Abstract
We sought to document the clinical performance of the 1st American Academy of Orthopaedic Surgeons (AAOS) guideline on the prevention of symptomatic pulmonary embolism (PE) after total knee arthroplasty (TKA) in Korean patients, in terms of the proportions of the each risk-stratified group, efficacy and safety. Consecutive 328 patients underwent TKA were preoperatively assessed for the risks of PE and bleeding and categorized into 4 groups: 1) standard risk, 2) high risk for PE, 3) high risk for bleeding, and 4) high risks both for PE and bleeding. One of three options was administered according to the groups (aspirin in group 1 or 4; enoxaparin and following aspirin in group 2; antithrombotic stocking in group 3). Incidences of symptomatic deep vein thrombosis (DVT) and PE, and major or minor bleeding complications were evaluated. Majority of the patients (86%) were assessed to be with standard risks both for PE and bleeding. No patient experienced symptomatic DVT or PE and major bleeding. Eleven percent of the patients discontinued chemoprophylaxis because of bleeding-related wound complication. In conclusion, the 1st AAOS guideline functions successfully in Korean patients undergoing TKA in terms of prevention of symptomatic DVT and PE while avoiding major bleeding complications.
Keywords: Arthroplasty, Replacement, Knee; Venous Thromboembolism; Pulmonary Embolism; Venous Thrombosis; Hemorrhage; Chemoprevention; Aspirin; Enoxaparin; AAOS Guideline
Graphical Abstract
INTRODUCTION
Venous thromboembolism (VTE), consisted of deep-vein thrombosis (DVT) and pulmonary embolism (PE), is a well-known serious complication after total knee arthroplasty (TKA) (1,2). Without prophylaxis after TKA, the overall incidence of DVT ranges from 41% to 85% and that of proximal thrombosis ranges from 5% to 22% (1). Especially, PE can be fatal if once developed, even though its incidence was reported much lower than DVT (3). Therefore, appropriate prophylaxis of VTE should be considered after TKA. However, adopting optimal prophylactic protocol in clinical practice remains challenging task for orthopedic surgeons (3,4).
As clinical guidelines for prevention of VTE by American College of Chest Physicians (ACCP) gained popularity, many orthopedic surgeons have experienced significant bleeding complications after TKA. It seemed largely to stem from the fact that this guideline regarded arthroplasty per se as high risk factor for VTE and emphasized a routine use of potent thromboprophylactic drugs after arthroplasty (1,5,6,7,8). In the meanwhile, the American Academy of Orthopaedic Surgeons (AAOS) clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip and knee arthroplasty, which was issued in 2007, was tried to balance the prevention of VTE and avoiding bleeding complications (2). According to the AAOS guideline, individualized prophylactic measures were recommended for each 4 types of risk-stratified groups based on preoperative assessment of the risks for symptomatic PE and major bleeding, paying equivalent attention to the prevention of both PE and major bleeding complication (4). Considering that the ideal prophylactic protocol should prevent VTE while avoiding bleeding complications related to the VTE prophylaxis, the AAOS guideline was theoretically appealing concept for orthopedic surgeons. Although the 1st AAOS guideline had been established based on the relatively weak evidences, and consequently the risk-stratified approach has been mostly retrieved on its recent 2nd edition, the 1st AAOS guideline has substantial value as the prototype of risk-stratified, individualized VTE prophylaxis strategy (9,10). However, proper evaluation of the 1st AAOS guideline has not been conducted well in the process of its renewal and transition to the 2nd guideline, and the results of its clinical application have not been reported in the literature.
This study aimed to demonstrate the clinical performance of the 1st AAOS guideline, in terms of the proportions of the each four risk-stratified group, efficacy (prevention of symptomatic PE and DVT) and safety (avoidance of bleeding complications). We hypothesized that the majority of the patients would have standard risks for both PE and bleeding and application of this AAOS guideline would provide satisfactory efficacy and safety.
MATERIALS AND METHODS
Study design and subjects
Retrospective cohort study using prospectively collected data of consecutive 328 patients was performed who undergoing primary TKA due to advanced osteoarthritis, from April 2008 to March 2009 at our institution. No patients were excluded for any reasons. There were 299 females (91.2%) and 29 males (8.8%) with the mean age of 69.2 yr (SD, 6.3; range, 54-78) (Table 1). The mean body mass index (BMI) was 27.2 kg/m2(SD, 3.6; range, 21.8-36.9). One hundred sixty-nine patients (51.5%) underwent unilateral TKA whereas simultaneous or staged bilateral TKAs were performed on 99 (30.2%) and 60 patients (18.3%) respectively.
Table 1. Demographic feature of the patients in the three groups according to the AAOS guideline.
| Variables | Total n = 328 (100%) |
Standard risk n = 282 (86.0%) |
High PE risk n = 32 (9.8%) |
Highbleedingrisk n = 14 (4.3%) |
P value |
|---|---|---|---|---|---|
| Female (%) | 299 (91.2) | 254 (90.1) | 32 (100.0) | 13 (92.9) | 0.168 |
| Age (yr) | 69.2 ± 6.3 | 69.2 ± 6.4 | 69.3 ± 6.3 | 69.8 ± 4.7 | 0.951 |
| Height (cm) | 152.6 ± 6.8 | 152.8 ± 7.0 | 149.7 ± 5.1 | 154.8 ± 6.8 | 0.024* |
| Weight (kg) | 63.4 ± 10.0 | 62.9 ± 9.8 | 66.3 ± 12.5 | 67.0 ± 8.3 | 0.075 |
| BMI (kg/m2) | 27.2 ± 3.6 | 26.9 ± 3.5 | 29.5 ± 4.8 | 27.9 ± 2.9 | 0.001† |
| Operation type (%) | 0.030‡ | ||||
| Unilateral TKA | 169 (51.5) | 144 (85.2) | 18 (10.7) | 7 (4.1) | |
| Simultaneous bilateral TKA | 99 (30.2) | 92 (92.9) | 6 (6.1) | 1 (1.0) | |
| Staged bilateral TKA | 60 (18.3) | 46 (76.7) | 8 (13.3) | 6 (10.0) |
Data of age, height, weight, and BMI are presented as mean value±standard deviation. *The patients with high risk for PE have short stature than patients of other groups; †The patients with high risk for PE have greater BMI than patients of other groups; ‡There were more patients with standard risks for pulmonary embolism and bleeding in the simultaneous bilateral TKA group than unilateral or staged bilateral TKA groups. AAOS, American Academy of Orthopaedic Surgeons; PE, pulmonary embolism; BMI, Body Mass Index; TKA, total knee arthroplasty.
Surgical procedure and postoperative care
All TKAs were performed by a single surgeon using the standard medial parapatellar arthrotomy with a tourniquet. One of the two posteriorly stabilized prostheses (Genesis II; Smith and Nephew, Memphis, USA and e.motion; B.Braun Aesculap, Tuttlingen, Germany) was implanted for every patient. In all cases, patellar resurfacing and cement fixation were done. A closed suction drainage was placed subcutaneously before wound closure. A compressive dressing was applied with an immobilizer during the first 24 hr after surgery, and patients were encouraged to perform quadriceps strengthening exercises after they had returned to the ward from the recovery unit. After the first 24 hr, all patients were allowed to walk as tolerated using with a walker and start active and passive range-of-motion (ROM) exercises. The continuous passive motion (CPM) machine was used twice a day, and ROM was increased as tolerated until discharge. Patients were discharged between 7 to 10 days after surgery.
The same protocol for anesthesia, pain control, antibiotics and blood transfusion were applied to all patients. Neuraxial anesthesia such as spinal anesthesia for unilateral or staged bilateral TKA and combined spinal-epidural anesthesia for simultaneous bilateral TKA was carried out. For pain control, preemptive analgesics were administered preoperatively, and multiple modalities, such as continuous femoral nerve block, intravenous patient controlled analgesia (PCA) or epidural PCA, oral analgesics, and acute pain rescuer injections, were employed postoperatively. Allogenic blood transfusion was done only when hemoglobin level dropped below 7.0 g/dL or when it fell to a level between 7.0 and 8.0 g/dL with anemic symptoms and signs, such as drop in systolic blood pressure below 100 mmHg, tachycardia defined as heart rate over 100 beats/min, or decreased urine output of less than 30 mL/hr, even after volume replacement with 500 mL of normal saline.
Risk stratification and thromboprophylaxis regimen
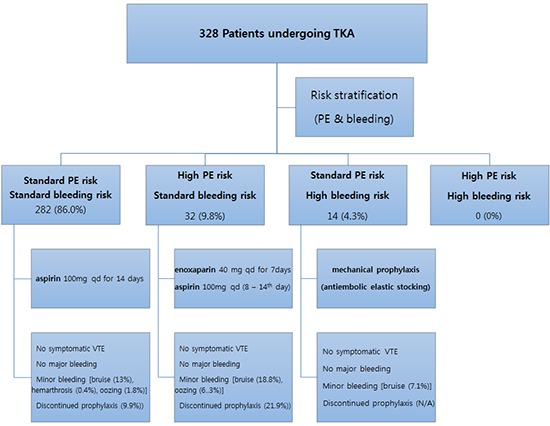
We preoperatively assessed the risks of pulmonary embolism and bleeding complications in all patients using medical history and laboratory results. Then all patients were prospectively stratified into 4 groups, based on the thorough preoperative risks assessments: 1) standard risks for both PE and bleeding,2) increased risk for PE and standard risk for bleeding,3) standard risk for PE and increased risk for bleeding, and 4) increased risks for both PE and bleeding. Risk assessment criteria were based on the 1st AAOS guideline and National Institute for Health and Clinical Excellence (NICE) guideline issued in 2007 (2,11). Detailed risk factors for PE and bleeding are listed in the Appendix Table 1.
The individualized postoperative thromboprophylaxis for 4 risk-stratified groups were followed by the 1st AAOS guideline (2). In patients of the elevated risk for PE and standard risk for bleeding group, enoxaparin 40 mg was administrated subcutaneously on postoperative 1 to 7 days, followed by aspirin 100 mg once daily until postoperative 14 days. In patients of the elevated risk of major bleeding with standard risk for PE group, only mechanical prophylaxis (antiembolic elastic stocking) was applied without chemoprophylaxis. In patients of the standard or elevated risk for both PE and major bleeding, aspirin 100 mg was administrated once daily on postoperative 1st to 14th day.
Outcome evaluation
All patients were evaluated until 3 months after surgery, concerning the efficacy and safety outcome. Primary efficacy outcome was defined as the overall incidence of symptomatic DVT or PE. All patients were routinely required to visit outpatient clinic at 2 weeks, 6 weeks and 3 months after surgery, and educated to visit emergency unit if any suspicious symptoms of PE developed. If a patient has symptoms which can make suspicion of DVT or PE, computerized tomographic (CT) angiography is performed to confirm the diagnosis of DVT, and CT angiography or ventilation-perfusion lung scan is performed to diagnose PE. Suspicious symptoms or signs of DVT are pain, edema, warmth or erythema of the leg or thigh and Homan's sign, and those of PE are chest pain or discomfort and dyspnea. Primary safety outcome was the overall incidence of major bleeding, which include bleeding in critical organ (retroperitoneal, intracranial, intraocular and intraspinal bleeding), fatal bleeding, bleeding leading to reoperation or other intervention, extrasurgical site bleeding that leading to fall in the hemoglobin level of ≥2 g/dL or that leading to transfusion of ≥2 units of packed red blood cells or whole blood (12,13). Secondary safety outcomes were minor bleeding complications and hematological changes such as the hemoglobin drop and the rate of transfusion. While changing wound dressing, we checked any bleeding-related wound problems, such as bruise (extending more than 3 cm from the incision margin), subcutaneous hematoma or hemarthrosis, oozing (persisting over 2 days after surgery which soaking 3 pieces of gauze with over 1 cm width). Surgical site infection, either superficial or deep infection, was also evaluated during wound evaluation. Whether to discontinue chemoprophylaxis was decided on the risk of wound complication that anticipated progressive bleeding or complicated infection. For the hematological evaluation, the hemoglobin level was checked on the 2nd and 5th postoperative days (POD2 and POD5) and hemoglobin drop was calculated by subtracting those of POD2 or POD5 from the preoperative hemoglobin level. In addition, allogenic or autologous transfusion rate was also noted.
Statistical analysis
Statistical analyses were conducted using SPSS for Windows (version 20.0; IBM, Chicago, IL, USA). The Kolmogorov-Smirnov test was used first to determine whether parameters were normally distributed. The proportions of the each risk-stratified group were assessed and the demographics of each group were compared. The proportions of each group were further evaluated by subgrouping the patients based on the surgical timing. The overall incidence of symptomatic DVT and PE or bleeding complications was assessed, as well as the rate of discontinuation of the chemoprophylaxis. The incidences of complications were also compared with each group. The overall hematologic results such as hemoglobin drop and allogenic transfusion rate were evaluated and the parameter were compared between the risk-stratified groups with the patient further divided into the unilateral or 1st knees in the staged bilateral TKAs and simultaneous bilateral TKAs. Statistical significance of the difference between the groups was determined by analysis of variance (ANOVA) with the Bonferroni test as the post-hoc test for continuous variables and chi-square test for categorical variables, regarding P values <0.05 as statistically significant.
Ethics statement
This study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB No. B-1110/138-107). Informed consent was waived by the board.
RESULTS
The distribution of the patients over 4 risk-stratified groups was obviously disproportionate and the demographic features are somewhat different between the groups. Of the 328 patients, 282 (86.0%) were categorized to the group with standard risks for both PE and bleeding, 32 (9.8%) to the group with increased risk for PE and standard risk for bleeding, and 14 (4.3%) to the group with standard risk for PE and increased risk for bleeding (Table 1). There were no patients with increased risks for both PE and bleeding. There were significant differences in height (P=0.024) and BMI (P=0.001), whereas gender, age and weight were not significantly different. The patients with simultaneous bilateral TKAs were categorized more to the standard risks group and less to the high risk for bleeding groups (P=0.030) (Table 1). There were no patients experienced symptomatic DVT or PE in this series (Table 2).
Table 2. Incidence of complications according to the risk groups.
| Complications | Total (n = 328) | Standard risk (n = 282) | High PE risk (n = 32) | High bleeding risk (n = 14) | P value |
|---|---|---|---|---|---|
| VTE | |||||
| Symptomatic PE | 0 | 0 | 0 | 0 | NA |
| Symptomatic DVT | 0 | 0 | 0 | 0 | NA |
| Major bleeding | 0 | 0 | 0 | 0 | NA |
| Minor bleeding | |||||
| Bruise (%) | 43 (13.1) | 37 (13.1) | 6 (18.8) | 1 (7.1) | 0.680 |
| Subcutaneous hematoma | 0 | 0 | 0 | 0 | NA |
| Hemarthrosis (%) | 1 (0.3) | 1 (0.4) | 0 (0) | 0 (0) | 0.910 |
| Oozing (%) | 7 (2.1) | 5 (1.8) | 2 (6.3) | 0 (0) | 0.187 |
| Infection (%) | 1 (0.3) | 1 (0.4) | 0 (0) | 0 (0) | 0.910 |
| Discontinued prophylaxis (%) | 35 (11.1) | 28 (9.9) | 7 (21.9) | NA | 0.079 |
PE, pulmonary embolism; VTE, venous thromboembolism; DVT, deep vein thrombosis; NA, not applicable.
There were no patients with any major bleeding complication whereas several minor bleeding complications were noted. There were 43 patients (13.1%) with bruise and 7 patients (2.1%) with oozing. There was 1 patient (0.3%) with hemarthrosis and deep infection, respectively, but no patients experienced sub-cutaneous hematoma. Among the patients having minor bleeding complications, we decided to stop chemoprophylaxis in 35 patients (10.7%) to prevent progressing into more serious complications. Statistical significance was not reached by any of the differences in the incidences of any complications among three groups (Table 2).
There were no significant differences in hematologic parameters, such as hemoglobin drop and transfusion rate, among the three risk-stratified groups (Table 3). The high bleeding risk group was showed greater hemoglobin drop on POD 2 in unilateral or 1st staged bilateral TKA patients than other groups, but the differences did not reach statistical significance. In the simultaneous bilateral group, the hemoglobin drop and transfusion rate were significantly higher than the unilateral or 1st knee of staged bilateral TKA patients.
Table 3. Hematologic results according to the risk groups.
| Parameters | Total | Standard risk | High PE risk | High bleeding risk | P value |
|---|---|---|---|---|---|
| Unilateral or 1st staged bilateral | n = 198 | n = 167 | n= 21 | n= 10 | |
| Preoperative hemoglobin (g/dL) | 12.9 ± 1.4 | 12.8 ± 1.1 | 13.1 ± 1.1 | 12.9 ± 1.4 | 0.796 |
| Hemoglobin on POD2 (g/dL) | 10.0 ± 1.4 | 10.1 ± 1.4 | 9.8 ± 1.5 | 9.4 ± 1.5 | 0.306 |
| Hemoglobin drop on POD2 (g/dL) | 2.9 ± 1.1 | 2.9 ± 1.1 | 3.0 ± 1.2 | 3.7 ± 0.9 | 0.079 |
| Hemoglobin on POD5 (g/dL) | 9.4 ± 1.3 | 9.4 ± 1.3 | 9.0 ± 0.9 | 8.9 ± 1.7 | 0.197 |
| Hemoglobin drop on POD5 (g/dL) | 3.5 ± 1.2 | 3.5 ± 1.2 | 3.7 ± 1.0 | 4.2 ± 1.1 | 0.138 |
| Allogenic transfusion (%) | 7 (3.5) | 6 (3.6) | 1 (4.8) | 0 (0) | 0.702 |
| Autologous transfusion (PABD, %) | 1 (0.6) | 1 (0.6) | 0 (0) | 0 (0) | 1.000 |
| Simultaneous bilateral | n= 99 | n= 92 | n=6 | n=1 | |
| Preoperative hemoglobin (g/dL) | 13.1 ± 1.1 | 13.1 ± 1.1 | 12.2 ± 0.5 | 12.7 (NA) | 0.107 |
| Hemoglobin on POD2 (g/dL) | 8.1 ± 1.1 | 8.1 ± 1.1 | 7.9 ± 1.2 | 8.3 (NA) | 0.850 |
| Hemoglobin drop on POD2 (g/dL) | 5.0 ± 1.5 | 5.0 ± 1.2 | 4.3 ± 1.0 | 4.4 (NA) | 0.459 |
| Hemoglobin on POD5 (g/dL) | 8.1 ± 0.8 | 8.1 ± 0.8 | 7.9 ± 0.8 | 8.7 (NA) | 0.592 |
| Hemoglobin drop on POD5 (g/dL) | 5.0 ± 1.3 | 5.0 ± 1.3 | 4.3 ± 0.6 | 4.0 (NA) | 0.273 |
| Allogenic transfusion (%) | 14 (14.1) | 13 (14.1) | 1 (16.7) | 0 (0) | 1.000 |
| Autologous transfusion (PABD, %) | 22 (22.2) | 20 (21.7) | 2 (33.3) | 0 (0) | 0.700 |
Data are presented as means±standard deviations or number of event with their percentage in the parenthesis. POD, postoperative day; PABD, preoperative autologous blood donation; NA, not applicable.
DISCUSSION
The most important findings of this study are that the majority of patients have standard risk for both PE and bleeding according to the risk stratification recommended by the 1st AAOS guideline, and that of efficacy and safety are favorable, although this study was performed in single tertiary hospital in Korea. The group with standard risks for both PE and bleeding accounted for 86.0%, while the patients with increased risk for PE or bleeding comprised only 9.8% or 4.3%, respectively. Symptomatic DVT and PE, major bleeding complications are not occurred when followed by the 1st AAOS guideline, although substantial minor bleeding complications were noted.
Our findings coincide with the hypothesis that the majority of Korean patients undergoing TKA have standard risks for both PE and bleeding while only minor proportions have high risks for PE or bleeding according to the risk assessment system of the 1st AAOS guideline. It was not feasible to compare our result directly with previous studies because of different risk assessment criteria. However, our results may be able to be interpreted that the proportion of the patients with high risk of PE is not very high in Korean patients, which is consistent with the previous reports stated low risk of VTE among Asians (14,15,16). It might also imply that routine use of potent thromboprophylactic drug in all patients may be excessive treatment which can bring bleeding complication, considering that the majority of patients have standard risk of PE and bleeding. Therefore, the strategy of risk assessment and individualized approach in the 1st AAOS guideline would be theoretically appealing to the orthopedic surgeons who want to balance the effective VTE prophylaxis and the safety related to the prophylactic drugs.
This study also supported our hypothesis that the application of the AAOS guideline would provide satisfactory efficacy. No patients were suffered the complications of symptomatic DVT or PE in all groups, and those incidences were significantly lower than earlier studies followed the ACCP guideline (5,6). In addition, our results are comparable to those of other studies from Korea, which reported very low incidence of symptomatic DVT or PE (Table 4) (16,17,18,19,20,21,22). Since this study was focused on only symptomatic events after TKA, the numbers of VTE events were comparable or even lower than those of 9th ACCP guideline (1.8% of VTE, 0.55% of PE and 1.25% of DVT after major orthopedic surgery with LMWH thromboprophylaxis) (23). As our results, lower incidence of DVT and PE in Asian TKA patients compared to Caucasian population were reported, even without VTE prophylaxis (14,15,16,24). It has been suggested that it is related to the low prevalence of obesity, venous disease and the absence of some genetic factors in Asian patient populations (14,25). In addition, clinical suspicion for VTE after surgery might not be as high as Western countries in Korea, owing to the relative low incidence of VTE (22). This difference of clinical interest about VTE among countries could affect the diagnosis of symptomatic VTE. However, considering that even the patients without VTE prophylaxis are reported having very low incidence of symptomatic DVT or PE, we cannot reveal that chemoprophylaxis can be beneficial in terms of prevention of symptomatic VTE in Korean patients undergoing TKA (17,19,20). Nonetheless, our findings can be interpreted, at the very least, that the different postoperative thromboprophylactic regimen based on an individual risk assessment according to the 1st AAOS guideline was not insufficient for prevention of symptomatic DVT and PE for in the Korean patients undergoing TKA.
Table 4. Incidence of symptomatic venous thromboembolism and bleeding complications in recent studies from Korea.
| Author (Reference No) | Year | Prophylaxis | No. of patients | Symptomatic DVT | Symptomatic PE | Major bleeding | Minor bleeding |
|---|---|---|---|---|---|---|---|
| Kim (16) | 2007 | No | 264 | 2 (0.8%) | 0 | NA | NA |
| Kim (19) | 2011 | No | 297 | 0 | 0 | NA | NA |
| Park (21) | 2012 | No | 126 | 4 (3.2%) | 0 | NA | NA |
| Kim (18) | 2013 | No | 429 | 6 (1.4%) | 1 (0.2%) | 1 (0.2%) | 1 (0.2%) |
| Enoxaparin | 907 | 4 (0.4%) | 2 (0.2%) | 5 (0.6%) | 8 (0.9%) | ||
| Cho (17) | 2013 | No | 74 | 0 | 0 | 8 (10.8%) | 4 (5.4%) |
| Fondaparinux | 74 | 0 | 0 | 13 (17.6%) | 24 (32.4%) | ||
| Lee (20) | 2013 | Fondaparinux + TXA | 36 | 0 | 0 | 0 | 5 (13.9%) |
| Fondaparinux | 36 | 0 | 0 | 0 | 13 (36.1%) | ||
| Yhim (22) | 2014 | NA | 190,341 | 0.71% | 0.37% | NA | NA |
NADVT, deep vein thrombosis; PE, pulmonary embolism; NA, not applicable; TXA, tranexamic acid.
The safety of the 1st AAOS guideline was also supported by our findings that reported no major bleeding, although several bleeding related wound complications were noted. In the literatures, chemoprophylaxis for VTE increases the risk of bleeding after total joint arthroplasty (4,5). The incidence of major bleeding variably rises to as high as 5% in patients given chemoprophylaxis for VTE (12,26). Surgeons regard major bleeding complications after TKA as unacceptable, because those can lead to more serious complications compromising the surgical outcome such as infection, wound healing problems, functional disability, and loosening. The 7th and 8th ACCP guideline did not accept aspirin with minimal risk for bleeding for VTE prophylaxis after Total joint arthroplasty. In addition, these guidelines did not consider significantly the possibility of bleeding complication of low molecular weight heparin (LMWH) and warfarin (5). In our study, aspirin was selected as thromboprophylactic regimen in standard risk group patients to keep the balance between efficacy and safety. Only mechanical measures were applied in high risk for bleeding group to focus more on safety outcome. In our series, only 1 patient (0.3%) suffered from hemarthrosis in standard risks group and no patient suffered from subcutaneous hematoma in all three groups. However, we found high incidence of bleeding-related wound complications such as bruise (13.1%) and subsequent discontinuation of chemoprophylaxis (11.1%). A previous study from Western reported 3.4% of clinically important operative-site hemorrhage in warfarin group, and 6.9% in enoxaparin group, which are lower than our results (12). Moreover, the mean hemoglobin drop of three groups was more than 2 g/dL in POD2 in our study, which was considered as a major bleeding event in some other studies (12,13). These findings showed the possibility that we might have underestimate bleeding complications. On the other hand, two Korean studies reported over 30% of minor bleeding complications in patients treated with factor Xa inhibitor, which incidences are higher than the current study (17,20). However, owing to the variability of the safety outcome measurement in other clinical studies, it was difficult to compare the safety outcomes of our study directly with those of other studies, especially the bleeding-related wound complications. Of the bleeding-related wound complications, there were not very much significant clinically detrimental events other than 9 patients (2.7%) of hemarthrosis, oozing or infection. We thought these results may be contributed by means of well-timed clinical decision on discontinuation of the prophylactic drugs. More bleeding related complication would be occurred if the chemoprophylaxis would have continued regardless of the clinical course. There were more discontinuation of the prophylaxis in the high risk of PE group than the standard risk group (21.9% vs. 9.9%) in spite of the statistical insignificance (P=0.079). It could be understood as probability of more bleeding related complications with the monotonous administration of Enoxaparin, which has known as much potent anticoagulant than aspirin, without regarding the risk groups. Our findings suggest that less aggressive thromboprophylaxis might be suitable for the Korean patients in terms of the safety. Although AAOS guideline was considered as less aggressive guideline and more balanced regarding the efficacy and safety than ACCP guideline, considerable number of bleeding-related wound complications should still be noted when applying AAOS guideline to the Korean patients undergoing TKA.
To interpret our findings properly, several limitations should be noted. First, 91.2% of our patients were women, which the gender proportion is distinct from Western series. However, a nation-wide data of Korea confirms that this female predominance is general feature of the patients' population undergoing TKA in Korea (27). For gender can influence on the incidence of VTE, this unique gender composition should be considered before extrapolating our findings to other population. Second, this is a single center study and the subject number is too small considering the very low incidence of symptomatic PE. Caution should be exercised to generalize the results of this study. Third, the risk assessment criteria were not only based on the 1st AAOS guideline, but also on the NICE guideline issued in 2007, and the criteria were not sufficiently validated by strong evidence. In the 2nd AAOS guideline, only previous history of VTE is accepted as a risk factor of VTE, and hemophilia and active liver disease are adopted as risk factors of bleeding. However, we decided to overestimate the patients' risks for both PE and bleeding rather than to neglect them by admitting many criteria in the NICE guideline, in order to more actively counteract to their risks, whether for PE or bleeding. Although our risk assessment criteria overestimate the risk of the patients, majority of the patients in our series were categorized into standard risk group both for PE and bleeding. It may imply that racial characteristics of Korean patients are not prone to have risk factors for PE or bleeding. Fourth, we did not have control group in this study so the direct comparison of the efficacy and safety of the 1st AAOS guideline with other control guideline was not available. However, our finding may have implication because there was no patient who experienced symptomatic DVT or PE and major bleeding complications. Furthermore, our study may reflect the real clinical situation, because it was performed in all consecutive patients undergoing primary TKA during 1 yr, not in selected study population. Fifth, there was no patient with high risks for both PE and bleeding in this series, so we could not show the result of the clinical performance of 1st AAOS guideline in that kind of patients. We thought that this unique risk group proportion may be linked with racial characteristics of Korea. Different risk assessment results are anticipated in other countries, and it might affect the overall incidence of symptomatic DVT and PE or bleeding complications. Sixth, the dosage and duration of aspirin administration was different from the recommendation of the 1st AAOS guideline. In the guideline, it was recommended to use 325 mg of aspirin twice a day for 6 weeks. However, the aspirin form of 325 mg is not available in Korean market and 100 mg form is only produced. We used 100 mg of aspirin once a day for 2 weeks considering the lower incidence of DVT and PE in Asia than in westerns. The shorter duration of aspirin administration could have increased the incidence of symptomatic DVT or PE, but those did not occur in our series. Furthermore, a dose of 100 mg, same as in our study, was used in a study about the efficacy of low-dose aspirin in prevention of recurrent VTE (28). So we thought the dose and duration of aspirin did not affect the result significantly, although that had not strictly followed the 1st AAOS guideline. Finally, routine screening studies, such as venography or ultrasonography were not performed in the current study, so the incidence of asymptomatic DVT was not evaluated. However, the routine screening test is not recommended in the latest clinical practice guidelines regarding VTE prophylaxis (23,29,30).
In conclusion, this study demonstrated that the risk-stratified thromboprophylaxis based on the 1st AAOS guideline on prevention of symptomatic pulmonary embolism after total knee arthroplasty functions successfully in Korean patients undergoing TKA regarding prevention of symptomatic DVT and PE while avoiding major bleeding complications. However, occurrence of minor wound complication should be considered when applying the guideline in clinical practice. Individualized, risk-stratified thromboprophylaxis might be a reasonable option in Korean patients undergoing TKA, which might be able to balance the efficacy and safety than routine use of potent chemoprophylaxis.
Appendix Table 1
Risk assessment criteria

Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Kim TK. Data collection: Kim YH, Cho KJ, Fang R. Data interpretation: Kim TK, Na YG. Statistical analysis: Na YG, Fang R. Writing: Na YG, Kim YH, Cho KJ. Review & revision: Kim TK, Fang R. Approval of final manuscript: all authors.
References
- 1.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 2.Johanson NA, Lachiewicz PF, Lieberman JR, Lotke PA, Parvizi J, Pellegrini V, Stringer TA, Tornetta P, 3rd, Haralson RH, 3rd, Watters WC., 3rd Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Am Acad Orthop Surg. 2009;17:183–196. doi: 10.5435/00124635-200903000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Haas SB, Barrack RL, Westrich G, Lachiewicz PF. Venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2008;90:2764–2780. [PubMed] [Google Scholar]
- 4.Parvizi J, Azzam K, Rothman RH. Deep venous thrombosis prophylaxis for total joint arthroplasty: American Academy of Orthopaedic Surgeons guidelines. J Arthroplasty. 2008;23:2–5. doi: 10.1016/j.arth.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Burnett RS, Clohisy JC, Wright RW, McDonald DJ, Shively RA, Givens SA, Barrack RL. Failure of the American College of Chest Physicians-1A protocol for lovenox in clinical outcomes for thromboembolic prophylaxis. J Arthroplasty. 2007;22:317–324. doi: 10.1016/j.arth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan JJ, Dorr LD, Engh GA, Hanssen AD, Healy WL, Lachiewicz PF, Lonner JH, Lotke PA, Ranawat CS, Ritter MA, et al. American Colleg of Chest Physicians. Prophylaxis for thromboembolic disease: recommendations from the American College of Chest Physicians--are they appropriate for orthopaedic surgery? J Arthroplasty. 2005;20:273–274. doi: 10.1016/j.arth.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 8.Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does "Excessive" Anticoagulation Predispose to Periprosthetic Infection? J Arthroplasty. 2007;22:24–28. [Google Scholar]
- 9.Eikelboom JW, Karthikeyan G, Fagel N, Hirsh J. American Association of Orthopedic Surgeons and American College of Chest Physicians Guidelines for Venous Thromboembolism Prevention in Hip and Knee Arthroplasty Differ: What Are the Implications for Clinicians and Patients? Chest. 2009;135:513–520. doi: 10.1378/chest.08-2655. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JJ, Mont MA, Bozic KJ, Della Valle CJ, Goodman SB, Lewis CG, Yates AC, Jr, Boggio LN, Watters WC, 3rd, Turkelson CM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Bone Joint Surg Am. 2012;94:746–747. doi: 10.2106/JBJS.9408.ebo746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NICE. NICE clinical guideline 46: venous thromboembolism-Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery. National Institute for Health and Clinical Excellence (United Kindom); 2007. [Google Scholar]
- 12.Fitzgerald RH, Jr, Spiro TE, Trowbridge AA, Gardiner GA, Jr, Whitsett TL, O'Connell MB, Ohar JA, Young TR Enoxaparin Clinical Trial Group. Prevention of venous thromboembolic disease following primary total knee arthroplasty. A randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am. 2001;83-A:900–906. [PubMed] [Google Scholar]
- 13.Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE Apollo Investigators. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost. 2007;5:1854–1861. doi: 10.1111/j.1538-7836.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 14.Klatsky AL, Armstrong MA, Poggi J. Risk of pulmonary embolism and/or deep venous thrombosis in Asian-Americans. Am J Cardiol. 2000;85:1334–1337. doi: 10.1016/s0002-9149(00)00766-9. [DOI] [PubMed] [Google Scholar]
- 15.Stein PD, Kayali F, Olson RE, Milford CE. Pulmonary thromboembolism in Asians/Pacific Islanders in the United States: analysis of data from the National Hospital Discharge Survey and the United States Bureau of the Census. Am J Med. 2004;116:435–442. doi: 10.1016/j.amjmed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22:974–980. doi: 10.1016/j.arth.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Cho KY, Kim KI, Khurana S, Bae DK, Jin W. Is routine chemoprophylaxis necessary for prevention of venous thromboembolism following knee arthroplasty in a low incidence population? Arch Orthop Trauma Surg. 2013;133:551–559. doi: 10.1007/s00402-013-1691-z. [DOI] [PubMed] [Google Scholar]
- 18.Kim GH, Park BY, Bae TY, Kang JW, In Y. Can enoxaparin reduce thromboembolism related events after primary TKA in Asian patients? J Arthroplasty. 2013;28:1862–1867. doi: 10.1016/j.arth.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Kim KI, Cho KY, Jin W, Khurana SS, Bae DK. Recent Korean perspective of deep vein thrombosis after total knee arthroplasty. J Arthroplasty. 2011;26:1112–1116. doi: 10.1016/j.arth.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21:2611–2617. doi: 10.1007/s00167-012-2213-1. [DOI] [PubMed] [Google Scholar]
- 21.Park KH, Cheon SH, Lee JH, Kyung HS. Incidence of venous thromboembolism using 64 channel multidetector row computed tomography-indirect venography and anti-coagulation therapy after total knee arthroplasty in Korea. Knee Surg Relat Res. 2012;24:19–24. doi: 10.5792/ksrr.2012.24.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yhim HY, Jang MJ, Bang SM, Kim KH, Kim YK, Nam SH, Bae SH, Kim SH, Mun YC, Kim I, et al. Incidence of venous thromboembolism following major surgery in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2014;12:1035–1043. doi: 10.1111/jth.12611. [DOI] [PubMed] [Google Scholar]
- 23.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW, Jr American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leizorovicz A, Turpie AGG, Cohen AT, Wong L, Yoo MC, Dans A The Smart Study Group. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART Study. J Thromb Haemost. 2005;3:28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 25.Zivelin A, Rosenberg N, Faier S, Kornbrot N, Peretz H, Mannhalter C, Horellou MH, Seligsohn U. A single genetic origin for the common prothrombotic G20210A polymorphism in the prothrombin gene. Blood. 1998;92:1119–1124. [PubMed] [Google Scholar]
- 26.Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833–1840. doi: 10.1001/archinte.162.16.1833. [DOI] [PubMed] [Google Scholar]
- 27.Koh IJ, Kim TK, Chang CB, Cho HJ, In Y. Trends in Use of Total Knee Arthroplasty in Korea From 2001 to 2010. Clin Orthop Relat Res. 2012 doi: 10.1007/s11999-012-2622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, et al. Aspire Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–1987. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 29.Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19:777–778. doi: 10.5435/00124635-201112000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Bang SM, Jang MJ, Kim KH, Yhim HY, Kim YK, Nam SH, Hwang HG, Bae SH, Kim SH, Mun YC, et al. Korean Society of Thrombosis and Hemostasis. Prevention of venous thromboembolism, 2nd edition: Korean Society of Thrombosis and Hemostasis Evidence-based Clinical Practice Guidelines. J Korean Med Sci. 2014;29:164–171. doi: 10.3346/jkms.2014.29.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]


