Abstract
Molecular events that drive disc damage and low back pain (LBP) may precede clinical manifestation of disease onset and can cause detrimental long-term effects such as disability. Biomarkers serve as objective molecular indicators of pathological processes. The goal of this study is to identify systemic biochemical factors as predictors of response to treatment of LBP with epidural steroid injection (ESI). Since inflammation plays a pivotal role in LBP, this pilot study investigates the effect of ESI on systemic levels of 48 inflammatory biochemical factors (cytokines, chemokines, and growth factors) and examines the relationship between biochemical factor levels and pain or disability in patients with disc herniation (DH), or other diagnoses (Other Dx) leading to low back pain, which included spinal stenosis (SS) and degenerative disc disease (DDD). Study participants (n = 16) were recruited from a back pain management practice. Pain numerical rating score (NRS), Oswestry Disability Index (ODI), and blood samples were collected pre- and at 7 to 10 days post-treatment. Blood samples were assayed for inflammatory mediators using commercial multiplex assays. Mediator levels were compared pre- and post-treatment to investigate the potential correlations between clinical and biochemical outcomes. Our results indicate that a single ESI significantly decreased systemic levels of SCGF-β and IL-2. Improvement in pain in all subjects was correlated with changes in chemokines (MCP-1, MIG), hematopoietic progenitor factors (SCGF-β), and factors that participate in angiogenesis/fibrosis (HGF), nociception (SCF, IFN-α2), and inflammation (IL-6, IL-10, IL-18, TRAIL). Levels of biochemical mediators varied based on diagnosis of LBP, and changes in pain responses and systemic mediators from pre- to post-treatment were dependent on the diagnosis cohort. In the DH cohort, levels of IL-17 and VEGF significantly decreased post-treatment. In the Other Dx cohort, levels of IL-2Rα, IL-3, and SCGF-β significantly decreased post-treatment. In order to determine whether mediator changes were related to pain, correlations between change in pain scores and change in mediator levels were performed. Subjects with DH demonstrated a profile signature that implicated hematopoiesis factors (SCGF-β, GM-CSF) in pain response, while subjects with Other Dx demonstrated a biomarker profile that implicated chemokines (MCP-1, MIG) and angiogenic factors (HGF, VEGF) in pain response. Our findings provide evidence that systemic biochemical factors in patients with LBP vary by diagnosis, and pain response to treatment is associated with a unique profile of biochemical responses in each diagnosis group. Future hypothesis-based studies with larger subject cohorts are warranted to confirm the findings of this pilot exploratory study.
Keywords: Back pain, Inflammation, Intervertebral disc, Epidural steroid injection
Introduction
Up to 84 % of adults have low back pain (LBP) at some time in their lives [1, 2]. The long-term outcome of LBP is generally favourable [3], but given the prevalence of LBP, persistent symptoms affect millions of individuals. Approximately 5 % of people with LBP disability account for 75 % of the costs associated with this condition [4]. Multiple treatment options for subacute and chronic LBP are available, including pharmacologic and non-interventional treatments, non-surgical interventional treatments, and surgical treatments. Non-surgical interventional treatments, such as epidural steroid injections (ESIs), are generally recommended for patients with LBP who have failed conservative management.
ESIs have been used for pain control in patients with radiculopathy due to herniated disc, spinal stenosis, and non-specific low back pain due to degenerative disc disease. However, the efficacy is unclear, due to heterogeneous populations and specifics of the ESI interventions in randomized trials. A systematic review of randomized, placebo-controlled trials found that ESI was associated with a small improvement in leg pain (6.2 %) and disability (3.1 %) for up to 3 months, but no improvement at 1 year [5–7]. The trials included heterogeneous groups of patients in terms of symptom duration, and both therapeutic and placebo treatments varied considerably between trials. Despite the relatively small average benefit observed in clinical trials, clinical experience suggests that some patients do obtain more significant relief, making it reasonable to offer a trial of ESIs for patients who have not found pain relief with more conservative approaches, particularly for patients who are not candidates for surgery or are not interested in surgery. In light of this, there is a significant need for developing diagnostic tools that are predictive of response to treatment with ESI.
Molecular events that drive disc damage and LBP may precede clinical manifestation of disease onset and can cause detrimental long-term effects such as disability. Biomarkers serve as objective molecular indicators of pathological processes. Owing to the heterogeneous aetiologies of back pain, identifying a single biomarker predictive of disc damage would be unlikely. Consequently, there is great interest in identifying a panel of biomarkers that could be incorporated into a signature predictive of symptom or disease progression. For example, a signature profile of disc destruction could facilitate more informed clinical decisions and help guide personalized medical approaches.
The goal of this pilot study is to explore the correlation of systemic biochemical factors and clinical response to treatment of LBP with ESI. Since inflammation plays a pivotal role in LBP [8, 9], and endogenous glucocorticoids are anti-inflammatory, this study investigates the effect of ESI on systemic levels of inflammatory biochemical factors (cytokines, chemokines, and growth factors) and examines the relationship between biochemical factors and pain or disability in patients with LBP due to various aetiologies. The three aetiologies of LBP included here are: disc herniation (DH), or other diagnoses such as degenerative disc disease (DDD), and spinal stenosis (SS). DH is the focal displacement of disc material beyond the limits of the disc space and can irritate nearby nerves resulting in pain, numbness, or weakness in the legs. SS is a pathological narrowing of the spinal canal due to hypertrophy of the ligamentum flavum and is associated with paresthesia, pain, and symptoms of neurologic compromise. DDD includes disc pathology such as desiccation and loss of disc height, diffuse disc bulging, fissures, osteophytes, or end-plate sclerosis without specific indication for herniation or stenosis. We hypothesize that patients responsive to treatment via local delivery of steroids into the epidural space, as defined by reduction in pain scores, will exhibit a coordinated change in systemic biochemical factors that may be associated with the ESI treatment. Since ESI treatment is indicated for various causes of LBP, we investigated levels and heterogeneity of systemic biochemical factors in differing diagnostic groups.
Methods
Participants
This study was performed with the approval of the IRB of the North Shore-LIJ Health System. Written informed consent was obtained from all subjects prior to enrolment. Samples were obtained from subjects undergoing ESI by a pain management physician for LBP with or without radiculopathy. Treatment consisted of an interlaminar ESI of 120 mg methylprednisolone mixed with 2cc of 1 % lidocaine. Patients were referred for ESI after having failed more conservative treatments including physical therapy and/or oral anti-inflammatory pain medications. According to clinical guidelines, ESI treatment is indicated before a patient is referred for surgery. Study subjects have reported pain symptoms for a median duration of 10.5 months (min: 0.25 months, max: 60 months), prior to enrolment in this study.
The following inclusion criteria were used: 18 years of age or older, requiring interlaminar ESI in the lumbar spine region (L1–L2 to L5–S1) for diagnoses including SS, DH, and DDD. Subjects were excluded if they had: history of previous lumbar surgery, epidural corticosteroid injection within the last 6 months, known inflammatory condition (e.g. rheumatoid arthritis, systemic lupus erythematosus, gout, osteomyelitis, other infections), history of cancer, and were pregnant or breastfeeding at time of enrolment.
Blood sample and clinical pain and disability surveys were obtained prior to epidural injection (pre-treatment) and at 7–10 days following epidural injection (post-treatment). Whole blood was collected (BD Vacutainer 367874) and maintained at 4 °C until processing. Blood samples were centrifuged, aliquoted, and stored at −80 °C until biochemical analysis. Pain was recorded at each visit using the numerical rating scale (NRS), an 11-point unidimensional measure of pain intensity ranging from 0 to 10, with 0 representing ‘no pain’ and 10 representing ‘most severe pain’. Disability was measured using a validated questionnaire (Oswestry Low Back Pain Disability Questionnaire) and was quantified by computing the Oswestry Disability Index (ODI) at pre- and post-treatment. ODI values in 0–20 % represent minimal disability, and values over 80 % represent complete disability. Clinical data such as basic demographics, underlying diagnosis (DH, SS, or DDD), prior treatment history, and duration of pain symptoms, categorized as acute (<6 months) or chronic (>6 months) were obtained from subjects’ medical records.
Biochemical analysis
Systemic plasma levels of 48 mediators were assayed by a multiplex bead-based assay (Bio-Plex Pro Human Cytokine 27-plex Assay, Group I, #M500KCAF0Y, and Bio-Plex Pro Human Cytokine 21-plex Assay, Group II, #MFO-005KMII, Bio-Rad, Hercules, CA) using the Luminex LX100/200 platform and xPONENT software. The assay was performed according to manufacturer’s recommendations. Samples were run in duplicate. The factors assayed were: interleukin (IL)-1 alpha (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-2 receptor alpha (IL-2Rα), IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-18, cutaneous T cell-attracting chemokine (CTACK), eotaxin, basic fibroblast growth factor (FGF-basic), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth-related protein alpha (GROα), hepatocyte growth factor (HGF), interferon (IFN) alpha 2 (IFN-α2), IFN gamma (IFN-γ), C-X-C motif chemokine 10 (IP-10/CXCL10), leukemia inhibitory factor (LIF), monocyte chemoattractant protein (MCP-1/CCL2), MCP-3/CCL7, macrophage colony-stimulating factor (M-CSF), macrophage migration inhibitory factor (MIF), monokine induced by IFN-gamma (MIG), macrophage inflammatory protein (MIP)-1 alpha (MIP-1α/CCL3), MIP-1β/CCL4, beta-nerve growth factor (β-NGF), platelet-derived growth factor subunit B (PDGF-BB), C-C motif chemokine 5 (RANTES/CCL5), stem cell factor (SCF), stem cell growth factor beta (SCGF-β), stromal-derived factor alpha (SDF-1α), tumour necrosis factor (TNF) alpha (TNF-α), TNF beta (TNF-β), TNF-related apoptosis-inducing ligand (TRAIL), and vascular endothelial growth factor (VEGF). The ranges of each factor assayed, including upper and lower limits of detection, were determined. For statistical purposes, values that were below the range of the assay were extrapolated, and values that were below the lowest limit of detection were assigned the value of the lowest limit of detection.
Statistical analysis
Levels of cytokines, chemokines, and growth factors were compared between pre- and post-treatment in all subjects using a Wilcoxon signed-rank (WSR) test. Subjects were grouped by diagnosis, as either disc herniation (DH) or Other Dx [including spinal stenosis (SS) and degenerative disc disease (DDD)]. The Mann–Whitney test was used to compare diagnostic groups (DH vs. Other Dx) with respect to pre-treatment levels. Differences within diagnosis cohorts from pre- to post-treatment were examined using WSR test. Additional analysis was conducted to examine whether the percent change in pain NRS was correlated with the percent change in each biochemical factor measurement or ODI. Spearman’s correlation coefficients (ρ) and corresponding p values are reported for significant correlations between the percent change in pain NRS and percent change in each of the biochemical factor. Spearman’s correlation analysis was performed on all subjects and separately by diagnosis cohort (DH, Other Dx). All statistical analyses were generated using SAS with significance level set at α = 0.05. No adjustments were applied for multiple comparisons because this was a pilot exploratory study. The use of α = 0.05 is acceptable in pilot studies; however, we recognize that type-I error may be inflated in the absence of a multiple comparison correction. In pilot exploratory studies, such as the current one, adjustments for multiple testing may not be required and often may not be practical [10, 11].
Results
Subject characteristics
Levels of systemic mediators in subjects with LBP were analysed from subjects undergoing ESI (n = 16), due to a diagnosis of DH (n = 10) or Other Dx, including SS or DDD (n = 6). Age, gender, and body mass index (BMI) demographics are presented in Table 1. The mean age of recruited subjects was 55 ± 15 years. Subjects were 70 % female and 30 % male, with similar distribution observed between DH and Other Dx groups. The average BMI was 30 ± 6 (Table 1). In the DH cohort, five of ten subjects reported chronic pain (duration of pain symptoms for 6 months or longer). In Other Dx group, five of six subjects reported chronic pain.
Table 1.
Summary of demographics of all study subjects (total) and by diagnosis groups (DH or Other Dx)
| Subject demographics | DH | Other Dx | Total | p value |
|---|---|---|---|---|
| Number of subjects (N) | 10 | 6 | 16 | |
| Age (years, mean ± SD) | 54 ± 15 | 57 ± 16 | 55 ± 15 | 0.74 |
| BMI (mean ± SD) | 30 ± 7 | 29 ± 4 | 30 ± 6 | 0.96 |
| Male | 3 (30 %) | 2 (33 %) | 5 (31 %) | 0.96 |
| Female | 7 (70 %) | 4 (67 %) | 11 (69 %) |
Clinical and biochemical changes from pre- to post-treatment
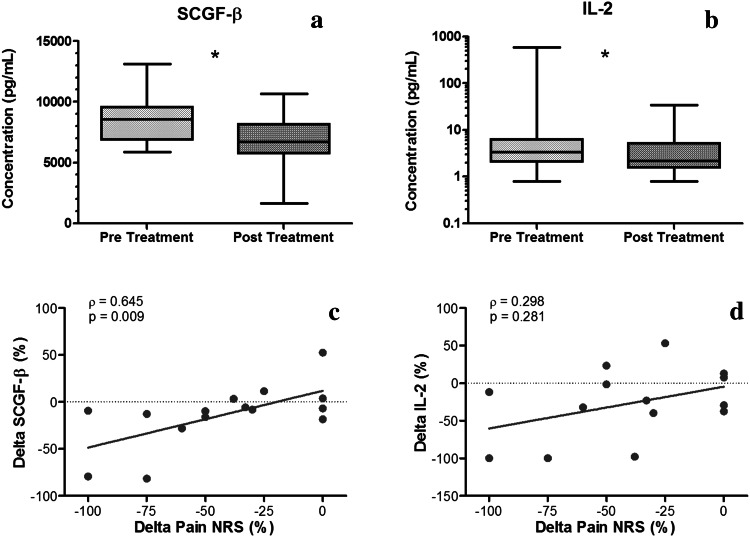
At pre-treatment, the mean pain NRS was 9.3 ± 0.9, and the ODI was 45 ± 15 %. At post-treatment, NRS levels were observed to be 44 % lower than pre-treatment (post-treatment: 5.2 ± 3.4, p < 0.001, Table 2). A trend for slightly lower ODI (34 ± 18 %) was also observed post-treatment (Table 2). Across all study subjects, circulating levels of SCGF-β and IL-2 decreased significantly post-treatment relative to pre-treatment (Fig. 1a, b). We examined the relationship between NRS and SCGF-β or IL-2, to determine whether changes from baseline to post-treatment in NRS are associated with corresponding changes in biochemical factors. We observed a significant correlation between change in NRS and change in SCGF-β in all subjects (ρ = 0.645, p = 0.009, Fig. 1c). However, no significant correlation between change in IL-2 levels was found with NRS response (Fig. 1d).
Table 2.
Summary of clinical measures of pain (NRS) and disability (ODI) in all subjects (N = 16), in DH subjects (N = 10), and in Other Dx subjects (N = 6) at pre- and post-treatment
| Clinical outcomes | Pre-treatment | Post-treatment | p value (WSR) |
|---|---|---|---|
| Pain NRS (all subjects) | 9.3 ± 0.9; 10 (9–10) |
5.2 ± 3.4; 5 (2.5–8) |
0.00048 |
| Pain NRS (DH) | 9.5 ± 0.9; 10 (9–10) |
5.5 ± 2.9; 5.5 (2.5–7) |
0.0078 |
| Pain NRS (Other Dx) | 9.0 ± 1.1; 9 (9–10) |
4.8 ± 4.3; 4.8 (0–9) |
0.13 |
| ODI (all subjects) | 45 ± 15; 49 (34–56) |
34 ± 18; 31 (21–52) |
0.064 |
| ODI (DH) | 44 ± 15; 41 (32–52) |
37 ± 16; 38 (26–51) |
0.42 |
| ODI (Other Dx) | 48 ± 15; 54 (48–58) |
30 ± 22; 23 (20–53) |
0.094 |
Data are presented as mean ± standard deviation; median (Q1–Q3), where Q1 and Q3 are the lower and upper quartiles, respectively
Fig. 1.
a–b Mediator levels of SCGF-β and IL-2 were significantly different in pre- versus post-treatment in all subjects (*p < 0.05, plot line: median, box: 25–75 % percentiles, error bars: range). c–d Spearman’s correlations (ρ and p value) between % change in pain NRS and % change in SCGF-β and IL-2 in all subjects. A significant correlation between % change in SCGF-β and % change in NRS was observed, while % change in IL-2 levels was not significantly correlated with % change in pain NRS
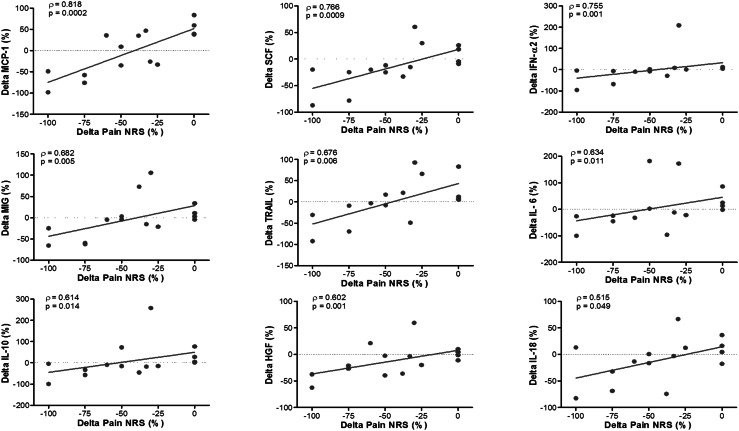
Correlations between NRS and other biochemical factors or ODI were also investigated, to determine whether multiple factors are coordinately varying with change in NRS. We found that changes in ODI were not significantly correlated with changes in NRS (ρ = 0.176, p = 0.5). However, changes in NRS were significantly correlated with changes in the levels of MCP-1, SCF, IFN-α2, MIG, TRAIL, SCGF-β, IL-6, IL-10, HGF, and IL-18 when analysed in all subjects (Fig. 2).
Fig. 2.
Spearman’s correlation coefficient (ρ) and p value for significant correlations observed between % change in pain NRS and % change in systemic mediator levels of MCP-1, SCF, IFN-α2, MIG, TRAIL, IL-6, IL-10, HGF, and IL-18 in all subjects. Each point on scatter plot represents one subject, and line represents the correlation fit
Differences in biochemical profiles and response by diagnosis
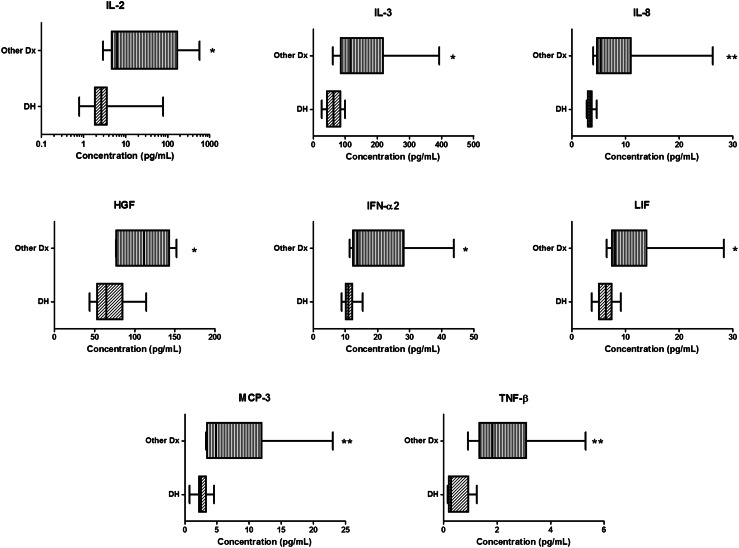
Subjects who had LBP due to other diagnoses (SS or DDD, grouped as Other Dx) had significantly higher levels of many biochemical factors compared to subjects with LBP attributed to a DH diagnosis (Fig. 3). Circulating levels of IL-2, IL-3, IL-8, HGF, IFN-α2, LIF, MCP-3, and TNF-β were significantly higher in subjects with Other Dx compared to DH (Fig. 3). No significant differences in age, BMI, or gender were observed between the DH cohort and the Other Dx cohort.
Fig. 3.
Levels of some systemic mediators prior to treatment differed based on diagnosis. Levels of IL-2, IL-3, IL-8, HGF, IFN-α2, LIF, MCP-3, and TNF-β, were significantly higher in Other Dx subjects compared to DH subjects. *p < 0.05, **p < 0.01. Plot line represents the median, plot box represents the 25–75 % percentiles, and error bars represent the range
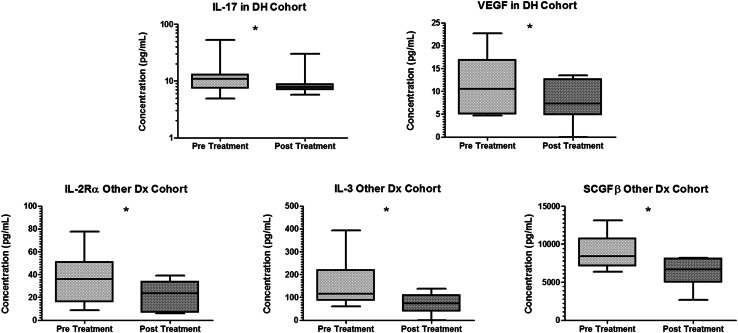
Changes in pain responses from pre- to post-treatment were dependent on the diagnosis cohort. In DH cohort, the average pain NRS was significantly lower post-treatment versus pre-treatment (Table 2). The NRS in Other Dx was 50 % lower in post- versus pre-treatment, although this was not statistically significant (Table 2). Biochemical changes in response to treatment also varied by diagnosis. In DH, a significant decrease in levels of IL-17 and VEGF was observed post-treatment (Fig. 4). In the Other Dx cohort, levels of IL-2Rα, IL-3, and SCGF-β significantly decreased post-treatment (Fig. 4).
Fig. 4.
Levels of mediators that signficantly changed after ESI treatment by diagnosis. In DH cohort (top row), levels of IL-17 and VEGF were significantly lower in post- versus pre-treatment. In Other Dx cohort (bottom row), levels of IL-2Rα, IL-3, and SCGF-β were significantly lower in post- versus pre-treatment (*p < 0.05). Plot line represents the median, plot box represents the 25–75 % percentiles, and error bars represent the range
We investigated potential factors that might be associated with clinical response status in the two diagnosis cohorts. Each of the 48 biomarkers was individually examined for a correlation between changes in mediator level and changes in pain NRS. Figures 1 and 2 show the mediators found to be correlated when analysing all subjects together, and Table 3 summarizes the list of mediators significantly correlated in the two diagnosis cohorts. Of the ten factors correlated with clinical responses in all subjects, four factors were also significantly correlated only in the DH group (IL-6, IL-10, IL-18, and SCGF-β, Table 3), while four other factors were significantly correlated only in the Other Dx subjects (MCP-1, HGF, MIG, and TRAIL, Table 3). Changes in IFN-α2 and SCF were significantly correlated in both individual diagnoses cohorts, and when all subjects were analysed as a single group. Analysis by diagnosis also yielded factors that were significantly correlated within each diagnosis cohort, but not when cohorts were combined into a single group (DH: GM-CSF, IL-2Rα, IL-12p40; Other Dx: IL-1β, IL-1ra, IL-9, IL-12, VEGF, Table 3).
Table 3.
Spearman’s correlation coefficient (ρ) and p value for significant correlations between % change in pain NRS and % change in systemic mediator levels by diagnosis (DH cohort, Other Dx cohort)
| Significantly correlated in all subjects (Figs. 1, 2) | Significantly correlated in DH cohort | Significantly correlated in Other Dx cohort | |||
|---|---|---|---|---|---|
| Spearman’s coefficient (ρ) | p value | Spearman’s coefficient (ρ) | p value | ||
| GM-CSF | 0.701 | 0.035 | – | NS | |
| HGF | x | – | NS | 0.837 | 0.038 |
| IFN-α2 | x | 0.773 | 0.015 | 0.837 | 0.038 |
| IL-1β | – | NS | 0.837 | 0.038 | |
| IL-1ra | – | NS | 0.837 | 0.038 | |
| IL-2Rα | 0.748 | 0.021 | – | NS | |
| IL-6 | x | 0.731 | 0.025 | – | NS |
| IL-9 | – | NS | 0.837 | 0.038 | |
| IL-10 | x | 0.731 | 0.025 | – | NS |
| IL-12 | – | NS | 0.837 | 0.038 | |
| IL-12p40 | 0.840 | 0.005 | – | NS | |
| IL-18 | x | 0.765 | 0.016 | – | NS |
| MCP-1 | x | – | NS | 0.956 | 0.003 |
| MIG | x | – | NS | 0.956 | 0.003 |
| SCF | x | 0.782 | 0.013 | 0.837 | 0.038 |
| SCGF-β | x | 0.874 | 0.002 | – | NS |
| TRAIL | x | – | NS | 0.837 | 0.038 |
| VEGF | – | NS | 0.837 | 0.038 | |
Changes in mediators found to be correlated in all subjects, in DH, and in Other Dx are marked in italics
Discussion
The goal of this pilot study was to examine systemic biochemical factors in patients undergoing ESI for lumbar disc disorders in order to explore their utility as predictors of pain and disability response to treatment of LBP with ESI. Our results indicate that a single interlaminar ESI in the lumbar spine significantly decreased circulating levels of SCGF-β and IL-2. Improvement in pain in all subjects was correlated with decreases in chemokines (MCP-1, MIG), hematopoietic progenitor factors (SCGF-β), and factors that participate in angiogenesis/fibrosis (HGF), nociception (SCF, IFN-α2), and inflammation (IL-6, IL-10, IL-18, TRAIL). SCGF-β is a cytokine for primitive hematopoietic progenitor cells. Its role in pain or disc degeneration is unknown, although recently it has been associated with neuroinflammatory conditions, such as spinal cord injury [12]. To our knowledge, this is the first study to demonstrate that changes in systemic levels of SCGF-β are positively correlated with changes in pain levels in patients with low back pain.
We observed additional significant correlations between changes in pain NRS and biochemical mediators post-treatment. While pain NRS is a commonly used clinical tool, the NRS is subjective and evaluates only one component of the pain experience, pain intensity, and therefore does not capture the complexity and idiosyncratic nature of the pain experience or improvements due to symptom fluctuations. We observed that changes in systemic biochemical mediators in patients with LBP correlate with change in NRS after a local epidural space steroid delivery. Of the factors identified, changes in IFN-α2 and SCF were correlated with changes in pain NRS independent of diagnosis. Stem cell factor (SCF) is a neurotrophic factor for sensory neurons [13]. SCF is a ligand of the c-kit receptor that is expressed in dorsal root ganglia (DRG) neurons and in the superficial layer of the spinal cord [14, 15]. Activation of c-kit receptor by SCF in DRGs induces neurite outgrowth and supports the survival of the c-kit-expressing neurons. Intrathecal injection of SCF induces mechanical allodynia in mice [16], and SCF activation of c-kit receptor controls the transduction properties of sensory neurons [17]. Our finding that decreases in SCF levels are strongly correlated with decrease in pain post-treatment is consistent with the known role of SCF in nociception. Interestingly, other factors that promote nerve growth and pain, such as NGF, were not significantly modulated post-treatment with ESI or with diagnosis.
IFN-α is a family of proteins with immunomodulatory properties and exerts effects on the neuroendocrine, CNS, and immune system [18, 19]. IFN-α has a significant analgesic action in the CNS [18], where in preclinical studies, the analgesic effects of IFN-α were found to be related to opioid receptor binding [20–22]. In the current study, we observed that systemic levels of IFN-α2 decreased significantly with decreasing pain, an effect that is different from the anti-nociceptive effects of IFN-α is in the brain. Further studies on the effects of IFN-α2 in the epidural space and in response to steroid treatment are warranted to further elucidate its role in spinal pain mechanisms. Interestingly, the immunoreactivity of another interferon (IFN-γ) was elevated in epidural lavage of degenerative discs and absent in asymptomatic control disc lavage [23]. IFN-γ immunoreactivity in epidural lavage is also highly correlated with reported reduction in pain at 3 months after ESI [24], consistent with this study’s observation regarding the relationship between systemic IFN-α2 and pain post-treatment.
Some of the mediators correlated with changes in pain levels in all subjects are known to participate in pain or disc aetiology (e.g. MCP-1 and IL-6) [25, 26]. Human disc tissue is capable of spontaneously producing the chemokine MCP-1 that acts as a chemotactic factor for macrophages and may explain the ingrowth of granulation tissue seen in DH and resorption [27]. Local production of MCP-1 in vivo and in vitro by disc cells from the annulus fibrosis suggests that annulus cells mediate physiological immune-related processes during disc degeneration by both autocrine and paracrine signalling [28]. MCP-1 is also elevated in the blood at the chronic stage of complex regional pain syndrome [29] and has been proposed as a candidate biomarker that can distinguish between mechanical and infectious causes of back pain [30]. A correlation in changes of IL-6 levels and pain levels represents the complex role of this cytokine in disc diseases and pain. IL-6 can be spontaneously produced in vitro by human DH tissue [31], and IL-6 stimulation of human NP cells inhibits matrix gene expression and synthesis [32]. In preclinical models, IL-6 increases production of the pro-inflammatory cytokine, TNF-α, by neurons in the DRG, and sensitizes DRG neurons to painful stimuli [33, 34]. In our prior clinical study, we observed higher levels of IL-6 in subjects with LBP relative to controls [35]. Subjects with chronic lumbar radicular pain have also been shown to have persistent increases in systemic IL-6 [36]. Elevation of IL-6 levels at presentation has been associated with worse outcomes in recovery from DH at 1-year follow-up [37]. Our current finding that improvement in pain is correlated with decreases in IL-6 levels further confirms its role in pain mechanisms. We did not evaluate changes in neuropeptide Y, a factor recently found to be associated with improvement in pain in elderly individuals with isolated back pain without radiculopathy [38].
Biochemical profiles of subjects varied by diagnosis; elevated levels of factors that participate in inflammation (IL-2, IL-3, IL-8, TNF-β), angiogenesis/fibrosis (HGF), nociception (IFN-α2, LIF), and chemokines (MCP-3) were observed in subjects with SS or DDD (Other Dx) compared to DH subjects. In Other Dx cohort, elevated systemic cytokine levels may be due to the greater incidence of chronic pain relative to DH cohort (83 vs. 50 %, respectively). Elevated cytokine levels in Other Dx are consistent with our prior study that examined cytokine levels in ~ 100 subjects with LBP relative to control subjects [35]. We previously observed that levels of IL-6 and TNF-α were higher in Other Dx relative to DH or control subjects [35]. The current study extends the scope of profiling systemic biochemical factors in subjects with Other Dx versus DH. Our current findings of elevated levels of LIF and TNF-β in Other Dx subjects reinforce the well-known roles of the IL-6 and TNF cytokine families in disc disease and back pain, respectively.
The biochemical response to treatment also varied based on diagnoses. Levels of IL-17 and VEGF decreased in DH subjects post-treatment, whereas IL-2Rα, IL-3, and SCGF-β levels decreased in subjects with Other Dx. In DH, IL-17 has been proposed as a potential player in the pathomechanism of degeneration, with a potentially greater contribution from infiltrating inflammatory cells than disc cells [39–41]. Herniation severity may also modulate the relative contribution of IL-17 in DH, with greater IL-17 immunoreactive cells found in ruptured DH specimens than specimens contained within the disc boundary [42]. We did not evaluate changes in mediator levels based on severity of herniation, though our findings of decreased IL-17 levels in DH subjects post-ESI treatment supports the evidence for its potential role in the pathomechanism. The known interactions between the IVD and immune system in DH may promote recovery (i.e. decrease in levels) of inflammatory mediators post-ESI treatment. Biochemical responses to treatment may be related to the predisposition of patients with Other Dx, especially spinal stenosis, to generally poorer outcomes with respect to ESI treatment [43] and to higher likelihood of disease progression [44].
In order to determine whether the changes we observed in mediators levels from pre- to post-treatment were related to pain, we performed further analyses to assess potential correlations between change in pain scores and change in mediator levels. The factors significantly correlated with changes in pain (Table 3) did not overlap considerably with the factors that changed on average from pre- to post-treatment within diagnosis cohorts. For example, IL-3 levels were significantly elevated at pre-treatment in Other Dx group, and IL-3 levels significantly decreased post-treatment, yet IL-3 levels were not correlated with pain response in Other Dx cohort (ρ = −0.119, p = 0.82). We postulate that mediator-level changes observed in cohort averages (Fig. 4) may be due to changes in disease condition, response to steroids or epidural intervention, patient demographic factors, or a combination of all of these variables. In addition, changes in activity levels of subjects due to pain relief may add a further level of complexity in interpreting overall changes in systemic mediators.
In an effort to identify potential predictors of pain response to treatment, we examined the changes in mediators with change in pain separately in the DH and Other Dx cohorts. Each cohort was associated with a unique profile of factors that were significantly correlated with change in pain. Loss of pain in Other Dx was correlated with decreases in chemokines (MCP-1, MIG) and factors that participate in mechanisms of angiogenesis (HGF, VEGF), inflammation (IL-1β, IL-1ra, IL-9, IL-12, TRAIL), and nociception (SCF, IFN-α2). Changes in HGF levels were uniquely correlated with change in pain in Other Dx, in addition to being significantly higher than in DH at pre-treatment. HGF is an angiogenic factor that was recently found to slow down the process of intervertebral disc degeneration by stimulating an anti-fibrosis process in degenerative discs [45]. Decreases in nerve degeneration and scar tissue formation around the sciatic nerve in a constriction injury model have also been associated with HGF and VEGF [46]. Interestingly, VEGF, another angiogenic factor, was also significantly correlated with change in pain in Other Dx group. HGF has known anti-inflammatory effects [47] and has been found to mediate anti-inflammatory effects of platelet-rich plasma (PRP) [48, 49]. Gene therapy with HGF has also been found to provide long-term symptomatic relief and quality of life improvements in patients with painful diabetic peripheral neuropathy [50]. Our findings suggest that elevated levels of HGF in Other Dx may be due to disc angiogenic changes known to participate in the degenerative cascade [51].
Systemic inflammation is accompanied by changes in pain perception. We observed significant correlations between change in pain and levels of the pluripotent pro-inflammatory cytokine IL-1β, and the receptor antagonist, IL-1ra, in Other Dx. Elevations of IL-1β in cerebrospinal fluid have been correlated with pain in a variety of chronic painful conditions [52, 53], and locally in osteoarthritis [54]. IL-1ra is a natural inhibitor of the pro-inflammatory effects of IL-1β. Decreased levels of IL-1ra have been observed in patients with pain due to a variety of diseases, including in local sites of inflammation in patients with complex regional pain syndrome [55] and in the cerebrospinal fluid of patients with rheumatoid arthritis [56]. Conversely, systemic elevations of IL-1ra were observed with hyperalgesia in humans with induced systemic inflammation [57]. These alterations suggest that these factors have a direct influence on the perception and regulation of pain both in the tissue and in the nervous system. The correlation between systemic levels of both factors reinforces the inflammatory mechanism underlying pain, and the tightly coordinated regulation between IL-1β and IL-1ra [58, 59].
In DH subjects, improvement in pain was correlated with decreases in factors that participate in hematopoiesis (SCGF-β, GM-CSF), nociception (SCF, IFN-α2), and inflammation (IL-6, IL-10, IL-18, IL-2Rα, IL-12p40). IL-10 is well documented to act as an anti-inflammatory cytokine, dampening the expression of pro-inflammatory cytokines and blocking NF-κB activity. IL-10 promoter polymorphisms are associated with increased susceptibility to lumbar disc degeneration [60]. GM-CSF, a hematopoietic growth factor, also acts on mature myeloid cells and acts as a pro-inflammatory cytokine [61]. Depletion of GM-CSF can have profound effects on disease severity and progression in inflammatory arthritis models [62]. GM-CSF is a key to the development of osteoarthritis associated pain, and its neutralization can abolish arthritic pain [63]. Our findings on correlations between pain levels and changes in IL-10 and GM-CSF are consistent with the factors’ respective roles as anti-inflammatory and anti-nociceptive factors.
In summary, our pilot study findings indicate that treatment of LBP subjects with local ESI is associated with improvement in pain and coordinated decreases in systemic biochemical factors associated with nociception, inflammation, angiogenesis, chemokines, and hematopoietic progenitor factors. Factor profiles varied by diagnosis of LBP, and consequently, changes in systemic biochemical factors were uniquely correlated in diagnosis cohorts. Two factors (SCF and IFN-α2) known to participate in nociception were correlated with pain changes independent of diagnosis, while others were unique to subjects with LBP due to DH or Other Dx. Subjects with DH demonstrated a profile that uniquely implicated hematopoiesis factors in pain response. Subjects with Other Dx demonstrated a profile that uniquely implicated chemokines and angiogenic factors in pain response.
Some limitations of this pilot study include the relatively small number of subjects especially in the Other Dx cohort, and an unequal gender distribution, although similar gender distributions were observed in both diagnosis cohorts. We recognize that multiple testing increases the rate of false positives, and therefore, results should be cautiously interpreted. However, this pilot study was designed to be an exploratory, hypothesis-generating endeavour. Therefore, we believe that the precise adjustment of p values is not practical in the context of the goal of this exploratory study. Future hypothesis-based studies with larger subject cohorts are warranted to confirm the findings of this pilot study. We did not evaluate the relationship of disease severity on MRI with biochemical factors, although our prior study indicated that MRI grade is neither correlated with nor predictive of biochemical levels [35]. Similarly, no significant correlation between the Pfirrmann grade, pain scores, and biochemical markers was observed in other studies that evaluated the suitability of a cartilage matrix degradation product as a predictor of disease severity [64]. Our findings suggest that some of the variation in clinical outcomes observed in studies or trials of heterogeneous populations of LBP subjects may be partly due to heterogeneous biochemical profiles that may differ by diagnosis. Refinement of inclusion criterion by diagnosis is warranted in future biomarker studies of LBP. In addition, future studies will examine longitudinal changes in biochemical factors in response to ESI in a larger cohort of LBP subjects.
Acknowledgments
The authors thank Dr. Bruce Volpe for his helpful discussion and Angelos Papatheodorou for excellent technical assistance. This study was supported in part by New York State Department of Health Empire Clinical Research Program (ECRIP), NSF CAREER Award 1151605, and NIH R41AG050021.
Nadeen O. Chahine

References
- 1.Manchikanti L, et al. Epidemiology of low back pain in adults. Neuromodulation. 2014;17(Suppl 2):3–10. doi: 10.1111/ner.12018. [DOI] [PubMed] [Google Scholar]
- 2.Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976) 1987;12(3):264–268. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Croft PR, et al. Outcome of low back pain in general practice: a prospective study. BMJ. 1998;316(7141):1356–1359. doi: 10.1136/bmj.316.7141.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22(2):263–271. [PubMed] [Google Scholar]
- 5.Arden NK, et al. A multicentre randomized controlled trial of epidural corticosteroid injections for sciatica: the WEST study. Rheumatology (Oxford) 2005;44(11):1399–1406. doi: 10.1093/rheumatology/kei028. [DOI] [PubMed] [Google Scholar]
- 6.Pinto RZ, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med. 2012;157(12):865–877. doi: 10.7326/0003-4819-157-12-201212180-00564. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SP, et al. Epidural steroid injections compared with gabapentin for lumbosacral radicular pain: multicenter randomized double blind comparative efficacy study. BMJ. 2015;350:h1748. doi: 10.1136/bmj.h1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLain RF, Kapural L, Mekhail NA. Epidural steroid therapy for back and leg pain: mechanisms of action and efficacy. Spine J. 2005;5(2):191–201. doi: 10.1016/j.spinee.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 9.Cannon DT, Aprill CN (2000), Lumbosacral epidural steroid injections. Arch Phys Med Rehabil, 81(3 Suppl 1): S87–S98; quiz S99–S100. [PubMed]
- 10.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/S0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 11.Sainani KL. The problem of multiple testing. PM R. 2009;1(12):1098–1103. doi: 10.1016/j.pmrj.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Stein A, et al. Pilot study: elevated circulating levels of the proinflammatory cytokine macrophage migration inhibitory factor in patients with chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94(8):1498–1507. doi: 10.1016/j.apmr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Carnahan JF, Patel DR, Miller JA. Stem cell factor is a neurotrophic factor for neural crest-derived chick sensory neurons. J Neurosci. 1994;14(3 Pt 2):1433–1440. doi: 10.1523/JNEUROSCI.14-03-01433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata T, et al. Stem cell factor induces outgrowth of c-kit-positive neurites and supports the survival of c-kit-positive neurons in dorsal root ganglia of mouse embryos. Development. 1993;119(1):49–56. doi: 10.1242/dev.119.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Hirata T, et al. Characterization of c-kit-positive neurons in the dorsal root ganglion of mouse. Brain Res Dev Brain Res. 1995;85(2):201–211. doi: 10.1016/0165-3806(94)00205-E. [DOI] [PubMed] [Google Scholar]
- 16.Takagi K, et al. Involvement of stem cell factor and its receptor tyrosine kinase c-kit in pain regulation. Neuroscience. 2008;153(4):1278–1288. doi: 10.1016/j.neuroscience.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 17.Milenkovic N, et al. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron. 2007;56(5):893–906. doi: 10.1016/j.neuron.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Vazquez C, Prieto-Gomez B, Dafny N. Interferon modulates central nervous system function. Brain Res. 2012;1442:76–89. doi: 10.1016/j.brainres.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Besedovsky HO, del Rey A. Introduction: immune-neuroendocrine network. Front Horm Res. 2002;29:1–14. doi: 10.1159/000061055. [DOI] [PubMed] [Google Scholar]
- 20.Jiang CL, et al. Analgesic effect of interferon-alpha via mu opioid receptor in the rat. Neurochem Int. 2000;36(3):193–196. doi: 10.1016/S0197-0186(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang YX, et al. Distinct domains of IFNalpha mediate immune and analgesic effects respectively. J Neuroimmunol. 2000;108(1–2):64–67. doi: 10.1016/S0165-5728(00)00271-X. [DOI] [PubMed] [Google Scholar]
- 22.Wang JY, et al. Mu- but not delta- and kappa-opioid receptor mediates the nucleus submedius interferon-alpha-evoked antinociception in the rat. Neurosci Lett. 2006;397(3):254–258. doi: 10.1016/j.neulet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 23.Cuellar JM, et al. Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J. 2010;10(3):212–218. doi: 10.1016/j.spinee.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Scuderi GJ, et al. Epidural interferon gamma-immunoreactivity: a biomarker for lumbar nerve root irritation. Spine (Phila Pa 1976) 2009;34(21):2311–2317. doi: 10.1097/BRS.0b013e3181af06b6. [DOI] [PubMed] [Google Scholar]
- 25.Thacker MA, et al. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105(3):838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 26.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke JG, et al. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human lumbar intervertebral disc. Spine (Phila Pa 1976) 2002;27(13):1402–1407. doi: 10.1097/00007632-200207010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Gruber HE, et al. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. Exp Mol Pathol. 2015;98(1):102–105. doi: 10.1016/j.yexmp.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Parkitny L, et al. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology. 2013;80(1):106–117. doi: 10.1212/WNL.0b013e31827b1aa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Sanchez C, et al. Serum monocyte chemotactic protein-1 concentrations distinguish patients with ankylosing spondylitis from patients with mechanical low back pain. J Spinal Disord Tech. 2011;24(3):202–207. doi: 10.1097/BSD.0b013e3181e15cc8. [DOI] [PubMed] [Google Scholar]
- 31.Kang JD, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1996;21(3):271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Studer RK, et al. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-alpha. Spine (Phila Pa 1976) 2011;36(8):593–599. doi: 10.1097/BRS.0b013e3181da38d5. [DOI] [PubMed] [Google Scholar]
- 33.Murata Y, et al. Local application of interleukin-6 to the dorsal root ganglion induces tumor necrosis factor-alpha in the dorsal root ganglion and results in apoptosis of the dorsal root ganglion cells. Spine (Phila Pa 1976) 2011;36(12):926–932. doi: 10.1097/BRS.0b013e3181e7f4a9. [DOI] [PubMed] [Google Scholar]
- 34.Wei XH, et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp Neurol. 2013;241:159–168. doi: 10.1016/j.expneurol.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Weber K, et al. (2015) Serum levels of the pro-Inflammatory cytokines IL-6 and TNF-alpha vary based on diagnoses in individuals with low back pain. Arthritis Res Ther (in review). [DOI] [PMC free article] [PubMed]
- 36.Pedersen LM, et al. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav Immun. 2015;46:132–136. doi: 10.1016/j.bbi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Schistad EI, et al. Association between baseline IL-6 and 1-year recovery in lumbar radicular pain. Eur J Pain. 2014;18(10):1394–1401. doi: 10.1002/j.1532-2149.2014.502.x. [DOI] [PubMed] [Google Scholar]
- 38.Sowa GA, et al. Associations between serum biomarkers and pain and pain-related function in older adults with low back pain: a pilot study. J Am Geriatr Soc. 2014;62(11):2047–2055. doi: 10.1111/jgs.13102. [DOI] [PubMed] [Google Scholar]
- 39.Shamji MF, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabr MA, et al. Interleukin-17 synergizes with IFNgamma or TNFalpha to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J Orthop Res. 2011;29(1):1–7. doi: 10.1002/jor.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruber HE, et al. Increased IL-17 expression in degenerated human discs and increased production in cultured annulus cells exposed to IL-1ss and TNF-alpha. Biotech Histochem. 2013;88(6):302–310. doi: 10.3109/10520295.2013.783235. [DOI] [PubMed] [Google Scholar]
- 42.Tian P, et al. Role of interleukin-17 in chondrocytes of herniated intervertebral lumbar discs. Exp Ther Med. 2015;10(1):81–87. doi: 10.3892/etm.2015.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedly JL, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014;371(1):11–21. doi: 10.1056/NEJMoa1313265. [DOI] [PubMed] [Google Scholar]
- 44.Johnsson KE, Rosen I, Uden A. The natural course of lumbar spinal stenosis. Clin Orthop Relat Res. 1992;279:82–86. [PubMed] [Google Scholar]
- 45.Zou F, et al. Efficacy of intradiscal hepatocyte growth factor injection for the treatment of intervertebral disc degeneration. Mol Med Rep. 2013;8(1):118–122. doi: 10.3892/mmr.2013.1450. [DOI] [PubMed] [Google Scholar]
- 46.Murakami K, et al. Vein wrapping for chronic nerve constriction injury in a rat model: study showing increases in VEGF and HGF production and prevention of pain-associated behaviors and nerve damage. J Bone Joint Surg Am. 2014;96(10):859–867. doi: 10.2106/JBJS.L.01790. [DOI] [PubMed] [Google Scholar]
- 47.Gong R, et al. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol. 2004;15(11):2868–2881. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- 48.Bendinelli P, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-kappaB inhibition via HGF. J Cell Physiol. 2010;225(3):757–766. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, et al. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS ONE. 2013;8(6):e67303. doi: 10.1371/journal.pone.0067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ajroud-Driss S, et al. Phase 1/2 open-label dose-escalation study of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with painful diabetic peripheral neuropathy. Mol Ther. 2013;21(6):1279–1286. doi: 10.1038/mt.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karamouzian S, et al. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine (Phila Pa 1976) 2010;35(8):881–886. doi: 10.1097/BRS.0b013e3181b9c986. [DOI] [PubMed] [Google Scholar]
- 52.Backonja MM, et al. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195(1–2):157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Kosek E, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain–interleukin-8 in fibromyalgia and interleukin-1 beta in rheumatoid arthritis. J Neuroimmunol. 2015;280:49–55. doi: 10.1016/j.jneuroim.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee AS, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenz M, et al. Local cytokine changes in complex regional pain syndrome type I (CRPS I) resolve after 6 months. Pain. 2013;154(10):2142–2149. doi: 10.1016/j.pain.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 56.Lampa J, et al. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci USA. 2012;109(31):12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Goeij M, et al. Systemic inflammation decreases pain threshold in humans in vivo. PLoS ONE. 2013;8(12):e84159. doi: 10.1371/journal.pone.0084159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28(8):2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 59.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–340. doi: 10.1016/S1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 60.Lin WP, et al. Interleukin-10 promoter polymorphisms associated with susceptibility to lumbar disc degeneration in a Chinese cohort. Genet Mol Res. 2011;10(3):1719–1727. doi: 10.4238/vol10-3gmr1283. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 62.Cook AD, et al. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3(5):293–298. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cook AD, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14(5):R199. doi: 10.1186/ar4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuellar JM, et al. (2013) Does a fibronectin and aggrecan complex play a role in painful vertebral disks? PM R, 5(4): 297–302; quiz 302. [DOI] [PubMed]