Abstract
Purpose
Physical activity can improve health of cancer survivors. To increase physical activity levels among colorectal cancer (CRC) survivors, we need to understand which factors affect physical activity. Therefore, this study examined the longitudinal relationship between symptom-related, functioning-related, and psychological barriers and socio-demographic and clinical factors with physical activity among CRC survivors.
Methods
CRC survivors identified from the population-based Eindhoven Cancer Registry (ECR) diagnosed between 2000 and 2009 were included. Survivors completed validated questionnaires measuring moderate-to-vigorous physical activity (MVPA) and barriers in 2010(T1), 2011(T2), and 2012(T3). Linear-mixed models and linear regression techniques were used.
Results
Response rates were 74 % (N = 2451, T1); 47 % (N = 1547, T2); and 41 % (N = 1375, T3). Several factors were negatively associated with MVPA: symptom-related barriers (e.g., fatigue, dyspnea, chemotherapy side effects, pain, appetite loss, and weight loss); psychological barriers (i.e., depressive symptoms and anxiety); functioning-related barriers (e.g., low physical or role functioning, unfavorable future perspective); socio-demographic (i.e., older age, female, no partner); and clinical factors (i.e., obesity). However, no within-subject effects were significantly associated with MVPA. Groups of functioning-related barriers, socio-demographic factors, symptom-related barriers, psychological barriers, and clinical factors explained 11, 3.9, 3.8, 2.4, and 2.2 % of the variance in MVPA at T1, respectively.
Conclusions
Several functioning-related and symptom-related barriers and few socio-demographic factors were associated with physical activity among CRC survivors. Future interventions to promote physical activity among CRC survivors could benefit by taking into account functioning aspects and symptoms of cancer and its treatment, and assess the causal direction of these associations.
Keywords: Colorectal cancer, Cancer survivorship, Physical activity, Exercise, Population based, Longitudinal study
Introduction
Colorectal cancer (CRC) is one of the leading causes of death worldwide and the third most common cancer type in the Netherlands, with 13,300 new cases diagnosed in 2013 [1]. Physical activity is important for CRC survivors because it decreases the risk of recurrence and comorbidities and has beneficial effects on certain health-related quality of life (HRQoL) domains [2–6]. However, less than one third of the CRC survivors comply with the physical activity guidelines (>2.5 h/week moderate-to-vigorous physical activity (MVPA)) [3, 7].
Many social cognitive models of human behavior, including the widely used Theory of Planned Behavior and Health Belief Model (HBM) [8, 9], contain constructs related to behavior change barriers. The HBM identifies “perceived barriers,” which refer to the patient’s assessment of obstacles to change physical activity. These perceived barriers may act as obstacles to undertake the recommended level of physical activity and could be classified into symptom-related barriers (e.g., fatigue, diarrhea, nausea, and having a stoma) [10–13]; functioning-related barriers (e.g., physical and social functioning) [14]; and psychological barriers (e.g., anxiety and depressive symptoms) (Table 1) [8, 10–13, 15]. Furthermore, physical activity behavior could also be affected by socio-demographic and clinical factors. For instance, studies show that CRC survivors who were younger, male, have a partner, a lower BMI, who were diagnosed with colon cancer, treated with chemotherapy, with a longer time since diagnosis and no comorbidities were more physically active [10, 12].
Table 1.
Classification of possible factors associated with physical activity among CRC survivors, according to the Health Belief Model
| Perceived control beliefs | Symptom-related barriers | Fatigue |
| Pain | ||
| Nausea | ||
| Dyspnea | ||
| Insomnia | ||
| Appetite loss | ||
| Financial problems | ||
| Micturition problems | ||
| Chemotherapy side effects | ||
| Gastro-intestinal problems | ||
| Stoma-related problems | ||
| Defecation problems | ||
| Weight loss | ||
| Functioning-related barriers | Physical functioning | |
| Role functioning | ||
| Social functioning | ||
| Emotional functioning | ||
| Cognitive functioning | ||
| Global quality of life | ||
| Body image | ||
| Future perspective | ||
| Psychological barriers | Anxiety | |
| Depressive symptoms | ||
| Socio-demographic factors | Gender | |
| Age | ||
| Partner | ||
| Educational level | ||
| Clinical factors | Years since diagnosis | |
| Localization of cancer | ||
| Tumor stage | ||
| Treatment | ||
| Stoma | ||
| Number of comorbidities | ||
| Body mass index (BMI) |
Nevertheless, previous studies had low response rates or cross-sectional designs, highlighting the need for larger studies and longitudinal designs. Longitudinal analysis allows to examine the relationship between barriers and MVPA levels over time. Hence, a longitudinal study has the potential to inform future intervention development to increase physical activity by understanding what barriers of change need to be targeted in a growing proportion of people surviving CRC. Although a longitudinal study is insufficient to distinguish true causality, it aids studying causal associations.
Therefore, the aims of the current study were to examine (1) the longitudinal relationship between symptom-related, functioning-related, and psychological barriers and socio-demographic and clinical factors with physical activity among CRC survivors and (2) which group of factors contained the largest barriers of physical activity. Besides the individual associations between aforementioned barriers and MVPA, it seems plausible that some barriers are related with each other and together influence MVPA [10–13]. In this study, effects of groups of barriers are defined as “conjoint effects.” Examining the conjoint effects of symptom-related, functioning-related, and psychological barriers and socio-demographic and clinical factors on physical activity could be used to investigate which group of factors contains the largest barriers for survivors.
We hypothesized that the following factors are negatively associated with physical activity: symptom-related barriers (e.g., fatigue, dyspnea, stoma-related problems, nausea, insomnia, chemotherapy side effects, pain, appetite loss, micturition problems, gastro-intestinal problems, defecation problems, weight loss, and financial problems); psychological barriers (i.e., depressive symptoms and anxiety); functioning-related barriers (e.g., low physical role; social, emotional, and cognitive functioning; unfavorable future perspective, body image and a low global quality of life).
Methods
Setting and participants
A longitudinal population-based cohort study was performed among CRC survivors registered within the Eindhoven Cancer Registry (ECR) of the Comprehensive Cancer Centre, The Netherlands. The ECR records data on all newly diagnosed cancer patients in the Southern part of the Netherlands, an area with 2.4 million inhabitants, ten hospital locations, and two large radiotherapy institutes. Individuals diagnosed between 2000 and 2009 with colon or rectal cancer stage I–III (N = 3323) as registered in the ECR, were eligible for participation (see flowchart: http://www.profilesregistry.nl/dataarchive/study_units/view/22). CRC survivors with stage IV were excluded (N = 114) from the analyses to obtain a more homogeneous study population. This study was approved by the local Medical Ethics committee of the Maxima Medical Centre. All included participants signed an informed consent.
Data collection
Data collection took place in December 2010 (T1), 2011 (T2), and 2012 (T3) by using questionnaires. Survivors received a letter from their (ex-) attending specialist to inform them about the study. Data collection was done within Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship (PROFILES). PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short- and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from the ECR [16].
Measures
Physical activity was assessed with the European Prospective Investigation into Cancer (EPIC) Physical Activity Questionnaire [17]. Participants were asked how many hours per week they spent on average on walking, bicycling, gardening, housekeeping, and sports in summer and winter. Six sports could be reported. First, the mean duration spent on these activities in summer and winter were computed. Second, to include an estimate of intensity, metabolic equivalent intensity values (1 MET = 4.184 kJ/kg body weight/h) were assigned to each activity, according to the compendium of physical activities [18, 19]. Finally, the duration of moderate-to-vigorous physical activity (MVPA) was assessed as time (h/week) spent on walking, bicycling, gardening and sports (≥3 MET). Housekeeping and light intensity sports were excluded from calculating the duration of MVPA [18, 19].
Clinical information was obtained from the ECR (i.e., gender, date of birth, years since diagnosis, localization of cancer, tumor stage, and primary treatment). Other relevant socio-demographic and clinical factors were obtained via questions concerning marital status/partner and educational level. Furthermore, comorbidity in the last 12 months was assessed with the Self-administered Comorbidity Questionnaire (SCQ) [20].
Anxiety and depressive symptoms were assessed with the Hospital Anxiety and Depression Scale (HADS) [21]. The questionnaire consist of 14 items which can be answered on a 4-point scale. The HADS yields separate scale scores for anxiety and depressive symptoms. The total score for each scale can range from 0 to 21. Cut-off values for anxiety or depressive symptoms were indicated by a score of ≥8. [21, 22].
Participants’ functioning and symptoms were assessed with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 [23]. This questionnaire consists of 30 items and questions can be answered on a 4-point scale. The following scales were included in the analysis: physical, social, emotional, cognitive, and role functioning scales; global quality of life scale; pain and nausea scales; and the single items dyspnea, insomnia, appetite loss, and financial problems.
Fatigue was measured with the Fatigue Assessment Scale (FAS) [24]. The questionnaire consists of 10 items which can be answered on a 5-point scale. The total score can range from 10 to 50. Cut-off values for fatigue were indicated by a score of ≥22 [25].
Colorectal cancer-specific symptoms and two functioning scales were measured with the EORTC QLQ-CR38 [26]. This questionnaire consists of 38 items and contains the following symptom scales: micturition problems, chemotherapy side effects, gastro-intestinal problems, defecation problems, having a stoma and stoma-related problems, weight loss, and two functioning scales: body image and future perspective. Scores for scales and items from the EORTC QLQ-C30 and EORTC QLQ-CR38 were linearly transformed to a 0 to 100 scale according to the guidelines [26]. High functioning scores indicate better functioning, while high symptom scores indicate higher symptom burden.
Statistical analyses
Differences in socio-demographic and clinical characteristics between respondents, non-respondents, and patients with unverifiable addresses, and between patients who completed one and those who completed more than one questionnaire were compared with a chi-square or ANOVA where appropriate to assess the representativeness of the sample. Slopes were made of MVPA over time for nine independent variables to investigate the change in MVPA over time for participants who completed all three questionnaires. Further analyses were based upon all participants and included all variables. Outliers of MVPA (>95th percentile) were imputed by the 95th percentile value. Missing items from multi-item scales were imputed according to the questionnaire guidelines [20, 21, 23, 24, 27].
The first part of the analyses included longitudinal data analyses. The individual association between each independent variable (Table 1) and MVPA over time was analyzed by using linear-mixed models. Linear-mixed models were used to adjust for the dependence of observations. Continuous independent variables were grand-mean centered to reduce multicollinearity. In the first step, we developed a longitudinal model, using linear-mixed models, by putting MVPA as dependent variable in the regression equation and one variable of interest, two dummies for time (T2 vs. T1 and T3 vs. T1) and the possible confounders as independent variables. A priori chosen possible confounders were the following: age, gender, having a partner, educational level, years since diagnosis, tumor stage, number of comorbidities, and BMI.
In the second step of the longitudinal data analyses, we examined the between-subject and within-subject effect for each continuous independent variable separately. The between-subject estimate was used to see if differences in the independent variable between participants resulted in differences in MVPA and was represented by a participants’ average amount of MVPA reported during the study across the three measurements. The within-subject estimate was used to study potential causal relations, by assessing if changes in the independent variable within a participant were related to changes in MVPA and was represented by the difference between a participants’ MVPA at a certain point in time and his/her average MVPA during the study. The between-subject and within-subject estimates were simultaneously entered in the linear-mixed models together with the possible confounders and two dummies for time. Statistically (non-)significant beta’s for the between-subject and within-subject estimates were presented for the clinical important difference (CID) in scale scores of the independent variables, based on the guidelines for interpretation of the EORTC QLQ-C30 [28]. The CID in EORTC QLQ-CR38, FAS, and HADS scores was obtained by Norman’s “rule of thumb,” whereby a ±0.5 SD difference in scores indicates a threshold for discriminating change in HRQoL scores of a chronic illness [29]. In the third step of the longitudinal data analyses, we included an interaction term to investigate if associations with MVPA were different for CRC survivors with stage I, II, or III. Due to these different treatments, i.e., surgery or adjuvant chemotherapy, CRC survivors may have different symptoms which influenced MVPA.
The second part of the analyses included cross-sectional data analyses, using multiple linear regression techniques to assess the conjoint association between multiple independent variables and MVPA at T1. The explained variance at T1 was assessed for the following domains: socio-demographic factors, clinical factors, psychological barriers, all functional-related barriers, and all symptom-related barriers. Linear regression analyses were more appropriate than linear-mixed models to assess the explained variance and therefore, we examined the conjoint association at a time point instead of over time. All statistical analyses were conducted using SAS version 9.3 (Statistical Analysis System); p values of <0.01 were considered statistically significant as multiple associations were tested.
Results
Characteristics of respondents and non-respondents
At T1, the response rate was 74 % (N = 2451), 47 % (N = 1547) completed the questionnaire at T2, and 41 % (N = 1375) at T3. Respondents were 69.6 (SD = 9.5) years, 55 % were male and years since diagnosis was on average 5.3 (SD = 2.8) and there were minimal 1.4 year since diagnosis at T1. Respondents were 3 years younger, more often male, and underwent more often surgery only compared with non-respondents (all p < 0.01; Table 2).
Table 2.
Socio-demographic and clinical characteristics of the respondents, non-respondents and patients with unverifiable addresses at T1
|
N (%) All respondents N = 2451 |
N (%) Non-respondents N = 566 |
N (%) Patients with unverifiable addresses N = 306 |
p value | |
|---|---|---|---|---|
| Socio-demographic factors | ||||
| Gender | <0.01 | |||
| Male | 1339 (54.6) | 275 (47.7) | 148 (48.4) | |
| Female | 1112 (45.4) | 301 (52.3) | 158 (51.6) | |
| Age at time of survey: mean (SD) | 69.6 (9.5) | 72.8 (9.4) | 68.9 (12.4) | <0.01 |
| <55 years | 186 (7.6) | 31 (5.4) | 42 (13.7) | <0.01 |
| 55–74 years | 1470 (60.0) | 267 (46.4) | 144 (47.1) | |
| ≥ 75 years | 795 (32.4) | 278 (48.3) | 120 (39.2) | |
| Partner (yes) | 1848 (76.1) | |||
| Educational level a | ||||
| Low | 1184 (48.8) | |||
| Medium | 773 (31.9) | |||
| High | 466 (19.2) | |||
| Clinical factors | ||||
| Years since diagnosis/mean (SD) | 5.3 (2.8) | 5.3 (2.9) | 5.7 (2.9) | 0.02 |
| Localization | 0.02 | |||
| Colon cancer | 1510 (61.6) | 390 (67.7) | 191 (62.4) | |
| Rectal cancer | 941 (38.4) | 186 (32.3) | 115 (37.6) | |
| Tumor stage | 0.05 | |||
| I | 780 (31.8) | 156 (27.1) | 91 (29.7) | |
| II | 947 (38.6) | 258 (44.8) | 132 (43.1) | |
| III | 724 (29.5) | 162 (28.1) | 83 (27.1) | |
| Treatment | <0.01 | |||
| Surgery only | 1215 (49.6) | 343 (59.6) | 176 (57.9) | |
| Surgery and RT | 565 (23.1) | 95 (16.5) | 52 (17.1) | |
| Surgery and CT | 497 (20.3) | 97 (16.8) | 51 (16.8) | |
| Surgery, RT and CT | 171 (7.0) | 38 (6.6) | 23 (7.6) | |
| Comorbidities | ||||
| No comorbidities | 673 (25.0) | |||
| 1 comorbid condition | 659 (28.8) | |||
| 2 or more comorbid conditions | 1060 (46.3) | |||
| BMI/mean (SD) | 26.7 (4.3) | |||
| Normal | 800 (33.5) | |||
| Overweight | 1159 (48.5) | |||
| Obesity | 430 (18.0) | |||
RT radiotherapy, CT chemotherapy, BMI body mass index
aEducational level: High University or high education, Medium Vocational training, Low secondary, primary or less
CRC survivors who completed only one questionnaire were older at time of first enrollment (71.4 vs. 68.4; p < 0.01), were more often female (49 vs. 43 %; p = 0.01) and had a lower educational level (27 vs. 17 %; p < 0.01) compared with CRC survivors who completed more then one questionnaire. No differences were found in years since diagnosis, BMI, and number of comorbid conditions. Furthermore, CRC survivors who completed only one questionnaire often did not meet the guidelines of MVPA (25 vs. 10 %; p < 0.01) and spent on average less hours per week on MVPA (9.1 vs. 12.0 h/week; p < 0.01).
Socio-demographic and clinical factors and MVPA
Levels of MVPA were relatively stable over time; however, they were different between male and female CRC survivors (Fig. 1). Male survivors reported, on average, 1.90 h/week more MVPA than female survivors over time (Table 3). Furthermore, survivors who were 55–74 years old reported 3.34 (p < 0.01) h/week more MVPA then survivors who were ≥75 years old. CRC survivors who had a partner reported 1.11 (p < 0.01) h/week more MVPA than CRC survivors without a partner. Other significant differences in MVPA over time were found for normal weight vs. obesity (B = 2.38) and overweight vs. obesity (B = 1.67) (Table 3). In addition, no associations were found for having a stoma, educational level, years since diagnosis, treatment, localization of cancer, and number of comorbidities.
Fig. 1.
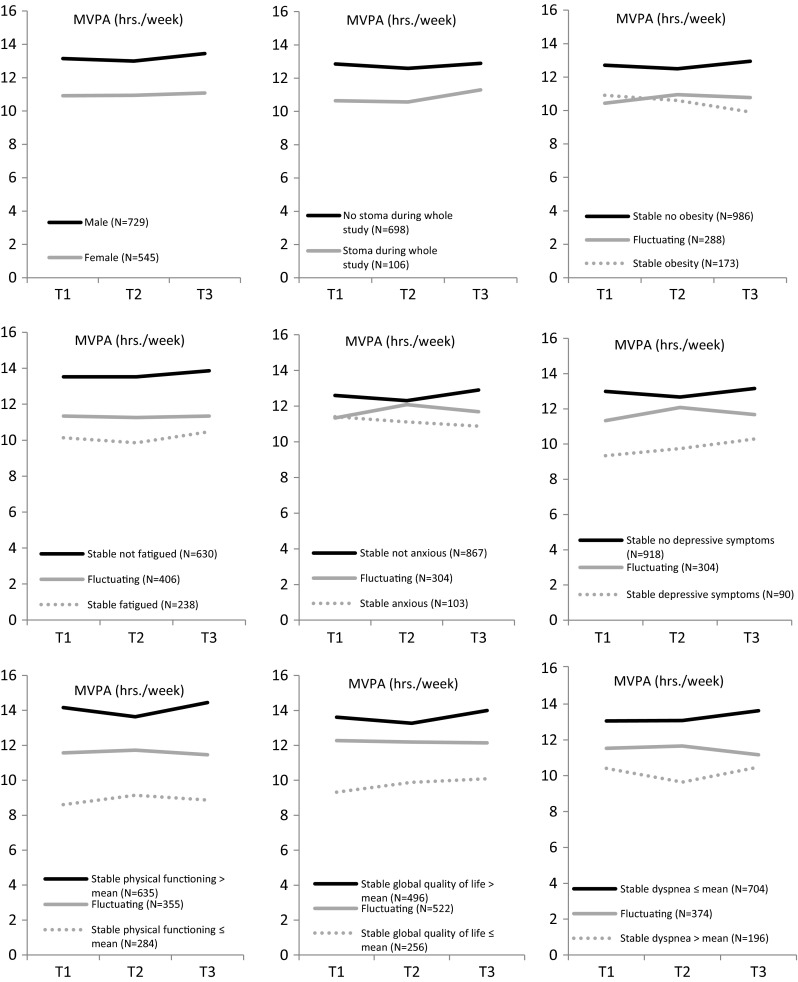
Longitudinal changes in mean score of MVPA (h/week) over time for gender, having a stoma, BMI, fatigue, anxiety, depressive symptoms, physical functioning, global quality of life, and dyspnea among participants who completed all three questionnaires (N = 1274). MVPA moderate-to-vigorous physical activity. Higher mean scores of physical functioning and global quality of life implicate better functioning, whereas higher mean scores of dyspnea indicate a higher symptom burden
Table 3.
Adjusted linear-mixed models estimating the individual associations between each independent factor and MVPA (h/week) over time (T1 N = 2451; T2 N = 1547; T3 N = 1375)
| Number in analysis* | CID | B** | 95 % CI Lower limit | 95 % CI Upper limit | p value | ||
|---|---|---|---|---|---|---|---|
| Socio-demographic factors | |||||||
| Gender (male vs. female) | 4768 | 1.90 | 1.23 | 2.58 | <0.01 | ||
| Age | 4768 | ||||||
| <55 vs. ≥75 years | 1.10 | −0.22 | 2.20 | 0.08 | |||
| 55–74 vs. ≥75 years | 3.34 | 2.73 | 4.04 | <0.01 | |||
| Partner (yes vs. no) | 4768 | 1.11 | 0.36 | 1.87 | <0.01 | ||
| Educational levela | 4768 | ||||||
| Low vs. high | −0.19 | −1.01 | 0.64 | 0.65 | |||
| Medium vs. high | 0.65 | −0.02 | 1.59 | 0.06 | |||
| Medium vs. low | 0.97 | 0.35 | 1.60 | <0.01 | |||
| Clinical factors | |||||||
| Years since diagnosis | 4768 | 0.05 | −0.05 | 0.17 | 0.37 | ||
| Colon vs. rectal cancer | 4768 | 0.37 | −0.30 | 1.04 | 0.28 | ||
| Tumor stage | 4768 | ||||||
| II vs. I | −1.11 | −1.88 | −0.34 | <0.01 | |||
| III vs. I | −0.58 | −1.40 | 0.23 | 0.16 | |||
| Treatment | 4926 | ||||||
| Surgery + CT + RT vs. surgery only | −0.79 | −2.13 | 0.54 | 0.24 | |||
| Surgery and RT vs. surgery only | −0.31 | −1.12 | 0.49 | 0.44 | |||
| Surgery and CT vs. surgery only | 0.58 | −0.46 | 1.62 | 0.27 | |||
| CT vs. RTb | 2482 | 0.37 | −0.76 | 1.49 | 0.52 | ||
| Stoma (yes vs. no) | 2874 | −1.24 | −2.10 | −0.38 | <0.01 | ||
| Comorbidities | 4768 | ||||||
| No vs. ≥2 comorbid conditions | 0.70 | 0.03 | 1.36 | 0.04 | |||
| 1 vs. ≥2 comorbid conditions | 0.49 | −0.07 | 1.05 | 0.09 | |||
| BMI | 4726 | ||||||
| Normal weight vs. obesity | 2.38 | 1.51 | 3.24 | <0.01 | |||
| Overweight vs. obesity | 1.67 | 0.89 | 2.44 | <0.01 | |||
| Symptom-related barriersc | |||||||
| Fatigue | Between | 4698 | 3.4 | −0.79 | −0.96 | −0.61 | <0.01 |
| Within | 3.4 | −0.25 | −0.50 | 0.01 | 0.05 | ||
| Pain | Between | 4742 | 6 | −0.22 | −0.32 | −0.13 | <0.01 |
| Within | 6 | 0.01 | −0.08 | 0.11 | 0.75 | ||
| Nausea | Between | 4725 | 3 | −0.15 | −0.25 | −0.05 | <0.01 |
| Within | 3 | −0.08 | −0.16 | −0.01 | 0.05 | ||
| Dyspnea | Between | 4698 | 4 | −0.21 | −0.27 | −0.15 | <0.01 |
| Within | 4 | −0.04 | −0.11 | 0.03 | 0.24 | ||
| Insomnia | Between | 4715 | 4 | −0.07 | −0.13 | −0.02 | <0.01 |
| Within | 4 | −0.03 | −0.09 | 0.02 | 0.28 | ||
| Appetite loss | Between | 4720 | 5 | −0.29 | −0.40 | −0.17 | <0.01 |
| Within | 5 | −0.08 | −0.18 | 0.03 | 0.14 | ||
| Financial difficulties | Between | 4706 | 3 | −0.08 | −0.14 | −0.02 | <0.01 |
| Within | 3 | −0.01 | −0.05 | 0.07 | 0.81 | ||
| Micturition problems | Between | 4669 | 8.7 | −0.39 | −0.57 | −0.20 | <0.01 |
| Within | 8.7 | −0.03 | −0.22 | 0.16 | 0.76 | ||
| Chemo side effects | Between | 4693 | 7.9 | −0.41 | −0.60 | −0.22 | <0.01 |
| Within | 7.9 | −0.04 | −0.23 | 0.14 | 0.65 | ||
| Gastro-intestinal problems | Between | 4655 | 7.2 | −0.12 | −0.30 | 0.06 | 0.20 |
| Within | 7.2 | −0.03 | −0.18 | 0.23 | 0.80 | ||
| Defecation problems | Between | 3609 | 6.0 | −0.21 | −0.41 | −0.01 | 0.04 |
| Within | 6.0 | −0.03 | −0.27 | 0.22 | 0.83 | ||
| Stoma-related problems | Between | 967d | 10.4 | −0.04 | −0.41 | 0.34 | 0.85 |
| Within | 10.4 | −0.10 | −0.45 | 0.65 | 0.71 | ||
| Weight loss | Between | 4716 | 7.8 | −0.26 | −0.46 | −0.07 | <0.01 |
| Within | 7.8 | −0.12 | −0.27 | 0.02 | 0.09 | ||
| Functioning-related barrierse | |||||||
| Physical functioning | Between | 4741 | 5 | 0.66 | 0.58 | 0.75 | <0.01 |
| Within | 5 | 0.10 | −0.03 | 0.24 | 0.13 | ||
| Role functioning | Between | 4726 | 6 | 0.38 | 0.29 | 0.46 | <0.01 |
| Within | 6 | 0.04 | −0.05 | 0.12 | 0.41 | ||
| Social functioning | Between | 4721 | 5 | 0.26 | 0.18 | 0.35 | <0.01 |
| Within | 5 | 0.05 | −0.04 | 0.13 | 0.29 | ||
| Emotional functioning | Between | 4722 | 4 | 0.20 | 0.13 | 0.28 | <0.01 |
| Within | 4 | 0.01 | −0.09 | 0.08 | 0.93 | ||
| Cognitive functioning | Between | 4728 | 3 | 0.09 | 0.04 | 0.15 | <0.01 |
| Within | 3 | 0.06 | 0.00 | 0.12 | 0.07 | ||
| Global quality of life | Between | 4739 | 4 | 0.37 | 0.29 | 0.45 | <0.01 |
| Within | 4 | 0.02 | −0.06 | 0.11 | 0.57 | ||
| Body image | Between | 4692 | 10.9 | 0.19 | 0.01 | 0.36 | 0.03 |
| Within | 10.9 | 0.02 | −0.23 | 0.18 | 0.82 | ||
| Future perspective | Between | 4728 | 13.7 | 0.38 | 0.20 | 0.57 | <0.01 |
| Within | 13.7 | 0.02 | −0.20 | 0.17 | 0.87 | ||
| Psychological barriers | |||||||
| Anxiety | Between | 4685 | 1.8 | −0.29 | −0.46 | −0.12 | <0.01 |
| Within | 1.8 | −0.08 | −0.31 | 0.15 | 0.51 | ||
| Depressive symptoms | Between | 4704 | 1.8 | −0.73 | −0.90 | −0.55 | <0.01 |
| Within | 1.8 | −0.21 | −0.45 | 0.03 | 0.09 | ||
Linear-mixed models are adjusted for gender, age, educational level, having a partner, years since diagnosis, tumor stage, number of comorbidities, and BMI
MVPA moderate-to-vigorous physical activity; CID clinical important difference in scores of the scales and items of the EORTC QLQ-C30, EORTC QLQ-CR38, FAS, and HADS; CI confidence interval; CT chemotherapy; RT radiotherapy
aEducational level: High university or high education, Medium vocational training, Low secondary, primary or less
bPatients underwent surgery as primary treatment
cHigher scores implicate higher symptom burden
dOnly patients with a stoma were asked to fill in items concerning stoma-related problems
eHigher scores implicate better functioning
*Each row in the analyses represents a patient at one time point, resulting in a maximum of three rows per patient in a long data file used for the linear-mixed models
**B is calculated for the clinical important difference (CID) in scale scores of the independent variables
Psychological barriers and MVPA
Figure 1 shows that the levels of MVPA were relatively stable over time; however, they were different between CRC survivors who were anxious or reported depressive symptoms and their counterparts. Furthermore, statistically significant between-subject estimates were found for anxiety (B = −0.29) and depressive symptoms (B = −0.73), meaning that a 1.8 point (±0.5 SD) higher score on these scales compared with another participant was associated with 0.29 or 0.73 h/week less MVPA (Table 3).
Functioning-related barriers and MVPA
Statistically significant differences were found for physical functioning (B = 0.66; between-subject estimate), meaning that a 5-point higher score on this scale compared with another participant was associated with 0.66 h/week more MVPA. Other significant differences were found for role functioning (B = 0.38), future perspective, and global quality of life (all between-subject estimates, Table 3).
Symptom-related barriers and MVPA
Levels of MVPA were lower for CRC survivors who were fatigued vs. those who were not fatigued or reported above vs. below average scores on the dyspnea scale during the whole study (Fig. 1). Furthermore, statistically significant differences were found for fatigue (B = −0.79; between-subject estimate) meaning that a 3.4 point (±0.5 SD) higher score on this scale compared with another participant was associated with 0.79 h/week less MVPA. Other significant results were found for chemotherapy side effects, micturition problems, appetite loss, weight loss, pain, and dyspnea (all between-subject estimates, Table 3).
An interaction term was added to investigate if associations with MVPA were different for CRC survivors with stage I, II, or III. Solely, the interaction term for cognitive functioning was significant (p < 0.01). Subgroup analysis demonstrated that there was a significant association between cognitive functioning and MVPA for stage I survivors (B = 0.06; between-subject estimate), whereas not for stage II and III survivors.
Conjoint associations with MVPA
At T1, 10.6 % of the differences in MVPA could be explained by differences in functioning-related barriers (Table 4). Differences in functioning-related barriers could explain the highest variance in MVPA compared with other groups of factors. Of the functioning-related barriers, physical functioning explained the highest variance in MVPA. Differences in symptom-related barriers explained 3.8 % of the differences in MVPA and most variance could be attributed to differences in fatigue, dyspnea, and chemotherapy side effects. Because of the large number of variables and some variables being correlated, we could not include all variables of interest in one linear regression analysis to assess the explained variance. Therefore, we decided to analyze groups of related barriers to assess explained variance per group. As a consequence, these explained variances cannot be summed, since they could have overlapping variance.
Table 4.
Linear regression techniques estimating the explained variance for groups of factors in MVPA at T1
| Domains | Numbera | R 2 |
|---|---|---|
| Socio-demographic factors | 2407 | 0.039 |
| Clinical factors | 2061 | 0.022 |
| Symptom-related barriersb | 1680 | 0.038 |
| Functioning-related barriersc | 2340 | 0.106 |
| Psychological barriers | 2345 | 0.024 |
MVPA moderate-to-vigorous physical activity, R 2 explained variance
aNumber of patients included in the analysis
b R 2 of all symptom-related scales in one model (EORTC QLQ-30, EORTC QLQ-CR38, and FAS)
c R 2 of all functioning scales and global quality of life in one model (EORTC QLQ-30 and EORTC QLQ-CR38)
Discussion
This longitudinal population-based cohort study examined the individual and conjoint association of factors with physical activity among CRC survivors. According to our results, several factors were negatively associated with physical activity over time: symptom-related barriers (e.g., fatigue, dyspnea, chemotherapy side effects, pain, appetite loss, and weight loss); psychological barriers (i.e., depressive symptoms and anxiety); functioning-related barriers (e.g., low physical or role functioning, unfavorable future perspective, low global quality of life); socio-demographic (i.e., being ≥75 years old, being female, having no partner); and clinical factors (i.e., being overweight or obese). Furthermore, conjoint associations indicated that most differences in physical activity can be attributed to differences in functioning aspects and experienced symptoms of cancer and its treatment.
Our results regarding individual associations with physical activity are in line with previous studies. Courneya et al. [11] found that fatigue was an important barrier for physical activity among CRC survivors. Furthermore, previous studies have also found that being female, having a higher BMI and being anxious were associated with a lower physical activity among CRC survivors [10, 15]. In contrast with previous studies, in the present study, the number of comorbidities and tumor stage were not significantly associated with physical activity [10, 12].
In the present study, conjoint associations demonstrated that differences in functioning-related barriers and symptom-related barriers seem to be important in explaining differences in MVPA, whereas socio-demographic and clinical factors seem to be less important. These results are in accordance with results of Lynch et al. [13], who found that disease-specific side effects are perceived as the greatest barriers to physical activity for CRC survivors.
A few results require further explanation. Besides significant between-subject effects, no within-subject effects were significant which indicates that the factors included in the analyses were significantly associated with MVPA over time between respondents but not within respondents. This could be caused by the fact that factors assumed to be related with MVPA were relatively stable over time within participants, which could be related to the fact that respondents were, on average, 5 years after diagnosis. Second, there was no association between having a stoma or stoma-related problems and MVPA. An explanation could be that the severity of stoma-related problems was generally low among the CRC survivors with a stoma in the present study and therefore may not affect MVPA. The low severity of stoma-related problems reported by CRC survivors with a stoma could be explained by the high number of long-term survivors (more than 5 years after diagnosis) and a decrease of stoma-related problems over time [13, 30, 31]. Third, our results demonstrated that survivors who were 55–74 years old were more physically active then survivors who were <55 years old. This relatively high level of physical activity among survivors who were 55–74 years old could be caused by early retirement or not having a paid job at the time of study whereby patients have more leisure time to be physically active [32]. Finally, this study showed that survivors who had surgery and chemotherapy were more physically active then survivors who only had surgery. An explanation could be that patients who were treated with chemotherapy received more advice on the health benefits of physical activity from health care professionals [33].
Besides socio-demographic factors, clinical factors, and perceived barriers, other behavioral factors could also affect physical activity among CRC survivors [9]. Such behavioral factors could be the intention to be physically active, the subjective norm (a person’s own estimate of the social pressure to be physically active), the instrumental attitude (the expected benefits of being physical active), and the affective attitude (the expected enjoyment of being physical active) [34, 35]. Furthermore, lack of time or enjoyment; facilities to be physically active; encouragement from family, friends, and health professionals could affect physical activity [13]. Future research should assess these factors and relate them to important correlates found in the present study.
Response rates decreased during the present study because respondents stopped participating or deceased. The decrease in response rates may have influenced the results. Respondents who completed only one questionnaire were less physically active compared with patients who completed two or three questionnaires which may led to an overestimation of MVPA at T2 and T3. Due to selection bias, respondents may be a more homogenous group than the target population, which could lead to underestimations of the effect estimates. However, this should not affect the direction of the associations. Incentives might have improved the compliance [36].
The current study has some limitations. There were differences between respondents and non-respondents, which may decrease the generalizability of our results. Furthermore, this study presented statistically significant results, whereas it was not possible to present clinically relevant results because no guidelines were available for the minimal clinically significant difference in MVPA. Another limitation is the use of self-report measures to assess MVPA. This may have led to systematic overestimation of MVPA levels [37]. Nevertheless, this study is one of the few available studies that reported a comprehensive view on factors associated with physical activity among CRC survivors. Moreover, this study is a large longitudinal population-based cohort study.
In conclusion, multiple individual factors from all domains were negatively associated with physical activity over time among CRC survivors. However, barriers and MVPA were relatively stable over time and therefore, this study found no association between changes in barriers and changes in MVPA (within-subject effects). Furthermore, conjoint associations indicated that most differences in physical activity can be attributed to differences in functioning aspects and experienced symptoms of cancer and its treatment. Future interventions to increase physical activity levels among CRC survivors should take into account functioning aspects and symptoms of cancer and its treatment.
Acknowledgments
We would like to thank the patients and their doctors for their participation in the study. This study would not have been possible without their valuable time and willingness to share personal information. We also thank Dr. W. Zijlstra (Center of Research on Psychology in Somatic diseases, Department of Medical and Clinical Psychology, Tilburg University, the Netherlands) for feedback on our statistical approach to the analyses.
Funding
The present research was supported by a VENI grant (#451-10-041) from the Netherlands Organization for Scientific Research (The Hague, The Netherlands) awarded to Floortje Mols. Dr. N.P.M. Ezendam is supported by a grant from the Dutch Cancer Society, and Prof. Dr. L.V. van de Poll-Franse is supported by a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349). These funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the paper for publication.
Conflict of interest
All authors declare that they have no financial or non-financial conflict of interest.
References
- 1.Cijfers over kanker. (2014). www.cijfersoverkanker.nl.
- 2.Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila) 2011;4(4):502–511. doi: 10.1158/1940-6207.CAPR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43(1):32–38. doi: 10.1136/bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 4.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2010;19(11):2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 5.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51(8):1242–1248. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 7.Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian community health survey. Cancer. 2008;112(11):2475–2482. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- 8.Champion VL, Skinner CS. The health belief model. Health Behav Health Educ Theory Res Pract. 2008;4:45–65. [Google Scholar]
- 9.Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. Health Behav Health Educ Theory Res Pract. 2008;4:67–95. [Google Scholar]
- 10.Buffart LM, Thong MS, Schep G, Chinapaw MJ, Brug J, van de Poll-Franse LV. Self-reported physical activity: its correlates and relationship with health-related quality of life in a large cohort of colorectal cancer survivors. PLoS One. 2012;7(5):e36164. doi: 10.1371/journal.pone.0036164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Vallance JK, Fairey AS. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med. 2005;29(2):147–153. doi: 10.1207/s15324796abm2902_9. [DOI] [PubMed] [Google Scholar]
- 12.D’Andrea AP, Fernandez CA, Tannenbaum SL, Clarke TC, McClure LA, Leblanc WG, Lee DJ. Correlates of leisure time physical activity compliance in colorectal cancer survivors. Prev Med. 2014;62:78–82. doi: 10.1016/j.ypmed.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Lynch BM, Owen N, Hawkes AL, Aitken JF. Perceived barriers to physical activity for colorectal cancer survivors. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2010;18(6):729–734. doi: 10.1007/s00520-009-0705-4. [DOI] [PubMed] [Google Scholar]
- 14.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, Cerin E, Chan WY, Leung IP, Lam SH, Taylor AJ, Cheng KK. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers SK, Lynch BM, Aitken J, Baade P. Relationship over time between psychological distress and physical activity in colorectal cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(10):1600–1606. doi: 10.1200/JCO.2008.18.5157. [DOI] [PubMed] [Google Scholar]
- 16.van de Poll-Franse LV, Horevoorts N, Eenbergen M, Denollet J, Roukema JA, Aaronson NK, Vingerhoets A, Coebergh JW, de Vries J, Essink-Bot M-L, Mols F. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188–2194. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Pols MA, Peeters PH, Ocke MC, Slimani N, Bueno-de-Mesquita HB, Collette HJ. Estimation of reproducibility and relative validity of the questions included in the EPIC physical activity questionnaire. Int J Epidemiol. 1997;26(Suppl 1):S181–S189. doi: 10.1093/ije/26.suppl_1.S181. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Olsson I, Mykletun A, Dahl AA. The hospital anxiety and depression rating scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 24.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the fatigue assessment scale. J Psychosom Res. 2003;54(4):345–352. doi: 10.1016/S0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 25.Michielsen HJ, Drent M, Peros-Golubicic T, De Vries J. Fatigue is associated with quality of life in sarcoidosis patients. Chest. 2006;130(4):989–994. doi: 10.1378/chest.130.4.989. [DOI] [PubMed] [Google Scholar]
- 26.Sprangers MAG, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38) Eur J Cancer. 1999;35(2):238–247. doi: 10.1016/S0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 27.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer study group on quality of life. Eur J Cancer (Oxford, England: 1990) 1999;35(2):238–247. doi: 10.1016/S0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 28.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire core 30. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(1):89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Rini B, Garcia JA, Baz RC, Abou-Jawde RM, Thakkar SG, Elson P, Mekhail TM, Zhou M, Bukowski RM. Prognostic factors associated with long-term survival in previously untreated metastatic renal cell carcinoma. Annals Oncol Off J Eur Soc Med Oncol/ESMO. 2007;18(2):249–255. doi: 10.1093/annonc/mdl371. [DOI] [PubMed] [Google Scholar]
- 31.Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, Nordlinger B. Long-term survival following resection of colorectal hepatic metastases. Association Francaise de Chirurgie. Br J Surg. 1997;84(7):977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 32.Barnett I, van Sluijs EM, Ogilvie D. Physical activity and transitioning to retirement: a systematic review. Am J Prev Med. 2012;43(3):329–336. doi: 10.1016/j.amepre.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Husson O, Thong MS, Mols F, Oerlemans S, Kaptein AA, van de Poll-Franse LV. Illness perceptions in cancer survivors: what is the role of information provision? Psycho-Oncology. 2013;22(3):490–498. doi: 10.1002/pon.3042. [DOI] [PubMed] [Google Scholar]
- 34.Forbes CC, Blanchard CM, Mummery WK, Courneya KS. A comparison of physical activity correlates across breast, prostate and colorectal cancer survivors in Nova Scotia, Canada. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2013 doi: 10.1007/s00520-013-2045-7. [DOI] [PubMed] [Google Scholar]
- 35.Speed-Andrews AE, McGowan EL, Rhodes RE, Blanchard CM, Culos-Reed SN, Friedenreich CM, Courneya KS. Identification and evaluation of the salient physical activity beliefs of colorectal cancer survivors. Cancer Nurs. 2013 doi: 10.1097/NCC.0b013e3182813972. [DOI] [PubMed] [Google Scholar]
- 36.Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, Rait G. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open. 2014;4(2):e003821. doi: 10.1136/bmjopen-2013-003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1–S14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]


