Abstract
The global incidence of chronic kidney disease (CKD) is increasing among individuals of all ages. Despite advances in proteomics, genomics and metabolomics, there remains a lack of safe and effective drugs to reverse or stabilize renal function in patients with glomerular or tubulointerstitial causes of CKD. Consequently, modifiable risk factors that are associated with a progressive decline in kidney function need to be identified. Numerous reports have documented the adverse effects that occur in response to graded exposure to a wide range of environmental chemicals. This Review summarizes the effects of such chemicals on four aspects of cardiorenal function: albuminuria, glomerular filtration rate, blood pressure and serum uric acid concentration. We focus on compounds that individuals are likely to be exposed to as a consequence of normal consumer activities or medical treatment, namely phthalates, bisphenol A, polyfluorinated alkyl acids, dioxins and furans, polycyclic aromatic hydrocarbons and polychlorinated biphenyls. Environmental exposure to these chemicals during everyday life could have adverse consequences on renal function and might contribute to progressive cumulative renal injury over a lifetime. Regulatory efforts should be made to limit individual exposure to environmental chemicals in an attempt to reduce the incidence of cardiorenal disease.
Introduction
The impact of environmental chemicals on public health and clinical well-being has long been recognized, with a historical focus on heavy metals and molecules that are produced in the work place.1 Increasing data have, however, indicated that the general public is unknowingly exposed to a wide range of chemicals as a consequence of normal consumer activities. These activities include dietary intake of food, domestic and commercial food preparation, household maintenance procedures, and routine medical and dental care.2–5
A major food safety incident in 2009 exemplified the potential scope of the adverse renal consequences that can occur following population-wide exposure to organic contaminants.6 Melamine is an organic nitrogenous compound used in the industrial production of plastics, dyes, fertilizers and fabrics that was considered safe on the basis of standard animal studies. This molecule was deliberately added to diluted raw milk at milk-collecting stations in China, with the presumed aim of falsely elevating assay results for protein content.7,8 Melamine was later detected in numerous food and milk-containing products that were exported from China to many countries worldwide.7,8 More than 300,000 infants and children exposed to milk-based formulas contaminated with melamine developed radiolucent stones, as well as impaired renal function and renal growth.6,9 The long-term consequences of melamine exposure in infancy and early childhood remain unknown,10 but exposure to melamine during adulthood might increase the risk of urolithiasis.11 The melamine story, therefore, provides a striking cautionary note regarding the potential serious adverse consequences of exposure to environmental organic chemicals during normal consumer activity.
The liver has long been considered the major target organ for most of the chemicals implicated in eliciting toxic effects following environmental exposure. Nevertheless, emerging data suggest that the kidney is also an important site of injury after chemical exposure, although substantial gaps remain regarding the effects of environmental chemicals on specific aspects of kidney function (Table 1).
Table 1.
Associations between environmental toxins and cardiorenal disease
| Chemical class | Albuminuria | eGFR | Blood pressure | Serum uric acid concentration |
|---|---|---|---|---|
| Phthalates | ↑ | No studies | ↑ | No studies |
| Bisphenol A (BPA) | ↑ | ↑ | ↑ | No studies |
| Perfluoroalkyl acids (PFAAs) | No studies | ↓ | ↑ | ↑ |
| Dioxins | ↑ | ↓ | ↑ | ↑ |
| Polycyclic aromatic hydrocarbons (PAHs) | No studies | Unknown | No studies | No studies |
| Polychlorinated biphenyls (PCBs) | ↑ | ↓ | ↑ | ↑ |
Abbreviation: eGFR, estimated glomerular filtration rate.
In this Review, we analyse the clinical effects of a select list of organic compounds on measures of cardiorenal function, including changes in blood pressure, albuminuria, glomerular filtration rate (GFR), and uric acid concentration. These parameters should not be considered an all-inclusive definition of cardiorenal function, and other clinical indicators, including vascular stiffness and left ventricular mass, could play a part. In addition, we distinguish the cardiorenal effects from cardiometabolic effects, such as insulin resistance and obesity. The toxicokinetic and toxicodynamic characteristics of environmental chemicals, and inter-species differences in susceptibility to injury, are beyond the scope of this Review. Furthermore, the ethical constraints imposed on clinical investigations in this field limit our capacity to discuss the underlying mechanisms of kidney injury in full. Here, we highlight both the scope of potential adverse renal effects and gaps in our knowledge for individual chemicals.
Phthalates
Sources of contamination
Phthalates are esters of phthalic acid that can be either low-molecular weight (LMW) or high-molecular weight (HMW) (Table 2, Supplementary Figure 1a and b). Phthalates of LMW are frequently added to shampoos, cosmetics, lotions and other personal hygiene products to preserve scent.6 Phthalates of HMW are typically used in the production of vinyl plastics used in flooring, food packaging and intravenous tubing.12 Within the HMW category, di-2-ethylhexylphthalate (DEHP) is commonly found in plastic products used during industrial food production,13,14 and its metabolites are often considered a subcategory of phthalates.3
Table 2.
Molecular structure, common derivatives, and source of contamination of environmental chemicals
| Environmental toxin | Common derivatives | Molecular structure | Source |
|---|---|---|---|
| Phthalates | DEHP | Esters of phthalic acid | Shampoos, cosmetics, personal hygiene products, vinyl plastics, food packaging, intravenous tubing |
| Bisphenol A | NA | Synthetic compound with two phenol rings connected by a methyl bridge, with two methyl groups attached to the bridge | Polycarbonate plastics, epoxy resins, intravenous tubing |
| Perfluoroalkyl acids | PFOS, PFOA, PFHxA, PFNA, PFHxS, PFHS | Synthetic organic chlorinated compounds, in which all hydrogen atoms of the hydrocarbon backbone are substituted with fluorine | Electrochemical fluorination, telomerization, surface protection agents, sealants, surfactants, food packaging, non-stick cooking surfaces, stain-resistant sprays, fire-retarding foams |
| Dioxins | PCDD | Synthetic halogenated aromatic hydrocarbon | Pesticides, bleaching of wood pulp, waste incineration |
| Furans | PCDF | Synthetic halogenated aromatic hydrocarbon | Pesticides, bleaching of wood pulp, waste incineration |
| Polycyclic aromatic hydrocarbons | Benzo[a]pyrene | Composed purely of carbon and hydrogen atoms in multiple aromatic rings | Incomplete combustion of coal, oil, and gas; tobacco smoke, charbroiled meat |
| Polychlorinated biphenyls | NA | Two benzene rings with varying degrees of saturation with chlorine moieties on the ringed backbone | Capacitants and coolants in electrical equipment |
Abbreviations: DEHP, di-2-ethylhexylphthalate; NA, not applicable; PCDD, polychlorinated dibenzo-p-dioxin; PCDF, polychlorinated dibenzo-p-furan; PFHS, perfluorohexane sulfonate; PFHxA, perfluorohexanoic acid; PFNA, perfluorononanoic acid; PFOA, perfluoro-octanoic acid; PFOS, perfluoro-octane sulfonic acid.
Cross-sectional and interventional studies have identified strong associations between diet and urinary levels of DEHP metabolites.4,5 A trial that intended to reduce the consumption of food contaminated with DEHP through dietary replacement led to unexpected increases of DEHP metabolites in the urine of individuals administered a controlled diet.15 These findings occurred as a result of unwitting contamination of food products included in the controlled diet with DEHP, and thus suggest that DEHP contamination is wider than expected. Substituting packaged and/or canned foods with fresh food products can reduce the presence of DEHP metabolites in the urine by 53–56%.16
DEHP can leach into solutions that are administered to patients through polyvinyl chloride medical devices, including endotracheal, orogastric and nasogastric tubes.17 Infants maintained on parenteral nutrition exhibit variable increases in serum concentrations of DEHP at the end of the treatment period.18 Given these findings, use of polyvinyl chloride-free medical devices is recommended in neonatal intensive care units.17 Leaching of DEHP from intravenous blood lines might be especially severe among patients undergoing dialysis. In a study of 21 patients with end-stage renal disease undergoing chronic haemodialysis, the plasma level of DEHP rose during a 4 h dialysis session and an estimated 75 mg DEHP was extracted from the dialyzer.19 In vitro studies confirmed that ~7.7 μg/ml DEHP migrates each hour from haemodialysis tubing into the blood without modifying the plasma concentration.20 Another study of adult patients on haemodialysis found that urinary excretion of 4-heptanone, a metabolite of DEHP, was increased more than ninefold after a standard treatment session.21 The amount of DEHP transferred during one haemodialysis session can be as much as the total annual exposure for patients receiving chronic ambulatory peritoneal dialysis.22
Metabolism
Pharmacokinetic studies of DEHP have not been performed in children and/or adolescents. Studies in animals suggest that DEHP is rapidly metabolized into three primary products: mono-2-ethylhexylphthalate; mono-2-ethyl-5-carboxypentylphthalate; and mono-2-ethyl-5-hydroxyhexylphthalate. These three metabolites have been identified as excretory products in human urine.23 Evidence obtained from animal studies suggests that some phthalates might be secreted by organic anion transporters,24 but comparable data are not available for humans.
Metabolites of phthalates undergo modest enterohepatic recirculation and intestinal excretion.25 Monoesters of phthalates have a biological half-life of 12–48 h, which can be lengthened owing to phthalate deposition in adipose tissue. The presence of phthalates in the urine probably represents short-term, current exposure rather than chronic exposure, although a single sample of urine can provide moderate sensitivity (54–69%) and high specificity (76–84%) for DEHP metabolites to estimate the level of phthalate exposure over a 3-month period.26–28
Albuminuria
Low-grade albuminuria might be a marker of endothelial dysfunction and is a risk factor for the development of cardiovascular disease (Table 3). A study of 667 children, aged 6–19 years, who participated in the National Health and Nutrition Examination Survey between 2009 and 2010 (NHANES 2009–2010), found that for each approximate threefold increase in DEHP metabolites detected in the urine, a 0.55 mg/g increase in the albumin-to-creatinine ratio was identified.29 Phthalates of LMW had no effect on albuminuria.29 Short-term exposure to DEHP during neonatal extra-corporeal membrane oxygenation therapy was not associated with any abnormalities in kidney function, including albuminuria.30
Table 3.
Effects of environmental chemicals on albuminuria
| Chemical class | Reference | Country (study) | Participants | Main findings |
|---|---|---|---|---|
| Phthalates | Trasande et al. (2014)29 | USA (NHANES 2009–2010) | 667 children | A three-fold increase in DEHP metabolites caused a 0.55 mg/g creatinine increment in albuminuria |
|
| ||||
| BPA | Li et al. (2012)50 | China | 3,055 adults | Graded increase in BPA exposure caused an increase in low-grade albuminuria |
| Trasande et al. (2013)51 | USA (NHANES 2009–2010) | 710 children | Graded increase in BPA exposure caused an increase in low-grade albuminuria | |
| Trachtenberg et al. (2014)53 | USA | 534 children | No effect of BPA associated with dental use on albuminuria | |
|
| ||||
| PCDDs and PCDFs | Everett et al. (2014)93 | USA (NHANES 1999–2004) | 2,588 adults with diabetes mellitus | Increased risk of nephropathy in association with select PCDDs and PCDFs |
|
| ||||
| PCBs | Everett et al. (2014)93 | USA (NHANES 1999–2004) | 2,588 adults with diabetes mellitus | Increased risk of nephropathy in association with select PCBs |
Abbreviations: BPA, bisphenol A; DEHP, di-2-ethylhexylphthalate; NHANES, National Health and Nutrition Examination Survey; PCB, polychlorinated biphenyl; PCDD, polychlorinated dibenzo-p-dioxin; PCDF, polychlorinated dibenzo-p-furan.
Blood pressure
A cross-sectional analysis of children aged 6–19 years who participated in NHANES 2003–2008 found an increase in the systolic blood pressure z-score of 0.041 standard deviation units for each approximate threefold increase in DEHP metabolite excretion (Table 4).31 For school age children and adolescents, this change in z-score translates into a 0.4–0.6 mmHg increase in systolic blood pressure. In contrast, exposure to LMW forms of phthalates had no effect on blood pressure.31 In line with the effect of HMW phthalates on blood pressure, the degree of exposure to these contaminants has been linked to the severity of cardio vascular disease. In 1,016 individuals aged ≥70 years, phthalate exposure correlated with the size of overt coronary artery atherosclerotic plaques.32 Interestingly, evidence from animal studies also suggests that DEHP might induce arrhythmia,33 alter metabolic profiles and elicit cardiomyocyte dysfunction.34 Other studies using the same population, however, failed to demonstrate any association between four phthalate derivatives and coronary risk, assessed using the Framingham Risk Score.35,36
Table 4.
Effects of environmental chemicals on blood pressure
| Chemical class | Reference | Country (study) | Participants | Main findings |
|---|---|---|---|---|
| Phthalates | Trasande et al. (2013)31 | USA (NHANES 2003–2008) | 2,447 children | Log unit increase in DEHP exposure were associated with a 0.041 unit increase in BP z-score |
|
| ||||
| BPA | Ahmadkhaniha et al. (2014)56 | Iran | 239 adults | Urinary BPA above the mean associated with increased risk of hypertension |
| Bae et al. (2012)57 | Korea | 560 adults | Highest quartile BPA excretion associated with 1.27-fold increased risk of hypertension | |
|
| ||||
| Perfluoroalkyl acids (PFAAs) | Geiger et al. (2014)81 | USA (NHANES 1999–2000 and 2003–2008) | 1,665 children | No association between serum PFOA or PFOS levels and BP |
|
| ||||
| Dioxins | Chang et al. (2010)96 | Taiwan | 1,490 adults | High dioxin levels associated with increases in BP |
| Ha et al. (2009)95 | USA (NHANES 1999–2002) | 524 adults | High dioxin levels associated with increases BP in women | |
| Lee et al. (2007)98 | USA | 721 adults | High dioxin levels associated with increases in BP | |
| Nakamoto et al. (2013)99 | Japan | 2,264 adults | High dioxin levels associated with increases in BP | |
| Uemura et al. (2009)100 | Japan | 1,374 adults | High dioxin levels associated with an increases in BP | |
|
| ||||
| PCBs | Goncharov et al. (2011)129 | USA (NHANES 1999–2002) | 394 adults | High occupational exposure to PCB associated with elevated BP |
| Everett et al. (2008)130 | USA (NHANES 1999–2002) | 2,556 adults | Seven PCBs associated with an increased risk of hypertension (highest risk score = 2.4) | |
| Ha et al. (2009)95 | Sweden | 524 adults | High PCB exposure associated with an increased risk of incident hypertension | |
| Lind et al. (2014)132 | Iceland | 1,016 elderly adults | High PCB levels associated with high BP | |
| Valera et al. (2013)135,136 | Greenland | 315 adults 1,614 adults | High PCB levels associated with fish consumption associated with increased BP levels | |
Abbreviations: BP, blood pressure; BPA, bisphenol A; DEHP, di-2-ethylhexylphthalate; NHANES, National Health and Nutrition Examination Survey; PCB, polychlorinated biphenyl; PFOA, perfluoro-octanoic acid; PFOS, perfluoro-octane sulfonic acid.
Bisphenol A
Sources of contamination
Bisphenol A (BPA) is a synthetic chemical comprised of two phenol rings connected by a methyl bridge, with two methyl groups attached to the bridge (Table 2, Supplementary Figure 1c). BPA was initially designed as a synthetic oestrogen, but is now widely used for its cross-linking properties in the manufacture of polycarbonate plastics and epoxy resins, and is present in intravenous tubing, including dialysis circuits.37 Incomplete polymerization and polymer degradation of BPA causes it to leach out of food and beverage containers and dental sealants.
Ubiquitous environmental exposure to nano-concentrations of BPA can occur through various routes, including ingestion, respiration, and absorption through the skin, and consequently detectable levels of BPA are found in the urine of >93% of adults.38 Serum BPA levels are high among males and smokers,39 and are inversely associated with socioeconomic status.40 A study of 22 patients with predialysis chronic kidney disease (CKD) found that serum BPA levels were inversely associated with renal function.41 Patients receiving dialysis have increased exposure to BPA owing to their near daily use of dialysis tubing. BPA levels are higher in patients undergoing haemodialysis (5.3 ± 0.3 ng/ml) and peritoneal dialysis (3.8 ± 0.2 ng/ml), than in healthy controls (2.6 ± 0.1 ng/ml).37 Elution of BPA might be enhanced from polysulfone and polyester-polymer alloy hollow fibres.41
Metabolism
Studies in animals have shown that BPA is rapidly and efficiently absorbed across the oral mucosa, especially after sublingual exposure.42 This efficient systemic route of entry via saliva, which bypasses first-pass hepatic clearance, might lead to greater BPA exposure than can be accounted for solely on the basis of absorption from the gastrointestinal tract.42 This observation, however, has not been confirmed in humans and requires further study.43 In humans, free BPA is metabolized by rapid glucurono-conjugation or sulfo-conjugation and is eliminated by the kidneys.44 Physiologically based pharmacokinetic models suggest that renal tubular reabsorption of BPA conjugates contribute to serum levels of BPA detected in humans,45 but the contribution of this pathway to renal injury has not been studied. Enterohepatic recirculation and lipid storage can also influence the rate of BPA elimination by the kidney,46,47 and might explain the differences between measured serum BPA levels in humans and those predicted from pharmacokinetic data.48 A minor pathway of BPA metabolism involves oxidation by hydroxylation to a catechol followed by transformation to an o-quinolone,44,49 which might elicit oxidative stress and augment BPA toxicity.49
Albuminuria
The first study to document albuminuria in healthy individuals exposed to BPA involved 3,055 adults living in Shanghai, China.50 Participants with the highest level of BPA exposure based on urinary excretion were at greater risk of low-grade albuminuria (<30 mg/g creatinine), regardless of whether BPA excretion was evaluated as a continuous or categorical variable. This association was confirmed in a study of 710 children enrolled in NHANES 2009–2010.51 Children in the highest quartile of urinary BPA levels had a statistically significant 0.91 mg/g higher albumin-to-creatinine ratio compared to those in the lowest quartile. The effect of BPA on albuminuria was similar to that noted for phthalates (Table 3). No association between BPA exposure and microalbuminuria or macroalbuminuria was observed in either of these studies in children or adults.50,51 Nevertheless, a study of 534 children aged 6–10 years found no difference in the urinary excretion of albumin or N-acetyl-β-D-glucosaminidase (both measures of renal damage) in those who were randomly assigned to dental restoration using BPA-free amalgam or BPA-containing resin.52,53 BPA exposure associated with dental restoration materials or preventive dental sealants might not be as important as dietary intake in determining the renal consequences of this molecule.
Injection of mice with 50 mg/kg BPA per day for 5 weeks causes albuminuria and podocytopaenia.54 Although the exact cause of BPA-induced albuminuria is unclear and could arise from oxidative stress-induced endothelial dysfunction, these data suggest that BPA has adverse effects on the glomerulus. In vitro exposure of podocytes to low (10 nM) or high (100 nM) concentrations of BPA promotes cellular hypertrophy, reduces cellular viability, induces apoptosis and diminishes expression of podocin and nephrin.54
Estimated GFR
One study has evaluated the effect of BPA exposure on kidney function.55 A reduction in urinary excretion of BPA and triclosan (a synthetic antibacterial agent found in many household products, including antimicrobial hand soaps) with declining GFR was identified in 2,573 adults without known kidney disease who were enrolled in NHANES 2003–2006.55 Use of the CKD–EPI formula to estimate GFR demonstrated no association between BPA excretion and GFR (Table 5).55
Table 5.
Effects of environmental chemicals on eGFR
| Chemical class | Reference | Country (study) | Participants | Main findings |
|---|---|---|---|---|
| BPA | You et al. (2011)55 | USA (NHANES 2003–2006) | 2,573 adults | Low BPA excretion associated with lowered eGFR |
|
| ||||
| Perfluoroalkyl acids | Shankar et al. (2011)78 | USA (NHANES 1999–2000) | 4,587 adults | Highest quartile of PFOA and PFOS associated with a 1.8-fold increased risk of CKD |
| Watkins et al. (2013)79 | USA | 9,660 children | Interquartile rise in PFOA excretion associated with an 0.75 ml/min/1.73 m2 decline in eGFR | |
| A. Kataria, et al. unpublished work | USA (NHANES 2003–2010) | 1,961 children | Highest quartiles of PFOA and PFOS excretion associated with a 6.6–9.5 ml/min/1.73 m2 decline in eGFR | |
|
| ||||
| Dioxins | Chang et al. (2013)94 | Taiwan | 1,531 adults | Highest quartile exposure associated with a 15–22 ml/min/1.73 m2 reduction in eGFR |
|
| ||||
| PAH | Stefanovic et al. (2006)118 | NA | NA | Possible association of PAH exposure with Balkan endemic nephropathy |
|
| ||||
| PCBs | Baker et al. (1980)127 | USA | 148 adults | No association between PCB and eGFR |
Abbreviations: BPA, bisphenol A; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; PAH, polycyclic aromatic hydrocarbon; PCB, polychlorinated biphenyl; PFOA, perfluoro-octanoic acid; PFOS, perfluoro-octane sulfonic acid.
Blood pressure
A study of 239 adults (mean age 52 years), found that participants with urinary excretion of BPA >0.85 μg/l had an increased risk of hypertension and diabetes mellitus, compared to those with urinary excretion of BPA <0.85 μg/l.56 A study of 560 non-institutionalized adults aged ≥60 years similarly showed that the odds ratio of having a systolic blood pressure >140 mmHg or a diastolic blood pressure >90 mmHg was 1.27 for those in the fourth quartile versus the first quartile of urinary BPA excretion.57 Similarly, an analysis of 1,380 participants in NHANES 2003–2004 showed that high urinary BPA levels were associated with development of hypertension, defined as blood pressure >140/90 mmHg.38 The multivariate adjusted odds ratio for hypertension associated with the third tertile (BPA >4.0 ng/ml) was 1.50 versus the first tertile (BPA <1.5 ng/ml).38 BPA exposure in a small cohort (n = 39) of obese children aged 3–8 years was associated with elevated diastolic blood pressure.58 In a randomized crossover study of 60 Korean adults, drinking soy milk from a can versus a glass container led to a 4–5 mmHg rise in blood pressure and a marked increase in urinary BPA excretion, 2 h after consumption (Table 4).59
The effect of BPA on blood pressure has encouraged longitudinal studies to investigate cardiovascular outcomes. The UK-based European Prospective Investigation of Cancer–Norfolk study, which included 758 individuals aged 40–74 years who were previously free of cardiovascular disease, found that participants with higher levels of BPA at entry had an increased risk of incident coronary artery disease during 10 years of follow-up compared to participants who had low BPA levels at entry. Specifically, each standard deviation (4.56 ng/ml) increment in urinary BPA excretion was associated with an odds ratio of 1.13 for coronary artery disease.60 Graded exposure to BPA among a subset of 745 participants in this cohort was associated with an increased likelihood of developing clinically detectable peripheral arterial disease.61 Coronary risk, based on the Framingham Risk Score, did not correlate with serum BPA levels in 1,016 patients aged 70 years who were included in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study.35 Moreover, other studies utilizing the NHANES database have failed to confirm associations between exposure to BPA and cardiovascular outcomes.62 Investigators have, therefore, questioned the validity of cross-sectional analyses of NHANES to derive conclusions about the effects of short-lived environmental chemicals on chronic complex diseases.62,63
Perfluoroalkyl acids
Sources of contamination
Perfluoroalkyl acids (PFAAs) are synthetic organic fluorinated compounds in which all the hydrogens of the hydrocarbon backbone are substituted with fluorine. This chemical structure imparts high stability and thermal resistance (Table 2, Supplementary Figure 1d). Potential precursor compounds for PFAAs derive from two major technologies: electrochemical fluorination (a method for the preparation of fluorocarbon-based organofluorine compounds) and telomerization (a radical polymerization reaction). These processes are used in the production of various specialized surface protection agents, sealants and surfactants. PFAAs have wide utility in stain-resistant sprays for carpets and upholstery, fire-retarding foams, non-stick cooking surfaces and food packaging.64,65 National biomonitoring surveys have revealed that >98% of the US population (from age 12 years to ≥60 years) have detectable levels of PFAAs in their blood.66 Similar to BPA, levels of PFAA are greatest among males, individuals ≥40 years of age, and in those with a high BMI, as shown among the general population of Daegu, Korea.67 In contrast to BPA exposure, serum PFAA levels are directly associated with socioeconomic status, such that those on high incomes exhibit lower body burdens of PFAA.40 Differences in food purchasing patterns by income might account for the variable association between socioeconomic status and exposure to environmental chemicals.
Perfluoro-octane sulfonic acid (PFOS) and perfluoro-octanoic acid (PFOA) are 8-carbon perfluoroalkyl chemicals that have been in widespread use for several decades. The utilization of PFOS was phased out of circulation in the USA in 2002 and long-chain PFAAs were to be phased out in the USA by 2015.68 The effects of previous exposure to long-chain PFAAs remain relevant owing to their 7–15 year half-life. Short-chain PFAAs with short half-lives, such as perfluorohexanoic acid (PFHxA) and perfluorononanoic acid (PFNA), have also been in circulation in the environment and might also cause short-term and long-term health problems. Sustained increases in serum PFNA concentrations were detected among participants of NHANES 2003–2004 compared to the levels detected during NHANES 1999–2000.66 Median serum levels of perfluorohexane sulfonic acid (PFHxS), the primary PFHxA metabolite, remained stable through NHANES 2009–2010 and 2007–2008.69 A Swedish study of trends in serum concentrations of PFAA between 1996 and 2010 found a 4.3% increase in PFHxS per year, and an 11% increase per year in perfluoroalkylbutane sulfonate (PFBS)—a short half-life metabolite of a four-carbon PFAA and a substitute for PFOS that is increasingly found in food.70 These data suggest that the potential adverse cardiorenal effects of PFAAs are likely to be an enduring health problem for several years to come.
Metabolism
PFAAs have the potential for marked bioaccumulation in the brain, liver, lung, bone and kidney.71 PFAAs are exceptionally stable to metabolic and environmental degradation, owing to the strength of their carbon–fluorine bond.72 The persistence of PFAAs in human serum over time probably reflects a combination of materials released from final products, materials used in the manufacturing process, and environmental and metabolic degradation of precursor compounds.
The persistence of PFAAs with long whole body half-life estimates in humans and animals is well known,73 but their toxicokinetic profiles and underlying mechanisms for such persistence are not fully understood. Excretion of PFAAs primarily occurs via the kidney and is a slow and concentration-dependent process.74,75 The carbon chain length of a given PFAA can influence its pharmacokinetic properties, with smaller molecules having a tendency to be eliminated more efficiently by the kidney than larger molecules.74 Differences in PFAA levels between species and by sex might reflect differences in the expression of organic anion transporters and postulated renal tubular reabsorption.73
Low levels of PFAAs can persist in tissues for prolonged periods of time.76 A study of 26 adults who had retired from employment in fluorochemical production and were free of other occupational PFAA exposures showed that the geometric serum elimination half-lives of the PFAAs were 4.8 years for PFOS, 7.3 years for perfluorohexane sulfate (PFHS) and 3.5 years for PFOA.77 Despite the increasing number of studies on PFAAs, major gaps still exist in our understanding of the pharmacology and toxicity of these compounds, which are important for the assessment of PFAA risk (Table 1).
Estimated GFR
The association between exposure to PFAAs and perturbed kidney function is not as strong as for some of the other organic contaminants covered in this Review. The existing literature does suggest, however, that PFAAs can have adverse renal effects and more research is needed in this area (Table 5).
A study of 4,587 adults included in NHANES 1999–2000 and 2003–2008 identified that those with PFOA or PFOS levels in the fourth quartile (>5.9 ng/ml) had a 1.73-fold and 1.82-fold higher risk of CKD (defined as GFR <60 ml/min/1.73 m2), respectively, than those in the first quartile (<2.8 ng/ml).78 Studies in children have verified a comparable adverse effect on kidney function. In a cohort of 9,600 children aged 1–18 years, an inter-quartile range increase in PFOA serum concentration was associated with a 0.75 ml/min/1.73 m2 decrease in estimated GFR.79 Cross-sectional analyses have found that measured PFOS, PFNA and PFHxS serum levels are associated with a decrease in GFR, but the predicted serum PFOA concentrations at the time of enrolment were not statistically associated with GFR. These data raise the possibility that cross-sectional associations between PFAA and GFR might be a consequence, rather than a cause, of reduced kidney function.79 A longitudinal study of PFAA exposure among individuals living near a hazardous waste site failed to show an effect on GFR, based on residential address and distance from the waste site.79 Finally, a study of 1,961 12–19-year-old adolescents included in NHANES 2003–2010 identified that those in the highest quartile for PFOA and PFOS excretion had a 6.6–9.5 ml/min/1.73 m2 lower estimated GFR than those in the lowest quartile (A. Kataria et al., unpublished work).
Blood pressure
Pre-clinical data have linked PFAA exposure to vascular injury and hypertension.80 An association between serum PFOA and PFOS concentrations and hypertension (systolic and/or diastolic blood pressure >95th percentile), however, was not identified in a cohort of 1,665 children from NHANES 1999–2000 and 2003–2008 (Table 4).81
Uric acid concentration
Serum levels of PFOA and PFOS are positively associated with hyperuricaemia in adults (Table 6).82 Adults included in the NHANES 1999–2000 and 2003–2008 who were in the highest quartile for PFOA and PFOS serum levels had a multivariate-adjusted odds ratio for hyperuricaemia (uric acid ≥ 357 μmol/l [≥6 mg/dl]) of 1.97.82 The same association was observed in 1,772 children in NHANES 1999–2000 and 2003–2008, with a multivariate-adjusted odds ratio for hyperuricaemia of 1.62.83 In the NHANES 2003–2010 study of 1,961 participants aged 12–19 years, those in the highest PFAA quartile had a statistically significant 12 μmol/l (0.20 mg/dl) increase in serum uric acid concentration compared to those in the lowest quartile (A. Kataria et al., unpublished work).
Table 6.
Effects of environmental chemicals on uric acid
| Chemical class | Reference | Country (study) | Participants | Main findings |
|---|---|---|---|---|
| Perfluoroalkyl acids | Shankar et al. (2011)82 | USA (NHANES 1999–2000, 2003–2008) | 3,883 adults | Highest quartile of PFOA associated with a 1.97-fold increased risk of hyperuricaemia |
| Geiger et al. (2013)83 | USA (NHANES 1999–2000, 2003–2008) | 1,772 children | Highest quartile of PFOA associated with 1.62-fold increased risk of hyperuricaemia | |
| A. Kataria, et al. unpublished work | USA (NHANES 2003–2010) | 1,961 children | Highest quartiles of PFOA associated with a 0.19–12.5 μmol/l (0.21 mg/dl) increase in serum uric acid level | |
|
| ||||
| Dioxins | Lee et al. (2013)102 | USA (NHANES 2003–2004) | 1,331 adults | Adjusted odds ratio for hyperuricaemia 2.3–3.0 depending on compound |
| Chang et al. (2013)94 | Taiwan | 1,531 adults | Men with dioxin levels greater than reference group levels had a 2.2-fold increased risk of hyperuricaemia | |
|
| ||||
| PCBs | Imamura et al. (2009)137 | Japan | 477 adults | Direct correlation between serum PCB and uric acid concentrations |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; PCB, polychlorinated biphenyl; PFOA, perfluorooctanoic acid.
Dioxins and furans
Sources of contamination
Dioxins, such as polychlorinated dibenzo-p-dioxins (PCDD), and furans, such as polychlorinated dibenzo-p-furans (PCDFs), are synthetic halogenated aromatic hydrocarbons (Table 2, Supplementary Figure 1e). PCDDs and PCDFs are ubiquitous environmental contaminants that are formed as waste products from the manufacture of pesticides, bleaching of wood pulp and waste incineration.84 PCDDs and PCDFs were banned worldwide under the Stockholm Convention in 2001, but still remain in items that were produced before the ban, as well as in the environment and in humans owing to their resistance to degradation by biological processes.84 The half-lives of PCDDs and PCDFs range from 2–15 years.85 The majority of human exposure to dioxins occurs as a result of eating products containing meat, milk, eggs and fish due to accumulation of dioxins in animal fat.86
The effects of dioxins are chiefly mediated by the aryl hydrocarbon receptor (AHR)—a ligand-activated transcription factor that regulates gene expression through binding the dioxin response element in DNA sequences together with AHR nuclear translocator (ARNT).87 The WHO developed the Toxic Equivalency Factor scale for this diverse group of chemicals based on the potency of their agonistic effects on the AHR. The Toxic Equivalency Factor expresses the relative AHR mediated potency of PCDDs and PCDFs compared to 2,3,7,8 tetrachloro-dibenzo-p-dioxin (TCDD), the most potent and well-studied molecule in this class. Induction of PCDD-mediated organ damage and cardiotoxicity correlates with the activation of the AHR.87,88 Although these effects were first characterized for dioxins and polychlorinated biphenyls (PCBs), cardiotoxicity has also been demonstrated for polybrominated diphenyl ether (PBDE) through AHR mechanisms in developing zebrafish embryos.89,90
Metabolism
PCDDs and PCDFs are readily absorbed through the digestive tract, which is enhanced through the ingestion of fatty foods. The lipophilic characteristics of PCDDs and PCDFs allows for slow excretion in bile and urine.91 These compounds become toxic through activity of the CYP1A1 enzyme, a well-characterized target of AHR/ARNT and is often used as a marker of AHR activation.92 The intra-renal mechanisms involved in the handling of PCCDs and PCDFs have not been fully established.
Albuminuria
No studies have provided information regarding the effects of PCDDs and PCDFs on albuminuria among healthy individuals (Table 3). A study of 2,588 adults with diabetes mellitus enrolled in NHANES 1999–2004 found that serum levels of three different PCDFs were associated with diabetic nephropathy, as defined by the presence of microalbuminuria (albumin-to-creatinine ratio >30 mg/g) or macroalbuminuria. When levels of at least four of the 23 chemicals analyzed in this study were elevated in the serum, the odds ratio was 7.00 (95% CI, 1.80–27.20) for diabetic nephropathy and 2.13 (95% CI, 0.95–4.78) for diabetes mellitus without nephropathy (Table 3).93
Estimated GFR
Moderate-to-high level exposure to dioxins is associated with decreased kidney function (Table 5). A cross-sectional study of 1,531 healthy adults living in close proximity to a pentachlorophenyl factory that was no longer in use found a strong monotonic inverse association between PCDD exposure and estimated GFR.94 Compared to the lowest quartile, the highest quartile of toxin exposure resulted in a 14.8 ml/min/1.73 m2 and a 21.5 ml/min/1.73 m2 reduction in estimated GFR in men and women, respectively.93 The association between low levels of dioxin exposure on kidney function in otherwise healthy adults or children without occupational exposure is unknown.95
Blood pressure
A study of 1,490 adults without diabetes mellitus living near a dioxin-contaminated area showed that increasing serum dioxin levels are correlated with elevated diastolic blood pressure.96 Furthermore, the prevalence of hypertension was correlated with serum PCDD and PCDF levels in adults with suspected dioxin exposure due to proximity to an uncontrolled and abandoned hazardous waste site.97 A modest association between dioxin exposure and hypertension was also noted in a US study of 721 adults without diabetes mellitus, living far from evident sources of contamination.98 Follow-up studies in adult populations residing in Japan have confirmed this finding (Table 4).99,100
Uric acid concentration
The study of adults living near a deserted pentachlorophenyl factory previously discussed also found that healthy men, but not women, in the highest quartile for toxin exposure had a 35 μmol/l (0.59 mg/dl) increase in serum uric acid concentration. Furthermore, men with serum dioxin concentrations above the reference group had a 2.20-fold higher risk of hyperuricaemia.93 A similar observation was made among 94 workers at the Nose Bika Center incinerator in Osaka, Japan, which was heavily contaminated with PCDD.101 Adults with only background exposure to dioxins are also at heightened risk of hyperuricaemia. The adjusted odds ratio for hyperuricaemia in 1,331 adults from NHANES 2003–2004, ranged from 2.3 to 3.0, depending upon the specific dioxin compound exposure (Table 6).102
Polycyclic aromatic hydrocarbons
Sources of contamination
Polycyclic aromatic hydrocarbons (PAHs) are a group of >100 different chemicals composed purely of carbon and hydrogen atoms that are arranged in multiple aromatic rings (Table 2, Supplementary Figure 1f). The majority of PAHs are formed during the incomplete burning of coal, oil, and gas, and from other organic substances, such as tobacco and meat grilled over charcoal.103 Although exposure to these compounds predominantly occurs in the work environment, such as in chemical and coke (fuel) factories, the ubiquity of motorized vehicles with internal combustion engines and increased industrial activity has resulted in substantial environmental exposure, especially in urban areas.104 Incomplete burning of carbon-based fuels produces oxidized PAHs that can be highly mutagenic and carcinogenic.105 Benzo[a]pyrene is among the most well studied of the PAHs and is a prime carcinogen in tobacco smoke.106 PAH has been detected in the serum of pregnant non-smokers that reside in the New York City area, and the level of exposure correlated with time spent outdoors, residential heating, and indoor burning of incense.107 Urinary excretion of PAH among children living in New York declined in response to legislative regulations targeting air pollution derived from traffic.108
Metabolism
PAHs are activated by CYP1A1 and polymorphisms in the gene encoding this enzyme are associated with alterations in PAH metabolism.109 Glutathione S-transferases are involved in the conjugation of PAHs with glutathione, and genetic variations in these proteins also contribute to the observed differences in PAH metabolism.110 Benzo[a] pyrenediol epoxide forms adducts with albumin111 and DNA,112 which have been identified in parallel with PAH exposure. The formation of these chemical adducts might provide a more accurate assessment of PAH exposure and potential nephrotoxicity.
Estimated GFR
High PAH exposure was associated with a 3.60-fold odds ratio of elevated levels of C-reactive protein in a study of 999 NHANES 2003–2004 participants.113 In view of the effect of inflammation in the development of atherosclerosis,114 these results are consistent with a role for PAH in cardiovascular disease. Very few studies, however, have addressed the effect of PAH exposure on either glomerular (estimated GFR and albuminuria) or tubular markers of kidney injury (Table 5).
Balkan endemic nephropathy is a chronic tubulointerstitial disease that is associated with an enhanced risk of urothelial cancer and has been attributed to PAH exposure.115 Drinking water contaminated with PAHs that have leached from pliocene lignite coal and coke factories into the water supply, has been implicated in renal parenchymal disease and urological malignancies.116–118 Additional epidemiological studies and preclinical investigations are needed to confirm the role of PAHs in Balkan endemic nephropathy.
Blood pressure
A single, small-scale study of 88 adult non-smokers residing in Belgium found that the serum levels of select PAH compounds were linearly associated with systolic and pulse pressure.119 Further studies with larger diverse cohorts are now needed to verify an association between PAH exposure and risk of hypertension (Table 4).
Polychlorinated biphenyls
Sources of contamination
Polychlorinated biphenyls (PCBs) are molecules composed of two benzene rings with varying degrees of saturation with chlorine moieties on the ringed backbone. This variation can result in 209 unique related chemicals (Table 2, Supplementary Figure 1g). PCBs were widely used in capacitors and coolants in electrical equipment, until the recognition of their persistence in the environment and their bioaccumulation and toxicity in animals and humans.120 PCB production was subsequently banned in the USA in 1979 and by the Stockholm Convention in 2001, but PCBs remain ubiquitous in human populations as a result of their persistence in the environment, incomplete disposal, and ongoing use of PCB-containing products. The Hudson River is contaminated with PCBs due to intentional disposal of PCB-containing products in the 1970s. This river is, therefore, the largest US Environmental Protection Agency Superfund site—covered by a US federal law that is designed to clean up sites contaminated with hazardous substances. Standing advisories consequently caution against fish consumption from this stretch of water. Serum levels of PCBs in those residing in the USA without occupational exposure to PCBs range from 0.6–4.0 ng/g in adolescents (12–19 years old) and 8.9–60.8 ng/g in the elderly (>60 years old).121 PCB serum levels in those with high consumption of fish from contaminated waters are several times higher than in those without PCB exposure, and are comparable to workers in PCB plants.122,123
Metabolism
PCB metabolism primarily occurs in the liver, where it must first be hydroxylated to increase the polarity of the molecule, before it is excreted in the bile.124 The rate of metabolism varies depending on the degree of chlorination of the congener.125 Metabolism of PCBs can also produce toxicologically active agents, such as arene oxides that can be enzymatically detoxified and excreted, or can form toxic adducts.126
Albuminuria
No data have been reported on the effect of PCBs on proteinuria in individuals with no known kidney disease. In a study of 2,588 patients with diabetes mellitus enrolled in NHANES 1999–2004, higher levels of exposure to PCB congeners were associated with an increased risk of diabetic nephropathy (Table 3).93
Estimated GFR
An event that occurred at an electrical capacitor manufacturing plant in Bloomington, USA, resulted in PCB discharge into the municipal sewage system. PCB compounds were detected in the sewage sludge that was used as fertilizer.127 In a limited follow-up study, serum PCB levels were elevated only among sewage workers exposed to PCB. No association was found between PCB serum concentration and kidney function,127 but no large-scale investigations of the effect of PCBs on kidney function or albuminuria have been performed (Table 5).
Blood pressure
Associations between high serum PCB levels and elevated systolic and diastolic blood pressure have been identified in residents near an agrochemical plant that manufactured PCB (Table 4).128,129 The association between PCB serum levels and blood pressure has also been confirmed among individuals with no occupational exposure. In a study of 2,556 adults in NHANES 1999–2002 who were assessed for serum levels of 11 different PCBs, the risk of hypertension was increased for seven of the compounds, with the highest odds ratio being 2.45.130 Elevated levels of one or more PCB in the serum were identified in ~25% of the population, with an odds ratio of 1.84 for elevated blood pressure.130 A similar study of 524 adults from the same NHANES cohort demonstrated that the extent of PCB exposure was markedly associated with the incidence of new-onset hypertension among men.95 The association between PCBs and hypertension has been verified in an independent evaluation of the NHANES 1999–2004 participants using clustering analyses.131 The PIVUS study also verified the association between PCB serum levels and blood pressure in a cohort of Swedish individuals aged 70 years.132 A follow-up investigation of this cohort noted an association between PCB exposure and disturbances in left ventricular systolic and diastolic function.133 Furthermore, a study of US adults in NHANES 1999–2008 aged >20 years found that although exposure to lead was most predictive of diastolic and mean blood pressure, serum levels of PCB were most predictive of systolic blood pressure.134 An association between high PCB serum levels as a consequence of fish consumption and an increased risk of hypertension has been reported in a study of adult Inuits residing in Greenland and Canada.135,136
Uric acid concentration
An industrial incident that occurred in Japan in 1968 resulted in large-scale exposure to PCBs. Serum levels of PCBs were directly correlated with uric acid concentration, with higher PCB concentration being associated with increased risk of hyperuricaemia (Table 6).137
Impact on the developing world
This Review has focused primarily on the effect of environmental chemicals on the residents of affluent, developed countries. Nonetheless, the molecules discussed might also contribute to renal disease in developing regions of the world. Over the past 10 years, there has been growing recognition of the major health impact of an epidemic of CKD in Central America, known as Mesoamerican nephropathy. This condition occurs primarily in hot, tropical, agricultural communities located along the Pacific coast.138,139 A similar endemic outbreak of CKD has been documented among farmers living in the north central province of Sri Lanka.140 Conflicting reports have been made regarding the potential role of cadmium, arsenic and pesticide exposure on the occurrence of CKD in farmers in Sri Lanka.141,142 In both locales, the affected patients are predominantly men who present with minimal symptoms, including marginally elevated blood pressure, low-grade proteinuria, non-inflammatory urinalysis and azotaemia (abnormally high levels of nitrogen-containing compounds in the blood).138,139 Several hypotheses have been proposed to explain this multifactorial condition, including recurrent dehydration and activation of polyol-fructokinase and vasopressin pathways.143 No conclusive evidence has linked Mesoamerican nephropathy to pesticides, herbal toxins, heavy metals, or certain medications, such as NSAIDs, but the role of environmental chemicals, including pesticides, has not been systemically addressed. Given the findings in developed countries where exposure is endemic, the environmental chemicals discussed in this Review might be a contributing factor to this emerging epidemic of CKD. This issue is especially pressing in developing countries where regulatory systems to control agrochemical use of hazardous compounds are not routinely implemented and where compliance with existing rules and standards to protect the health of the work force are not aggressively enforced.144
Limitations of published reports
Cross-sectional data
Much of the literature on the cardiorenal effects of environmental chemicals is based on cross-sectional data that associate exposure to chemicals with the development of the outcome of interest. Most studies rely on single measurements without serial sample collection. Although not a major concern for persistent organic compounds that exhibit stable serum levels over extended periods of time, such as PFAA, single measurements become problematic when considering short-lived molecules, such as BPA and phthalates. Short-term variations in exposure can dramatically influence urinary excretion of these described compounds and can lead to a misclassification of the degree of cumulative long-term exposure.145
Acute versus chronic exposure
Exposure to environmental chemicals can be acute or chronic in nature. In this Review, we have reported data accumulated following industrial exposure that is presumed to be chronic or after major accidents that presumably reflects acute high-level exposure. The majority of published studies have, however, been cross-sectional in design and cannot discriminate between short-term and long-term exposures to organic contaminants. Prospective cohort studies using serial biosample collection methods are needed to address this important issue. Furthermore, no studies have detailed the consequences of combined exposure to different classes of chemicals, which requires prospective sample collection with simultaneous measurements of numerous analytes.
Biomarkers of renal injury
No standardized protocol has been devised to assess the effects of environmental chemicals on renal parameters, including albuminuria, estimated GFR, blood pressure, and serum uric acid concentration. Consequently, the full scope of these effects have not been fully studied for all classes of compounds (Tables 3–6). Specific markers of glomerular injury (for example, urinary excretion of podocytes) and tubular injury (for example, levels of NGAL, KIM-1 and IL-18) could also be studied, but these biomarkers do not identify the cause of renal damage and are not specific for environmental chemical exposure.146,147 Abnormal serum concentrations and urinary excretion of these biomarkers might, however, precede routine clinical tests, such as measurement of serum creatinine concentration, and be sensitive for the detection of acute kidney injury and CKD.148,149 Furthermore, these biomarkers could enable precise localization of the site of renal damage as a consequence of environmental toxins. To date, such measurements have not been routinely performed because of the reliance on cross-sectional observational cohort studies. Future work in this area should incorporate these novel biomarkers to clarify the full effect of organic contaminants on kidney injury and dysfunction.
Reverse causation
The kidney excretes many of the organic chemicals that have been discussed in this Review. By the concept of reverse causation, serum levels of organic chemicals might be elevated as a consequence of a reduction in GFR that is secondary to other factors. Moreover, a primary decline in GFR should decrease the urinary excretion of the organic contaminants. For this reason, some investigators devalue the data derived from NHANES and dismiss a causative effect of environmental chemicals on kidney function.150 Various statistical approaches that incorporate multivariate analyses have been used to address this important point.151 Prospective longitudinal cohort studies are the next step to corroborate findings from cross-sectional studies, which are supported by clinical data. Such analyses will require serial sampling for environmental chemicals and measurements of kidney function, and will enable precise delineation of the association between environmental toxins and renal function. In this way, the effect of the environmental chemicals as modifiable risk factors for albuminuria, hypertension, hyperuricaemia and CKD can be determined.
Potential mechanisms of injury
Oxidative stress
Prospective or interventional studies have not been performed to confirm the association between environmental chemicals and potential cardiorenal injury. A biological plausibility is required first to link these two phenomena. Oxidative stress is a major pathophysiologic mechanism that underlies cardiometabolic risk and renal injury and can be induced by exposure to environmental chemicals.152 Peroxidation of lipids induces cellular damage and inflammation, and oxidative stress perturbs the endothelial relaxant nitric oxide, thus promoting vasoconstriction, platelet adhesion and release of inflammatory cytokines.153,154 Production of free radicals from oxygen is increased in the glomerular podocyte in animal models of CKD.155 Excessive oxidative stress alters the podocyte cytoskeleton and can lead to albuminuria,156 podocyte loss and tubular injury, which are prominent pathologies in the progression of primary glomerulopathies.157 Tubulointerstitial fibrosis, which develops in patients with all forms of CKD secondary to oxidative stress, is a better predictor of prognosis and progressive decline of renal function than are glomerular abnormalities.158
Environmental chemicals such as BPA, phthalates and biphenyls have been identified as possible contributors to cardiometabolic risk, independent of an increased risk of obesity.159 Animal studies indicate that BPA induces oxidative stress160–162 and inhibits the release of the adiponectin from human adipose tissue.163 Laboratory studies have found that metabolites of phthalates can enhance the release of IL-6164 and elevate the expression of integrin in neutrophils.165 Biomarkers of phthalate exposure have also been associated with increases in C-reactive protein, γ-glutamyltransferase,166 and markers of oxidative stress, such as malondialdehyde and 8-hydroxydeoxyguanosine.31,167 Cell culture studies have shown that exposure of microvascular endothelial cells to PFAA increases the production of reactive oxidative species and induces endothelial permeability,168 which have a critical function in ischaemic renal injury.169 Exposure of animals to PBDE can induce oxidative stress-mediated hepatotoxicity and nephrotoxicity.170 PAHs are potent oxidant stressors171 that can enhance lipid oxidation and cause subsequent inflammation. PAHs also reduce production of nitric oxide,172 and promote vasoconstriction, platelet adhesion, and release of inflammatory cytokines by human coronary artery endothelial cells.173,174
As illustrated above, oxidative stress might represent a common pathway that mediates renal injury associated with exposure to environmental chemicals. This mechanism has biological plausibility and justifies further investigation when examining the adverse effects of these chemicals. The possibility remains that other functional disturbances contribute to the adverse cardiorenal effects elicited by the described compounds, including effects on modifiable patient-associated factors, such as obesity (Figure 1).
Figure 1.
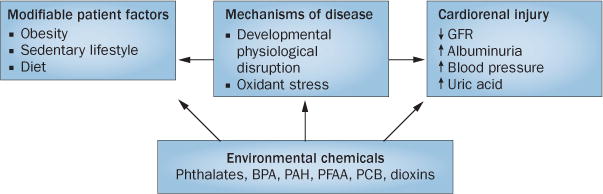
The integrated effects of environmental chemicals on cardiorenal function. The schematic summarizes the potential effects of environmental chemicals on renal and/or vascular function and integrates these effects with the known adverse consequences of obesity and the metabolic syndrome. Abbreviations: BPA, bisphenol A; GFR, glomerular filtration rate; PAH, polycyclic aromatic hydrocarbon; PCB, polychlorinated biphenyl; PFAA, perfluoroalkyl acid.
Intrauterine environment
The ‘thrifty phenotype’ hypothesis175,176 suggests that early life adaptations to poor in utero nutritional conditions can produce a profile of maladaptation ex utero, where the ability to acquire energy results in increased adiposity.153,154 This effect can present in childhood and contributes to an elevated risk of cardiometabolic and renal disorders in later life.175–178 An association between phthalates and low birth weight,179 as well as data associating increases in maternal BPA levels with reductions in estimated fetal weight,180 is consistent with the hypothesis that prenatal exposure to environmental oxidant stressors could contribute to cardiorenal risks through intrauterine maladaptation.
The influence of organic contaminants during human development has been studied for several classes of compounds. Prenatal exposure to BPA might be associated with an increased risk of respiratory disease during childhood.181 Maternal exposure to phthalates during the prenatal period182 (based on urinary excretion) and PAH183 (based on maternal and umbilical cord serum levels), have been associated with altered mental, psychomotor, and behavioural function at 3 years of age. A review of eight epidemiologic studies, (including six non-occupational and two occupational exposure studies) found no consistent association between maternal blood and/or umbilical cord concentrations of PFOS and PFOA and birth weight or other anthropometric measurements, such as head circumference and developmental milestones at 6 and 18 months of age.184 In contrast to studies that have documented adverse pulmonary and neurocognitive effects of prenatal exposure to organic contaminants, no studies have investigated the effects of exposure to organic contaminants during the prenatal period or during infancy on renal structure or function.
Conclusions
The effects of environmental chemicals on GFR described in this Review are unlikely to cause clinically overt adverse effects. Prolonged cumulative lifetime exposure to an array of compounds, however, in conjunction with age-associated decline in kidney function and other comorbid conditions, might accelerate the rate of deterioration in kidney function and progression to CKD. The effects on albuminuria are also modest and might reflect generalized endothelial dysfunction rather than alterations in the glomerular filtration barrier. Regardless of the under lying mechanism, changes in low-grade albuminuria that are attributed to environmental chemicals are associated with an increased risk of cardiovascular events.185 The modest changes noted in blood pressure could be associated with an increased incidence of cardio vascular events, if achieved at the population level. Worsening hyperuricaemia after exposure to toxins might cause hypertension, aggravate endothelial dysfunction, and elicit effects consistent with metabolic syndrome.186 The co-expression of such alterations might increase the risk of CKD over an individual’s lifespan.
Reducing exposure to environmental chemicals can be achieved by comprehensive modification of the regulations governing the use of these compounds, and would produce large economic benefits as compared to the costs incurred in preventing cardiorenal disease.187,188 BPA-associated cardiovascular diseases are estimated to cost US$1.5 billion annually in the United States.189 Importantly, the adverse cardiorenal effects of environmental chemicals is likely to persist into the future as industry shifts its use from regulated compounds to alternative replacements, such as bisphenol S, di-isodecyl, and di-isononylphthalate.
The majority of data presented in this Review reflect cross-sectional assessments of environmental toxins. The effect of long-term exposure to these toxins, either singly or in combination and at different stages of development, on longitudinal changes in kidney function have not been determined. Longitudinal studies are now needed to guide regulatory strategies for heightened control or complete elimination of these molecules to reduce or prevent human exposure and lower the risk of target organ damage including the kidney. We recommend that industry, regulatory agencies, medical societies and the scientific community prospectively implement protocols to determine the safety of organic contaminants before their introduction into widespread use. Furthermore, ongoing surveillance is required to detect the emergence of unanticipated adverse effects.
Supplementary Material
Key points.
Exposure to organic contaminants occurs through normal daily and routine medical procedures, and is ubiquitous among healthy children and adults
Exposure to environmental chemicals that can damage the kidney can occur via dietary intake or as a consequence of medical interventions, such as haemodialysis or parenteral nutrition
The full impact of persistent exposure to environmental chemicals on renal function has not been addressed in great detail
Cross-sectional data from numerous studies worldwide indicate that exposure to organic chemicals can have adverse effects on glomerular filtration rate, albuminuria, blood pressure and serum uric acid concentration
Oxidative stress is a contributing factor to the adverse effects of environmental chemicals on cardiorenal function; additional studies are required to clarify the mechanism and long-term effects of environmental chemicals
Acknowledgments
The preparation of this review was supported in part by an NIH-NIDDK grant (number DK100307) awarded to L.T. and H.T., and an NIH–NIEHS grant (number ES022972) awarded to L.T. The authors would like to thank Judy Chen, Bryn Mawr College, Bryn Mawr, PA, USA for her assistance in finalizing the literature review that was conducted for preparation of this manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors researched data, provided substantial contribution to discussion of content and contributed equally to writing the article and to review and/or editing of the manuscript before submission.
Supplementary information is linked to the online version of the paper at www.nature.com/nrnephrol.
References
- 1.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. EXS. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malaguarnera G, et al. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol. 2012;18:2756–2766. doi: 10.3748/wjg.v18.i22.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sathyanarayana S. Phthalates and children’s health. Curr Probl Pediatr Adolesc Health Care. 2008;38:34–49. doi: 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13:43. doi: 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trasande L, et al. Phthalates and the diets of US children and adolescents. Environ Res. 2013;126:84–90. doi: 10.1016/j.envres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Hau AK, Kwan TH, Li PK. Melamine toxicity and the kidney. J Am Soc Nephrol. 2009;20:245–250. doi: 10.1681/ASN.2008101065. [DOI] [PubMed] [Google Scholar]
- 7.Skinner CG, Thomas JD, Osterloh JD. Melamine toxicity. J Med Toxicol. 2010;6:50–55. doi: 10.1007/s13181-010-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gossner CM, et al. The melamine incident: implications for international food and feed safety. Environ Health Perspect. 2009;117:1803–1808. doi: 10.1289/ehp.0900949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan N, et al. Melamine-contaminated powdered formula and urolithiasis in young children. N Engl J Med. 2009;360:1067–1074. doi: 10.1056/NEJMoa0809550. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, et al. Four years follow-up of 101 children with melamine-related urinary stones. Urolithiasis. 2013;41:265–266. doi: 10.1007/s00240-013-0548-9. [DOI] [PubMed] [Google Scholar]
- 11.Liu CC, et al. Low exposure to melamine increases the risk of urolithiasis in adults. Kidney Int. 2011;80:746–752. doi: 10.1038/ki.2011.154. [DOI] [PubMed] [Google Scholar]
- 12.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 13.Fromme H, et al. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Int. 2007;33:1012–1020. doi: 10.1016/j.envint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::aid-ajim10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Sathyanarayana S, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23:378–384. doi: 10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- 16.Rudel RA, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su PH, et al. Exposure to di(2-ethylhexyl) phthalate in premature neonates in a neonatal intensive care unit in Taiwan. Pediatr Crit Care Med. 2012;13:671–677. doi: 10.1097/PCC.0b013e3182455558. [DOI] [PubMed] [Google Scholar]
- 18.Kambia K, et al. Comparative study of the leachability of di(2-ethylhexyl) phthalate and tri(2-ethylhexyl) trimellitate from haemodialysis tubing. Int J Pharm. 2001;229:139–146. doi: 10.1016/s0378-5173(01)00840-7. [DOI] [PubMed] [Google Scholar]
- 19.Faouzi MA, et al. Exposure of hemodialysis patients to di-2-ethylhexyl phthalate. Int J Pharm. 1999;180:113–121. doi: 10.1016/s0378-5173(98)00411-6. [DOI] [PubMed] [Google Scholar]
- 20.Pollack GM, Buchanan JF, Slaughter RL, Kohli RK, Shen DD. Circulating concentrations of di(2-ethylhexyl) phthalate and its de-esterified phthalic acid products following plasticizer exposure in patients receiving hemodialysis. Toxicol Appl Pharmacol. 1985;79:257–267. doi: 10.1016/0041-008x(85)90347-3. [DOI] [PubMed] [Google Scholar]
- 21.Wahl HG, et al. 4-Heptanone is a metabolite of the plasticizer di(2-ethylhexyl) phthalate (DEHP) in haemodialysis patients. Nephrol Dial Transplant. 2004;19:2576–2583. doi: 10.1093/ndt/gfh425. [DOI] [PubMed] [Google Scholar]
- 22.Nassberger L, Arbin A, Ostelius J. Exposure of patients to phthalates from polyvinyl chloride tubes and bags during dialysis. Nephron. 1987;45:286–290. doi: 10.1159/000184165. [DOI] [PubMed] [Google Scholar]
- 23.Frederiksen H, et al. Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected between 2006 and 2012. Reproduction. 2014;147:555–565. doi: 10.1530/REP-13-0522. [DOI] [PubMed] [Google Scholar]
- 24.Tremaine LM, Quebbemann AJ. The renal handling of terephthalic acid. Toxicol Appl Pharmacol. 1985;77:165–174. doi: 10.1016/0041-008x(85)90277-7. [DOI] [PubMed] [Google Scholar]
- 25.Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- 26.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mes J, Coffin DE, Campbell DS. Di-n-butyl- and di-2-ethylhexyl phthalate in human adipose tissue. Bull Environ Contam Toxicol. 1974;12:721–725. doi: 10.1007/BF01685921. [DOI] [PubMed] [Google Scholar]
- 29.Trasande L, Sathyanarayana S, Trachtman H. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin J Am Soc Nephrol. 2014;9:100–109. doi: 10.2215/CJN.04570413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rais-Bahrami K, Nunez S, Revenis ME, Luban NL, Short BL. Follow-up study of adolescents exposed to di(2-ethylhexyl) phthalate (DEHP) as neonates on extracorporeal membrane oxygenation (ECMO) support. Environ Health Perspect. 2004;112:1339–1340. doi: 10.1289/ehp.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trasande L, et al. Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr. 2013;163:747–753.e1. doi: 10.1016/j.jpeds.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lind PM, van Bavel B, Salihovic S, Lind L. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environ Health Perspect. 2012;120:38–43. doi: 10.1289/ehp.1103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posnack NG, Lee NH, Brown R, Sarvazyan N. Gene expression profiling of DEHP-treated cardiomyocytes reveals potential causes of phthalate arrhythmogenicity. Toxicology. 2011;279:54–64. doi: 10.1016/j.tox.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posnack NG, Swift LM, Kay MW, Lee NH, Sarvazyan N. Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ Health Perspect. 2012;120:1243–1251. doi: 10.1289/ehp.1205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen L, Lind L, Lind PM. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol Environ Saf. 2012;80:179–183. doi: 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Olsen L, Lampa E, Birkholz DA, Lind L, Lind PM. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Ecotoxicol Environ Saf. 2012;75:242–248. doi: 10.1016/j.ecoenv.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Kanno Y, Okada H, Kobayashi T, Takenaka T, Suzuki H. Effects of endocrine disrupting substance on estrogen receptor gene transcription in dialysis patients. Ther Apher Dial. 2007;11:262–265. doi: 10.1111/j.1744-9987.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- 38.Shankar A, Teppala S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J Environ Public Health. 2012;2012:481641. doi: 10.1155/2012/481641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y, et al. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ Res. 2009;109:629–633. doi: 10.1016/j.envres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Nelson JW, Scammell MK, Hatch EE, Webster TF. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003–2006. Environ Health. 2012;11:10. doi: 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami K, et al. Accumulation of bisphenol A in hemodialysis patients. Blood Purif. 2007;25:290–294. doi: 10.1159/000104869. [DOI] [PubMed] [Google Scholar]
- 42.Gayrard V, et al. High bioavailability of bisphenol A from sublingual exposure. Environ Health Perspect. 2013;121:951–956. doi: 10.1289/ehp.1206339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teeguarden JG, et al. 24-hour human urine and serum profiles of bisphenol A: evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Fenichel P, Chevalier N, Brucker-Davis F. Bisphenol A: an endocrine and metabolic disruptor. Ann Endocrinol (Paris) 2013;74:211–220. doi: 10.1016/j.ando.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Fisher JW. Unraveling bisphenol A pharmacokinetics using physiologically based pharmacokinetic modeling. Front Pharmacol. 2014;5:292. doi: 10.3389/fphar.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto H, Yokota H, Kibe R, Sayama Y, Yuasa A. Excretion of bisphenol A-glucuronide into the small intestine and deconjugation in the cecum of the rat. Biochim Biophys Acta. 2002;1573:171–176. doi: 10.1016/s0304-4165(02)00418-x. [DOI] [PubMed] [Google Scholar]
- 47.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch HM, Kolossa-Gehring M, Schroter-Kermani C, Angerer J, Bruning T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: a retrospective exposure evaluation. J Expo Sci Environ Epidemiol. 2012;22:610–616. doi: 10.1038/jes.2012.39. [DOI] [PubMed] [Google Scholar]
- 49.Kovacic P. How safe is bisphenol A? Fundamentals of toxicity: metabolism, electron transfer and oxidative stress. Med Hypotheses. 2010;75:1–4. doi: 10.1016/j.mehy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Li M, et al. Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int. 2012;81:1131–1139. doi: 10.1038/ki.2012.6. [DOI] [PubMed] [Google Scholar]
- 51.Trasande L, Attina TM, Trachtman H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013;83:741–748. doi: 10.1038/ki.2012.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGinley EL. Summary of: dental composite materials and renal function in children. Br Dent J. 2014;216:80–81. doi: 10.1038/sj.bdj.2014.17. [DOI] [PubMed] [Google Scholar]
- 53.Trachtenberg FL, Shrader P, Barregard L, Maserejian NN. Dental composite materials and renal function in children. Br Dent J. 2014;216:E4. doi: 10.1038/sj.bdj.2014.36. [DOI] [PubMed] [Google Scholar]
- 54.Olea-Herrero N, et al. Bisphenol-A induces podocytopathy with proteinuria in mice. J Cell Physiol. 2014 doi: 10.1002/jcp.24665. [DOI] [PubMed] [Google Scholar]
- 55.You L, et al. Renal function, bisphenol A, and alkylphenols: results from the National Health and Nutrition Examination Survey (NHANES 2003–2006) Environ Health Perspect. 2011;119:527–533. doi: 10.1289/ehp.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmadkhaniha R, et al. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng. 2014;12:64. doi: 10.1186/2052-336X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bae S, Kim JH, Lim YH, Park HY, Hong YC. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension. 2012;60:786–793. doi: 10.1161/HYPERTENSIONAHA.112.197715. [DOI] [PubMed] [Google Scholar]
- 58.Khalil N, et al. Bisphenol A and cardiometabolic risk factors in obese children. Sci Total Environ. 2014:470–471. doi: 10.1016/j.scitotenv.2013.09.088. [DOI] [PubMed] [Google Scholar]
- 59.Bae S, Hong YC. Exposure to bisphenol A from drinking canned beverage increases blood pressure: randomized crossover trial. Hypertension. 2015;65:313–319. doi: 10.1161/HYPERTENSIONAHA.114.04261. [DOI] [PubMed] [Google Scholar]
- 60.Melzer D, et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 61.Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120:1297–1300. doi: 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaKind JS, Goodman M, Naiman DQ. Use of NHANES data to link chemical exposures to chronic diseases: a cautionary tale. PLoS ONE. 2012;7:e51086. doi: 10.1371/journal.pone.0051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lind L, Lind PM. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J Intern Med. 2012;271:537–553. doi: 10.1111/j.1365-2796.2012.02536.x. [DOI] [PubMed] [Google Scholar]
- 64.Trudel D, et al. Estimating Consumer Exposure to PFOS and PFOA. Risk Analysis. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 65.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicology Profiles. 2015 [online], http://www.atsdr.cdc.gov/
- 66.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji K, et al. Major perfluoroalkyl acid (PFAA) concentrations and influence of food consumption among the general population of Daegu, Korea. Sci Total Environ. 2012;438:42–48. doi: 10.1016/j.scitotenv.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 68.US Environmental Protection Agency. Long-chain perfluorinated chemicals (PFCs) action plan. 2009 [online] http://www.epa.gov/opptintr/existingchemicals/pubs/pfcs_action_plan1230_09.pdf.
- 69.Wen LL, Lin LY, Su TC, Chen PC, Lin CY. Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007–2010. J Clin Endocrinol Metab. 2013;98:E1456–E1464. doi: 10.1210/jc.2013-1282. [DOI] [PubMed] [Google Scholar]
- 70.Glynn A, et al. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol. 2012;46:9071–9079. doi: 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- 71.Perez F, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Krafft MP, Riess JG. Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie. 1998;80:489–514. doi: 10.1016/s0300-9084(00)80016-4. [DOI] [PubMed] [Google Scholar]
- 73.Andersen ME, et al. Perfluoroalkyl acids and related chemistries—toxicokinetics and modes of action. Toxicol Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- 74.Ohmori K, Kudo N, Katayama K, Kawashima Y. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology. 2003;184:135–140. doi: 10.1016/s0300-483x(02)00573-5. [DOI] [PubMed] [Google Scholar]
- 75.Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 76.Seals R, Bartell SM, Steenland K. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ Health Perspect. 2011;119:119–124. doi: 10.1289/ehp.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olsen GW, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol. 2011;174:893–900. doi: 10.1093/aje/kwr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watkins DJ, et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect. 2013;121:625–630. doi: 10.1289/ehp.1205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol. 2009;56:338–349. doi: 10.1007/s00244-008-9194-6. [DOI] [PubMed] [Google Scholar]
- 81.Geiger SD, Xiao J, Shankar A. No association between perfluoroalkyl chemicals and hypertension in children. Integr Blood Press Control. 2014;7:1–7. doi: 10.2147/IBPC.S47660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and elevated serum uric acid in US adults. Clin Epidemiol. 2011;3:251–258. doi: 10.2147/CLEP.S21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geiger SD, Xiao J, Shankar A. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am J Epidemiol. 2013;177:1255–1262. doi: 10.1093/aje/kws392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.US Environmental Protection Agency. EPA’s reanalysis of key issues related to dioxin toxicity and response to NAS comments, volume 1. 2012 [online] http://www.epa.gov/iris/supdocs/dioxinv1sup.pdf.
- 85.Milbrath MOG, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.International Agency for Research on Cancer. Chemical agents and related occupations: 2, 3, 7, 8 tetrachlorodibenzo-para-dioxin, 2, 3, 4, 7, 8-pentachlorodibenzofuran, and 3, 3, 4, 4′5-pentachlorobiphenyl. IARC Monogr Eval Carcinog Risks Hum. 2012;100F:339–378. [Google Scholar]
- 87.Rocas M, Jakubauskiene E, Kanopka A. Polymorphism of the aryl-hydrocarbon receptor gene in intron 10 of human cancers. Braz J Med Biol Res. 2011;44:1112–1117. doi: 10.1590/s0100-879x2011007500120. [DOI] [PubMed] [Google Scholar]
- 88.Heid SE, Walker MK, Swanson HI. Correlation of cardiotoxicity mediated by halogenated aromatic hydrocarbons to aryl hydrocarbon receptor activation. Toxicol Sci. 2001;61:187–196. doi: 10.1093/toxsci/61.1.187. [DOI] [PubMed] [Google Scholar]
- 89.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major pentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci. 2013;133:144–156. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- 90.van Ede KI, Gaisch KP, van den Berg M, van Duursen MB. Differential relative effect potencies of some dioxin-like compounds in human peripheral blood lymphocytes and murine splenic cells. Toxicol Lett. 2014;226:43–52. doi: 10.1016/j.toxlet.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 91.Kitamura K, et al. Dioxins in bile in relation to those in the human liver and blood. J Toxicol Sci. 2001;26:327–336. doi: 10.2131/jts.26.327. [DOI] [PubMed] [Google Scholar]
- 92.Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- 93.Everett CJ, Thompson OM. Dioxins, furans and dioxin-like PCBs in human blood: causes or consequences of diabetic nephropathy? Environ Res. 2014;132:126–131. doi: 10.1016/j.envres.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 94.Chang JW, Ou HY, Chen HL, Su HJ, Lee CC. Hyperuricemia after exposure to polychlorinated dibenzo-p-dioxins and dibenzofurans near a highly contaminated area. Epidemiology. 2013;24:582–589. doi: 10.1097/EDE.0b013e318294ef68. [DOI] [PubMed] [Google Scholar]
- 95.Ha MH, Lee DH, Son HK, Park SK, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23:274–286. doi: 10.1038/jhh.2008.124. [DOI] [PubMed] [Google Scholar]
- 96.Chang JW, et al. Interrelationship between exposure to PCDD/Fs and hypertension in metabolic syndrome in Taiwanese living near a highly contaminated area. Chemosphere. 2010;81:1027–1032. doi: 10.1016/j.chemosphere.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 97.Karouna-Renier NK, Rao KR, Lanza JJ, Davis DA, Wilson PA. Serum profiles of PCDDs and PCDFs, in individuals near the Escambia Wood Treating Company Superfund site in Pensacola, FL. Chemosphere. 2007;69:1312–1319. doi: 10.1016/j.chemosphere.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 98.Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007;50:1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- 99.Nakamoto M, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86:849–859. doi: 10.1007/s00420-012-0819-8. [DOI] [PubMed] [Google Scholar]
- 100.Uemura H, et al. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Environ Health Perspect. 2009;117:568–573. doi: 10.1289/ehp.0800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitamura K, et al. Health effects of chronic exposure to polychlorinated dibenzo-P-dioxins (PCDD), dibenzofurans (PCDF) and coplanar PCB (Co-PCB) of municipal waste incinerator workers. J Epidemiol. 2000;10:262–270. doi: 10.2188/jea.10.262. [DOI] [PubMed] [Google Scholar]
- 102.Lee YM, Bae SG, Lee SH, Jacobs DR, Jr, Lee DH. Persistent organic pollutants and hyperuricemia in the U.S. general population. Atherosclerosis. 2013;230:1–5. doi: 10.1016/j.atherosclerosis.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Agency for Toxic Substances and Disease Registry. Public Health Statement: polycyclic aromatic hydrocarbons (PAHs) 1995 [online] http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf. [PubMed]
- 104.Fang GC, Wu YS, Fu PP, Yang IL, Chen MH. Polycyclic aromatic hydrocarbons in the ambient air of suburban and industrial regions of central Taiwan. Chemosphere. 2004;54:443–452. doi: 10.1016/S0045-6535(03)00706-9. [DOI] [PubMed] [Google Scholar]
- 105.Harvey RG, Dai Q, Ran C, Penning TM. Synthesis of the o-quinones and other oxidized metabolites of polycyclic aromatic hydrocarbons implicated in carcinogenesis. J Org Chem. 2004;69:2024–2032. doi: 10.1021/jo030348n. [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Canton C, Anadon A, Meredith C. Genotoxicity evaluation of individual cigarette smoke toxicants using the in vitro gammaH2AX assay by high content screening. Toxicol Lett. 2013;223:81–7. doi: 10.1016/j.toxlet.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 107.Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect. 2004;112:754–759. doi: 10.1289/ehp.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung KH, et al. Time trends of polycyclic aromatic hydrocarbon exposure in New York City from 2001 to 2012: assessed by repeat air and urine samples. Environ Res. 2014;131:95–103. doi: 10.1016/j.envres.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sultana S, et al. Association of CYP1A1 Gene Polymorphism with Ischemic Stroke in South Indian Population. Trans Stroke Res. 2011;2:26–32. doi: 10.1007/s12975-010-0059-8. [DOI] [PubMed] [Google Scholar]
- 110.Nerurkar PV, et al. CYP1A1, GSTM1, and GSTP1 genetic polymorphisms and urinary 1-hydroxypyrene excretion in non-occupationally exposed individuals. Cancer Epidemiol Biomarkers Prev. 2000;9:1119–1122. [PubMed] [Google Scholar]
- 111.Tas S, Buchet JP, Lauwerys R. Determinants of benzo[a]pyrene diol epoxide adducts to albumin in workers exposed to polycyclic aromatic hydrocarbons. Int Archives Occup Environ Health. 1994;66:343–348. doi: 10.1007/BF00378368. [DOI] [PubMed] [Google Scholar]
- 112.Tuominen R, et al. Susceptibility factors and DNA adducts in peripheral blood mononuclear cells of aluminium smelter workers exposed to polycyclic aromatic hydrocarbons. Arch Toxicol. 2002;76:178–186. doi: 10.1007/s00204-002-0331-0. [DOI] [PubMed] [Google Scholar]
- 113.Everett CJ, et al. Association of urinary polycyclic aromatic hydrocarbons and serum C-reactive protein. Environ Res. 2010;110:79–82. doi: 10.1016/j.envres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 114.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 115.Voice TC, et al. Evaluation of the hypothesis that Balkan endemic nephropathy is caused by drinking water exposure to contaminants leaching from Pliocene coal deposits. J Expo Sci Environ Epidemiol. 2006;16:515–524. doi: 10.1038/sj.jes.7500489. [DOI] [PubMed] [Google Scholar]
- 116.Stefanovic V. Balkan endemic nephropathy: a need for novel aetiological approaches. QJM. 1998;91:457–463. doi: 10.1093/qjmed/91.7.457. [DOI] [PubMed] [Google Scholar]
- 117.Stefanovic V, Cukuranovic R, Miljkovic S, Marinkovic D, Toncheva D. Fifty years of Balkan endemic nephropathy: challenges of study using epidemiological method. Ren Fail. 2009;31:409–418. doi: 10.1080/08860220902839097. [DOI] [PubMed] [Google Scholar]
- 118.Stefanovic V, Toncheva D, Atanasova S, Polenakovic M. Etiology of Balkan endemic nephropathy and associated urothelial cancer. Am J Nephrol. 2006;26:1–11. doi: 10.1159/000090705. [DOI] [PubMed] [Google Scholar]
- 119.Jacobs L, et al. Acute changes in pulse pressure in relation to constituents of particulate air pollution in elderly persons. Environ Res. 2012;117:60–67. doi: 10.1016/j.envres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 120.McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sjodin A, et al. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey: 2003–2008. Environ Sci Technol. 2014;48:753–60. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kreiss K. Studies on populations exposed to polychlorinated biphenyls. Environ Health Perspect. 1985;60:193–199. doi: 10.1289/ehp.8560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kreiss K, et al. Association of blood pressure and polychlorinated biphenyl levels. JAMA. 1981;245:2505–2509. [PubMed] [Google Scholar]
- 124.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polychlorinated Biphenyls (PCBs) 2000 [online] http://www.atsdr.cdc.gov/toxfaqs/tfacts17.pdf. [PubMed]
- 125.Megson D, et al. Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci Total Environ. 2013:461–462. doi: 10.1016/j.scitotenv.2013.04.082. [DOI] [PubMed] [Google Scholar]
- 126.Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic activation of PCBs to carcinogens in vivo—a review. Environ Toxicol Pharmacol. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baker EL, Jr, et al. Metabolic consequences of exposure to polychlorinated biphenyls (PCB) in sewage sludge. Am J Epidemiol. 1980;112:553–563. doi: 10.1093/oxfordjournals.aje.a113024. [DOI] [PubMed] [Google Scholar]
- 128.Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28:2053–2060. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- 129.Goncharov A, Pavuk M, Foushee HR, Carpenter DO. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect. 2011;119:319–325. doi: 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Everett CJ, Mainous AG, 3rd, Frithsen IL, Player MS, Matheson EM. Commentary on the association of polychlorinated biphenyls with hypertension. Environ Res. 2008;108:428–429. doi: 10.1016/j.envres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 131.Yorita Christensen KL, White P. A methodological approach to assessing the health impact of environmental chemical mixtures: PCBs and hypertension in the National Health and Nutrition Examination Survey. Int J Environ Res Public Health. 2011;8:4220–4237. doi: 10.3390/ijerph8114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p′-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27–31. doi: 10.1016/j.envres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 133.Sjoberg Lind Y, Lind PM, Salihovic S, van Bavel B, Lind L. Circulating levels of persistent organic pollutants (POPs) are associated with left ventricular systolic and diastolic dysfunction in the elderly. Environ Res. 2013;123:39–45. doi: 10.1016/j.envres.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 134.Peters JL, Patricia Fabian M, Levy JI. Combined impact of lead, cadmium, polychlorinated biphenyls and non-chemical risk factors on blood pressure in NHANES. Environ Res. 2014;132c:93–99. doi: 10.1016/j.envres.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 135.Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282–289. doi: 10.1016/j.envint.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 136.Valera B, Jorgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65–73. doi: 10.1016/j.envres.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 137.Imamura T, et al. Cutaneous symptoms such as acneform eruption and pigmentation are closely associated with blood levels of 2, 3, 4, 7, 8-penta-chlorodibenzofurans in Yusho patients, using data mining analysis. BMC Res Notes. 2009;2:27. doi: 10.1186/1756-0500-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weiner DE, McClean MD, Kaufman JS, Brooks DR. The Central American epidemic of CKD. Clin J Am Soc Nephrol. 2013;8:504–511. doi: 10.2215/CJN.05050512. [DOI] [PubMed] [Google Scholar]
- 140.Nanayakkara S, et al. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med. 2012;17:213–221. doi: 10.1007/s12199-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jayasumana C, Gajanayake R, Siribaddana S. Importance of arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC Nephrol. 2014;15:124. doi: 10.1186/1471-2369-15-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jayatilake N, Mendis S, Maheepala P, Mehta FR. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson RJ, et al. Hyperosmolarity drives hypertension and CKD—water and salt revisited. Nat Rev Nephrol. 2014;10:415–420. doi: 10.1038/nrneph.2014.76. [DOI] [PubMed] [Google Scholar]
- 144.Ordunez P, et al. Chronic kidney disease epidemic in Central America: urgent public health action is needed amid causal uncertainty. PLoS Negl Trop Dis. 2014;8:e3019. doi: 10.1371/journal.pntd.0003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311:859–860. doi: 10.1001/jama.2013.283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ramirez-Sandoval JC, et al. Urinary neutrophil gelatinase-associated lipocalin predicts graft loss after acute kidney injury in kidney transplant. Biomarkers. 2014;19:63–69. doi: 10.3109/1354750X.2013.867536. [DOI] [PubMed] [Google Scholar]
- 147.Gluhovschi G, et al. Familial versus environmental factors in Balkan endemic nephropathy in Mehedinti county, Romania, by means of albuminuria and tubular biomarkers: preliminary study. Ren Fail. 2015;37:219–224. doi: 10.3109/0886022X.2014.982476. [DOI] [PubMed] [Google Scholar]
- 148.Pickering JW, Endre ZH. Acute kidney injury urinary biomarker time-courses. PLoS One. 2014;9:e101288. doi: 10.1371/journal.pone.0101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sabbisetti VS, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weaver VM, Kotchmar DJ, Fadrowski JJ, Silbergeld EK. Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment? J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2015.8. http://dx.doi.org/10.1038/jes.2015.8. [DOI] [PubMed]
- 151.Suliman M, et al. The reverse epidemiology of plasma total homocysteine as a mortality risk factor is related to the impact of wasting and inflammation. Nephrol Dial Transplant. 2007;22:209–217. doi: 10.1093/ndt/gfl510. [DOI] [PubMed] [Google Scholar]
- 152.Alberti KGMM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 153.Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13:129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 154.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 155.Marshall CB, Pippin JW, Krofft RD, Shankland SJ. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 2006;70:1962–1973. doi: 10.1038/sj.ki.5001965. [DOI] [PubMed] [Google Scholar]
- 156.Piwkowska A, Rogacka D, Jankowski M, Kocbuch K, Angielski S. Hydrogen peroxide induces dimerization of protein kinase G type Iα subunits and increases albumin permeability in cultured rat podocytes. J Cell Physiol. 2012;227:1004–1016. doi: 10.1002/jcp.22810. [DOI] [PubMed] [Google Scholar]
- 157.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2:529–542. doi: 10.2215/CJN.04121206. [DOI] [PubMed] [Google Scholar]
- 158.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 159.Everett CJ, Mainous AG, 3rd, Frithsen IL, Player MS, Matheson EM. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2008;108:94–97. doi: 10.1016/j.envres.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 160.US Environmental Protection Agency. Bisphenol A (BPA) action plan summary. 2013 [online] http://www.epa.gov/opptintr/existingchemicals/pubs/actionplans/bpa.html.
- 161.Hasselberg L, Meier S, Svardal A. Effects of alkylphenols on redox status in first spawning Atlantic cod (Gadus morhua) Aquat Toxicol. 2004;69:95–105. doi: 10.1016/j.aquatox.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 162.Atkinson A, Roy D. In vivo DNA adduct formation by bisphenol A. Environ Mol Mutag. 1995;26:60–66. doi: 10.1002/em.2850260109. [DOI] [PubMed] [Google Scholar]
- 163.Hugo ER, et al. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol In Vitro. 2004;18:265–269. doi: 10.1016/j.tiv.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 165.Gourlay T, Samartzis I, Stefanou D, Taylor K. Inflammatory response of rat and human neutrophils exposed to di-(2-ethyl-hexyl)-phthalate-plasticized polyvinyl chloride. Artif Organs. 2003;27:256–260. doi: 10.1046/j.1525-1594.2003.07107.x. [DOI] [PubMed] [Google Scholar]
- 166.Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environ Res. 2011;111:718–726. doi: 10.1016/j.envres.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qian Y, et al. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Res Health. 2010;73:819–836. doi: 10.1080/15287391003689317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sutton TA, et al. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285:F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 170.Albina ML, et al. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology. 2010;271:51–56. doi: 10.1016/j.tox.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 171.Araujo JA, Barajas B, Kleinman M, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chuang HC, Fan CW, Chen KY, Chang-Chien GP, Chan CC. Vasoactive alteration and inflammation induced by polycyclic aromatic hydrocarbons and trace metals of vehicle exhaust particles. Toxicol Lett. 2012;214:131–136. doi: 10.1016/j.toxlet.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 173.Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13:129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 174.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 175.Barker DJ, Godfrey KM, Osmond C, Bull A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr Perinat Epidemiol. 1992;6:35–44. doi: 10.1111/j.1365-3016.1992.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 176.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 177.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 179.Zhang Y, et al. Phthalate levels and low birth weight: a nested case-control study of chinese newborns. J Pediatr. 2009;155:500–504. doi: 10.1016/j.jpeds.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 180.Snijder CA, et al. Fetal growth and prenatal exposure to bisphenol A: the generation R study. Environ Health Perspect. 2013;121:393–398. doi: 10.1289/ehp.1205296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gascon M, et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 182.Whyatt RM, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120:290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Perera FP, et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect. 2012;120:921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol. 2009;27:212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 185.Schmieder RE, et al. Low-grade albuminuria and cardiovascular risk: what is the evidence? Clin Res Cardiol. 2007;96:247–257. doi: 10.1007/s00392-007-0510-3. [DOI] [PubMed] [Google Scholar]
- 186.DeBosch BJ, Kluth O, Fujiwara H, Schurmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642. doi: 10.1038/ncomms5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Trasande L, Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff (Millwood) 2011;30:863–70. doi: 10.1377/hlthaff.2010.1239. [DOI] [PubMed] [Google Scholar]
- 188.Trasande L, et al. How developing nations can protect children from hazardous chemical exposures while sustaining economic growth. Health Aff (Millwood) 2011;30:2400–2409. doi: 10.1377/hlthaff.2010.1217. [DOI] [PubMed] [Google Scholar]
- 189.Trasande L. Further limiting bisphenol a in food uses could provide health and economic benefits. Health Aff (Millwood) 2014;33:316–323. doi: 10.1377/hlthaff.2013.0686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


