Abstract
Cosmetic facial filler-related ophthalmic artery occlusion is rare but is a devastating complication, while the exact pathophysiology is still elusive. Cerebral angiography provides more detailed information on blood flow of ophthalmic artery as well as surrounding orbital area which cannot be covered by fundus fluorescein angiography. This study aimed to evaluate cerebral angiographic features of cosmetic facial filler-related ophthalmic artery occlusion patients. We retrospectively reviewed cerebral angiography of 7 patients (4 hyaluronic acid [HA] and 3 autologous fat-injected cases) showing ophthalmic artery and its branches occlusion after cosmetic facial filler injections, and underwent intra-arterial thrombolysis. On selective ophthalmic artery angiograms, all fat-injected patients showed a large filling defect on the proximal ophthalmic artery, whereas the HA-injected patients showed occlusion of the distal branches of the ophthalmic artery. Three HA-injected patients revealed diminished distal runoff of the internal maxillary and facial arteries, which clinically corresponded with skin necrosis. However, all fat-injected patients and one HA-injected patient who were immediately treated with subcutaneous hyaluronidase injection showed preserved distal runoff of the internal maxillary and facial arteries and mild skin problems. The size difference between injected materials seems to be associated with different angiographic findings. Autologous fat is more prone to obstruct proximal part of ophthalmic artery, whereas HA obstructs distal branches. In addition, hydrophilic and volume-expansion property of HA might exacerbate blood flow on injected area, which is also related to skin necrosis. Intra-arterial thrombolysis has a limited role in reconstituting blood flow or regaining vision in cosmetic facial filler-associated ophthalmic artery occlusions.
Keywords: Retinal Artery Occlusion, Hyaluronic Acid, Autologous Fat, Facial Filler, Cerebral Angiography
Graphical Abstract
INTRODUCTION
Increasing numbers of aesthetic procedures-especially cosmetic nonsurgical injection procedures-are performed every year (1). These procedures are generally safe, although cosmetic facial filler injections could lead to devastating complications such as blindness. There are several reports (most of which were case reports) of cosmetic facial filler injection-associated retinal artery occlusion (2,3,4,5,6,7,8,9,10,11). A nation-wide survey was recently performed and a thorough analysis was performed on 44 patients regarding the clinical features of this condition with respect to its fluorescein angiographic findings. Our group participated in that study and classified occlusion of the ophthalmic artery and its branches into 6 types on the basis of fluorescein angiographic findings and found that the more diffuse occlusion type was associated with poor visual outcomes (12). Several researchers proposed that the occlusion was caused by retrograde embolism by the injected material (3,7,8). The supratrochlear and supraorbital arteries are thought to be the possible route for retrograde embolism in the glabellar region and the anastomosis of the dorsal nasal artery and angular artery seems to be the possible inlet of nasally injected embolic material (8). However, the exact mechanism of retrograde embolism is still elusive and precise localization of emboli is difficult when it is evaluated only by fundus fluorescein angiography, especially when the presumed occlusion site is proximal to central retinal arteries.
In the previous studies, the two most commonly injected materials, autologous fat and hyaluronic acid (HA) showed different clinical features. Autologous fat injection was interestingly more frequently associated with diffuse occlusion, compared to HA injection (8,12), while skin necrosis associated with facial filler injection was more common in HA injected cases (12). The size difference between autologous fat and HA seems to be related in part to the differences in the clinical features of retinal artery occlusions associated with cosmetic facial injections of these materials; however, the exact pathophysiology has never been thoroughly scrutinized. Cerebral angiography provides more detailed information on blood flow of ophthalmic artery as well as surrounding orbital area which cannot be covered by fundus fluorescein angiography. In this study, we aimed to evaluate cerebral angiographic features of cosmetic facial filler-related ophthalmic and retinal artery occlusion patients who had undergone intra-arterial thrombolysis (IAT), thus elucidate the pathophysiology of the disease.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of patients who were diagnosed as having ophthalmic artery and/or retinal artery occlusion associated with cosmetic facial filler injections of autologous fat or HA and underwent IAT along with transfemoral cerebral angiography at the Seoul National University Bundang Hospital (Seongnam, Korea) between January 1, 2008 and August 31, 2014.
We performed IAT after obtaining written informed consent. In particular, IAT was considered for patients who presented less than 24 hr after symptom onset and for patients without any systemic conditions that restricted thrombolysis, such as uncontrolled hypertension, coagulation disorders, or a history of intracranial or extracranial hemorrhages within 6 months before presenting to the hospital. After a detailed ophthalmologic evaluation that included fundus fluorescein angiography, IAT was performed with transfemoral cerebral angiography using a biplane angiographic unit (Integris Allura; Philips Medical Systems, Eindhoven, the Netherlands). A microcatheter (Excelsior SL-10; Stryker Neurovascular, Fremont, CA, USA) was placed in the proximal part of the ophthalmic artery, and a thrombolytic agent-urokinase (in patients with fat embolism, Green Cross, Yongin, Korea) or hyaluronidase (in patients with HA embolism, Kuhnil Pharm, Seoul, Korea) or both- was slowly injected with mechanical disruption by microwire to dislodge the emboli distally. The hyaluronidase dosages ranged between 1,000 units and 9,000 units, and the urokinase dosage was up to 500,000 units.
We reviewed patient demographics, clinical characteristics including fundus fluorescein angiographic findings. We evaluated cerebral angiography by localizing the embolic obstruction site on ophthalmic angiogram and characterizing the pattern of distal angiographic runoff on external carotid angiogram, which was corresponded to skin necrosis lesion.
Ethics statement
This study was approved by the institutional review board of Seoul National University Bundang Hospital (Seongnam, Korea, No. B-1510-320-109). The study adhered to the tenets of the Declaration of Helsinki. The board waived the need for informed consent for study participation.
RESULTS
A total of 17 patients (10 HA-injected patients and 7 autologous fat-injected patients) sustained ophthalmic artery and/or retinal artery occlusion associated with cosmetic facial filler injections during the period. Of these patients, 7 patients (4 HA-injected patients and 3 autologous fat-injected patients) underwent IAT along with cerebral angiography, and were finally included in this study. The clinical findings for 4 of 7 patients had already been published in the literature (6,8,9,12). Table 1 summarizes the demographics, clinical characteristics and cerebral angiographic findings of the study patients. All patients were women and most were young (mean age of 40.6±12.7 yr). They underwent facial filler injection on glabella area and/or nasal dorsum for rhinoplasty. Diffuse occlusions such as complete ophthalmic artery occlusion or occlusion of its branches were noted in all patients. Three of 4 HA-injected patients showed skin necrosis, whereas all fat-injected patients and one HA-injected patient who were immediately treated with subcutaneous hyaluronidase injection by the physician who performed cosmetic filler injection showed none or mild skin lesion. One patient in fat-injected group showed concomitant middle cerebral artery territory infarct on brain magnetic resonance image. All patients arrived at the emergency room immediately (i.e., 4 hr or less) after symptom onset and underwent IAT on the same day. However, the result of thrombolysis was unfavorable because only 2 patients showed partial recanalization of the obstruction (Table 1). The visual outcome was poor that all patients had final vision of no light perception.
Table 1. Demographic and cerebral angiographic findings of patients.
| No. | Age (yr) | Sex | Eye | Cosmetic Injection | Diagnosis | Skin Necrosis | MRI Brain | Angiographic findings | Time to Visit/IAT | Thrombolysis | Visual acuity baseline/final | Follow-up Period | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | Site | Dose | Ophthalmic Angiogram Obstruction level | ECA Angiogram Distal angiographic runoff | |||||||||||
| 1 | 24 | F | R | HA | Glabella, nose | 0.2 mL | OAO | Yes | Normal | 2nd branch (Ciliary branch) |
Diminished | 1 hr/3.5 hr | Hyaluronidase 1,000 units/recanalization fail | NLP/NLP | 7 mo |
| 2 | 34 | F | R | HA | Glabella, nose | 0.7 mL | OAO | Yes | Normal | Distal to 2nd branch | Diminished | 4 hr/5 hr | Hyaluronidase 1,500 units + UK 20,000 units/recanalization fail | NLP/NLP | 3 mo |
| 3 | 39 | F | L | HA | Glabella, nose | Unknown | OAO | Yes | Normal | Distal to 2nd branch | Diminished | 1 hr/3 hr | Hyaluronidase 9,000 units/partial recanalization | HM/NLP | 7 mo |
| 4* | 41 | F | R | HA | Glabella, nose | 0.4 mL | OAO | No | Normal | 2nd branch | Preserved | 2 hr/4.5 hr | Hyaluronidase 1,600 units/recanalization fail | NLP/NLP | 7 day |
| 5 | 40 | F | L | Fat | Nose | Unknown | OAO | No | Normal | OphA before branching | Preserved | 1 hr/2.5 hr | UK 500,000 units + tirofiban 500 µg /partial recanalization | NLP/NLP | 17 mo |
| 6 | 66 | F | L | Fat | Glabella, nose | Unknown | OAO | No | Normal | OphA before branching | Preserved | 2 hr/3 hr | UK 40,000 units/recanalization fail | NLP/NLP | 5 day |
| 7 | 40 | F | R | Fat | Glabella | Unknown | CRAO | No | MCA infarct | OphA before branching | Preserved | 4 hr/5 hr | UK 40,000 units/recanalization fail | NLP/NLP | 5 mo |
*This patient was treated with subcutaneous hyaluronidase injection immediately after the onset of visual symptom by the physician who performed cosmetic filler injection. CRAO, central retinal artery occlusion; ECA, external carotid artery; F, female; IAT, intra-arterial thrombolysis; L, left; HA, hyaluronic acid; HM, hand motion; MCA, middle cerebral artery; MRI, magnetic resonance imaging; NLP, no light perception; OAO, ophthalmic artery occlusion; OphA, Ophthalmic artery; R, right; UK, urokinase.
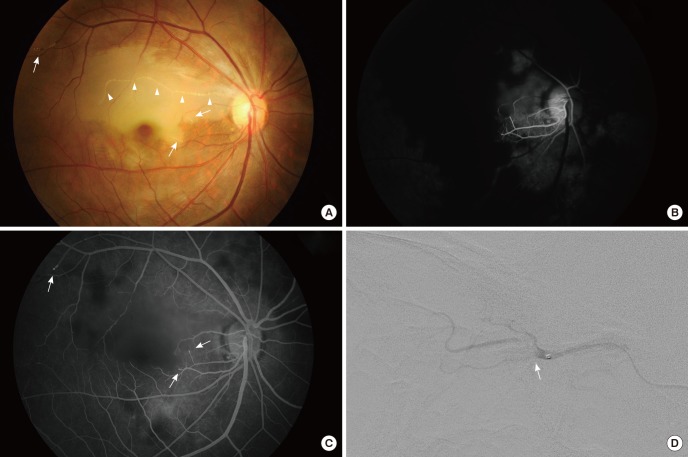
A case of autologous fat injection-associated retinal artery occlusion is shown in Fig. 1. On fundus photography, initially it appeared as occlusion of a branch of the retinal artery because one branch of the artery is occluded and retinal edema was present in the involved area (Fig. 1A). However, with fluorescein angiography, retinal perfusion and the choroidal perfusion were severely delayed (Fig. 1B). There is abrupt cut off of some of the arteriolar ends and showed focal hyperfluorescence in the late phase of fluorescein angiography, which suggests direct embolic obstruction of the arterioles (Fig. 1C). The selective ophthalmic angiogram revealed a large filling defect in the proximal ophthalmic artery, which explains the retinal and choroidal hypoperfusion in fluorescein angiography (Fig. 1D).
Fig. 1. Angiographic findings of an autologous fat-injected patient. A 40-yr-old woman after autologous fat injection in the glabella. (A) The fundus photograph shows an obstructed retinal artery with white infiltrations (arrow heads) and retinal edema at the corresponding area. Some whitish infiltration is observed at the end of arterioles (arrows). (B) Fluorescein angiography reveals markedly delayed retinal and choroidal perfusion. (C) There is abrupt cut off of some of the arteriolar ends (arrows), and demonstrates focal hyperfluorescence in the late phase, which suggests direct embolic obstruction of the arteriole. (D) The selective ophthalmic artery angiogram shows a large filling defect (arrow) in the proximal ophthalmic artery.
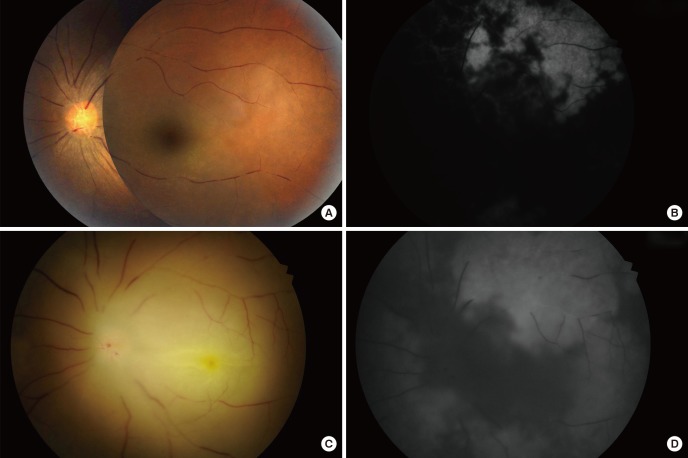
A case of HA-injection associated retinal artery occlusion is shown in Fig. 2. On initial fundus photography, multiple attenuated and segmented retinal vessels were observed (Fig. 2A). Fluorescein angiography revealed severe retinal and choroidal perfusion impediment (Fig. 2B). The day after IAT, retinal vessels were still segmented and the margin was blurred due to retinal edema secondary to ischemic injury (Fig. 2C). The choroidal perfusion was improved after IAT, while retinal perfusion remained compromised (Fig. 2D).
Fig. 2. Angiographic findings of a hyaluronic acid-injected patient. A 39-yr-old woman after hyaluronic acid injection in the glabella and nasal dorsum. (A) Fundus photograph reveals segmented and attenuated retinal vessels. (B) Fundus fluorescein angiography shows markedly compromised retinal and choroidal perfusion. (C) Fundus photograph taken at the day after intra-arterial thrombolysis. The retinal vessels were still segmented and the margin was blurred due to retinal edema secondary to ischemic injury. (D) The choroidal perfusion was improved after intra-arterial thrombolysis, while retinal perfusion remained compromised.
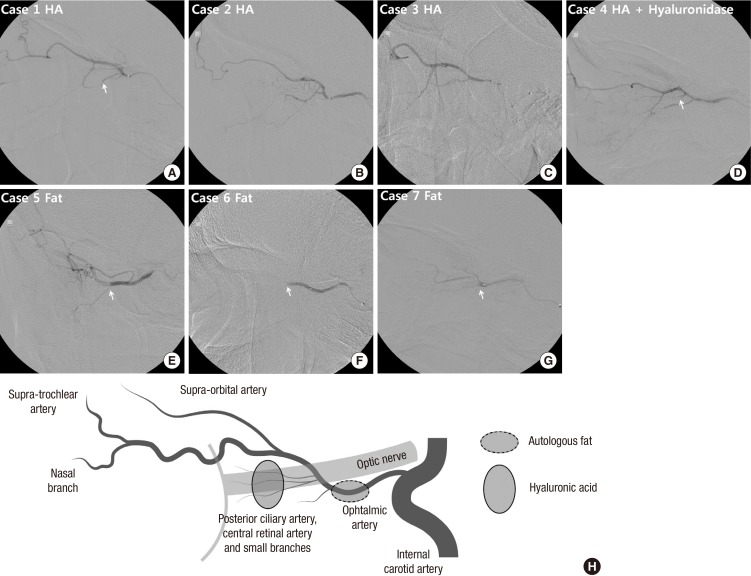
Cerebral angiography in all patients showed no choroidal blush. However, selective ophthalmic artery angiographic findings were different between HA-injected patients and fat-injected patients. A large filling defect was visible in the proximal part of the ophthalmic artery, and blood flow was compromised to the supratrochlear or supraorbital branch, and to the posterior ciliary branch in the fat-injected patients (Table 1 and Fig. 3E-G). On the other hand, in the HA-injected patients, although there was flow stagnation in the distal branches of ophthalmic artery on initial angiogram, selective, pressurized infusion of contrast dye revealed grossly no mechanical obstruction in the supratrochlear branch or the supraorbital branch, while blood flow to the eyeball was compromised (Fig. 3A-D). However, the exact obstruction level was obscure. In two patients, obstruction was present at the level of the second segment of ophthalmic artery including the posterior ciliary branch (Table 1, Fig. 3A and D), while the other 2 patients did not show definite obstruction point in the second segment of ophthalmic artery (Table 1, Fig. 3B and C).
Fig. 3. Selective ophthalmic artery angiogram. (A-D) In hyaluronic acid-injected patients, no mechanical obstruction is visible in the supratrochlear or supraorbital branch, while blood flow to the retina and the choroid is compromised. (A and D) Obstruction was visible in two patients at small branches of the second segment of ophthalmic artery including the posterior ciliary branch (arrow). (B and C) However, it could not be clearly delineated in the other two patients. (E-G) In autologous fat-injected patients, a large filling defect is visible in the proximal part of ophthalmic artery (arrow). Blood flow is compromised thereafter. (H) Schematic drawings of the vascular anatomy in selective ophthalmic artery angiogram. The lighter lines indicate the optic nerve and the posterior wall of the eye ball. Presumed obstruction level is shown as dashed circle (autologous fat) or lined circle (hyaluronic acid). Case numbers are identical to those in Table 1.
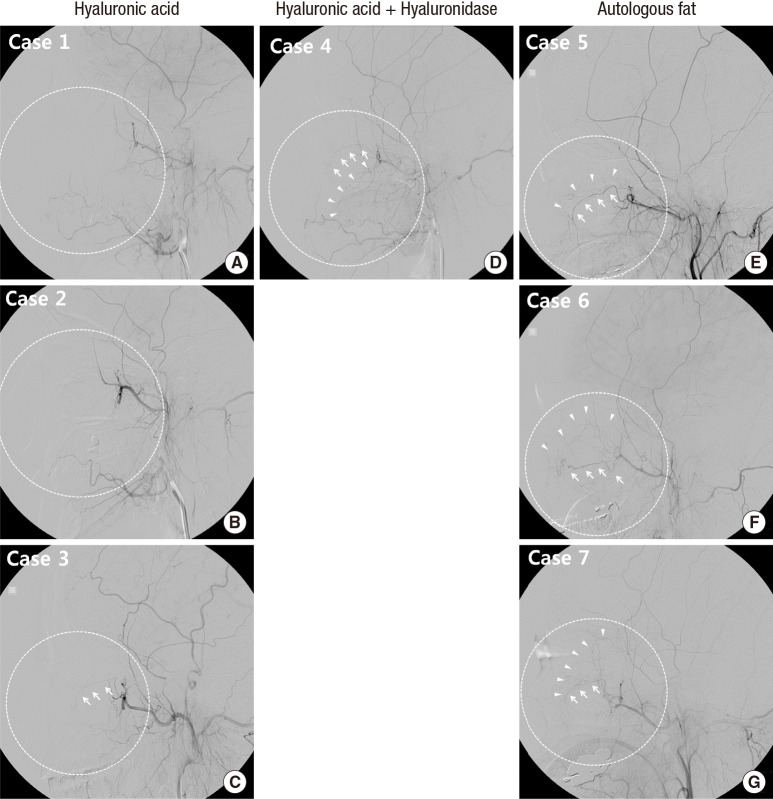
The selective angiographic findings for the external carotid artery were also distinctly different between the HA-injected and fat-injected groups. Three HA-injected patients showed diminished angiographic runoff in the distal branches of the internal maxillary and facial arteries, and decreased contrast staining in the periorbital area (Table 1 and Fig. 4A-C). This finding was corresponded with skin lesion of the patients, as these patients revealed skin necrosis on injected area (Fig. 5A-C). On the other hand, all fat-injected patients and one HA-injected patient who was also treated with subcutaneous hyaluronidase injection showed preserved distal runoff in the distal branches of the internal maxillary and facial arteries and more prominent contrast staining in the periorbital area (Table 1 and Fig. 4D-G). They also sustained only mild skin problems (Fig. 5D-F)
Fig. 4. Selective external carotid artery angiogram. (A-C) In hyaluronic acid-injected patients, the angiographic runoff is diminished in the distal branches of internal maxillary and/or facial arteries and contrast staining is decreased in the periorbital area (dashed circle). (C) Faint distal runoff of internal maxillary artery (arrows) is observed but periorbital contrast staining is diminished (dashed circle). (D-G) In hyaluronic acid-injected patient, who were also treated with subcutaneous hyaluronidase injection (D), and in autologous fat-injected patients (E-G), the distal angiographic runoff in the distal branches of internal maxillary and/or facial arteries (arrows) and contrast staining (arrow heads) are relatively preserved (dashed circle). Case numbers are identical to those in Table 1.
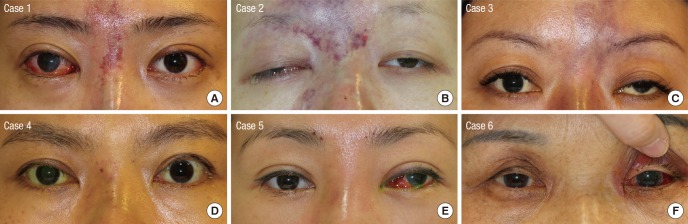
Fig. 5. Skin changes after cosmetic facial filler injections on the glabella and/or nasal dorsum. (A-C) Hyaluronic acid-injected patients have skin necrosis. (D) Hyaluronic acid-injected patient, who were also treated with subcutaneous hyaluronidase injection showed mild erythema in the injected area. Autologous fat-injected patients also have (E) mild erythema or (F) normal appearance in the injected area. Case numbers are identical to those in Table 1.
DISCUSSION
In this study, we carefully reviewed the angiographic findings of cosmetic facial filler injection-associated ophthalmic artery occlusions. Although it is known that both HA and autologous fat injection could result in various spectrum of ophthalmic and retinal arterial occlusion (12), all cases included in this study showed diffuse ophthalmic artery and its branches occlusion. Both HA and autologous fat injection group showed severely compromised choroidal and retinal perfusion on fundus fluorescein angiography. Despite these similar fundus findings, the cerebral angiographic findings were distinctively different between the HA-injected patients and the fat-injected patients. On selective ophthalmic angiography, all fat-injected patients had visible, large filling defects at the proximal part of the ophthalmic artery, whereas the HA-injected patients had obstruction at a more distal area than that observed in fat-injected patients. This obstruction site difference suggests a size difference between both materials.
Another interesting finding is that skin necrosis was mostly present in the HA-injected patients. In the previous study, our group also found the trend that 5 (23%) of 22 fat-injected patients had skin lesions, whereas 9 (69%) of 13 HA-injected patients had skin lesions (P=0.007) (12). We could not have clearly explained the reason for this phenomenon. However, by comparing the selective external carotid angiograms, we found that the angiographic runoff is diminished primarily in the distal branches of internal maxillary and facial arteries only in HA-injected group. This could have resulted from direct vascular obstruction by the injected filler material. On the other hand, the wide range of vascular runoff decrease around the filler-injected facial area also suggests another possibilities that the impediment of normal blood flow caused by elevation of distal intra-tissue pressure. Some authors suggest that injected HA expands as it attracts water; the facial artery and the angular artery or its branches become compressed, and skin necrosis ensues (13,14). In our cases, the skin necrosis lesion was most severe on a few days after cosmetic filler injection and this also supports the pressure necrosis mechanism secondary to local ischemic edema or to hydrophilic, volume-expansion properties of HA. Interestingly, the patient who underwent immediate subcutaneous hyaluronidase injection showed relatively preserved angiographic runoff in the distal branches of internal maxillary and facial arteries and she sustained only mild erythema in filler injected area. Hyaluronidase might have dissolved HA in injected area and reduced intra-tissue pressure, which improved vascular supply in the area and prevented skin necrosis. However, skin necrosis in HA-injected patients cannot be fully explained only by pressure necrosis mechanism, as only small proportions of HA-injected patients suffer these complications. It would be more reasonable to explain as mechanical interruption of the vasculature of injected area are further compromised by increased tissue pressure secondary to both ischemic tissue edema and hydrophilic, volume-expansion properties of HA.
In addition, increased distal tissue pressure could have worsened the blood flow into the periorbital area from the ophthalmic artery because the pressure gradient from ophthalmic artery to its peripheral branch is diminished. During the cerebral angiography in HA-injected patients, there was initial flow stagnation in the supratrochlear or supraorbital branches of ophthalmic artery, which was proven to be no mechanical obstruction after selective, pressurized infusion of contrast dye into ophthalmic artery. This finding also suggests that some proportion of flow impediment in HA-injected patients stems from decreased pressure gradient secondary to increased distal tissue pressure. Previously thought as partial recanalization of frontal branches of the ophthalmic artery after IAT might have been in reality, forced flow with pressure in the stagnated area (9).
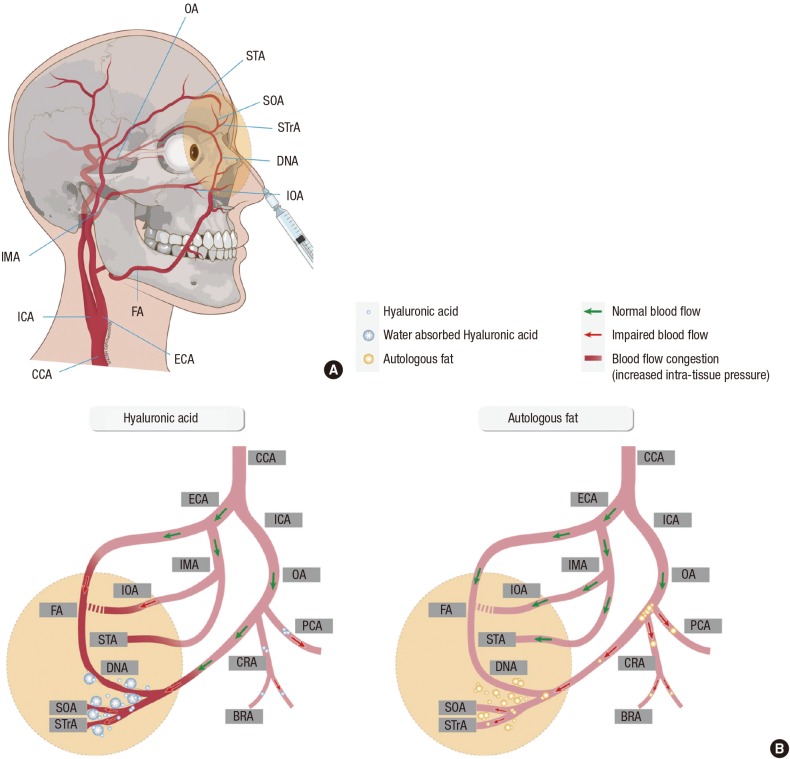
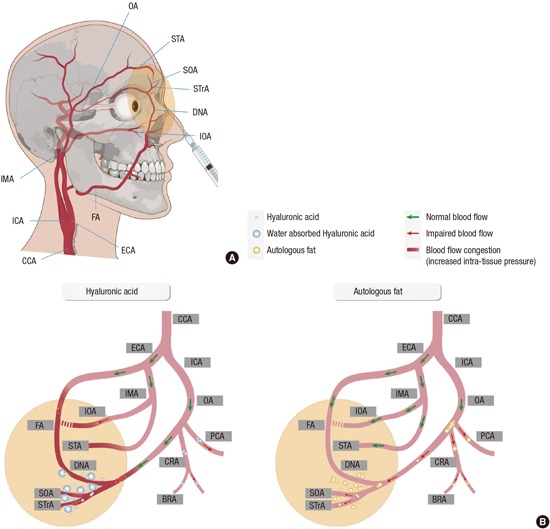
Based on all these findings, we summarized the different mechanisms of occlusion of the ophthalmic artery and its branches caused by HA and autologous fat in Fig. 6. The main difference between the 2 materials stems from differences in their particle sizes. Fat particles in an aggregated form could completely block the proximal part of the ophthalmic artery. Small fat particles could also obstruct smaller vessels, as proven by the presence of branch retinal artery occlusion cases in the previous literature (12), and arteriolar obstruction found in late fundus fluorescein angiography in this study. On the other hand, HA seems to be more uniform in size and could obstruct the more distal part, compared to a fat embolism. This could be the reason for the less prevalent diffuse occlusion of ophthalmic and its branches in HA-injected patients compared to autologous fat-injected group (12). Furthermore, the exact obstruction level would be different between HA and autologous fat injected group with diffuse occlusion, although the clinical manifestation is similar as complete ophthalmic artery or central retinal artery occlusion. It is likely that diffuse ophthalmic artery occlusions caused by HA injection may arise from multiple obstructions of the retinal and posterior ciliary vessels rather than by a large bolus of filler material completely obstructing the proximal part of the ophthalmic artery, as in the fat-injected patients. Another remarkable finding is that HA might increase distal intra-tissue pressure by attracting water, and thereby impede blood flow into these areas since the pressure gradient between the ophthalmic artery and its distal branches may be diminished (Fig. 6).
Fig. 6. Schematic drawing of vascular anatomy and the comparison of possible obstruction mechanism between hyaluronic acid and autologous fat. (A) Vascular anatomy of external and internal carotid artery and its ophthalmic and facial branches. (B) Hyaluronic acid (HA) molecules are small and uniform in size, compared to fat. HA may obstruct the central retinal artery, posterior ciliary arteries, and branch retinal arteries. In addition, the pressure gradient may be diminished between the ophthalmic artery and its distal ends because the distal intra-tissue pressure increases due to water absorption and volume expansion properties of HA, thereby decreasing blood flow. On the other hand, fat particles are composed of various sizes; thus, they can concomitantly obstruct from small arteries to the proximal part of the ophthalmic artery. Filler injected area and possible affected vascular area is drawn with yellow colored circle in A and B. BRA, branch retinal artery; CCA, common carotid artery; CRA, central retinal artery; DNA, dorsal nasal artery; ECA, external carotid artery; FA, facial artery; ICA, internal carotid artery; IMA, internal maxillary artery; IOA, infraorbital artery; OA, ophthalmic artery; PCA, posterior ciliary artery; SOA, supraorbital artery; STA, superficial temporal artery; STrA, supra-trochlear artery.
Molecular characteristics of HA is determined by the amount of molecular cross-linking (15). Recently, by adding low molecular weight HA polymer chains into high molecular weight HA, more efficient cross-linking capability was achieved. The resultant material became more cohesive and viscous, and showed good clinical outcomes (16). In addition to the size of the material, cohesiveness or viscosity might also affect material embolism. Unfortunately, the information on the exact composition or brand name of HA injected in this study is lacking, thus the influence of cohesiveness or viscosity of the injected material on embolism is obscure. Further study with regard to the cohesiveness or viscosity of injected material and its relationship with embolism is needed.
We performed IAT in 7 patients, but the results were not promising. Only 2 patients showed partial recanalization of the obstructed vessel, but they had no visual gain. Hyaluronidase is a soluble protein enzyme that breaks down and hydrolyzes HA by splitting the glucosaminidic bond of glucuronic acid (17,18). It is also used in cases of skin necrosis derived from HA filler injections, and results in a good clinical course (19). The experimental study also suggested that hyaluronidase may reduce skin necrosis if injected within the first 4 hr (20). In our study, in contrast to other three HA-injected patients, the patient who underwent subcutaneous hyaluronidase injection showed good vascular supply in periorbital area and no significant skin lesion. However, with regard to ophthalmic artery obstruction, the role of IAT and hyaluronidase seems to be very limited. The efficacy of IAT on fat embolism was likewise unfavorable. Complete obstruction by a large fat aggregate may be associated with a poor outcome, and no current treatment method can be recommended.
Our study is limited by the small number of patients included in this study. However, considering that cosmetic facial filler related ophthalmic and retinal artery occlusion is very rare complication, this is by far the largest case series on cerebral angiographic findings of ophthalmic complication following cosmetic facial filler injection. Furthermore, the distinctive angiographic characteristics between HA-injected and fat-injected patients noted even in this small case series provide valuable information on the pathophysiology of cosmetic facial filler injection-associated occlusion of the ophthalmic artery and its branches.
In conclusion, the size difference between injected materials is associated with different angiographic findings. Large, aggregated autologous fat particle is more prone to obstruct proximal part of ophthalmic artery, whereas small HA particle obstructs distal branches compared to fat embolism. In addition, hydrophilic and volume-expansion property of HA might exacerbate blood flow on injected area, either by compressing already disrupted vessels or by diminishing the pressure gradient between the ophthalmic artery and its distal end, which is also related to skin necrosis. Considering the angiographic findings of the involved cases, IAT has a limited role in reconstituting blood flow or regaining vision in cosmetic facial filler-associated ophthalmic artery occlusions.
Footnotes
Funding: This study was supported by a grant (CCP-13-02-KIST) from the Convergence Commercialization Project of National Research Council of Science and Technology, Seoul, Korea.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Kim YK, Jung C, Woo SJ. Acquisition of data: Kim YK, Jung C. Analysis and interpretation of data: all authors. Drafting of the manuscript: Kim YK, Jung C. Manuscript approval: all authors.
References
- 1.American Society of Plastic Surgeons. 2014 plastic surgery statistics report. [accessed on 12 June 2015]. Available at Http://www.Plasticsurgery.Org/news/plastic-surgery-statistics/2014-statistics.Html.
- 2.Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001;119:777–778. [PubMed] [Google Scholar]
- 3.Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin Ophthalmol. 2008;2:679–683. [PMC free article] [PubMed] [Google Scholar]
- 4.Sung MS, Kim HG, Woo KI, Kim YD. Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxylapatite filler. Ophthal Plast Reconstr Surg. 2010;26:289–291. doi: 10.1097/IOP.0b013e3181bd4341. [DOI] [PubMed] [Google Scholar]
- 5.Kim YJ, Kim SS, Song WK, Lee SY, Yoon JS. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27:e152–e155. doi: 10.1097/IOP.0b013e3182082f37. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Woo SJ, Park KH, Hwang JM, Hwang GJ, Jung C, Kwon OK. Partial recovery after intraarterial pharmacomechanical thrombolysis in ophthalmic artery occlusion following nasal autologous fat injection. J Vasc Interv Radiol. 2011;22:251–254. doi: 10.1016/j.jvir.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, Lazzeri S. Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012;129:995–1012. doi: 10.1097/PRS.0b013e3182442363. [DOI] [PubMed] [Google Scholar]
- 8.Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon OK. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154:653–662.e1. doi: 10.1016/j.ajo.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Oh BL, Jung C, Park KH, Hong YJ, Woo SJ. Therapeutic intra-arterial hyaluronidase infusion for ophthalmic artery occlusion following cosmetic facial filler (hyaluronic acid) injection. Neuroophthalmology. 2014;38:39–43. doi: 10.3109/01658107.2013.830134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carle MV, Roe R, Novack R, Boyer DS. Cosmetic facial fillers and severe vision loss. JAMA Ophthalmol. 2014;132:637–639. doi: 10.1001/jamaophthalmol.2014.498. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Ryoo NK, Park KH. Occlusion caused by cosmetic facial filler injection. JAMA Ophthalmol. 2015;133:224–225. doi: 10.1001/jamaophthalmol.2014.4243. [DOI] [PubMed] [Google Scholar]
- 12.Park KH, Kim YK, Woo SJ, Kang SW, Lee WK, Choi KS, Kwak HW, Yoon IH, Huh K, Kim JW, et al. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Ophthalmol. 2014;132:714–723. doi: 10.1001/jamaophthalmol.2013.8204. [DOI] [PubMed] [Google Scholar]
- 13.Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35:1635–1640. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 14.Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011;10:1007–1012. [PubMed] [Google Scholar]
- 15.Kim JE, Sykes JM. Hyaluronic acid fillers: history and overview. Facial Plast Surg. 2011;27:523–528. doi: 10.1055/s-0031-1298785. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey S, Carruthers J, Carruthers A. Clinical experience with 11,460 mL of a 20-mg/mL, smooth, highly cohesive, viscous hyaluronic acid filler. Dermatol Surg. 2015;41:1060–1067. doi: 10.1097/DSS.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 17.Cox SE, Adigun CG. Complications of injectable fillers and neurotoxins. Dermatol Ther. 2011;24:524–536. doi: 10.1111/j.1529-8019.2012.01455.x. [DOI] [PubMed] [Google Scholar]
- 18.Cavallini M, Gazzola R, Metalla M, Vaienti L. The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthet Surg J. 2013;33:1167–1174. doi: 10.1177/1090820X13511970. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007;33:357–360. doi: 10.1111/j.1524-4725.2007.33073.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, Dhong ES. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64:1590–1595. doi: 10.1016/j.bjps.2011.07.013. [DOI] [PubMed] [Google Scholar]