Abstract
The use of caffeine citrate for treatment of apnea in very low birth weight infants showed short-term and long-term benefits. A systematic review and meta-analysis of the literature was undertaken to document the effect providing caffeine early (0-2 days of life) compared to providing caffeine late (≥3 days of life) in very low birth weight infants on several neonatal outcomes, including bronchopulmonary dysplasia (BPD). We searched MEDLINE, the EMBASE database, the Cochrane Library, and KoreaMed for this meta-analysis. The quality of the included studies was assessed using the Newcastle-Ottawa Scale and Jadad's scale. Studies were included if they examined the effect of the early use of caffeine compared with the late use of caffeine. Two reviewers screened the candidate articles and extracted the data from the full-text of all of the included studies. We included a total of 59,136 participants (range 58,997-59,136; variable in one study) from a total of 5 studies. The risk of death (odds ratio [OR], 0.902; 95% confidence interval [CI], 0.828 to 0.983; P=0.019), bronchopulmonary dysplasia (BPD) (OR, 0.507; 95% CI, 0.396 to 0.648; P<0.001), and BPD or death (OR, 0.526; 95% CI, 0.384 to 0.719; P<0.001) were lower in the early caffeine group. Early caffeine use was not associated with a risk of necrotizing enterocolitis (NEC) and NEC requiring surgery. This meta-analysis suggests that early caffeine use has beneficial effects on neonatal outcomes, including mortality and BPD, without increasing the risk of NEC.
Keywords: Caffeine; Very Low Birth Weight; Bronchopulmonary Dysplasia; Infant, Newborn; Outcome
INTRODUCTION
Methylxanthines, caffeine citrate, or others have been used to reduce the frequency of apnea in preterm infants (1,2,3). Most neonates who are born at a gestational age<29 weeks or a birth weight<1,000 g experience apnea of prematurity (4), and caffeine is usually used until the post-menstrual age of 34 to 35 weeks when apnea of prematurity resolves (5). In addition to reducing the frequency of apnea, the use of caffeine in preterm infants weighing less than 1,250 g showed additional short-term and long-term benefits (5,6). Although caffeine acts as an antagonist of adenosine and has protective effects by reducing energy demands after cell injury or hypoxic stress (7), caffeine also shows a protective effect on the developing brain (8,9,10) and has anti-inflammatory effects (11,12,13). Moreover, the early initiation of caffeine therapy, earlier than 3 days of life, was associated with improved neonatal outcomes, including bronchopulmonary dysplasia (BPD), in recent reports (14,15,16,17). Due to these actions, early caffeine use may have beneficial effects in premature or immature organs during vulnerable periods.
We aimed to document the effect of the early initiation of caffeine on neonatal outcomes in this meta-analysis.
MATERIALS AND METHODS
Method of searching and identifying studies
Medline, EMBASE, Cochrane Library, and KoreaMed were searched by using the terms "methylxanthine," "aminophylline," "theophylline," or "caffeine" and the MESH term "infant," "infant, newborn disease," or "infant, premature disease." There were no restrictions on language, population, or publication year. The last search was performed on September 4, 2014. The titles and abstracts of the articles were initially screened, and the full-text articles were reviewed when any of the reviewers considered the abstract to be eligible. We excluded case reports, case series, review articles, editorials, and comments. All the studies were separately reviewed by two reviewers to determine their relevance in the meta-analysis by using specific selection criteria.
Study selection
The studies were included in this meta-analysis if they met the following criteria. 1) Study design: randomized controlled trial, case-control study, or prospective or retrospective matched cohort study; 2) Patients: very low birth weight (VLBW) infants (birth weight<1,500 g) who were treated with caffeine during hospitalization; 3) Interventions: the early use of caffeine (0-2 days of life) vs. the late use of caffeine (≥3 days of life); and 4) Outcomes: death, BPD, and BPD or death, or secondary outcomes of neonatal morbidity such as intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP) requiring laser photocoagulation, patent ductus arteriosus (PDA) requiring treatment, necrotizing enterocolitis (NEC) (medical or surgical), NEC requiring surgical treatment, and duration of mechanical ventilation.
Case reports, case series, or single arm cohort studies were excluded from the meta-analysis.
Data extraction and quality assessment
Two authors independently extracted the data from the full-text articles of all of the included studies. The following data were extracted: the first author, publication year, study design, study location, study period, study population, gestational age at birth, birth weight, sample size, proportion of males, duration of caffeine therapy, and all outcome measures (including death, BPD, PDA, NEC, PVL, IVH, ROP requiring laser photocoagulation, and duration of mechanical ventilation). Any discrepancies in the interpretations of the data were resolved by discussion with a third reviewer.
The two authors separately assessed the quality of the included studies. Any disagreement was resolved by discussion with a third reviewer, after which the study was reevaluated. We evaluated randomized controlled trials by using Jadad's scale (18) and cohort studies by using the Newcastle-Ottawa Scale (19).
The Newcastle-Ottawa Scale is composed of 8 items evaluating 3 domains: the selection (4 items), comparability (1 items), and outcome (3 items) in the cohort studies. Each domain that meets the criteria is given 1 star, except for the comparability domain wherein 2 stars can be given. Accordingly, the methodological quality of the studies was evaluated by using the total number of stars given, where the maximum total score was 9. A total score of≤3 was considered to represent "low quality," a score of 4 or 5 was considered to represent "moderate quality," and a score of ≥6 was considered to represent "high quality." Jadad's scale assesses the quality of randomized control trials based on whether the study reported information on randomization, double blinding, and drop-outs. The total Jadad score ranges from 0 (bad) to 5 (good), and a total score of 4/5 corresponds to a "good trial."
Data synthesis and analysis
We assessed the heterogeneity between studies by using I2 statistics. The value of I2 was expressed as a percentage of the total variation across studies, where I2 >50% is representative of significant heterogeneity. No heterogeneity was observed between studies, and the meta-analysis was performed by using a fixed effect model. If significant heterogeneity was noted between studies, a random effects model was used for the analysis. Sensitivity analysis was performed by removing each study sequentially to evaluate the robustness of the combined estimates and to examine its contribution to the results of the pooled odds ratio (OR).
The Begg and Mazumdar rank correlation test and Egger's regression test were used to determine publication bias. Publication bias was also evaluated according to the distribution of the effect sizes against the standard errors of a graphically displayed funnel plot. The meta-analysis was performed using Comprehensive Meta-Analysis software version 2.0 (Biostat Inc., Englewood, NJ, USA).
Definition of neonatal outcomes
The definitions of BPD used in the studies included the requirement of oxygen or respiratory support at a post-menstrual age of 36 weeks in studies by Patel et al. (17) and Taha et al. (16). Based on the gestational age at birth, in Dobson's study (15), infants born with a gestational age of<32 weeks were defined as having BPD, whereas infants born with a gestational age of ≥32 weeks were defined as requiring oxygen or respiratory support at 28-34 days of life. A diagnosis of NEC relied on the documentation of NEC in the patient charts or the documentation of surgery. PDA treatment was defined as pharmacologic treatment administered after 3 days of life, excluding the prophylactic use of indomethacin, in 2 studies (15,17). In the CAP trial, Davis et al. (14) defined PDA treatment as the use of drugs without any limitation on the timing of medication or surgical ligation to close the PDA (5).
RESULTS
Literature search and selection
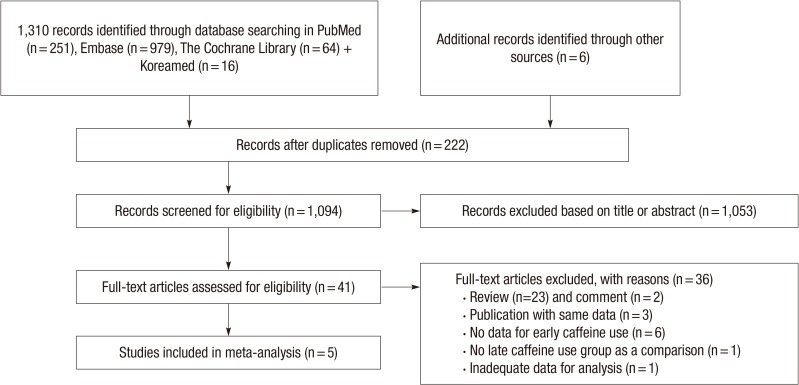
Fig. 1 shows how relevant studies were identified and why studies were excluded for this meta-analysis. Among a total of 1,316 studies that were identified from the initial search, 222 duplicates were removed. After reviewing the abstracts and titles of 1,094 studies, 41 articles remained. Through the full text review, 34 studies were excluded (the reasons are described in Fig. 1), and 5 reports were included in this meta-analysis.
Fig. 1. Flow diagram of the literature search and study selection process. The values in parentheses indicate the number of documents corresponding to each category.
Characteristics of the included studies
A total of 5 studies (14,15,16,17,20), 59,136 (range 58,997-59,136; a variable number of the study population among the reported outcomes from the study of Davis et al. (14), Table 1) infants born with a birth weight<1,500 g met the inclusion criteria of this meta-analysis. For the BPD outcome analysis, 59,093 infants (33,522 in the early caffeine group and 25,571 in the late caffeine group) were included. The characteristics of the included studies for this meta-analysis of the effect of the early initiation of caffeine therapy (before 3 days of life) on neonatal morbidity or mortality are shown in Table 1.
Table 1. Characteristics of the studies included in the meta-analysis.
| Authors/year | Study design | Location; study period | Population | Gestational age at birth (median or mean, week) | Birth weight at birth (median or mean, g) | Duration of caffeine therapy (day) |
|---|---|---|---|---|---|---|
| Patel et al., 2013 (17) | Retrospective cohort | USA; Jan. 2008-June 2010 | Birth weight ≤ 1,250 g total: 104 (EC: 83, LC: 57) |
Median, EC: 40, LC: 39.5 |
Median, EC: 940, LC:910 | Median, EC: 40, LC: 39.5 |
| Taha et al., 2014 (16) | Retrospective cohort | USA; June 2006-May 2011 | Birth weight ≤ 1,250 g treated with caffeine within 10 days of life total: 2,951 (EC: 1,986, LC: 965) | Mean, EC: 27.5, LC 27.2 | Mean, EC: 938, LC: 899 | Median, EC: 50, LC: 49 |
| Dobson et al., 2014 (15) | Retrospective cohort | USA; 1997-2010 | Birth weight < 1,500 g total: 54,707 (EC: 30,891, LC: 23,816) | Median, EC: 28.2, LC: 27.7 | Median, EC: 1,076, LC:1,009 | Median, EC: 36, LC: 30 Mean, EC: 37, LC: 33 |
| Abbasi et al., 2010 (20) | Retrospective cohort, (abstract) | USA, multicenter gene targets IVH study; NA | Birth weight 500-1,250 g 166 case/control pairs | NA | NA | NA |
| Davis et al., 2010 (14) | Randomized controlled trial | Caffeine for Apnea of Prematurity (CAP) Trial Group (Canada, US, Australia, Europe and Israel; Oct. 11, 1999-Oct. 22, 2004) | Birth weight of 500 to 1,250 g treated with caffeine within 10 days of life post hoc analysis with CAP trial total: 867-1,006 (EC:348-413, LC:519-593, variable among reported outcomes) | NA | NA | NA |
EC, early caffeine group; LC, late caffeine group; NA, not available.
Among the 4 retrospective cohort studies, the full text was available for 3 studies (15,16,17), and all were awarded 8 stars in the quality assessment by the Newcastle-Ottawa Scale, except for 1 study by Abbasi et al. (20), which was available as an abstract only and was awarded 4 stars because of the limited information that was available (Table 2). We assessed the study of Davis et al. (14), which was based on the previously reported Caffeine therapy for Apnea of Prematurity (CAP) trial (5) that was a randomized controlled trial. The Jadad's score for randomized control trials (18) was 4 because the information for double blinding was not available.
Table 2. Newcastle-Ottawa scale for the risk of bias and the quality assessment of the included studies.
| Authors | Year | Selection | Comparability | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Length of follow-up | Adequacy of follow-up of cohorts | |||
| Patel et al. (17) | 2013 | * | * | * | * | * | * | * | 8 | |
| Taha et al. (16) | 2014 | * | * | * | * | * | * | * | 8 | |
| Dobson et al. (15) | 2014 | * | * | * | * | * | * | * | 8 | |
| Abbasi et al. (20) | 2010 | NA | * | NA | NA | NA | * | * | * | 4 |
NA, not available.
In the 4 studies, except the Dobson study, the study population had birth weights≤1,250 g. Dobson's study included infants with birth weights<1,500 g. All of the studies defined the initiation of caffeine therapy before 3 days of life as the early caffeine group and the initiation of caffeine 3 days or after as the late caffeine group. The inclusion criteria of the studies restricted the timing of caffeine initiation to within 10 days of life in 2 studies (14,15,16). The study by Davis et al. (14) was a post hoc subgroup analysis of the previously reported CAP trial (5).
Primary outcome: BPD, death, and "BPD and death"
The percentage of mortality was 1,177 (3.8%) of 30,974 patients in the early caffeine group and 1,001 (4.2%) of 23,873 patients in the late caffeine group. The incidence of BPD was 6,667 of 33,356 (20.0%) patients in the early caffeine group compared to 8,785 (34.6%) of 25,405 in the late caffeine group. BPD or death occurred in 7,821 (23.7%) of 32,960 patients in the early caffeine group versus 9,415 (37.9%) among 24,838 patients in the late caffeine group. The early use of caffeine was associated with a decreased incidence of death (OR, 0.902; 95% CI, 0.828 to 0.983; P=0.019, Fig. 2A), BPD (OR, 0.507; 95% CI, 0.396 to 0.648; P<0.001, Fig. 2B), and BPD or death (OR, 0.526; 95% CI, 0.384 to 0.719; P<0.001, Fig. 2C). The results of the heterogeneity assessment of BPD (P<0.001; I2=85.48%) and BPD and death (P<0.001; I2=89.92%) indicate a heterogeneous property, and thus, a random effect model was used for the meta-analysis. However, there was no heterogeneity between studies (P=0.743; I2=0.0001%), so a fixed effect model was used for the meta-analysis for death.
Fig. 2. Meta-analysis for the relationship between the timing of caffeine therapy initiation (early vs. late) and the primary outcomes. (A) Death. (B) Bronchopulmonary dysplasia (BPD). (C) BPD or death.
The sensitivity analysis did not show significant changes in the results with the sequential exclusion of individual studies for any of the primary outcomes, including BPD, death, and BPD and death. Inspection of the funnel plot did not rule out publication bias because of the small number of included studies. However, the Begg-Mazumdar rank correlation test and Egger's regression test did not show evidence of publication bias for all of the primary outcomes.
Secondary outcomes
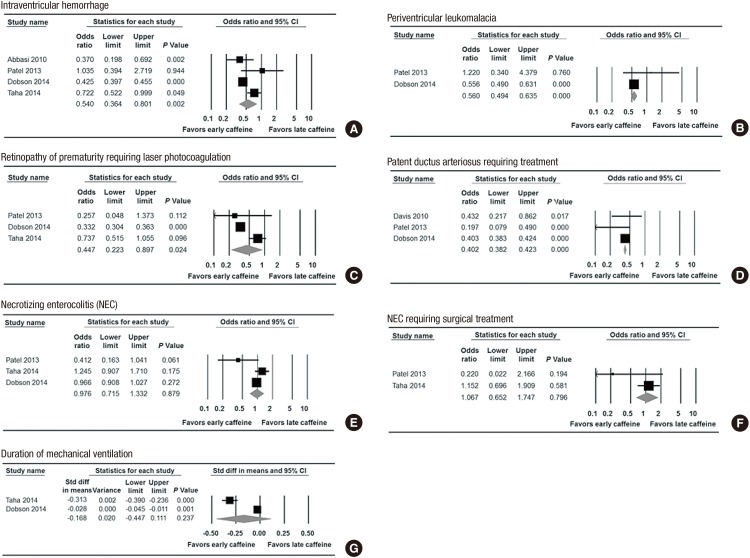
The incidence of the secondary outcomes was as follows: PVL (1.4% in the early caffeine group and 2.4% in the late caffeine group), PDA requiring treatment (8.8% in the early caffeine group and 19.3% in the late caffeine group), NEC (8.0% in the early caffeine group and 8.3% in the late caffeine group) and NEC requiring surgery (2.6% in the early caffeine group and 2.4% in the late caffeine group). The risk of IVH (OR, 0.540; 95% CI, 0.364 to 0.801; P=0.002, Fig. 3A), PVL (OR, 0.560; 95% CI, 0.494 to 0.635; P<0.001, Fig. 3B), ROP requiring laser photocoagulation (OR, 0.447; 95% CI, 0.223 to 0.897; P=0.024, Fig. 3C), and PDA requiring treatment (OR, 0.402; 95% CI, 0.380 to 0.423; P<0.001, Fig. 3D) were lower in the early caffeine group than in the late caffeine group.
Fig. 3. Meta-analysis for the relationship between the timing of caffeine therapy initiation (early vs. late) and the secondary outcomes. (A) Intraventricular hemorrhage. (B) Periventricular leukomalacia. (C) Retinopathy of prematurity. (D) Patent ductus arteriosus. (E) Necrotizing enterocolitis (NEC). (F) NEC requiring surgical treatment. (G) Duration of mechanical ventilation.
However, the risks of NEC (OR, 0.976; 95% CI, 0.715 to 1.332; P=0.879, Fig. 3E) in the random model analysis and NEC requiring surgery (OR, 1.067; 95% CI, 0.652 to 1.747; P=0.796, Fig. 3F) were not associated with the early use of caffeine. The results of the meta-analysis show that the early use of caffeine did not significantly reduce the duration of mechanical ventilation as a continuous variable (standard mean difference, -0.168; 95% CI, -0.447 to 0.111; P=0.237, Fig. 3G).
The results of the heterogeneity of IVH (P=0.004; I2=77.15%), duration of mechanical ventilation (P<0.001; I2=97.99%), and ROP requiring laser photocoagulation (P<0.001; I2=88.92%) showed heterogeneity among the included studies, and we used the random effect model for the meta-analysis. The analysis for PVL (P=0.230; I2=30.63%), PDA (P=0.301; I2=16.62%), and NEC requiring surgery (P=0.166; I2=47.99%) did not show heterogeneity, and a fixed effect model was used for the meta-analysis. For the analysis of the NEC risk (P=0.058; I2=64.79%), both a fixed effect model and a random effect model were used in this meta-analysis; however, there was no significant difference in the results of the analysis using the random (OR, 0.976; 95% CI, 0.715 to 1.332; P<0.879) and fixed effect models (OR, 0.971; 95% CI, 0.195 to 1.032; P=0.347).
We cannot assess the publication bias with tools such as the Begg-Mazumdar rank correlation test and Egger's regression test for PVL, NEC requiring surgery, and the duration of mechanical ventilation among the secondary outcomes because of the small number of included studies. None of the other outcomes showed a publication bias in the Begg-Mazumdar rank correlation test and Egger's regression test.
DISCUSSION
Methylated xanthines, such as theopylline, aminophylline and caffeine, and doxapram, have been used as respiratory stimulants to treat apnea of prematurity via the inhibition of the adenosine receptor (3,6,12) after Aranda et al. (21) first reported the efficacy of caffeine in reducing the frequency of apnea in preterm infants.
Although no difference was observed in the treatment failure rate between caffeine citrate and theophylline (2,3), caffeine citrate is superior to theophylline for apnea of prematurity because of the once daily dose, lack of need of monitoring the drug level, early onset of the effect, and minimal side effects (2,3,4,5,6). The use of doxapram could be harmful in the developing brain, with the possibility of cerebral dysfunction (7).
All of the included studies (14,15,16,17,20) in this meta-analysis used caffeine citrate to treat apnea of prematurity in infants weighting<1,500 g at birth. The dosage of caffeine was usually administered intravenously, with a loading dose of 20 mg/kg, followed by 5 mg/kg/day maintenance doses (2,4,14,21). Only one study (14) mentioned the dosage of caffeine in a previous report, the CAP trial (5).
Caffeine has the effect of reducing the frequency of apnea and improving respiratory function because, as a bronchodilator, it reduces diaphragmatic fatigue, it increases respiratory muscle strength, and it reduces resistance and increases compliance (2,21,22,23,24,25). Recently, the beneficial effects of caffeine in preterm infants, both in the long-term and short-term, have been reported. In addition, its effects on respiratory function, including apnea (5,6,10) and the efficacy of the early commencement of caffeine therapy (14,17,20), have also been reported. The beneficial effect of caffeine on other neonatal morbidities may result from the anti-inflammatory and immunomodulatory properties of caffeine (26,27) and the effect of caffeine during a critical period of disease progression or organ development in preterm infants (17). The use of caffeine in preterm infants could affect neonatal outcomes by reducing the frequency of apnea and intermittent hypoxia, which can activate the pro-inflammatory cascade (11,12,28) and the anti-inflammatory action of caffeine itself (11,12,13).
In the CAP trial (6), the death rates did not differ between the caffeine group and the non-caffeine group, but early caffeine use was associated with a reduced risk of death compared to late caffeine use in this meta-analysis (OR, 0.902; 95% CI, 0.828 to 0.983; P=0.019, Fig. 2A). Patel et al. (17) suggested that the early initiation of caffeine could decrease the incidence of BPD and BPD or death, especially in high-risk infants weighing<750 g. The proposed possible mechanism of caffeine of reducing the risk of BPD is the blockade of the adenosine 2 receptor, which acts on inflammation and increases permeability and remodeling in the lung (9). An increased number of neutrophils in bronchoalveolar lavage fluid was found soon after birth and within 4 days of life (29), and early initiation of caffeine treatment blocked the initiation of BPD development.
Schmidt et al. (5) reported that the evidence of brain injury on ultrasound did not significantly differ between the caffeine group and the non-caffeine group. However, the results of this meta-analysis showed a significant reduction of the risk of IVH (OR, 0.540; 95% CI, 0.364 to 0.801; P=0.002, Fig. 3A) and PVL (OR, 0.560; 95% CI, 0.494 to 0.635; P<0.001, Fig. 3B). Recently, in an animal study, Kilicdag et al. (8) found that the mechanism by which caffeine reduces brain injury in the developing brain was its ability to decrease neuronal apoptosis after a hypoxic-ischemic brain injury. Although adenosine is known to be neuroprotective, the use of caffeine (an adenosine antagonist) showed a neuroprotective effect in the developing brain via a blockade of the premature differentiation of oligodendrocyte precursor cells in PVL and decreased capillary leakage in the blood-brain barrier or reduced cerebral blood flow in IVH (9,10,30). Because it is the period of greatest vulnerability to brain injury, the protective effect of caffeine may only manifest in the early caffeine group (9,10).
The incidence of ROP in infants weighing 1,250 g or less was estimated to be 50%, and 10% of those experienced stage 3 ROP (2). Aranda et al. (2) reported the preliminary results of an animal study that found that the effect of caffeine on ROP prevention occurred through the up-regulation of the Shh (sonic hedgehog) signaling pathway via vascular endothelial growth factor and insulin-like growth factor and resulted in the preservation of retinal growth and angiogenesis. As shown by the results of this meta-analysis, early caffeine use was associated with a decreased risk of ROP requiring laser photocoagulation (OR, 0.447; 95% CI, 0.223 to 0.897; P=0.024, Fig. 3C).
This meta-analysis showed a decreased incidence of PDA requiring treatment (OR, 0.402; 95% CI, 0.380 to 0.423; P<0.001, Fig. 3D), similar to the report by Schmidt et al. (5). Patel et al. (17) explained that the decreased risk of PDA requiring treatment was due to the diuretic effect of caffeine and the improvement of lung function.
The use of caffeine was not associated with a difference in the incidence of NEC compared to the non-caffeine group (5,31), but there was no information on the time of initiation of caffeine therapy. The early initiation of caffeine did not reduce the risk of NEC (2) and was not associated with an increased risk of NEC (16). In this meta-analysis, early caffeine use did not increase the risk of NEC (OR, 0.976; 95% CI, 0.715 to 1.332; P=0.879, Fig. 3E) or NEC requiring surgery (OR, 1.067; 95% CI, 0.652 to 1.747; P=0.796, Fig. 3F) compared to the late caffeine group.
Methylxanthine prophylaxis increased the success of extubation compared to the placebo group in preterm infants (32), and the mean duration of mechanical ventilation was shorter in the early caffeine group than in the late caffeine group (23.7 days and 21 days, respectively) in two studies included in this meta-analysis (15,16). However, the results of the meta-analysis found no significant difference in the duration of mechanical ventilation between the early and late caffeine groups (standard mean difference, -0.168; 95% CI, -0.447 to 0.111; P=0.237, Fig. 3G) in the random effect model analysis. The day of caffeine initiation (mean value) was 0.9 days and 1 day in the early caffeine group and 5.3 days and 13 days in the late caffeine group in the two included studies (15,16). In the study by Taha et al. (16), infants who used caffeine starting after 3 days of life and earlier than 10 days of life were included in the late caffeine group, and thus, caffeine was started relatively early in life, even in the late caffeine group. Therefore, the duration of mechanical ventilation was shortened with early caffeine therapy (standard mean difference, -0.041; 95% CI, -0.058 to -0.025; P<0.001, the forest plots of the fixed model analysis are not shown) in the meta-analysis using the fixed model. Four studies (14,15,16,17) mentioned the duration of positive ventilation and were included in the meta-analysis on the duration of mechanical ventilation, and two studies (14,17) were excluded because they described the post-menstrual age at the last positive pressure ventilation or only mentioned the median value of mechanical ventilation. Only two studies were included in this meta-analysis on the duration of mechanical ventilation, and inconsistent results were shown between the random and fixed effect model. Therefore, further studies are needed to assess the effect of caffeine on the duration of mechanical ventilation.
The improvement of neonatal outcomes with the early initiation of caffeine may result from the synergistic interaction among the reduced risks of neonatal morbidities, including BPD, NEC, ROP, and PDA. The side effects following caffeine use did not differ compared to the placebo group, except for a temporary weight reduction (5,33) that was postulated to be an effect of the diuretic effect of caffeine (34). Therefore, Caffeine use was found to have short-term and long-term benefits in preterm infants (5,6). Early commencement of caffeine therapy, especially in preterm infants born at a gestational age of<29 weeks or with birth weights<1,000 g who were at high risk of apnea of prematurity and neonatal morbidities, such as BPD (4,17), could be helpful for improving clinical outcomes as well as for preventing apnea of prematurity.
Some limitations of the present study need to be addressed. First, only one RCT study (8) regarding the effects of the early administration of caffeine was included, and we used a retrospective study in the meta-analysis. Second, we could not report the effect of early caffeine use on the treatment of apnea. The analysis could not be performed for the effect on apnea due to the nature of the meta-analysis because the studies included did not report apnea as an outcome.
In conclusion, this meta-analysis suggests that early caffeine use (<3 days of life) in VLBW infants has beneficial effects on neonatal outcomes, including mortality, BPD, IVH, PVL, ROP laser photocoagulation, and PDA requiring treatment, without increasing the risk of NEC. Further studies, specifically well-designed randomized controlled trials, are needed to evaluate the effect of early and late caffeine use on neonatal outcomes, including long-term outcomes.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Literature review: Park HW, Lim G, Chung SH. Data acquisition & interpretation: Park HW, Lim G, Chung SH, Chung S, Kim KS. Statistical analysis: Kim SN. Writing: Park HW. Critical revision and final approval: all authors.
References
- 1.Gannon BA. Theophylline or caffeine: which is best for apnea of prematurity? Neonatal Netw. 2000;19:33–36. doi: 10.1891/0730-0832.19.8.33. [DOI] [PubMed] [Google Scholar]
- 2.Aranda JV, Beharry K, Valencia GB, Natarajan G, Davis J. Caffeine impact on neonatal morbidities. J Matern Fetal Neonatal Med. 2010;23:20–23. doi: 10.3109/14767058.2010.517704. [DOI] [PubMed] [Google Scholar]
- 3.Sacré L, Vandenplas Y. Xanthines in apnea of premature infants. Influence on gastroesophageal reflux. Arch Fr Pediatr. 1987;44:383–385. [PubMed] [Google Scholar]
- 4.Picone S, Bedetta M, Paolillo P. Caffeine citrate: when and for how long. A literature review. J Matern Fetal Neonatal Med. 2012;25:11–14. doi: 10.3109/14767058.2012.712305. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 7.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Kilicdag H, Daglioglu YK, Erdogan S, Zorludemir S. Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J Matern Fetal Neonatal Med. 2014;27:1470–1475. doi: 10.3109/14767058.2013.878694. [DOI] [PubMed] [Google Scholar]
- 9.Rivkees SA, Wendler CC. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr Res. 2011;69:271–278. doi: 10.1203/PDR.0b013e31820efbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, Rees S, Anderson PJ, Inder TE. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68:734–742. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- 11.Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111:877–892. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Martin RJ, Wang K, Köroğlu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez Valdez R, Ahlawat R, Wills-Karp M, Nathan A, Ezell T, Gauda EB. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. J Pediatr. 2011;158:57–64.e1. doi: 10.1016/j.jpeds.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis PG, Schmidt B, Roberts RS, Doyle LW, Asztalos E, Haslam R, Sinha S, Tin W Caffeine for Apnea of Prematurity Trial Group. Caffeine for Apnea of Prematurity trial: benefits may vary in subgroups. J Pediatr. 2010;156:382–387. doi: 10.1016/j.jpeds.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 15.Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, Herring A, Laughon MM, Carlton D, Hunt CE. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014;164:992–998.e3. doi: 10.1016/j.jpeds.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taha D, Kirkby S, Nawab U, Dysart KC, Genen L, Greenspan JS, Aghai ZH. Early caffeine therapy for prevention of bronchopulmonary dysplasia in preterm infants. J Matern Fetal Neonatal Med. 2014;27:1698–1702. doi: 10.3109/14767058.2014.885941. [DOI] [PubMed] [Google Scholar]
- 17.Patel RM, Leong T, Carlton DP, Vyas-Read S. Early caffeine therapy and clinical outcomes in extremely preterm infants. J Perinatol. 2013;33:134–140. doi: 10.1038/jp.2012.52. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyse. [accessed on 4 September 2014]. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 20.Abbasi S, Aden U, Allan W, Bada H, Barks J, Bauer C, Bizzarro M, Carlo W, Chen X, Cummings J. Early caffeine is associated with decreased IVH in very low birth weight neonate. Platform Session 2: (#9-16) [abstract] Ann Neurol. 2010;68:S88–S90. [Google Scholar]
- 21.Aranda JV, Gorman W, Bergsteinsson H, Gunn T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr. 1977;90:467–472. doi: 10.1016/s0022-3476(77)80718-x. [DOI] [PubMed] [Google Scholar]
- 22.Steer PA, Henderson-Smart DJ. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000273. CD000273. [DOI] [PubMed] [Google Scholar]
- 23.Henderson-Smart DJ, Steer PA. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000273. CD000273. [DOI] [PubMed] [Google Scholar]
- 24.Sreenan C, Etches PC, Demianczuk N, Robertson CM. Isolated mental developmental delay in very low birth weight infants: association with prolonged doxapram therapy for apnea. J Pediatr. 2001;139:832–837. doi: 10.1067/mpd.2001.119592. [DOI] [PubMed] [Google Scholar]
- 25.Kassim Z, Greenough A, Rafferty GF. Effect of caffeine on respiratory muscle strength and lung function in prematurely born, ventilated infants. Eur J Pediatr. 2009;168:1491–1495. doi: 10.1007/s00431-009-0961-9. [DOI] [PubMed] [Google Scholar]
- 26.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65:203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 27.Weichelt U, Cay R, Schmitz T, Strauss E, Sifringer M, Bührer C, Endesfelder S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur Respir J. 2013;41:966–973. doi: 10.1183/09031936.00012412. [DOI] [PubMed] [Google Scholar]
- 28.Martin RJ, Di Fiore JM, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol. 2012;758:351–358. doi: 10.1007/978-94-007-4584-1_47. [DOI] [PubMed] [Google Scholar]
- 29.Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984;130:817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- 30.Pryds O, Schneider S. Aminophylline reduces cerebral blood flow in stable, preterm infants without affecting the visual evoked potential. Eur J Pediatr. 1991;150:366–369. doi: 10.1007/BF01955942. [DOI] [PubMed] [Google Scholar]
- 31.Lampkin SJ, Turner AM, Lakshminrusimha S, Mathew B, Brown J, Fominaya CE, Johnson KK. Association between caffeine citrate exposure and necrotizing enterocolitis in preterm infants. Am J Health Syst Pharm. 2013;70:603–608. doi: 10.2146/ajhp120457. [DOI] [PubMed] [Google Scholar]
- 32.Henderson-Smart DJ, De Paoli AG. Prophylactic methylxanthine for prevention of apnoea in preterm infants. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD000432.pub2. CD000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erenberg A, Leff RD, Haack DG, Mosdell KW, Hicks GM, Wynne BA. Caffeine citrate for the treatment of apnea of prematurity: a double-blind, placebo-controlled study. Pharmacotherapy. 2000;20:644–652. doi: 10.1592/phco.20.7.644.35167. [DOI] [PubMed] [Google Scholar]
- 34.Bancalari E. Caffeine for apnea of prematurity. N Engl J Med. 2006;354:2179–2181. doi: 10.1056/NEJMe068028. [DOI] [PubMed] [Google Scholar]