Abstract
This study investigated the toxicity of commercial non-steroid anti-inflammatory drug (NSAID) eye solutions against corneal epithelial cells in vitro. The biologic effects of 1/100-, 1/50-, and 1/10-diluted bromfenac sodium, pranoprofen, diclofenac sodium, and the fluorometholone on corneal epithelial cells were evaluated after 1-, 4-, 12-, and 24-hr of exposure compared to corneal epithelial cell treated with balanced salt solution as control. Cellular metabolic activity, cellular damage, and morphology were assessed. Corneal epithelial cell migration was quantified by the scratch-wound assay. Compared to bromfenac and pranoprofen, the cellular metabolic activity of diclofenac and fluorometholone significantly decreased after 12-hr exposure, which was maintained for 24-hr compared to control. Especially, at 1/10-diluted eye solution for 24-hr exposure, the LDH titers of fluorometholone and diclofenac sodium markedly increased more than those of bromfenac and pranoprofen. In diclofenac sodium, the Na+ concentration was lower and amount of preservatives was higher than other NSAIDs eye solutions tested. However, the K+ and Cl- concentration, pH, and osmolarity were similar for all NSAIDs eye solutions. Bromfenac and pranoprofen significantly promoted cell migration, and restored wound gap after 48-hr exposure, compared with that of diclofenac or fluorometholone. At 1/50-diluted eye solution for 48-hr exposure, the corneal epithelial cellular morphology of diclofenac and fluorometholone induced more damage than that of bromfenac or pranoprofen. Overall, the corneal epithelial cells in bromfenac and pranoprofen NSAID eye solutions are less damaged compared to those in diclofenac, included fluorometholone as steroid eye solution.
Keywords: Bromfenac Sodium, Corneal Epithelial Cells, Diclofenac Sodium, Fluorometholone, Pranoprofen, Cytotoxicity
Graphical Abstract

INTRODUCTION
It is well known that topical steroid has more beneficial effect both on the subjective and objective clinical parameters compared to the non-steroid anti-inflammatory drugs (NSAIDs) eye solutions (1,2,3). Steroids have been the standard of care for the treatment of postoperative inflammation, but may contribute to inhibition of corneal wound healing, elevations in intraocular pressure, cataract formation, and increased risk of infection (1,2).
As topical steroid act as double-edged sword, NSAID eye solutions with less complications and comparable efficacy are replacing the needs for topical steroid (4,5,6,7). Generally, NSAIDs inhibit cyclooxygenases and thereby decrease peripheral and central prostaglandin production (5,6,7,8,9,10), these properties would render them ideal for application in case of we are afraid the undesirable effect of steroid after ocular surgeries. Over the past decade, the efficacy of NSAIDs has increased dramatically, because topical eye solutions of NSAIDs are comparable with topically applied corticosteroids in their ability to control postoperative inflammation.
Although numerous clinical studies have shown that topical NSAID eye solutions are well tolerated, many ophthalmologist are afraid that severe side effects such as impaired corneal sensation, persistent epithelial defects, superficial punctuate keratitis, corneal infiltrations, and corneal melt as previous reports mentioned (11,12,13).
Although some studies related with NSAID eye solutions showed the delayed corneal epithelial healing (13,14), to our knowledge, there is no report on the direct effect of NSAID eye solutions on corneal epithelial cell metabolism until now. Therefore, we investigated the toxicity of commercially available NSAID eye solutions against corneal epithelial cells compared with that of steroid eye solutions, with special attention to drug concentration and exposure time.
MATERIALS AND METHODS
Cell culture and preparation
Human corneal epithelial cell primary cultures were obtained using leftover human donor peripheral corneas after transplantation (15). Under a tissue culture hood, Descemet's membrane, the endothelium, and the posterior stroma were removed under a dissecting microscope using forceps. The anterior cornea with intact epithelium was covered with 1.2 U/mL of Dispase II (Boehringer Mannheim, Mannheim, Germany) in calcium- and magnesium-free phosphate-buffered saline (PBS) and incubated at 37℃ in a humidified 5% CO2 incubator for 1-hr. The epithelial cells were subsequently centrifuged at 1,000 revolutions per minute (rpm) for 5 min.
Primary cultures of corneal epithelial cells were prepared in 35-mm Petri tissue-culture dishes (Corning Incorporated, Corning, NY, USA), containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 20 ng/mL EGF, 100 U/mL penicillin, and 100 µg/mg streptomycin (all from Gibco BRL, Rockville, NY, USA). The cells were incubated at 37℃ under a humidified atmosphere of 95% air and 5% CO2. The culture medium was changed every 2 or 3 days. Corneal epithelial cell growth commenced after 4-7 days and reached confluence within 21-28 days. Subsequently, the cells were enzymatically detached with 0.25% trypsin and 0.002% EDTA (Irvine Scientific, Santa Ana, CA, USA) at 37℃ for 10 min after washing once with Dulbecco's PBS (D-PBS) (Gibco BRL, Rockville, NY, USA). The suspended epithelial cells were centrifuged at 400 rpm for 10 min. The supernatant was removed and fresh medium was added. The number of cells in the cell suspension was counted in a hemacytometer and 5×103 cells/well were plated in 96-well tissue culture plates. Second-passage human corneal epithelial cells were used in all experiments. The cells were incubated in 1 mL of culture medium at 37℃ (5% CO2, 95% air) and were allowed to attach to the bottom of the well for 24-hr before 3 different NSAID eye solutions; diclofenac sodium (Ofenac®, Tajoon Pharmaceutical Ltd., Seoul, Korea), bromfenac sodium (Bronuck®, Senju Pharmaceutical Ltd., Osaka, Japan), pranoprofen (Pranoprofen®, Senju Pharmaceutical Ltd., Osaka, Japan) and the fluorometholone (FML®; Santen Inc., Osaka, Japan) as control were added. Since the effect of a drug on corneal epithelial cells can be underestimated when cells are grown too dense, the cells were cultured for approximately 4-5 days to ensure 80%-90% confluence.
MTT assay for determining cellular metabolic activity
To determine the proliferation rate of human corneal epithelial cells, the colorimetric tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) test was performed (11). The MTT assay is based on the generation of purple formazan from the methyl tetrazolium salt by the mitochondrial enzymes of viable cells. Cultured cells were seeded in 96-well culture plates at a concentration of 4×103 cells/well and were allowed to form a monolayer within 24-hr. The cells were subsequently exposed to 150 µL of DMEM medium containing 1 of the 3 different NSAID eye solutions or the steroid drug 0.1% fluorometholone for 1-, 4-, 12-, and 24-hr. After drug exposure, the cells were washed twice with PBS and were incubated in culture medium for 24-hr, followed by the MTT assay. The balanced salt solution-treated group was used as a control. At the end of the incubation period, the MTT solution was carefully aspirated, with care taken not to disturb the purple formazan crystals formed at the bottom of each well. The formazan reaction product was dissolved by adding 150 µL dimethyl sulfoxide (Sigma, St. Louis, MO, USA) and the optical density of each well was measured using an automatic plate reader (Molecular Devices, Sunnyvale, CA, USA) at a 570- and 690-nm test and reference wavelength, respectively. In each experiment, 8 wells were used for each concentration. This procedure was repeated in triplicate (16).
Cellular metabolic activity was calculated using the means of the absorption rates at each exposure time and concentration. Cellular metabolic activity was calculated using the following formula: cellular metabolic activity (%)=absorption rate of each well/absorption rate of the control group×100. Data were analyzed using Kruskal-Wallis test and were considered statistically significant at P<0.05.
Lactate dehydrogenase assay for determining cellular toxicity
The lactate dehydrogenase (LDH) assay measures leakage of the cytoplasmic enzyme LDH into the extracellular medium. The presence of LDH in the cell culture medium represents cell membrane damage. For this assay, 4×103 corneal epithelial cells/mL were seeded in each well of 96-microtiter plates. Twenty-four hours after cell seeding, cells were exposed to 1 of the 3 different NSAID eye solutions or the fluorometholone. The LDH titer was assessed at 1-, 4-, 12-, and 24-hr after addition of the agent. After 24-hr, the supernatant was collected from each well. The cell monolayer was subsequently treated with a cell lysis solution for 30 min at room temperature and the cells and the lysates were collected. LDH activity was measured in both the supernatant and cell lysate fractions using CytoTox 96, a nonradioactive cytotoxicity assay kit (Promega, Madison, WI, USA), per the manufacturer's instructions. The absorbance was determined at 490 nm with a 96-well plate enzyme-linked immunosorbent assay (ELISA) reader (Moleculae Devices, Sunnyvale, CA, USA). LDH activity, which is proportional to color intensity, was expressed as optical density. This procedure was repeated in triplicate. The effect of the balanced salt solution-treated group was used as a control. To evaluate the significance of the differences in cytotoxicity between the treatments, the data were analyzed by the Kruskal-Wallis test and were considered statistically significant at P<0.05.
Analysis of the electrolyte composition, pH, and osmolarity of the eye solutions
The composition differences between the NSAID and fluorometholone eye solutions were analyzed using a LX-20 chemistry analyzer (Beckman Coulter, Fullerton, CA, USA). The pH and the osmolarity of the eye solutions were measured using the Metrohm 780 (Metrohm, Zofingen, Switzerland) and Micro-Sample Osmometer (Fiske Associate, Norwood, MA, USA), respectively. The 3 NSAID eye solutions as well as the fluorometholone eye solution were tested 5 times. Data were reported as the means±standard deviation of all tests. The Kruskal-Wallis test was used for statistical analysis with P<0.05 considered statistically significant.
Scratch-wound assay for determining cellular migration
A scratch-wound assay using human corneal epithelial cells was used to determine whether NSAIDs or the steroid could promote wound closure. Human corneal epithelial cells (5×103 cells/mL/well), which were grown to confluence in 24-well plates, were seeded at 500 µL/well in 6-well plates, and were subsequently incubated in DMEM under a 5% CO2/95% air atmosphere at 37℃. The human corneal epithelial cells were wounded by scratching the surface of the culture layer with a 100-µL pipette tip. The scratched human corneal epithelial cells were washed with fresh medium to remove detached cells and the cells were subsequently incubated in the medium in the presence of the NSAIDs or fluorometholone for 1-, 24-, and 48-hr under 1/10 diluted eye solution tested. Cell migration was measured using an inverted phase-contrast light microscope. The balanced salt solution-treated group was used as a control.
To ensure that wounds of a similar-size area were compared, multiple positioning marks were made at the center of the denuded surface with a small needle. Eighteen hours after wounding, the monolayers were fixed and stained, and the migrated cells' edges were imaged. The size of wound gapping was measured by ROI manager tool in Image J software (US National Institutes of Health, Bethesda, MD, USA). To have better recognition by ROI manager, corneal image was preprocessed with versatile magic wand tool in Image J to enhance the contrast between acellular gap zone. After preprocessing, acellular area was automatically selected by ROI manager and the number of pixels in selected area was calculated. This procedure was performed 10 times for the statistical analysis. The Mann-Whitney U-test was used for statistical analysis with P<0.05 considered statistically significant.
Cellular morphology analysis using transmission electron microscopy
Changes in cellular morphology were investigated using transmission electron microscopy (TEM, JEOL 1200EX; Jeol Ltd., Tokyo, Japan) by a well-trained technician. Cells (5×103 cells/mL/well) were grown to confluence in 24-well plates, were seeded at 500 µL/well in 6-well plates, and were subsequently incubated in DMEM under a 5% CO2/95% air atmosphere at 37℃. After exposure to the 3 different NSAIDs and fluorometholone for 12- and 24-hr under 1/50 diluted eye solution tested, the cells were incubated at 37℃ for another 24-hr after being rinsed with D-PBS. The cells were fixed with 2% glutaraldehyde in 0.1 M of phosphate buffer (pH 7.4) for 12-hr and post-fixed with 0.1% osmium tetroxide for 2-hr. After rinsing with 0.1 M of phosphate buffer, the fixed cells were embedded in an Epon® 812 mixture (SPI Supplies/Structure Probe, West Chester, PA, USA) and ultrathin sections were cut, which were stained with uranyl acetate and lead citrate, and examined by TEM.
Ethics statements
The study protocol was approved by the institutional review board (IRB) of Pusan National University Hospital (IRB No. E-2015067). Informed consent was waived by the IRB. All study conduct adhered to the tenets of the Declaration of Helsinki.
RESULTS
Cellular metabolic activity
The MTT values among the NSAID eye solutions and the control at 1-hr of exposure were not significantly different. However, they insignificantly decreased with concentration and after 4-hr of exposure time. Especially, the cellular metabolic activity of diclofenac sodium or fluorometholone markedly decreased after 1/10-diluted eye solution for 12-hr of exposure compared to control (P=0.037, P=0.021), and was significantly maintained for 24-hr (P=0.032, P=0.018), compared with those of bromfenac sodium and pranoprofen (Fig. 1). However, there were not statistically significant difference of MTT value between diclofenac sodium and fluorometholone after 12-hr and 24-hr exposure, respectively (P=0.86, P=0.72).
Fig. 1. The absorption rate of water-insoluble formazan in corneal epithelial cells as measured by scanning spectrometry. The cellular metabolic activity of diclofenac sodium or fluorometholone markedly decreased after 1/10-diluted eye solution for 12-hr of exposure compared to control (P = 0.037, P = 0.021), and was significantly maintained for 24-hr (P = 0.032, P = 0.018), compared with those of bromfenac sodium and pranoprofen (by Kruskal-Wallis test, statistically significant at P < 0.05).
Cytotoxicity
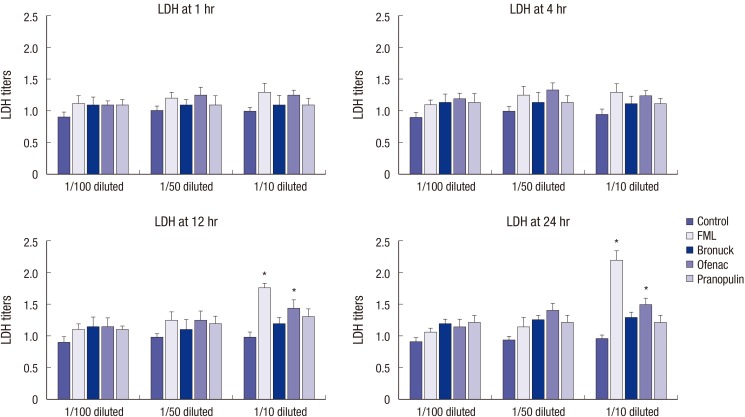
Exposure to 1/100- and 1/50-diluted eye drops did not significantly affect the LDH titer at all exposure times measured compared to human corneal epithelial cell treated with BSS. Especially, the LDH titers at 1/10-diluted fluorometholone and diclofenac significantly increased after 12-hr exposure rather than other two NSAIDs (P=0.029, P=0.039). The LDH titers of diclofenac sodium markedly increased after up to 24-hr of exposure (P=0.041), but that of fluorometholone was the highest among eye solutions tested (P=0.024) (Fig. 2).
Fig. 2. Lactate dehydrogenase (LDH) titers of cultured corneal epithelial cells by NSAIDs. Especially, the LDH titers at 1/10-diluted fluorometholone and diclofenac significantly increased after 12-hr exposure (P = 0.029, P = 0.039) and 24-hr exposure compared to control (P = 0.024, P = 0.041) rather than other two NSAIDs, respectively (by Kruskal-Wallis test, statistically significant at P < 0.05).
Electrolyte composition, pH, and osmolarity of the eye drop solutions
The preservative tested in the three NSAID and fluorometholone was 0.5% chlorbutanol in diclofenac sodium and 0.005% benzalkonium chloride (BAC) in the other 3 solutions. The Na+ concentration in diclofenac sodium was 9 mM/kg, lower than that of bromfenac sodium and pranoprofen. The concentration of Cl- and K+ was similar in all eye drop solutions. The pH and osmolarity of diclofenac sodium, bromfenac sodium, and pranoprofen was 7.0, 8.0, and 7.7 and 301, 300, and 309 mOsm/kg, respectively (Table 1).
Table 1. Comparison of the contents of the 4 anti-inflammatory eye drop solutions used.
| Parameters | Eye drop solutions | |||
|---|---|---|---|---|
| Bronuck® | Ofenac® | Pranoprofen® | FML® | |
| Na+ (mM/kg) | 101.0 | 86.5 | 98.8 | 164 |
| K+ (mM/kg) | 2.3 | 2.2 | 2.3 | 4.0 |
| Cl- (mM/kg) | 5.6 | 4.9 | 4.4 | 121 |
| pH | 8.0 | 7.0 | 7.7 | 7.1 |
| Osmolarity (mOsm/kg) | 300 | 301 | 309 | 315 |
| Preservatives, % | BAC, 0.005 | Chlorobutanol, 0.5 | BAC, 0.005 | BAC, 0.005 |
BAC, benzalkonium chloride.
Cellular migration
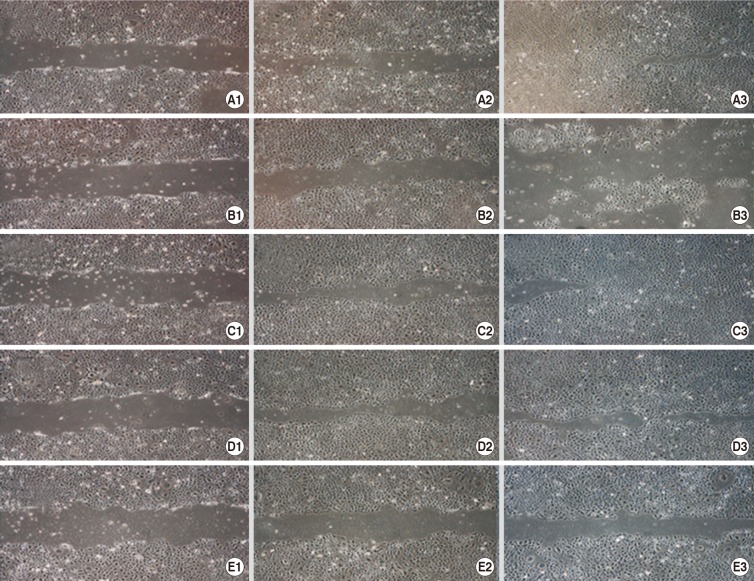
Using phase-contrast microscopy, human corneal epithelial cells treated with BSS were found to be densely distributed throughout the culture media with time. No treated cellular migration of control more migrated and covered to the wound gap region to 48-hr. With time, the diclofenac and fluorometholone-exposed cells did not migrate to the wound region compared with bromfenac and pranoprofen-treated cells. After 1/10 diluted eye solution for 48-hr exposure, the cell migration of diclofenac and fluorometholone significantly inhibited compared with that of control, respectively (P=0.001, P=0.002) (Fig. 3, Table 2). Compared with diclofenac and fluorometholone-treated cells, but there was no difference between control and two NSAIDs such as bromfenac and pranoprofen (P=0.328, P=0.486) (Fig. 3, Table 2).
Fig. 3. Scratch-wound assay after control (A), fluorometholone (B), pranoprofen (C), bromfenac (D), or diclofenac (E) exposure. Migration was assessed after a 1- (1), 24- (2), and 48- (3) hr exposure to A1-A3, B1-B3, C1-C3, D1-D3, or E1-E3. With time, the diclofenac and fluorometholone-exposed cells did not migrate to the wound region compared to bromfenac and pranoprofen-treated cells. After 1/10 diluted eye solution for 48-hr exposure, the cell migration of diclofenac and fluorometholone significantly inhibited compared with that of control.
Table 2. Comparison of the cell migration assay by all NSAIDs eye drop solutions tested.
| Exposed time | Eye drop solutions | |||||
|---|---|---|---|---|---|---|
| Control | Ofenac® | Pranoprofen® | Bronuck® | FML® | ||
| 1 hr | Acellular Zone (number of pixels) | 5,436 ± 158 | 6,369 ± 129 | 6,457±157 | 5,141 ± 151 | 5,879 ± 140 |
| P value | 0.26 | 0.833 | 0.986 | 0.746 | ||
| 24 hr | Acellular Zone (number of pixels) | 2,682 ± 149 | 4,951 ± 136 | 1,786 ± 104 | 2,188 ± 145 | 4,342 ± 139 |
| P value | 0.774 | 0.052 | 0.955 | 0.54 | ||
| 48 hr | Acellular Zone (number of pixels) | 453 ± 139 | 12,949 ± 144 | 674 ± 136 | 721 ± 111 | 3,427 ± 151 |
| P value | < 0.001* | 0.486 | 0.328 | 0.002* | ||
Acellular zone: values are mean±standard deviation. P value by Mann-Whitney U-test between control and medication group. *Significant value.
Cellular morphology
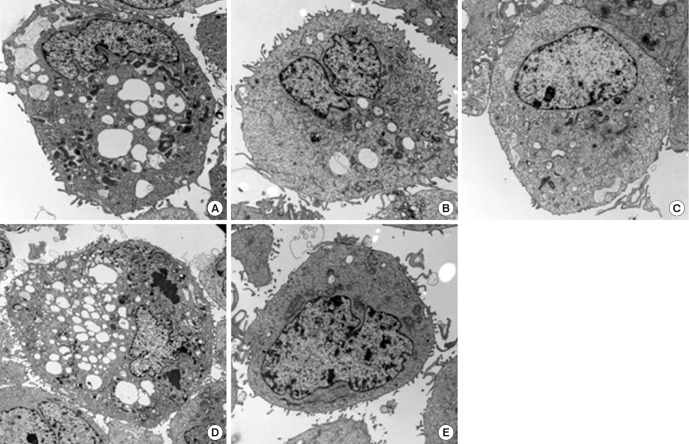
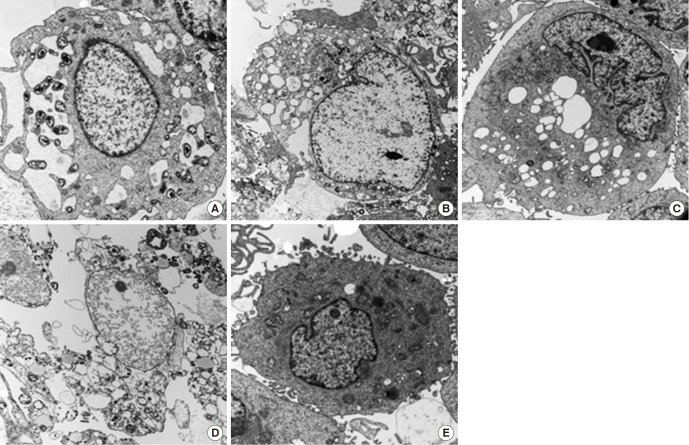
The cellular morphology of control corneal epithelial cells was not changed, although loss of microvilli was seen in some cells. All 1/50-diluted NSAID eye solutions and the fluorometholone eye solution induced the cytoplasmic and nuclear changes of the corneal epithelial cells after 24-hr of exposure, such as loss of microvilli, a dilated rough endoplasmic reticulum, enlarged mitochondria, a disrupted cytoplasmic membrane, and nuclear condensation (Fig. 4). At 48-hr exposure to 1/50-diluted diclofenac sodium or fluorometholone, there was more severe cellular morphology of corneal epithelial cell rather than those of bromofenac sodium or pranoprofen. Especially, certain types of nuclear change, such as nuclear marginal condensation, were observed after 48-hr exposure to 1/50 diluted fluorometholone (Fig. 5).
Fig. 4. Corneal epithelial cell transmission electron micrographs after 24-hr exposure to all NSAIDs and control. (A) Diclofenac. (B) Bromfenac. (C) Pranoprofen. (D) Fluorometholone. (E) Control. At 24-hr exposure to 1/50-diluted diclofenac sodium or fluorometholone compared with control, there was more severe cellular morphology of corneal epithelial cell such as microvilli disruption, vacuole formation, plasma membrane, and nuclear changes compared with those of bromofenac sodium or pranoprofen.
Fig. 5. Corneal epithelial cell transmission electron micrographs after 48-hr exposure to all NSAIDs and control. (A) Diclofenac. (B) Bromfenac. (C) Pranoprofen. (D) Fluorometholone. (E) Control. At 48-hr exposure to 1/50-diluted diclofenac sodium or fluorometholone compared with control, there was more severe cellular morphology of corneal epithelial cell rather than those of bromofenac sodium or pranoprofen.
DISCUSSION
The metabolic activity of cultured human corneal epithelial cells decreased after 4-hr of exposure to each drug evaluated in this study. While there was no significant difference between the two drugs, diclofenac sodium and fluorometholone significantly decreased the cellular metabolic activity, with the effect remaining after 24-hr exposure.
The cellular toxicity observed against cultured human corneal epithelial cells after exposure to each drug in this study increased in response to increase in the drug concentration or the exposure time. Fluorometholone exposure caused significantly higher cytotoxicity compared with that observed for the 3 NSAID eye solutions used in this study. While diclofenac sodium showed the highest cytotoxicity after 12- and 24-hr exposure compared to the other NSAID eye solutions, fluorometholone's cytotoxicity after 24-hr exposure was significantly higher than that of diclofenac sodium. The 1/10-diluted solution of all the eye drops tested had differential effects on cellular metabolic activity and cellular toxicity after 12-hr exposure.
By 1/10-diluted solution of all eye drops tested, cell migration was significantly inhibited by diclofenac sodium and fluorometholone compared with that observed for the other NSAID eye solutions, especially 48 hr later. The untreated cells (control group) migrated and covered the wound gap region more than bromfenac- and pranoprofen-treated cells; however, there was no significant difference between the control and the two NSAIDs.
We evaluated the cellular morphology treated with all eye drops by TEM. The concentration of 1/50 diluted eye solution tested was used, because 1/10 diluted solution of all eye drops induced severe cellular damage enough not to differentiate the morphological cellular change by same concentration of eye solution tested. By TEM, we found that the prominent cellular damage including a dilated rough endoplasmic reticulum, enlarged mitochondria, microvilli loss, and cytoplasmic vacuole formation was observed after 48-hr of exposure to diclofenac sodium and fluorometholone. Especially, fluorometholone induced more severe cytoplasmic membrane disruption and nuclear damage compared to the cellular damage by diclofenac sodium.
Experimental and clinical studies have shown that the long-term use of topical drugs may induce ocular discomfort, tear film instability, conjunctiva inflammation, subconjunctival fibrosis, epithelial apoptosis, and corneal surface impairment (17). The mechanisms underlying ocular surface damage include abnormalities in electrolyte composition, pH, osmolarity, and the preservatives used in ophthalmic solutions. The corneal epithelium is avascular tissue, which derives its electrolytes and oxygen from the tear film. Electrolyte balance of the tear film is, therefore, crucial for biological function. For this reason, unless the eye drop solution has an electrolyte balance that precisely matches that of the human tear film, damage might be caused to the corneal epithelial cells. Eye drop solutions include electrolytes such as Na+, Cl-, K+, and Ca2+. Na+ and Cl- are important ions that are related to cell membrane permeability and their optimal concentration in the extracellular matrix is 142-152.7 mEq/L and 104.0-117.4 mEq/L, respectively (18). The marked corneal epithelial cytotoxicity induced by diclofenac sodium as observed in the current study might be related to the eye drop solution's low Na+ concentration that alters cell membrane permeability. Indeed, Lee and Oum (19) showed that low Na+ and Cl- concentrations caused keratocyte toxicity secondary to changes in cell membrane permeability.
Artificial tears are used as a control group in these types of studies to assess cellular toxicity of eye drops; however, we evaluated the cytotoxicity of steroid and non-steroid eye solutions to corneal cells. Since steroid eye drops are frequently used in eye clinics, their toxic effects on cells or their side effects are known. Hence, we focused on the toxicity of or cellular morphological alterations induced by fluorometholone as well as NSAID eye solutions, and found that cellular toxic effects of commercially available NSAID eye drops differed from those of steroid eye drops. Further studies with these anti-inflammatory eye drops containing same concentrations of electrolytes and preservatives might be required to confirm the cellular toxicity of these two types of anti-inflammatory eye drops.
The preservatives used in eye solutions can also cause ocular surface toxicity. Currently, BAC is the most commonly used preservative in eye solutions. Several studies have reported on the corneal and conjunctival toxicity caused by BAC, including cell loss, disruption of tight junctions, apoptosis and preapoptosis, cytoskeleton changes, and immuno-inflammatory responses (20,21). Diclofenac sodium contains chlorobutanol 0.5%, a higher preservative concentration than that present in the other NSAIDs containing 0.005% BAC. Furthermore, 0.5% chlorobutanol is known to induce cytotoxicity to a degree similar to that observed for 0.01% BAC (22). This also explains the higher toxicity of diclofenac sodium against corneal epithelial cells. Fluorometholone induced the most severe cell damage than that induced by bromfenac sodium and pranoprofen. Although these 3 eye solutions contain the same amount of preservative, i.e., 0.005% BAC, fluorometholone is a steroid that not only inhibits the cyclooxygenase but also the lipoxygenase pathway, which presumably could result in the induction of severe cellular damage (6). In addition, high concentrations of diclofenac sodium have been shown to inhibit the lipoxygenase pathway (23), which might also explain the increased cell damage caused by diclofenac sodium in the current study.
The results of this study agree with previous reports on the toxicity of diclofenac sodium (7,8,24,25,26,27,28). Overall, the toxicity of the diclofenac sodium eye solution might be resulted from the following 3 underlying mechanisms: 1) the lower Na+ concentration, 2) the higher amount of preservatives, and 3) the blockage of the lipoxygenase pathway at high concentrations (22,23). Our study revealed diclofenac sodium has lower Na+ concentration and higher amount of preservatives rather than other NSAIDs. Owing to these disadvantages or mechanisms of diclofenac, clinicians do not usually prefer diclofenac for treatment of corneal inflammation. As contrasted with diclofenac sodium, the commercially available Bronuck® and Pranoprofen® NSAID eye solutions investigated in this study showed favorable outcomes regarding the cytotoxicity against corneal epithelial cells compared with that of diclofenac sodium or fluorometholone eye solution.
Overall, commercially available, recently developed Bronuck® and Pranoprofen® have less corneal epithelial toxicity compared to the fluorometholone eye solution. Thus, more indications of NSAID eye solutions are required in patients with specific inflammatory ocular conditions that cannot be treated with steroid eye solutions.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design: Lee JS. Acquisition of data: Kim YH. Analysis, interpretation of data and writing the manuscript: Lee JS, Park YM. Critical revision and final submission of manuscript: Lee JS, Park YM. Manuscript approval: all authors.
References
- 1.Chan CK, Lam DS. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2004;137:1157–1158. doi: 10.1016/j.ajo.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 2.McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2000;11:3–6. doi: 10.1097/00055735-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 3.McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Yülek F, Ozdek S, Gürelik G, Hasanreisoğlu B. Effect of topical steroids on corneal epithelial healing after vitreoretinal surgery. Acta Ophthalmol Scand. 2006;84:319–322. doi: 10.1111/j.1600-0420.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 5.Waterbury L, Kunysz EA, Beuerman R. Effects of steroidal and non-steroidal anti-inflammatory agents on corneal wound healing. J Ocul Pharmacol. 1987;3:43–54. doi: 10.1089/jop.1987.3.43. [DOI] [PubMed] [Google Scholar]
- 6.Gasset AR, Lorenzetti DW, Ellison EM, Kaufman HE. Quantitative corticosteroid effect on corneal wound healing. Arch Ophthalmol. 1969;81:589–591. doi: 10.1001/archopht.1969.00990010591023. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Jung W, Choi YR, Kim YS. The effects of steroids and nonsteroidal antiinflammatory agents on proliferation of human ocular fibroblast. J Korean Ophthalmol Soc. 1999;40:1496–1502. [Google Scholar]
- 8.Kapin MA, Yanni JM, Brady MT, McDonough TJ, Flanagan JG, Rawji MH, Dahlin DC, Sanders ME, Gamache DA. Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation. 2003;27:281–291. doi: 10.1023/a:1026024409826. [DOI] [PubMed] [Google Scholar]
- 9.Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000;24:357–370. doi: 10.1023/a:1007049015148. [DOI] [PubMed] [Google Scholar]
- 10.Assouline M, Renard G, Arne JL, David T, Lasmolles C, Malecaze F, Pouliquen YJ. A prospective randomized trial of topical soluble 0.1% indomethacin versus 0.1% diclofenac versus placebo for the control of pain following excimer laser photorefractive keratectomy. Ophthalmic Surg Lasers. 1998;29:365–374. [PubMed] [Google Scholar]
- 11.Lindstrom R. The pharmacologic and pathophysiologic rationale for using NSAIDs in ocular inflammatory disease and ocular surgery. Int Ophthalmol Clin. 2006;46:7–11. doi: 10.1097/01.iio.0000212131.98760.a9. [DOI] [PubMed] [Google Scholar]
- 12.Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108:936–944. doi: 10.1016/s0161-6420(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 13.Hersh PS, Rice BA, Baer JC, Wells PA, Lynch SE, McGuigan LJ, Foster CS. Topical nonsteroidal agents and corneal wound healing. Arch Ophthalmol. 1990;108:577–583. doi: 10.1001/archopht.1990.01070060125062. [DOI] [PubMed] [Google Scholar]
- 14.Ayaki M, Iwasawa A, Soda M, Yaguchi S, Koide R. Cytotoxicity of five fluoroquinolone and two nonsteroidal anti-inflammatory benzalkonium chloride-free ophthalmic solutions in four corneoconjunctival cell lines. Clin Ophthalmol. 2010;4:1019–1024. doi: 10.2147/opth.s12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982;23:269–273. [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Wee WR, Wang XW, McDonnell PJ. Effect of artificial tears on cultured keratocytes in vitro. Cornea. 1995;14:273–279. doi: 10.1097/00003226-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Park YS. Physiology of body fluid. In: Kang DH, editor. Physiology. 5th ed. Seoul: in-Kwang Publishing & Printing; 2000. pp. 585–606. [Google Scholar]
- 19.Lee JS, Oum BS. The effects of artificial tear formulations and anti-inflammatory agents on the cultured keratocytes of rabbit. J Korean Ophthalmol Soc. 1998;39:42–51. [Google Scholar]
- 20.Ramselaar JA, Boot JP, van Haeringen NJ, van Best JA, Oosterhuis JA. Corneal epithelial permeability after instillation of ophthalmic solutions containing local anaesthetics and preservatives. Curr Eye Res. 1988;7:947–950. doi: 10.3109/02713688808997251. [DOI] [PubMed] [Google Scholar]
- 21.Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25:113–119. doi: 10.1089/jop.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusano M, Uematsu M, Kumagami T, Sasaki H, Kitaoka T. Evaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivo. Cornea. 2010;29:80–85. doi: 10.1097/ICO.0b013e3181a3c3e6. [DOI] [PubMed] [Google Scholar]
- 23.Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med. 1986;80:18–23. doi: 10.1016/0002-9343(86)90074-4. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien TP, Li QJ, Sauerburger F, Reviglio VE, Rana T, Ashraf MF. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001;108:656–659. doi: 10.1016/s0161-6420(00)00590-x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu JK, Johnston WT, Read RW, McDonnell PJ, Pangalinan R, Rao N, Smith RE. Histopathology of corneal melting associated with diclofenac use after refractive surgery. J Cataract Refract Surg. 2003;29:250–256. doi: 10.1016/s0886-3350(02)01702-9. [DOI] [PubMed] [Google Scholar]
- 26.Ku EC, Kothari H, Lee W, Kimble EF, Liauw LH. Effects of diclofenac sodium on arachidonic acid metabolism. Agents Actions Suppl. 1985;17:189–193. doi: 10.1007/978-3-0348-7720-6_23. [DOI] [PubMed] [Google Scholar]
- 27.Uyemura SA, Santos AC, Mingatto FE, Jordani MC, Curti C. Diclofenac sodium and mefenamic acid: potent inducers of the membrane permeability transition in renal cortex mitochondria. Arch Biochem Biophys. 1997;342:231–235. doi: 10.1006/abbi.1997.9985. [DOI] [PubMed] [Google Scholar]
- 28.Qu M, Wang Y, Yang L, Zhou Q. Different cellular effects of four anti-inflammatory eye drops on human corneal epithelial cells: independent in active components. Mol Vis. 2011;17:3147–3155. [PMC free article] [PubMed] [Google Scholar]