Abstract
Purpose
The prospective pilot study was designed to evaluate the preventive effects of amino-acid-rich elemental diet (ED), Elental®, on chemotherapy-induced oral mucositis in patients with colorectal cancer. The factors influencing its efficacy are also investigated.
Methods
A total of 22 eligible patients with colorectal cancer experiencing grade 1–3 oral mucositis during treatment with fluorouracil-based chemotherapy entered the current study. Their average age was 67 years. There were 10 male and 12 female. The PS was 0 in the majority of patients. Patients received two courses of the same chemotherapy regimen and Elental® concurrently after recovery to grade 0 or 1 oral mucositis.
Results
FOLFOX6 + bevacizumab in 8 patients, FOLFIRI + bevacizumab in 8 patients, FOLFIRI + panitumumab in 1 patient, FOLFIRI in 1 patient, XELOX + bevacizumab in 2 patients, and S-1 + cetuximab in 2 patients were used as first-line (16 cases) or as second-line (6 cases) chemotherapy. Dose reduction of 5-fluorouracil (5-FU) or oral fluoropyrimidine was performed in the 2 patients achieving grade 3 oral mucositis and in the 3 patients achieving grade 2 oral mucositis. The maximum grade of oral mucositis decreased in 18 of the 22 patients during the first treatment course with Elental® (p = 0.0002) and in 20 of the 22 patients in the second course (p < 0.0001). Multivariate analyses found that the dose reduction in 5-FU or oral fluoropyrimidine, ED intake, and the prior administration of ED were each a significant factor for the preventive efficacy on oral mucositis.
Conclusion
The amino-acid-rich elemental diet Elental® may be useful as a countermeasure for 5-FU-based chemotherapy-induced oral mucositis in patients with colorectal cancer.
Keywords: Chemotherapy, Oral mucositis, Elemental diet (ED), Nourishment academic intervention, Colorectal cancer, Amino acid
Introduction
Mucositis is a common adverse effect of chemotherapy and/or radiotherapy. The oral mucositis that is the mouth mucous membrane injury causes anguish to the patient regardless of the severity of symptoms and induces a decrease in quality of life (QOL) and in desire for continuation of the treatment. After oral mucositis once occurs, adjustments must be made to the dose of the anticancer drug and to the administration schedule, as well as requiring new preventive care to the mouth [1]. Therefore, to continue chemotherapy as far as possible without decreasing the dose, some effective oral mucositis preventive measures are needed.
The mechanism of chemotherapy-induced mucositis has been described as occurring stepwise through a sequence of events [2]. First, chemotherapy causes direct DNA damage resulting in the death of basal epithelial cells and the generation of reactive oxygen species (ROS) that damage connective tissue, DNA, and cell membranes. Second, this cell damage causes the activation of several transcription factors including nuclear factor-kappa B (NF-κB), wnt, and p53, and their molecular pathways. Third, these pathways are further amplified via positive feedback loops. Finally, cell death causes mucosal thinning, resulting in the development of clinical and symptomatic ulcerated mucositis. The generation of the oral mucositis appears at around 5 to 10 days after the initiation of chemotherapy in relation to the cell cycle of the mouth mucous membrane epithelial cells. After the appearance of the mucous membrane injury, 2 to 3 weeks are required to achieve recovery, since the usual regeneration cycle of the oral mucosa is around 7 to 14 days. The period until recovery, the frequency, and the severity of oral mucositis each depends on the kind and the dose of the anticancer drug, the treatment regimen, and the general condition of the patient.
The incidence of oral mucositis, induced by colorectal cancer chemotherapy such as FOLFOX and FOLFIRI including bolus and continued infusion of 5-fluorouracil (5-FU), and XELOX that uses oral 5-FU, is high at 25–40 % [3–5]. Glutamine is a natural amino acid which functions as a substrate for nucleotide synthesis in most dividing cells [6]. There have been several randomized studies to date on the use of glutamine in chemotherapy- and radiotherapy-induced mucositis with varying results [7, 8]. Another study, focused on the action of mucous protection of the glutamine [9] in addition to the countermeasures for oral intake trouble, suggests a prospective pilot study to evaluate preventive effects of the elemental diet (ED) Elental®, (one packing 80 g/300 kcal) which contains 1932 mg of l-glutamine, on oral mucositis caused by chemotherapy. Elental® is composed of various amino acids, very little fat, vitamins, trace elements, and a major energy source, dextrin. The composition of Elental® is detailed elsewhere [10]. In the present study, the preventive effects of the ED on oral mucositis and the factors influencing its efficacy are followed prospectively, and the significance of such nourishment intervention as a countermeasure is discussed.
Patients and methods
Eligibility criteria
A total of 23 metastatic colorectal cancer patients were registered prospectively after developing grade 1–3 oral mucositis between September 2008 and December 2010 during 5-FU-based chemotherapy involving mFOLFOX6, FOLFIRI, XELOX, or S-1 with or without biologics. Each of these eligible patients had histologically confirmed advanced or metastatic cancer. No patient had received prior radiotherapy or concurrent radiotherapy. The criteria for entry into our study included age older than 20 years, Eastern Cooperative Oncologic Group (ECOG) performance status ≤2, neutrophils ≥1500/mm3, platelets ≥750,000/mm3, creatinine ≤1.5 times the upper normal limit or creatinine clearance of more than 60 mL/min, and liver function tests (serum bilirubin level ≤1.5 times the upper normal limit and AST/ALT <3 times the upper normal limit). All patients were required to be free of any signs of systemic infection and must not have taken antibiotics. Each patient gave written informed consent before entering the study. The study was performed in accordance with the Declaration of Helsinki.
Administration of Elental® and anticancer drugs
Two courses of the same chemotherapy that induced the oral mucositis were performed with or without dose reduction in 5-FU or oral fluoropyrimidine. The chemotherapy was then restarted according to the standard criteria of clinical trials such as neutrophil depletions of grade 1 or less and decrease in platelet of grade 1 or less as the hematological toxicity, and oral mucositis of grade 1 or less, peripheral neurological symptoms of grade 1 or less, protein urea and hemorrhage of grade 1 or less, and diarrhea of grade 1 or less as the non-hematological toxicity. In those patients experiencing grade 3 oral mucositis and in those patients experiencing grade 2 oral mucositis which persisted even after 7 days of next chemotherapy delay, the chemotherapy was restarted with a dose reduction in 5-FU/oral fluoropyrimidine after all oral mucositis recovered to grade 1 or less. The ED Elental® (80 g/300 kcal or more per day) was given perorally in addition to normal oral ingestion, together with chemotherapy in each course lasting 2 to 3 weeks (on days 1–14 or days 1–21). To help the patient, the ED was flavored and made into a jelly. Patients recorded the daily Elental® intake and the chief physician collected the data before the next course of chemotherapy.
Oral mucositis assessment
Chemotherapy-induced oral mucositis was graded basically according to version 3.0 of the Common Terminology Criteria for Adverse Events (CTCAE v3.0). The CTCAE v3.0 grades were defined as follows: grade 0, no mucositis; grade 1, minimal symptoms such as pain/throat pain and erythema of the mucosa; grade 2, obviously symptomatic but can eat, patchy ulceration, or pseudomembranes; grade 3, obviously symptomatic and cannot eat, confluent ulceration, or pseudomembranes, and bleeding with minor trauma; and grade 4, severely symptomatic in association with life-threatening consequences, tissue necrosis, and/or with significant spontaneous bleeding. Patients daily recorded mouth and throat soreness and activity score that was modified from the Oral Mucositis Daily Questionnaire (OMDQ) (questions 2 and 3) [11]. Finally, oral mucositis was assessed by independent physician based on the patient’s self-assessment of the mouth and throat soreness and activity score, the pretreatment patient interview by pharmacologists and chief physician’s decision every week on out-patient basis for the study period. The maximum grade of oral mucositis at individual chemotherapy course were recorded by the independent physician.
Endpoints and statistical methods
The ED intake in each course, the presence and grade of the oral mucositis, and the presence and grade of any adverse effect were monitored. Multivariate analysis for the factors (age, gender, PS 0 vs. 1–2, usage of antiepidermal growth factor receptor (EGFR) antibodies, pretreatment albumin value, pretreatment lymphocyte, number of anticancer drugs 2 vs. 3, regimen duration 2 vs. 3 weeks, treatment phase: first line vs. second line, improvement in neutrophil depletion, ED intake, prior treatment with ED, dose reduction in 5-FU or oral fluoropyrimidine) related to the efficacy of the ED on oral mucositis was performed using logistic regression analysis, and any differences were investigated using the χ2 test and Student’s t test. Differences between groups were evaluated by the χ2 test or G-test for categorical variables, and the Mann–Whitney U test was used for continuous variables. All analyses were performed using SPSS software (Version 18.0; SPSS, Inc., Chicago, IL), and the significance level was set at p < 0.05.
Results
Patient characteristics
Among the 23 patients, one patient who had developed grade 3 oral mucositis in a prior course could not receive the chemotherapy and the ED. Table 1 summarizes the characteristics of the other 22 patients who completed this study protocol. There were 10 male and 12 female, and their average age was 67 years, ranging from 44 to 84 years. The oral mucositis that had developed in the prior course was grade 3 in 2 patients, grade 2 in another 15 patients, and grade 1 in the other 5 patients.
Table 1.
Patient outline
| Case number | Prior grade of oral mucositis | Regimen | Regimen duration/q (in weeks) | Treatment phase | Gender | Age | PS | Dose reduction |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | FOLFIRI + Bmab | 2 | First line | Male | 68 | 0 | 5-FU |
| 2 | 3 | FOLFIRI + Bmab | 2 | First line | Female | 75 | 2 | 5-FU |
| 3 | 2 | FOLFOX + Bmab | 2 | First line | Male | 59 | 0 | |
| 4 | 2 | FOLFOX + Bmab | 2 | First line | Male | 73 | 1 | |
| 5 | 2 | FOLFOX + Bmab | 2 | First line | Female | 82 | 1 | 5-FU |
| 6 | 2 | FOLFOX + Bmab | 2 | First line | Female | 84 | 1 | |
| 7 | 2 | FOLFOX + Bmab | 2 | Second line | Female | 67 | 0 | |
| 8 | 2 | TS-1 + Cmab | 3 | Second line | Female | 59 | 1 | |
| 9 | 2 | TS-1 + Cmab | 3 | First line | Male | 76 | 0 | S-1 |
| 10 | 2 | FOLFOX + Bmab | 2 | First line | Male | 63 | 0 | |
| 11 | 2 | FOLFIRI + Bmab | 2 | First line | Female | 73 | 1 | |
| 12 | 2 | FOLFIRI + Bmab | 2 | First line | Female | 73 | 1 | |
| 13 | 2 | FOLFIRI + Bmab | 2 | Second line | Female | 63 | 0 | |
| 14 | 2 | XELOX + Bmab | 3 | First line | Male | 74 | 1 | Capecitabine |
| 15 | 2 | XELOX + Bmab | 3 | First line | Female | 75 | 1 | |
| 16 | 2 | FOLFIRI + Bmab | 2 | First line | Male | 66 | 0 | |
| 17 | 2 | FOLFIRI + Bmab | 2 | Second line | Female | 76 | 0 | |
| 18 | 1 | FOLFOX + Bmab | 2 | First line | Female | 62 | 0 | |
| 19 | 1 | FOLFIRI + Bmab | 2 | First line | Male | 61 | 0 | |
| 20 | 1 | FOLFOX + Bmab | 2 | First line | Male | 44 | 0 | |
| 21 | 1 | FOLFIRI | 2 | First line | Male | 66 | 0 | |
| 22 | 1 | FOLFIRI + Bmab | 2 | Second line | Female | 59 | 0 |
mFOLFOX6 + bevacizumab in 8 patient, FOLFIRI + bevacizumab in 8 patients, FOLFIRI + panitumumab in 1 patient, FOLFIRI in 1 patient, XELOX + bevacizumab in 2 patients, and S-1 + cetuximab in 2 patients were used as the first-line (16 cases) or second-line (6 cases) chemotherapy. Dose reduction in 5-FU was performed in 2 patients experiencing grade 3 oral mucositis in the prior chemotherapy using the mFOLFOX6 + bevacizumab regimen, and in 1 patient experiencing grade 2 oral mucositis in the prior chemotherapy using the FOLFIRI + bevacizumab regimen. Also, dose reduction in S-1 was performed in 1 patient experiencing grade 2 oral mucositis in the prior chemotherapy using the S-1 + cetuximab regimen, and in capecitabine in 1 patient experiencing grade 2 oral mucositis in the prior chemotherapy using the XELOX + bevacizumab regimen (Table 1).
The average ED intake in the first course was 600 g, ranging from 240 to 1120 g, and was 557 g in the second course, ranging from 240 to 1120 g (Table 2).
Table 2.
The efficacy on oral mucositis and neutropenia by treatment with the ED
| Case number | Oral mucositis (prior treatment) | Oral mucositis (first treatment) | Oral mucositis (second treatment) | Efficacy (first treatment) | Efficacy (second treatment) | Neutropenia (prior treatment) | Neutropenia (first treatment) | Neutropenia (second treatment) | ED intake (first treatment, in g) | ED intake (second treatment, in g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 1 | 0 | Excellent | Excellent | 2 | 1 | 0 | 720 | 560 |
| 2 | 3 | 2 | 1 | Good | Excellent | 3 | 1 | 1 | 560 | 480 |
| 3 | 2 | 0 | 0 | Excellent | Excellent | 2 | 0 | 0 | 720 | 480 |
| 4 | 2 | 2 | 1 | No change/poor | Good | 2 | 2 | 1 | 240 | 240 |
| 5 | 2 | 1 | 0 | Good | Excellent | 2 | 1 | 0 | 480 | 560 |
| 6 | 2 | 1 | 1 | Good | Good | 3 | 2 | 1 | 480 | 400 |
| 7 | 2 | 1 | 0 | Good | Excellent | 0 | 0 | 0 | 800 | 560 |
| 8 | 2 | 1 | 1 | Good | Good | 0 | 0 | 0 | 560 | 420 |
| 9 | 2 | 1 | 1 | Good | Good | 0 | 0 | 0 | 480 | 480 |
| 10 | 2 | 1 | 0 | Good | Excellent | 0 | 0 | 0 | 560 | 480 |
| 11 | 2 | 1 | 0 | Good | Excellent | 0 | 0 | 1 | 560 | 560 |
| 12 | 2 | 0 | 0 | Excellent | Excellent | 1 | 0 | 0 | 800 | 800 |
| 13 | 2 | 1 | 1 | Good | Good | 1 | 0 | 0 | 560 | 480 |
| 14 | 2 | 0 | 0 | Excellent | Excellent | 0 | 0 | 0 | 720 | 640 |
| 15 | 2 | 2 | 1 | No change/poor | Good | 0 | 2 | 1 | 1120 | 1120 |
| 16 | 2 | 1 | 0 | Good | Excellent | 1 | 2 | 0 | 880 | 800 |
| 17 | 2 | 1 | 3 | Good | No change/poor | 0 | 0 | 0 | 880 | 960 |
| 18 | 1 | 0 | 0 | Good | Good | 1 | 0 | 0 | 400 | 560 |
| 19 | 1 | 0 | 0 | Good | Good | 0 | 0 | 0 | 240 | 240 |
| 20 | 1 | 1 | 0 | No change/poor | Good | 0 | 0 | 0 | 320 | 320 |
| 21 | 1 | 1 | 1 | No change/poor | No change/poor | 0 | 0 | 0 | 400 | 640 |
| 22 | 1 | 0 | 0 | Good | Good | 1 | 0 | 0 | 720 | 480 |
The efficacy of the ED was categorized as “excellent” in those cases where the maximum grade of oral mucositis was reduced by two grades, as “good” where the maximum grade was reduced by one grade, and as “no change/poor” where there was no reduction in the maximum grade
Preventive effect on oral mucositis and neutrophil depletion
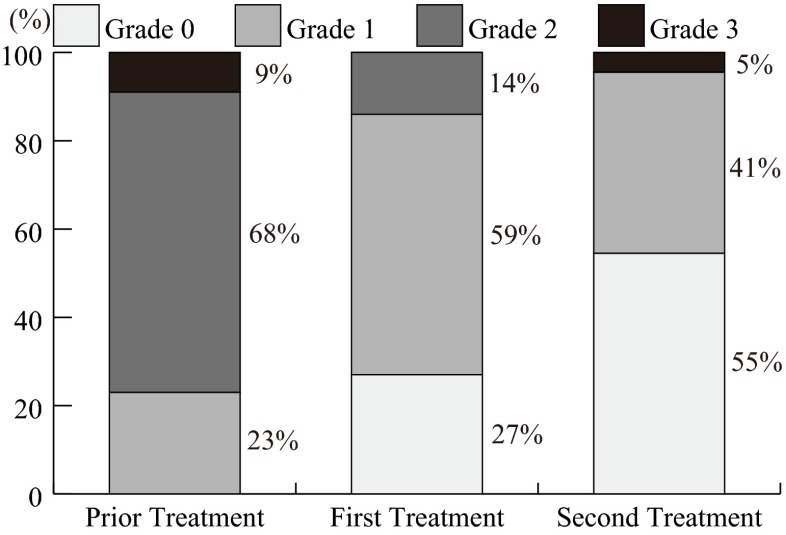
Table 2 shows the grades of oral mucositis and neutropenia before and after the ED treatment. The maximum grade of oral mucositis decreased in 18 of 22 patients during the first course and in 20 of 22 patients in the second course, compared to those in the prior chemotherapy course. The grade of oral mucositis was significantly reduced by the ED treatment (p = 0.0002, prior treatment vs. first treatment; p < 0.0001, prior treatment vs. second treatment) (Fig. 1). The efficacy of the ED was categorized as “excellent” in those cases where the maximum grade of oral mucositis was reduced by two grades or more, as “good” where the maximum grade was reduced by one grade, and as “no change/poor” where there was no reduction in the maximum grade. In those 18 patients who received chemotherapy of a 2-week cycle, we investigated the correlation, if any, between the efficacy of the ED (categories) and the ED intake. There was a significant difference in ED intake between the excellent vs. the no change/poor (747 ± 46 vs. 267 ± 46 g, p < 0.001) and between the good vs. the no change/poor (607 ± 172 vs. 267 ± 46 g, p < 0.006) in ED intake in the first course, and between the excellent vs. the good (600 ± 128 vs. 450 ± 128 g, p = 0.034) in ED intake in the second course, and between the excellent vs. the good (1290 ± 244 vs. 923 ± 239 g, p = 0.009) in total ED intake in the first plus second courses (Table 3).
Fig. 1.
Preventive effects by the ED on oral mucositis. The oral mucositis was significantly reduced (p = 0.004, prior treatment vs. first treatment; p < 0.001, prior treatment vs. second treatment) by the ED
Table 3.
The efficacy of ED on oral mucositis and the ED intake
| Efficacy | First treatment | Second treatment |
|---|---|---|
| Excellent | 3 cases/747 ± 46 g | 8 cases/600 ± 128 g (1290 g) |
| Good | 12 cases/607 ± 172 g | 8 cases/450 ± 128 g (923 g) |
| No change/poor | 3 cases/267 ± 46 g | 2 cases/600 ± 509 g (1160 g) |
(): total intake of ED (first plus second treatment courses). First treatment: p = 0.197 (excellent vs. good), p < 0.001 (excellent vs. no change/poor), p = 0.006 (good vs. no change/poor). Second treatment: p = 0.034 (excellent vs. good), p = 0.406 (good vs. no change/poor). First plus second treatments: p = 0.009 (excellent vs. good), p = 0.699 (excellent vs. no change/poor), p = 0.481 (good vs. no change/poor)
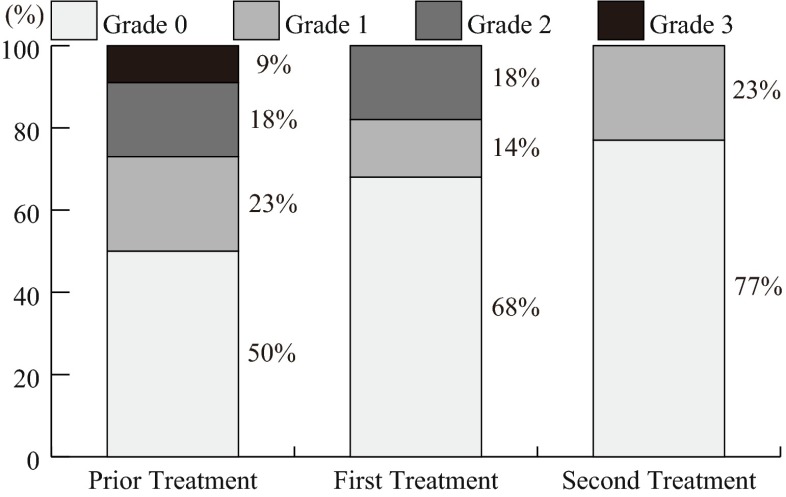
The severity of oral mucositis showed a tendency to increase with the severity of neutropenia, in the prior chemotherapy course (p = 0.083) (data not shown). The neutropenia was improved particularly by the second treatment with the ED (p < 0.327, prior treatment vs. first treatment; p = 0.038, prior treatment vs. second treatment) (Fig. 2).
Fig. 2.
Preventive effects by the ED on neutropenia. The neutropenia was significantly improved (p < 0.327, prior treatment vs. first treatment; p = 0.038, prior treatment vs. second treatment) by treatment with the ED
Logistic regression analysis for the factors relating to the efficacy by the ED on oral mucositis
Among the 44 courses of ED in the 22 patients, the efficacy was excellent in 14 courses, good in another 24 courses, and no change/poor in the other 6 course (Table 2). The logistic regression analysis revealed that prior administration of ED, the dose reduction in 5-FU or oral fluoropyrimidine, and the ED intake were each an independent significant factor statistically correlated to the preventive efficacy by the ED (the excellent) on the oral mucositis (Table 4).
Table 4.
Multivariate analysis for the factors related to the efficacy of the ED on oral mucositis
| Factor | p value | Hazard ratio | 95 % CI |
|---|---|---|---|
| Prior administration of ED | 0.023 | 99.423 | 1.879–5261 |
| Dose reduction of 5-FU/oral fluoropyrimidine | 0.034 | 1019.912 | 1.674–621,200 |
| ED intake | 0.039 | 1.015 | 1.001–1.030 |
| Regimen 2 weeks (vs. 3 weeks) | 0.051 | 5.886 | 0.986–3.418 |
| Male | 0.060 | 230.525 | 0.800–66,430 |
| Pretreatment albumin value | 0.094 | 75.445 | 0.482–11,820 |
Discussion
In the present pilot study, the findings indicated that the amino-acid-rich ED Elental® might reduce the incidence and severity of oral mucositis dose-dependently in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy. However, 5 of the 22 patients (23 %) received dose reductions of 5-FU or oral fluoropyrimidine in chemotherapy in the cycles after Elental® diet. This dose reduction itself would decrease the grade of oral mucositis and could be the reason for the significant difference. Our additional analysis only in those patients who received the same dose of chemotherapy before and after Elental® therapy confirmed the similar efficacy of Elental®. The maximum grade of oral mucositis decreased in 13 of 17 patients during the first course and in 15 of 17 patients in the second course, compared to those in the prior chemotherapy course. The grade of oral mucositis was significantly reduced by the ED treatment (p < 0.0001, prior treatment vs. first treatment; p < 0.0001, prior treatment vs. second treatment) (data not shown). The logistic regression multivariate analysis revealed that the pretreatment with the ED and the ED intake were important for prevention of oral mucositis by the ED as well as the dose reduction in fluorouracil. The results suggest a possible significance of the nourishment intervention for prevention of chemotherapy-induced oral mucositis.
It is known that cancer produces a state of glutamine deficiency [12], and many nutritional supplements are designed with cancer patients in mind to contain free l-glutamine, which is the preferred substrate for enterocytes within the GI tract. l-Glutamine encourages protein synthesis, enterocyte proliferation, and antiinflammatory effects—each of which plays a role in preventing mucositis [8, 13, 14]. In advanced colorectal cancer patients receiving 5-FU/LV chemotherapy, Choi et al. [15] reported the preventive efficacy of high-dose peroral glutamine (30 g/day) on oral mucositis. However in general, the systemic administration of l-glutamine alone is not recommended by the Clinical Practice Guidelines for Prevention and Treatment of Mucositis of the Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology (MASCC/ISOO) in 2007 [16]. In the present pilot study, we used a small dose of l-glutamine ranging from 5.8 to 27 g during the 14 or 21 days of chemotherapy. Therefore, the efficacy of the ED for preventing oral mucositis is thought to depend on general nutritional intervention involving a well-balanced amino acid mixture including l-glutamine, rather than on l-glutamine alone.
It is reported that the severity of the neutropenia has been associated with the incidence and severity of chemotherapy-induced oral mucositis [17]. Of interest, nourishment intervention using ED in the present study apparently controlled the neutrophil depletion, suggesting the potential of nourishment intervention as an adverse-event measure in cancer chemotherapy. It has been shown that anorexia promotes the generation of other adverse experiences [18], suggesting the importance of the nutritional intervention in cancer chemotherapy. Individual amino acids such as glutamine and arginine which are main constituents of the ED may prevent neutropenia via activation of cellular immunity [19]. However, treatment with glutamine or arginine alone seems to be insufficient for preventing neutropenia [14]. It is expected that the relationship between the nutrient state and the preventive effects of nutritional intervention on neutropenia and the oral mucositis may be clarified by future studies.
Recently, some studies are reported on keratinocyte growth factor (palifermin) [20, 21] and a sustained-release drug of glutamine (Saforis) [22] reducing the incidence of chemotherapy-induced mucositis. The Food and Drug Administration (FDA) of the United States Department of Health and Human Services approved palifermin as prevention and as a treatment for oral mucositis in blood cancer patients who develop marrow toxicity and need hemopoietic stem cell transplantation. However, another consideration is the high cost of these new drugs [23] for introduction into clinical practice. Therefore, nourishment intervention using ED for oral mucositis should be considered alongside other standard oral care for sustaining the patient’s QOL.
We conclude that the amino-acid-rich elemental diet Elental® may be useful as a countermeasure for 5-FU-based chemotherapy-induced oral mucositis in patients with colorectal cancer in association with controlling neutropenia. The findings from this prospective pilot study warrant the initiation of a new clinical study with nutritional intervention in colorectal cancer patients developing oral mucositis. However, this small study makes any conclusions limited. We have conducted a multicenter clinical study clarifying the preventive effects on oral mucositis by Elental® and its correlation with change of amino acid balance in the serum.
Acknowledgments
We thank all patients who consented to participate in this study.
Authors’ contributions
YO was the principal investigator and study coordinator. YO developed the protocol. NI, KY, SU, HK, GN, MT, YA, and YO collected, analyzed, and helped to interpret the data.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB, Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 2.Sonis ST. Pathobiology of oral mucositis; novel insights and opportunities. J Support Oncol. 2007;5:3–11. [PubMed] [Google Scholar]
- 3.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, Schöffski P, Sobrero A, Van Cutsem E, Díaz-Rubio E. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 6.Cory JG, Cory AH. Critical roles of glutamine as nitrogen donors in purine and pyrimidine nucleotide synthesis: asparaginase treatment in childhood acute lymphoblastic leukemia. In vivo. 2006;20:587–589. [PubMed] [Google Scholar]
- 7.Chattopadhyay S, Saha A, Azam M, Mukherjee A, Sur PK. Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: a prospective randomized study. South Asian J Cancer. 2014;3:8–12. doi: 10.4103/2278-330X.126501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blijlevens NM, Donnelly JP, Naber AH, Schattenberg AV, DePauw BE. A randomized, double-blinded, placebo-controlled, pilot study of parenteral glutamine for allogeneic stem cell transplant patients. Support Care Cancer. 2005;13:790–796. doi: 10.1007/s00520-005-0790-y. [DOI] [PubMed] [Google Scholar]
- 9.Noé JE. L-glutamine use in the treatment and prevention of mucositis and cachexia: a naturopathic perspective. Integr Cancer Ther. 2009;8:409–415. doi: 10.1177/1534735409348865. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis. 2005;11:580–588. doi: 10.1097/01.MIB.0000161307.58327.96. [DOI] [PubMed] [Google Scholar]
- 11.Stiff PJ, Erder H, Bensinger WI, Emmanouilides C, Gentile T, Isitt J, Lu ZJ, Spielberger R. Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT) Bone Marrow Transplant. 2006;37:393–401. doi: 10.1038/sj.bmt.1705250. [DOI] [PubMed] [Google Scholar]
- 12.Gaurav K, Goel RK, Shukla M, Pandey M. Glutamine: a novel approach to chemotherapy-induced toxicity. Indian J Med Paediatr Oncol. 2012;33:13–20. doi: 10.4103/0971-5851.96962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sornsuvit C, Komindr S, Chuncharunee S, Wanikiat P, Archararit N, Santanirand P. Pilot study: effects of parenteral glutamine dipeptide supplementation on neutrophil functions and prevention of chemotherapy-induced side-effects in acute myeloid leukemia patients. J Int Med Res. 2008;36:1383–1391. doi: 10.1177/147323000803600628. [DOI] [PubMed] [Google Scholar]
- 14.Skubitz KM, Anderson PM. Oral glutamine to prevent chemotherapy induced oral mucositis: a pilot study. J Lab Clin Med. 1996;127:223–228. doi: 10.1016/S0022-2143(96)90082-7. [DOI] [PubMed] [Google Scholar]
- 15.Choi K, Lee SS, Oh SJ, Lim SY, Lim SY, Jeon WK, Oh TY, Kim JW. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/oral mucositis assessed by intestinal permeability test. Clin Nutr. 2007;26:57–62. doi: 10.1016/j.clnu.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, DB McGuire, Hutchins RD, Peterson DE, Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–831. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 17.Barasch A, Peterson DE. Risk factors for ulcerative oral mucositis in cancer patients: unanswered questions. Oral Oncol. 2003;39:91–100. doi: 10.1016/S1368-8375(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 18.Goto A, Yamada Y, Yasui H, Kato K, Hamaguchi T, Muro K, Shimada Y, Shirao K. Phase II study of combination therapy with S-1 and irinotecan in patients with advanced colorectal cancer. Ann Oncol. 2006;17:968–973. doi: 10.1093/annonc/mdl066. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen MA, van de Poll MC, Ligthart-Melis GC, Dejong CH, van den Tol MP, Boelens PG, van Leeuwen PA. Specific amino acids in the critically ill patient—exogenous glutamine/arginine: a common denominator? Crit Care Med. 2007;35(9 Suppl):S568–S576. doi: 10.1097/01.CCM.0000278600.14265.95. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, Shea T, Yanovich S, Hansen K, Noga S, McCarty J, LeMaistre CF, Sung EC, Blazar BR, Elhardt D, Chen MG, Emmanouilides C. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 21.Rosen LS, Abdi E, Davis ID, Gutheil J, Schnell FM, Zalcberg J, Cesano A, Gayko U, Chen MG, Clarke S. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol. 2006;24:5194–5200. doi: 10.1200/JCO.2005.04.1152. [DOI] [PubMed] [Google Scholar]
- 22.Peterson DE, Jones JB, Petit RG., 2nd Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer. 2007;109:322–331. doi: 10.1002/cncr.22384. [DOI] [PubMed] [Google Scholar]
- 23.Raber-Durlacher JE, von Bültzingslöwen I, Logan RM, Bowen J, Al-Azri AR, Everaus H, Gerber E, Gomez JG, Pettersson BG, Soga Y, Spijkervet FK, Tissing WJ, Epstein JB, Elad S, Lalla RV, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21:343–355. doi: 10.1007/s00520-012-1594-5. [DOI] [PubMed] [Google Scholar]