BVR) in the normally appearing duodenal mucosa in 14 patients with PDA versus a control group. Preliminary evidence is very encouraging and suggests that in vivo measurement of normally appearing duodenal tissue can differentiate PDA patients from a distance with high accuracy [66].
It is obvious that pancreatic cancer is a challenge to treat and is an area of intensive research. It is very likely that, in the future, tumor DNA of each patient will be thoroughly analyzed and information thus gained be used for individualized cancer treatment. Early diagnosis seems to be the most reliable insurance for long survival in this surreptitious cancer.
Acknowledgments
I am very grateful to Dr. William Jarnagin, MD, FACS, Chief, Hepatopancreatobiliary Service; Benno C. Schmidt Chair in Surgical Oncology, Memorial Sloan Kettering Cancer Centre, New York, for going through this manuscript and for his guidance while I was in his unit as a UICC fellow.
Compliance with Ethical Standards
Conflict of Interest
The author declares that she has no competing interests.
Footnotes
Dr. Mallika Tewari, M.S., M.Ch. (Surgical Oncology), MRCS.Ed
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai M, Yamaue H. Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery. Surg Today. 2010;40:1011–1017. doi: 10.1007/s00595-009-4245-9. [DOI] [PubMed] [Google Scholar]
- 4.Winter JW, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Clores MJ, Thosani A, Buscaglia JM. Multidisciplinary diagnostic and therapeutic approaches to pancreatic cystic lesions. J Multidiscip Healthc. 2014;7:81–91. doi: 10.2147/JMDH.S43098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yachida S, Jones S, Bozic I, Antal T, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhim A, Mirek E, Aiello N, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365–374. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.CAN-03-3823. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res. 2005;11:7508–7515. doi: 10.1158/1078-0432.CCR-05-1048. [DOI] [PubMed] [Google Scholar]
- 13.Winder JM, Yeo CJ, Broody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol. 2013;107:15–22. doi: 10.1002/jso.23192. [DOI] [PubMed] [Google Scholar]
- 14.Tempero MA, Uchida E, Takasaki H, et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501–5503. [PubMed] [Google Scholar]
- 15.Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh DA, Durbin-Johnson B, Urayama S. Utility of serum CA19-9 levels in the diagnosis of pancreatic ductal adenocarcinoma in an endoscopic ultrasound referral population. J Gastrointest Cancer. 2014;45(1):74–79. doi: 10.1007/s12029-013-9563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien DP, Sandanayake NS, Jenkinson C, et al. Serum CA19-9 is significantly up-regulated up to 2 years prior to diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2015;21(3):622–631. doi: 10.1158/1078-0432.CCR-14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang TL, Liang Y, Chien KY, et al. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics. 2006;6:2259–2272. doi: 10.1002/pmic.200500345. [DOI] [PubMed] [Google Scholar]
- 20.Patwa TH, Li C, Poisson LM, et al. The identification of phosphoglycerate kinase-1 and histone H4 autoantibodies in pancreatic cancer patient serum using a natural protein microarray. Electrophoresis. 2009;30:2215–2226. doi: 10.1002/elps.200800857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Wang W, Liao P, et al. Identification of serum biomarkers for pancreatic adenocarcinoma by proteomic analysis. Cancer Sci. 2009;100:2292–2301. doi: 10.1111/j.1349-7006.2009.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong Y, Jin D, Hou C, et al. Proteomics analysis of serum protein profiling in pancreatic cancer patients by DIGE: up-regulation of mannose-binding lectin 2 and myosin light chain kinase 2. BMC Gastroenterol. 2010;10:68. doi: 10.1186/1471-230X-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue A, Scarlett CJ, Chung L, et al. Discovery of serum biomarkers for pancreatic adenocarcinoma using proteomic analysis. Br J Cancer. 2010;103:391–400. doi: 10.1038/sj.bjc.6605764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.OuYang D, Xu J, Huang H, Chen Z. Metabolomic profiling of serum from human pancreatic cancer patients using 1H NMR spectroscopy and principal component analysis. Appl Biochem Biotechnol. 2011;165(1):148–154. doi: 10.1007/s12010-011-9240-0. [DOI] [PubMed] [Google Scholar]
- 25.Napoli C, Sperandio N, Lawlor RT, et al. Urine metabolic signature of pancreatic ductal adenocarcinoma by (1)h nuclear magnetic resonance: identification, mapping, and evolution. J Proteome Res. 2012;11(2):1274–1283. doi: 10.1021/pr200960u. [DOI] [PubMed] [Google Scholar]
- 26.Davis VW, Schiller DE, Eurich D, Bathe OF, Sawyer MB. Pancreatic ductal adenocarcinoma is associated with a distinct urinary metabolomic signature. Ann Surg Oncol. 2013;20(Suppl 3):S415–S423. doi: 10.1245/s10434-012-2686-7. [DOI] [PubMed] [Google Scholar]
- 27.Gowda GA. Human bile as a rich source of biomarkers for hepatopancreatobiliary cancers. Biomark Med. 2010;4(2):299–314. doi: 10.2217/bmm.10.6. [DOI] [PubMed] [Google Scholar]
- 28.Schultz NA, Dehlendorff C, Jensen BV, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311(4):392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 29.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiorean EG, Coveler AL. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529–3545. doi: 10.2147/DDDT.S60328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi C, Fukushima N, Abe T, et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol Ther. 2008;7:353–360. doi: 10.4161/cbt.7.3.5362. [DOI] [PubMed] [Google Scholar]
- 32.Winter JM, Tang LH, Klimstra DS et al (2012) A novel survival- based tissue microarray of pancreatic cancer identifies clinical useful candidate biomarkers. PLoS One 7(7), e40157. doi:10.1371/journal.pone.0040157 [DOI] [PMC free article] [PubMed]
- 33.Koops A, Wojciechowski B, Broering DC, Adam G, Krupski- Berdien G. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239–244. doi: 10.1007/s00276-004-0229-z. [DOI] [PubMed] [Google Scholar]
- 34.Balachandran A, Darden DL, Tamm EP, Faria SC, Evans DB, Charnsangavej C. Arterial variants in pancreatic adenocarcinoma. Abdom Imaging. 2008;33(2):214–221. doi: 10.1007/s00261-007-9235-z. [DOI] [PubMed] [Google Scholar]
- 35.Perwaiz A, Singh A, Singh T, Chaudhary A. Incidence and management of arterial anomalies in patients undergoing pancreaticoduodenectomy. JOP. 2010;11(1):25–30. [PubMed] [Google Scholar]
- 36.Kimura W, Watanabe T. Anatomy of the pancreatic nerve plexuses and significance of their dissection. Nihon Geka Gakkai Zasshi. 2011;112(3):170–176. [PubMed] [Google Scholar]
- 37.Ceyhan GO, Demir IE, Altintas B, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374:442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218–223. doi: 10.1097/mpa.0b013e3180619677. [DOI] [PubMed] [Google Scholar]
- 39.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi T, Ishikura H, Motohara T, et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65:164–170. doi: 10.1002/(SICI)1096-9098(199707)65:3<164::AID-JSO4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Gulbinas A, Barauskas G, Pundzius J. Pancreaticojejunal anastomosis: the “Achilles heel” of pancreaticoduodenectomy. Medicina (Kaunas) 2004;40:927–934. [PubMed] [Google Scholar]
- 42.Tewari M, Hazrah P, Kumar V, Shukla HS. Options of restorative pancreaticoenteric anastomosis following pancreaticoduodenectomy: a review. Surg Oncol. 2010;19(1):17–26. doi: 10.1016/j.suronc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Birkmeyer JD, Siewers JE, Finlayson EVA, et al. Hospital volume and hospital mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Li Y, Ahmad A, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 45.Zalatnai A, Molnar J. Review. Molecular background of chemoresistance in pancreatic cancer. In Vivo. 2007;21:339–347. [PubMed] [Google Scholar]
- 46.Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9(8):454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 47.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60(6):861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 48.Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene. 2014;33(23):2956–2967. doi: 10.1038/onc.2013.257. [DOI] [PubMed] [Google Scholar]
- 49.Erkan M, Adler G, Apte MV, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–178. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demir IE, Ceyhan GO, Rauch U, et al. The microenvironment in chronic pancreatitis and pancreatic cancer induces neuronal plasticity. Neurogastroenterol Motil. 2010;22(480–490):e112–e483. doi: 10.1111/j.1365-2982.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 51.Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut. 2012;61(10):1377–1379. doi: 10.1136/gutjnl-2012-302604. [DOI] [PubMed] [Google Scholar]
- 52.Koong AC, Mehta VK, Le QT, Fisher GA, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48(4):919–922. doi: 10.1016/S0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 53.Komar G, Kauhanen S, Liukko K, et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15(17):5511–5517. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 54.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephenson J, Wolpin BM, Becerra C, et al. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer. J Clin Oncol. 2011;29(Suppl):4114. [Google Scholar]
- 56.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein D, El-Maraghi RH, Hammel P, et al. Analyses of updated overall survival (OS) and prognostic effect of neutrophil-to-lymphocyte ratio (NLR) and CA 19-9 from the phase III MPACT study of nab-paclitaxel (nab-P) plus gemcitabine (Gem) versus Gem for patients (pts) with metastatic pancreatic cancer (PC) [abstract] ASCO Meet Abstr. 2014;32:4027. [Google Scholar]
- 58.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neesse A, Frese KK, Chan DS, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63(6):974–983. doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frese K, Neesse A, Cook N, et al. nab-paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fattahi R, Balci N, Perman W, et al. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009;29:350–356. doi: 10.1002/jmri.21651. [DOI] [PubMed] [Google Scholar]
- 62.Yao X, Zeng M, Wang H, et al. Evaluation of pancreatic cancer by multiple breath-hold dynamic contrast-enhanced magnetic resonance imaging at 3.0T. Eur J Radiol. 2012;81:e917–e922. doi: 10.1016/j.ejrad.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 63.D’Onofrio M, Zamboni G, Malago R, et al. Resectable pancreatic adenocarcinoma: is the enhancement pattern at contrast-enhanced ultrasonography a pre-operative prognostic factor? Ultrasound Med Biol. 2009;35:1929–1937. doi: 10.1016/j.ultrasmedbio.2009.06.1100. [DOI] [PubMed] [Google Scholar]
- 64.Opačić D, Rustemović N, Kalauz M, et al. Endoscopic ultrasound elastography strain histograms in the evaluation of patients with pancreatic masses. World J Gastroenterol. 2015;21(13):4014–4019. doi: 10.3748/wjg.v21.i13.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bausch D, Thomas S, Mino-Kenudson M, et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res. 2011;17(2):302–309. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel M, Gomes A, Ruderman S, et al. Polarization gating spectroscopy of normal-appearing duodenal mucosa to detect pancreatic cancer. Gastrointest Endosc. 2014;80(5):786–93.e1-2. doi: 10.1016/j.gie.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]