Abstract
This study involved a cost-utility analysis of early diagnosis and treatment of diabetic retinopathy depending on the screening strategy used. The four screening strategies evaluated were no screening, opportunistic examination, systematic fundus photography, and systematic examination by an ophthalmologists. Each strategy was evaluated in 10,000 adults aged 40 yr with newly diagnosed diabetes mellitus (hypothetical cohort). The cost of each strategy was estimated in the perspective of both payer and health care system. The utility was estimated using quality-adjusted life years (QALY). Incremental Cost Effectiveness Ratio (ICER) for the different screening strategies was analyzed. After exclusion of the weakly dominating opportunistic strategy, the ICER of systematic photography was 57,716,867 and that of systematic examination by ophthalmologists was 419,989,046 from the perspective of the healthcare system. According to the results, the systematic strategy is preferable to the opportunistic strategy from the perspective of both a payer and a healthcare system. Although systematic examination by ophthalmologists may have higher utility than systematic photography, it is associated with higher cost. The systematic photography is the best strategy in terms of cost-utility. However systematic examination by ophthalmologists can also be a suitable policy alternative, if the incremental cost is socially acceptable.
Keywords: Diabetic Retinopathy, Markov Model, Cost-utility Analysis, Quality-Adjusted Life Years (QALY)
Graphical Abstract
INTRODUCTION
Diabetic retinopathy is one of the important complications of chronic diabetes and is characterized by abnormalities in the retinal vasculature. Approximately 2% of patients with diabetes for more than 15 yr can develop blindness, while 10% can develop serious visual impairments (1). However, the silent period during the initial stages, when no symptoms of abnormal vision appear, is quite long; therefore, early detection of diabetic retinopathy is difficult and highly dependent on regular examinations.
In Korea, according to Lim and Choi (2), 36.9% of patients with diabetes aged > 40 yr underwent screening for diabetic retinopathy in the Korea National Health and Nutrition Examination Survey IV. This proportion was markedly lower than that in advanced countries, including 51% in the USA and 59% in the UK (3,4). Several studies regarding screening strategies for diabetic retinopathy and the evaluation of their economic feasibility are being conducted in other countries. In Korea, however, opportunistic examination is most common, and patients with diabetes visit ophthalmologists directly and undergo screening.
Currently, fundus examination following mydriasis is the most widely used screening method for diabetic retinopathy. However, it has certain limitations. For example, patients cannot drive or perform tasks involving near vision on the day of examination, and the procedure can only be performed by experienced ophthalmologists. Recently, fundus photography using a digital nonmydriatic fundus camera was introduced as an alternative to fundus examination following mydriasis. This procedure has the following advantages: shortened examination time, no requirement of particular skills, and decreased discomfort.
However, the suitability of the fundus photograph screening method remains controversial because the sensitivity and specificity are reported to vary depending on the study method, such as the number of shots and the presence or absence of mydriasis (5).
This study aimed to evaluate various screening strategies for diabetic retinopathy and analyze their cost and utility using the Markov model to obtain an empirical estimate of cost and utility from the perspective of a payer and a health care system. We aim to provide basic data that can be used to establish an effective screening policy for this complication.
MATERIALS AND METHODS
Study design
Population
A hypothetical cohort for cost-utility analysis was determined for each screening strategy used in this study. Considering the starting age for national diabetes screening, 10,000 adults who were newly diagnosed with diabetes at the age of 40 yr were included as a hypothetical cohort for each screening strategy (6). Park et al. (7) stated that the average age of patients who die from diabetes is 66.3±10.7 yr, so we assumed that they all would survive until 80 yr when most of patients die. Jin et al. (8) claimed that gender had no particular correlation with this prognosis, so we did not consider the gender of patients.
That is, during the 40 yr of analysis after the initial diagnosis of diabetes at the age of 40, the cost and outcome of the natural progression of diabetic retinopathy estimated for these hypothetical cohorts were used for the cost-utility analysis.
Perspective
In economic analysis studies of health care, analysis perspectives can be classified as a payer perspective, a health care system perspective, and a societal perspective. Generally, a payer perspective includes medical cost, a health care system perspective involves non-medical costs, and a societal perspective involves the cost of lost productivity.
In this study, we basically conducted an analysis from the perspectives of a payer and a health care system to prepare for the possibility that screening for diabetic retinopathy will be implemented systemically at the national level in the future. Meanwhile, we did not conduct an analysis from a societal perspective because of the lack of domestic data regarding the loss of productivity and social expenses created by vision impairment.
Screening strategies
Four screening strategies were analyzed in this study: no screening (no screening group), opportunistic fundus examination by an ophthalmologist (opportunistic examination group), systematic fundus photography using a digital fundus camera (systematic photography group), and systematic examination by ophthalmologists (systematic examination by ophthalmologists group). Opportunistic fundus photography using a fundus camera was excluded, because it is rarely performed and can overlap with opportunistic examination by ophthalmologists.
Modeling
A cost-utility analysis was performed through the Markov model, using cost and outcome data obtained from domestic and international studies. Markov models are well recognized methods for analyzing clinical and economic consequences of medical decisions, particularly in long-term diseases characterized by repeating risks of events over time (9). TreeAge Pro 2013 (TreeAge Software Inc., Williamstown, MA, USA) was used for the analysis. The course of each screening strategy is described below.
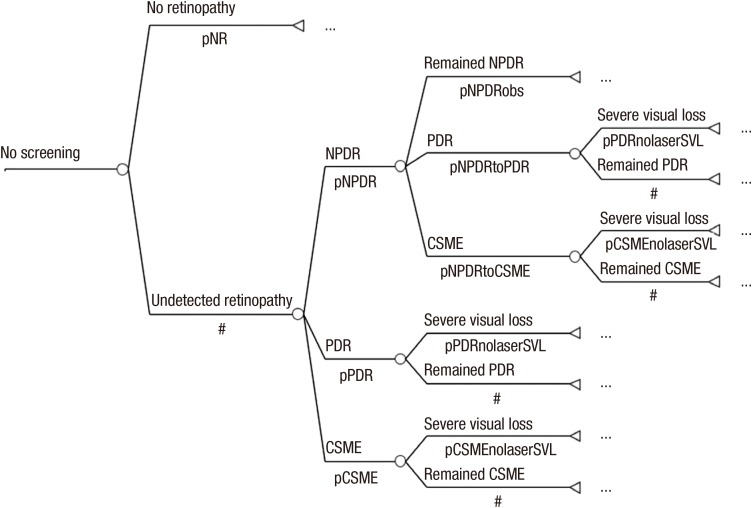
No screening group: The individuals of the no screening group who were diagnosed with diabetes at the age of 40 yr but did not receive a diagnosis of or any treatment for diabetic retinopathy, such they are expected to develop blindness from diabetic retinopathy according to the probabilities of the Markov model. This group was used as the baseline for this study (Fig. 1).
Fig. 1. Markov model of no screening group. A decision node (□) is the decision to test a contact by using the respective screening procedure. Branches from a change node (○) represent the possible outcomes of an event; terminal nodes (◃) are assigned the cost of a prior series of actions and events. Probabilities (p): see model specifications; #: complementary probability (all probabilities of chance node's branches to sum to 1.0). pNR, probability of no retinopathy; pNPDR, probability of NPDR; pPDR, probability of PDR; pCSME, probability of CSME; pNPDRobs, probability of remained NPDR; pNPDRtoPDR, probability of NPDR to PDR; pNPDRtoCSME, probability of NPDR to CSME; pPDRnolaserSVL, probability of PDR to SVL after no laser therapy; pCSMEnolaserSVL, probability of CSME to SVL after no laser therapy.
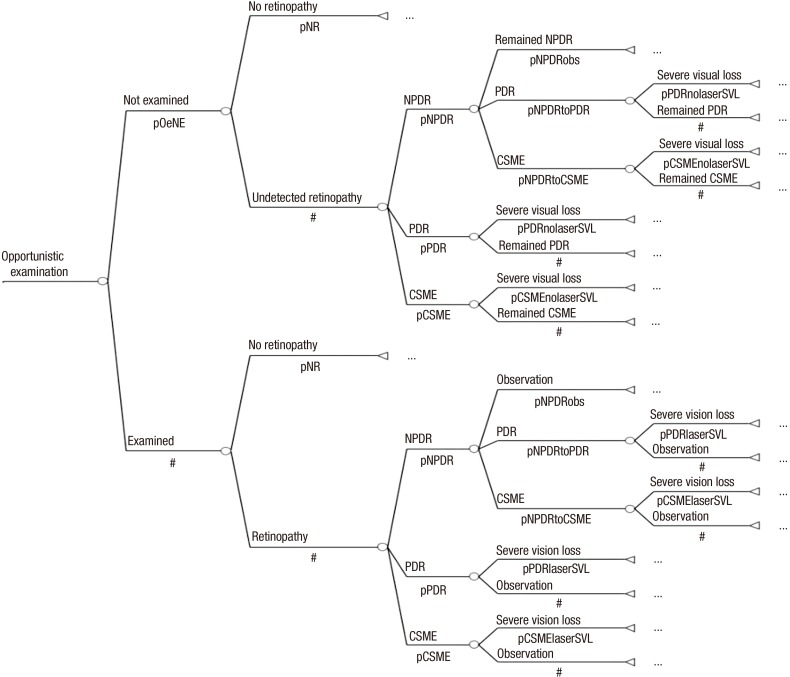
Opportunistic examination group: The opportunistic examination group included patients who visited ophthalmologists and underwent actual fundus examination (fundus examined subgroup), as well as patients who did not undergo fundus examination (not examined subgroup). After being diagnosed with diabetes at the age of 40 yr, this group was evaluated annually during the 40-yr analysis period. The examined subgroup had a reduced risk of blindness because they received proper treatment, but the not examined subgroup ran the same course as the no screening group. The no retinopathy subgroup of the examined subgroup and not examined subgroup were re-evaluated every year (Fig. 2).
Fig. 2. Markov model of opportunistic examination group. pOeNE, probability of not examined in opportunistic examination group; pPDRlaserSVL, probability of PDR to SVL after laser therapy; pCSMElaserSVL, probability of CSME to SVL after laser therapy; pSpNR, probability of no retinopathy in systematic photography group; pSpNPR, probability of negative predictive ratio in systematic photography group; pSpPPR, probability of positive predictive ratio in systematic photography group.
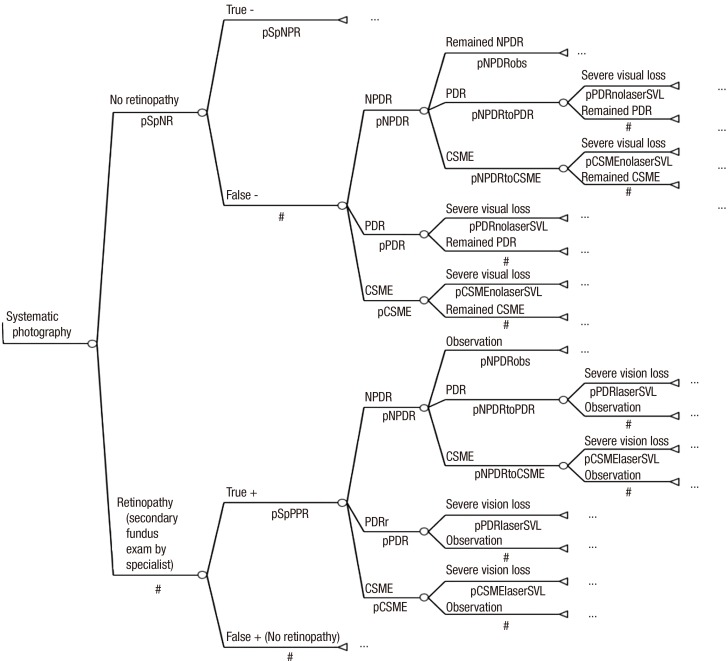
Systematic photography group: The systematic photography group included patients who were diagnosed with diabetes at the age of 40 yr, and it was assumed that everyone in this group underwent fundus photography to determine the maximum utility of systematic fundus photography. In addition, all patients in this group were presumed to undergo secondary fundus examination by ophthalmologists if the photography results were positive. The false positive subgroup and true negative subgroup were re-evaluated every year (Fig. 3).
Fig. 3. Markov model of systematic photography group.
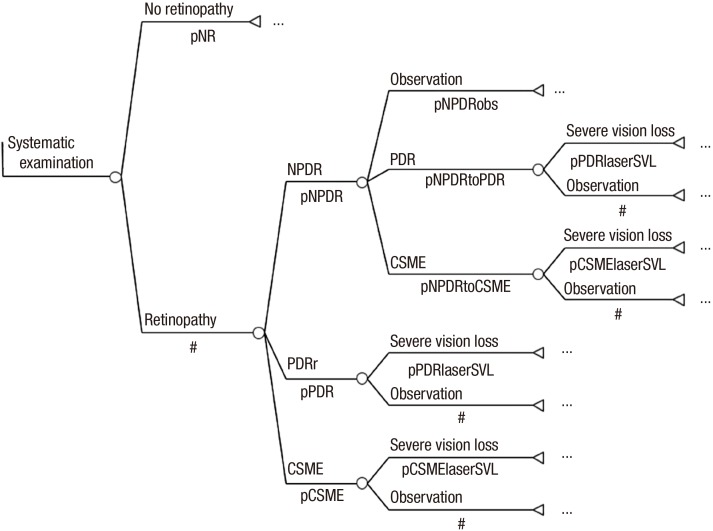
Systematic examination by the ophthalmologists group: The systematic examination by the ophthalmologists group included patients who were diagnosed with diabetes at the age of 40 yr, and it was also assumed that everyone in this group underwent fundus examination by ophthalmologists to determine the maximum utility of systematic fundus examination. The retinopathy subgroup had a reduced risk of blindness because they received proper treatment, and the no retinopathy subgroup was re-examined every year (Fig. 4).
Fig. 4. Markov model of systematic examination by ophthalmologists.
Clinical data sources
The Research Information Sharing Service (RISS, http://www.riss4u.net) provided by the Korea Education & Research Information Service (KERIS) was used to obtain domestic literature, while PubMed (http://ncbi.nih.gov), a biomedical information search engine provided by the U.S. National Institutes of Health National Library of Medicine (NIH/NLM), was used to obtain international literature. Searches were performed using common keywords. Domestic data were referenced as much as possible, while international data were referenced when domestic research data were unavailable.
Prevalence estimation
According to the Korea National Health and Nutrition Examination Survey III results, 28.2% of patients with diabetes for <5 yr and 48.3% of patients with diabetes for >5 yr were diagnosed with diabetic retinopathy (10). Therefore, considering the diabetic retinopathy incidence rate depending on the disease period, we hypothesized that the occurrence of diabetic retinopathy had a probability of 5.64% each year in the cohort of this study.
Kim et al. (11), who took into account the prevalence rate for each stage of diabetic retinopathy, analyzed 158 patients with diabetes who were admitted to an ophthalmologic clinic or a primary medical institute, and determined the incidence of each stage at the first fundus examination as follows: Nonproliferative Diabetic Retinopathy (NPDR), 70.27%; Proliferative Diabetic Retinopathy (PDR), 8.12%; and Clinically Significant Diabetic Retinopathy (CSME), 21.61%. We used these findings as domestic data for the incidence of each stage of diabetic retinopathy.
Meanwhile, Lim and Choi (2) analyzed Korea National Health and Nutrition Examination Survey IV results and stated that 464 of 1,257 patients underwent ophthalmological examination for diabetic retinopathy within a year, accounting for only 36.9% of patients. Therefore, the probability of opportunistic examination by ophthalmologists was presumed to be 36.9%.
Sensitivity and specificity of screening tools
Maberley et al. (12) conducted a systematic analysis of diabetic retinopathy and estimated the sensitivity of fundus examination by ophthalmologists as 0.95, because in Canada ophthalmologists rarely perform primary retinal examinations, and the sensitivity of fundus examination using a direct ophthalmoscope by general practitioners was low at 0.8. However, because of the high accessibility of ophthalmologists in Korea, most retinal examinations for diabetic retinopathy are performed directly by ophthalmologists, unlike in Canada. Therefore, our study assumed that a definite diagnosis of diabetic retinopathy using fundus examination by ophthalmologists is possible.
A study on the utility of nonmydriatic fundus photography for diabetic retinopathy screening showed that a retinal image obtained using a nonmydriatic fundus camera had a sensitivity of 47.6%, specificity of 97.6%, positive predictive value of 45.5%, and negative predictive value of 97.8% (4). Our study used these data to predict the sensitivity and specificity of a fundus camera and performed sensitivity analysis by comparison with studies from other countries.
Transition probability estimation
Well-designed cohort data are required to estimate the transition probability of diabetic retinopathy during each stage, but no studies have used domestic patients as subjects. Therefore, our study used the transition probability presented in the study by Aoki et al. (13), despite the limitation that it was conducted in a different country. In their study on cost-utility analysis of diabetic retinopathy, the results of the Liverpool Diabetic Eye Study, a long-term cohort study from the UK, were used to determine the probability of progression for each stage of diabetic retinopathy, and the results of 15-yr laser treatment in the Diabetic Retinopathy Study from the USA were used to determine the probability of a good prognosis of retinal laser treatment (14,15).
Utility data sources
In this study, utility was estimated from the QALY score. That is, the utility-weighted value for each stage of diabetic retinopathy was applied according to each screening strategy, and the increased QALY score was calculated. The utility-weighted value for each stage of diabetic retinopathy was estimated from existing domestic and international research data using the following method.
QALY of diabetic retinopathy stages
From the report by the Organization of Services for Diabetic Retinopathy Screening in the UK, Facey et al. (16) set the QALY score for no retinopathy as 0.89, that of NPDR as 0.89, and that of PDR as 0.72. Heintz et al. (17) reported the QALY score for no retinopathy as 0.88, that for NPDR as 0.86, that for PDR as 0.81, and that for CSME as 0.83 through a systematic literature search. This study used these data to estimate the QALY score for each stage of diabetic retinopathy.
QALY of severe vision loss
In the study by Brown et al. (18), the QALY score for severe visual loss (SVL) was 0.59 according to the TTO method and 0.70 according to the SG method. Maberley et al. (12) also referenced this study and assumed the QALY score for SVL caused by diabetic retinopathy to be 0.59. Our study also set the QALY score for SVL caused by diabetic retinopathy as 0.59.
Cost data sources
Medical cost
The screening cost for fundus photography was calculated by summing the health insurance fees for fundus photography, basic (E6670) and refraction tests (E6710). Because health insurance fees vary by the type of health care provider, we used the weighted average fees considering the frequencies of these tests for each type of health care provider. The frequencies were calculated using the 2011 Korea National Patient Sample (KNPS), which was constructed from health insurance claims data by the Health Insurance Review and Assessment Service (HIRA). The screening cost for examination using fundus photography derived by this method was KRW (Korean Won) 17,863 per patient (Table 1).
Table 1. Medical cost of diabetic retinopathy.
| Cost items | Cost (KRW) | ||
|---|---|---|---|
| Screening cost | Fundus photography method | Fundus photography basic (E6670) | 8,329 |
| Refraction test (E6710) | 9,534 | ||
| Fundus examination method | Fundus examination (E6660) | 7,299 | |
| Slit-lamp biomicroscopy (E6810) | 2,114 | ||
| Refraction test (E6710) | 9,534 | ||
| Tonometry (E6752) | 2,101 | ||
| Doctor's consultation for outpatient | 13,743 | ||
| Management of diabetic retinopathy cost | Annual NPDR follow-up | Fundus examination method * 2 | 91,434 |
| Fundus fluorescein angiography * 1/2 | 38,353 | ||
| Annual PDR follow-up | Fundus examination method * 4 | 182,869 | |
| Fundus fluorescein angiography * 1 | 76,707 | ||
| Optical coherence tomography * 1 | 165,000 | ||
| Annual CSME Follow-up | Fundus examination method * 4 | 182,869 | |
| Fundus Fluorescein angiography * 1 | 76,707 | ||
| Optical coherence tomography * 1 | 165,000 | ||
| PDR laser therapy | Fundus examination method * 10 | 457,172 | |
| Fundus photocoagulation * 10 | 906,098 | ||
| CSME laser therapy | Fundus examination method * 4 | 182,869 | |
| Fundus photocoagulation * 4 | 362,439 | ||
KRW, Korean won (average exchange rate in 2013 was 1 USD=1,094.70 KRW); NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; CSME, clinically significant macula edema.
The screening cost for fundus examination by ophthalmologists was calculated by summing the health insurance fees for fundus examination (E6660), slit lamp microscopy (E6810), refraction test (E6710), intraocular pressure examination (E6752), and doctor's consultation for an outpatient (AA154-AA157). As before, we used the weighted average fees considering the frequencies of each type of health care provider. The screening cost for fundus examination was calculated to be KRW 34,791 per patient (Table 1).
The management cost for diabetic retinopathy was calculated by summing the costs of the follow-up fundus examinations and treatments for diabetic retinopathy. In this study, we used standard guidelines from the Diabetic Retinopathy Study (DRS) and Early Treatment of Diabetic Retinopathy Study (ETDRS) to establish the process of monitoring and treatment of diabetic retinopathy at each stage: fundus examination every 6 months and fluorescence fundus photography every 2 yr for NPDR, fundus examination every 3 months and fluorescence fundus photography and Optical Coherence Tomography (OCT) every year for PDR and CSME, fundus examination and 10 panretinal photocoagulations (five per eye) for PDR, and fundus examination and four retinotopic photocoagulations for both eyes (two per eye) for CSME. As before, we used the weighted average fees for examinations and treatments considering the frequencies for each type of health care provider. In addition, we considered the cost of uninsured services that patients have to pay. According to the 2012 Health Insurance Patients' Actual Medical Expenses Survey, the uninsured out-of-pocket rate was 23.9% for outpatients, so we adjusted the cost of covered services by this rate. As a result, the cost of fundus examination by ophthalmologists, including the noninsured out-of-pocket fee, was KRW 45,717. The cost for fluorescence fundus angiography was KRW 76,707 for both eyes, while that for fundus photocoagulation was KRW 90,610. The cost for Optical Coherence Tomography (OCT) was calculated using the average customary fees charged by hospitals because a health insurance fee for this procedure does not exist. The cost for OCT per patient was KRW 165,000.
Non-medical cost
For transportation costs incurred during diabetic retinopathy screening, we took into account data from the Korea National Health and Nutrition Examination Survey and the inflation rate. The resulting cost of a round trip for an outpatient visit for diabetic retinopathy management was KRW 5,141. If fundus laser therapy was scheduled, an accompanying guardian may have been needed, and we assumed the accompanying guardian rate to be 80%. Therefore the cost of a round trip for an outpatient visit for fundus laser treatment was KRW 9,254 including the transportation costs of the guardian (Table 2).
Table 2. Non-medical cost of diabetic retinopathy.
| Cost items | Cost (KRW) | |
|---|---|---|
| Transportation cost | Fundus photography screening method | 2,571 |
| Fundus examination screening method | 5,141 | |
| Out-patient management | 5,141 | |
| Fundus laser therapy | 9,254 | |
| Time cost | Fundus photography screening method | 11,283 |
| Fundus examination screening method | 17,664 | |
| Out-patient management | 17,664 | |
| Fundus laser therapy | 22,557 | |
| Nursing cost of a guardian | Fundus laser therapy | 14,326 |
KRW, Korean won (average exchange rate in 2013 was 1 USD=1,094.70 KRW).
To calculate the cost of lost productive work time due to diabetic retinopathy screening, outpatient treatment, and outpatient laser treatment, we used the employment rate for each standard age from the Korean Statistical Information Service (KOSIS), provided by the National Statistical Office, and data on labor conditions, provided by an employment type survey, a national public health and medical condition survey, and the Korea National Health and Nutrition Examination Survey. The resulting costs were KRW 11,283 for fundus photography, KRW 17,664 for fundus examination by ophthalmologists, and KRW 22,557 for retinal laser treatment (Table 2).
To calculate the nursing cost, the cost of lost productive work time for a guardian who was from the economically active population (over 15 yr of age) was calculated. The resulting cost of lost productive work time for a guardian was KRW 17,908. We assumed the rate for an accompanying guardian for retinal laser treatment to be 80%; therefore, the final cost of lost productive work time for a guardian was KRW 14,326 (Table 2). We assumed that screening examinations and other diabetic retinopathy management do not require a guardian, and thus we did not consider nursing costs for these.
Our study applied a discount rate of 5% as a basic value and conducted a sensitivity analysis when applying other discount rates. For utility, a discount was not applied to enable direct interpretation.
RESULTS
Cost-utility analysis
When the Incremental Cost Effectiveness Ratio (ICER) for the different screening strategies was analyzed from a payer perspective, the value for the opportunistic examination group was 48,961,339, that for the systematic photography group was 43,575,592, and that for the systematic examination by the ophthalmologists group was 308,193,813 (Table 3). Because of a higher utility and lower ICER for systematic photography compared with opportunistic examination, the opportunistic examination group was weakly dominated. Therefore, when cost-utility analysis was performed after excluding the opportunistic examination group, which was the inferior alternative, ICER was 45,588,788 for the systematic photography group and 308,193,813 for the systematic examination by the ophthalmologists group (Table 3).
Table 3. Incremental cost effectiveness ratio of diabetic retinopathy screening methods according to a payer perspective.
| Comparators | ΔCost*(KRW) | ΔQALY† | ICER‡ (ΔC/ΔQ) |
|---|---|---|---|
| No screening (base line) | - | - | - |
| Opportunistic examination | 12,744,808,602 | 260.3 | 48,961,339 |
| Systematic photography | 19,001,854,196 | 436.07 | 43,575,592 |
| Systematic examination by ophthalmologists | 2,792,113,902 | 9.06 | 308,193,813 |
| No screening (base line) | - | - | - |
| Opportunistic examination | Weakly dominated | ||
| Systematic photography | 31,746,662,798 | 696.37 | 45,588,788 |
| Systematic examination by ophthalmologists | 2,792,113,902 | 9.06 | 308,193,813 |
Discounting rate 5%. *Incremental cost per 10,000 person; †Incremental quality-adjusted life years per 10,000 person; ‡Incremental cost effectiveness ratio per 10,000 person. KRW, Korean won.
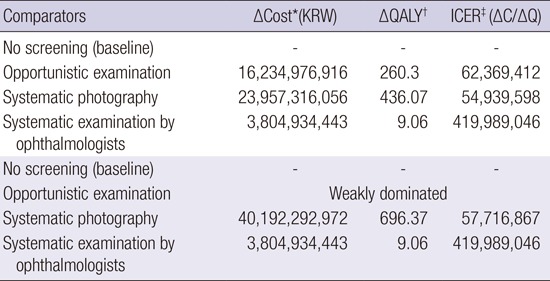
Meanwhile, from the perspective of a health care system, ICER was 62,369,412 for the opportunistic examination group, 54,939,598 for the systematic photography group, and 419,989,064 for the systematic examination by the ophthalmologists group. As before, the opportunistic examination group was excluded from the cost-utility analysis because it was weakly dominated. After exclusion, ICER for systematic photography was 57,716,867 (Table 4).
Table 4. Incremental cost effectiveness ratio of diabetic retinopathy screening methods according to health care system perspective.
| Comparators | ΔCost*(KRW) | ΔQALY† | ICER‡ (ΔC/ΔQ) |
|---|---|---|---|
| No screening (baseline) | - | - | - |
| Opportunistic examination | 16,234,976,916 | 260.3 | 62,369,412 |
| Systematic photography | 23,957,316,056 | 436.07 | 54,939,598 |
| Systematic examination by ophthalmologists | 3,804,934,443 | 9.06 | 419,989,046 |
| No screening (baseline) | - | - | - |
| Opportunistic examination | Weakly dominated | ||
| Systematic photography | 40,192,292,972 | 696.37 | 57,716,867 |
| Systematic examination by ophthalmologists | 3,804,934,443 | 9.06 | 419,989,046 |
Discounting rate 5%. *Incremental cost per 10,000 person; †Incremental quality-adjusted life years per 10,000 person; ‡Incremental cost effectiveness ratio per 10,000 person. KRW, Korean won.
Sensitivity analysis
Factors that can influence the cost-utility rate, which was the ultimate result of this study, include the compliance rate for diabetic retinopathy screening, prevalence of diabetic retinopathy, sensitivity and specificity of each screening strategy, and discount rate for costs.
Sensitivity analysis was conducted after setting the compliance rates for systematic photography and systematic examination by ophthalmologists at 95%, 80%, 65%, and 50%. There was no difference when calculated from the perspective of a payer versus a health care system. However, if the compliance rate for systematic examination by ophthalmologists becomes more than 10% lower than that for systematic photography, the cost and utility for systematic photography will increase, considerably affecting the interpretation of the results. Therefore, discussing the compliance rate for the examination as well as ICER is required for the decision-making process with regard to systematic screening strategies for diabetic retinopathy.
Sensitivity analysis regarding the incidence of diabetic retinopathy and the prevalence of each stage was conducted on the basis of the Korea National Health and Nutrition Examination Survey and the studies by Fong et al. (19) and Rema et al. (20). There were no differences in results when calculated from the perspective of a payer versus a health care system, with the exception of the cost difference.
Sensitivity and specificity analyses for digital fundus cameras were conducted based on the reports by Lee et al. (5), Usher et al. (21), and James et al. (22), and there were no differences in results when calculated from the perspective of a payer versus a health care system, with the exception of the cost difference.
A 5% discount rate for the cost was applied in the basic analysis, but when additional 3% and 7.5% discount rates were considered in the sensitivity analysis, there were no differences in results when calculated from the perspective of a payer versus a health care system, with the exception of the cost difference.
DISCUSSION
In Korea, national screening for diabetes is implemented by the National Health Insurance Services, and economic analyses for this disease are underway. However, with regard to screening for diabetic retinopathy, opportunistic examination, in which patients visit ophthalmologists directly for examination, remains the most prevalent strategy. Our study evaluated the different screening strategies for diabetic retinopathy to prevent a decrease in quality of life caused by blindness and empirically estimated the cost and utility for these strategies.
Many studies have demonstrated that a diabetic retinopathy screening program has more advantages than no diabetic retinopathy screening. Maberley et al. (12) reported that compared with no screening, the retina-specialist program showed a 14.6 QALY increase and the retinal-camera program a 17.4 QALY increase. Aoki et al. (13) reported 18.73 QALYs with the systematic teleophthalmology strategy and 18.58 QALYs using the opportunistic non-teleophthalmology strategy. In our study, the QALY score for diabetic retinopathy screening showed increased utility for all the strategies, compared with that for no diabetic retinopathy screening. This finding indicates that diabetic retinopathy screening can result in an improved quality of life.
Among diabetic retinopathy screening strategies, systematic examination is more cost-effective than opportunistic examination. James et al. (22) reported that the cost effectiveness of the systematic program was £209 per true positive, and that of the opportunistic program was £289 per true positive. Also, the incremental cost effectiveness of completely replacing the opportunistic program was £32 per true positive. Aoki et al. (13) reported that the average cost effectiveness was $882 per QALY for the systematic teleophthalmology and $947 for the opportunistic non-teleophthalmology strategies. Also, the systematic teleophthalmology strategy is dominant in the incremental cost-effectiveness analysis, because it costs less and leads to a greater QALY gain. In our study, when the ICER was analyzed, the opportunistic examination group was weakly dominated by the systematic examination groups both from the perspective of a payer and from that of a health care system. Therefore, systematic examination is more cost-effective than is opportunistic examination.
Maberley et al. (12) reported that the systematic camera program was more cost-effective, maintaining the highest number of sight years and being cheaper than the systematic specialist-based program. In our study, with regard to ICER, systematic photography was superior to systematic examination by ophthalmologists. That is to say, to screen 10,000 patients aged >40 yr and first diagnosed with diabetes through systematic examination by ophthalmologists, which has higher utility, approximately 282,000 USD (1 USD=1,094.70 Korean Won [KRW] in 2013) for each QALY from a payer perspective and approximately 384,000 USD for each QALY from a health care system perspective were required in addition to the amount required for systematic photography. Considering that the diabetic population is estimated to be 3 million in Korea, 82 million USD from a payer perspective and 115 million USD from a health care system perspective are additionally required. Therefore, social discussion is needed for the payment of additional costs based on the calculated ICER values.
Meanwhile, fundus photography and systematic examination by ophthalmologists showed differences in results depending on the compliance rate. Besides, many variables such as the presence or absence of mydriasis, fundus area included in the photograph, number of photographs, shooting method, and the examiner's experience may influence the results. Therefore, policymakers should consider these variables when selecting a screening strategy for diabetic retinopathy.
The limitations of this study are as follows. First, a large-scale cohort was lacking for the domestic research data, and thus we used international data to obtain the transition rate for each stage of diabetic retinopathy and the utility for each stage. Second, although clinically significant CSME can actually be present during all stages of diabetic retinopathy, our study classified it as CSME when it merged with NPDR and as PDR when it merged with PDR. Third, to examine the results when the goal of systematic examination was best achieved, the compliance rate for fundus photography and fundus examination by ophthalmologists was set at 100%. Fourth, because of the lack of domestic data regarding the loss of productivity and social costs resulting from unemployment due to visual impairment, the loss of productivity cost due to diabetic retinopathy could not be calculated. Fifth, we did not consider the cost or utility of the recent treatment methods for diabetic retinopathy complications, such as vitreous humor resection, intraocular steroid injection, and intraocular injection of antivascular endothelial growth factor (anti-VEGF). When data regarding the utility of these new treatments becomes available through large-scale experimental studies in the future, an analysis of additional costs for the increased utility will be necessary.
Despite these limitations, we can present the following proposals for diabetic retinopathy screening on the basis of our study. First, it is necessary to consider the introduction of systematic examination by fundus photography programs for diabetic retinopathy in national screening. However, this requires a proper guideline and techniques for improving the accuracy of fundus photography in patients with diabetic retinopathy. Second, efforts by health institutions and academia are needed to increase the current rate of opportunistic examination to the rates in advanced countries through intensive education on diabetic retinopathy and through the promotion of regular retinal examinations for patients with diabetes. Third, because the costs for examination by ophthalmologists are not very high in Korea, unlike in other countries, systematic examination by ophthalmologists can also be a suitable policy alternative in our country.
Footnotes
DISCLOSURE: The authors declare that no potential conflicts of interest exist.
AUTHOR CONTRIBUTION: Design of the study: Kim SW, Kang GW. Collection and management of data: Kim SW, Kang GW. Analysis and interpretation of data: Kim SW. Preparation of manuscript and statistical analysis: Kim SW. Review and approval of manuscript: Kim SW, Kang GW.
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14:S1–S85. [PubMed] [Google Scholar]
- 2.Lim HT, Choi KS. Factors associated with screening for diabetic retinopathy in diabetic patients aged > or = 40 years using the KNHANES IV. J Korean Ophthalmol Soc. 2012;53:516–521. [Google Scholar]
- 3.Beckles GL, Zhu J, Moonesinghe R Centers for Disease Control and Prevention (CDC) Diabetes - United States, 2004 and 2008. MMWR Surveill Summ. 2011;60:s90–s93. [PubMed] [Google Scholar]
- 4.Yudkin JS, Boucher BJ, Schopflin KE, Harris BT, Claff HR, Whyte NJ, Taylor B, Mellins DH, Wootliff AB, Safir JG, et al. The quality of diabetic care in a London health district. J Epidemiol Community Health. 1980;34:277–280. doi: 10.1136/jech.34.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Park CY, Song SJ. Study on survey of knowledge and awareness level of diabetic retinopathy in type 2 diabetes patients: results from Seoul metro-city diabetes prevention program survey. J Korean Ophthalmol Soc. 2011;52:1296–1301. [Google Scholar]
- 6.Jeong H, Kwon H, Han J, Kim Y, Lee A. Cost-effectiveness analysis of type 2 DM screening program of national health insurance corporation. Korean J Health Econ Policy. 2008;14:29–50. [Google Scholar]
- 7.Park SK, Park M-K, Suk JH, Kim MK, Kim YK, Kim IJ, Kang YH, Lee KJ, Lee HS, Lee CW. Cause-of-death trends for diabetes mellitus over 10 years. Korean Diabetes J. 2009;33:65–72. [Google Scholar]
- 8.Jin JH, Lee SJ, Lee HS, Kim SD. Prognostic factors of visual acuity in diabetes mellitus. J Korean Ophthalmol Soc. 2006;47:755–762. [Google Scholar]
- 9.Drummond MF. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005. [Google Scholar]
- 10.Shin KH, Chi MJ. Fundus examination rate in diabetics and the public health factors associated with fundus examination rate. J Korean Ophthalmol Soc. 2009;50:1319–1325. [Google Scholar]
- 11.Kim HK, Oh TS, Lee SM, Lee JB. The initial fundus examination and severity of diabetic retinopathy at a primary eye clinic. J Korean Ophthalmol Soc. 2005;46:982–988. [Google Scholar]
- 12.Maberley D, Walker H, Koushik A, Cruess A. Screening for diabetic retinopathy in James Bay, Ontario: a cost-effectiveness analysis. CMAJ. 2003;168:160–164. [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki N, Dunn K, Fukui T, Beck JR, Schull WJ, Li HK. Cost-effectiveness analysis of telemedicine to evaluate diabetic retinopathy in a prison population. Diabetes Care. 2004;27:1095–1101. doi: 10.2337/diacare.27.5.1095. [DOI] [PubMed] [Google Scholar]
- 14.Younis N, Broadbent DM, Vora JP, Harding SP Liverpool Diabetic Eye Study. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. 2003;361:195–200. doi: 10.1016/s0140-6736(03)12267-2. [DOI] [PubMed] [Google Scholar]
- 15.Blankenship GW. Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute's patients participating in the diabetic retinopathy study. Ophthalmology. 1991;98:125–128. doi: 10.1016/s0161-6420(91)32326-1. [DOI] [PubMed] [Google Scholar]
- 16.Facey K, Cummins E, Macpherson K, Morris A, Reay L, Slattery J. Health technology assessment report 1: Organisations of services for diabetic retinopathy screening. Glasgow: Health Technology Board for Scotland; 2002. [Google Scholar]
- 17.Heintz E, Wiréhn AB, Peebo BB, Rosenqvist U, Levin LA. QALY weights for diabetic retinopathy--a comparison of health state valuations with HUI-3, EQ-5D, EQ-VAS, and TTO. Value Health. 2012;15:475–484. doi: 10.1016/j.jval.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Brown MM, Brown GC, Sharma S, Shah G. Utility values and diabetic retinopathy. Am J Ophthalmol. 1999;128:324–330. doi: 10.1016/s0002-9394(99)00146-4. [DOI] [PubMed] [Google Scholar]
- 19.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, Klein R. Retinopathy in diabetes. Diabetes care. 2004;27:s84–s87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 20.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–2333. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 21.Usher D, Dumskyj M, Himaga M, Williamson TH, Nussey S, Boyce J. Automated detection of diabetic retinopathy in digital retinal images: a tool for diabetic retinopathy screening. Diabet Med. 2004;21:84–90. doi: 10.1046/j.1464-5491.2003.01085.x. [DOI] [PubMed] [Google Scholar]
- 22.James M, Turner DA, Broadbent DM, Vora J, Harding SP. Cost effectiveness analysis of screening for sight threatening diabetic eye disease. BMJ. 2000;320:1627–1631. doi: 10.1136/bmj.320.7250.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]