Abstract
Chronic pain creates a large socio-economic burden around the world. It is physically and mentally debilitating, and many sufferers are unresponsive to current therapeutics. Many drugs that provide pain relief have adverse side effects and addiction liabilities. Therefore, a great need has risen for alternative treatment strategies. One rich source of potential analgesic compounds that has emerged over the past few decades are conotoxins. These toxins are extremely diverse and display selective activity at ion channels. Voltage gated sodium (NaV) channels are one such group of ion channels that play a significant role in multiple pain pathways. This review will explore the literature around conotoxins that bind NaV channels and determine their analgesic potential.
Keywords: conotoxins, toxins, NaV, ion channels, pain, analgesia, inhibition
1. Introduction
Chronic pain is a major problem in the world today. The total cost of chronic pain in the United States was estimated to be between $560 and $635 billion per annum [1]. This cost is greater than cancer and heart diseases combined [1]. The current therapeutics that are available for chronic pain often provide only limited pain relief and have many side effects. Therefore, there is a great need for alternative therapies that provide analgesia to chronic pain sufferers. Animal toxins are one such alternative source for pain therapy. In particular, conotoxins from marine cone snails provide a very diverse pool of selective compounds that may form the foundation of future analgesic drugs. Conotoxins interacting selectively with NaV channels have potential as pain therapeutics, as reviewed previously [2,3]. The present review updates and provides the perspectives of the authors on the therapeutic potential of conotoxin NaV channel inhibitors for pain management.
The pain transmission pathway involves sensors known as nociceptors, which transmit the signal from the site of pain to primary sensory neurons in the spinal cord known as dorsal root ganglion (DRG) neurons. Central fibres of nociceptive DRG neurons project to the superficial laminae of the dorsal horn, particularly laminae I and II [4,5].
DRG neurons process many sensory modalities, such as proprioception and touch, with the exception of small DRG neurons, which primarily conduct pain signals. Thus, DRG neurons can be characterised into four dorsal root fibre types. Aα fibres conduct information from muscle and skeletal mechanoreceptors. Aβ fibres relay signals from cutaneous and subcutaneous mechanoreceptors. Aδ and C fibres conduct nociceptive information [6]. The central terminals from Aδ and C fibres innervate lamina I (marginal zone) and lamina II (substantia gelatinosa). Superficial (lamina I) and deep (lamina V) second order pain transmission neurons in the dorsal horn send projections to brainstem/midbrain nuclei and thalamic nuclei, respectively, then on to sensory and emotional cortical regions [4]. In addition, descending modulatory pathways from brain strongly modulate ascending information. Direct application of conotoxins to the spinal cord is a potential target for NaV channel inhibitors, where specific inhibition of neurotransmission in the central terminals of nociceptive primary afferent nerves may be achieved [7].
Within DRG neurons, there is a multitude of ion channels, receptors and peptides [8,9,10]. These channels and receptors are believed to serve many functions including transduction, conduction and modulation of synaptic transmission [8]. Transduction involves transient receptor potential channels, sodium channels, acid-sensing ion channels, and ATP-sensitive receptors that convert stimuli to electrical signals in the peripheral terminals of DRG neurons. Conduction involves potassium and sodium channels that help propagate action potentials. Synaptic transmission comprises of neurotransmitter release aided by voltage-gated calcium channels (CaV) and glutamate receptors located on presynaptic terminals of primary afferent fibres in the dorsal horn [8]. Among the diverse ion channels differentially expressed in sensory neurons, some NaV channel subtypes are expressed selectively in pain sensing neurons.
2. NaV Channels Structure
NaV channels are large complex structures made up of a primary alpha (α) subunit along with one or two auxiliary beta (β) subunits. The primary α subunit is about 260 kDa in size with four homologous domains each with six transmembrane segments (S1–S6), [11,12]. Much of the primary structure of the channel was revealed by crystallisation of the bacterial sodium channel, NaVAb [13]. NaVAb has a central pore surrounded by four pore forming modules made of S5 and S6 segments and a pore loop. The outer edge of the pore is associated with S1–S4 voltage sensing modules. There is also a wide outer vestibule, a narrow ion selectivity filter, a large central cavity and an intracellular activation gate made by crossing of S6 segments [11].
In contrast to the single α subunit, there can be one or two β subunits based on the location of the channel. The brain NaV channels are generally made of two β subunits [11]. The β subunit comprises of a N-terminal extracellular immunoglobulin-like fold, a single transmembrane segment and a short intracellular segment. The primary component of the β subunit helps regulate channel kinetics such as voltage dependence of activation and inactivation. The extracellular immunoglobulin domains of β subunits function as cell adhesion molecules that interact with extracellular matrix proteins and other cell adhesion molecules [11].
3. NaV Channel Subtypes
There are nine different mammalian sodium channel α-subunits labelled, NaV1.1 to 1.9. Despite different isoforms of NaV, they share at least 50 percent of their amino acid sequence [14]. When phylogenetically grouped, NaV1.1, 1.2, 1.3 and 1.7 show great similarity and can be found on human chromosome 2q23-24 [11,15]. In comparison, NaV1.5, 1.8 and 1.9 encoded on human chromosome 3p21-24 are also closely related with 64 percent similarity to NaV channels on chromosome 2. Distinguished from both these groups, NaV1.4 is primarily expressed in skeletal muscles and encoded by chromosome 17q23-25 while NaV1.6 is mainly found at nodes of ranvier and encoded by chromosome 12q13. However, both NaV1.4 and NaV1.6 show at least 84 percent similarity to NaV channels coded by chromosome 2 [11,14].
4. Roles of Sodium Channels in Nociception and Chronic Pain
In terms of neuropathic and inflammatory pain, it is unlikely that all NaV channels play a role. Within the diverse DRG neuronal population, NaV channels are localised in various subgroups based on function. Therefore, the properties of each NaV channel needs to be explored. NaV1.1 is primarily found in large diameter A-fibre DRG neurons suggesting a possible role in proprioception [8]. However, a proportion of small non-peptidergic neurons also contain NaV1.1. In fact, mutations of the SCN1A gene that codes for NaV1.1 have been associated with familial hemiplegic migraine suggesting a likely role in nociception [8,16]. In rodents that undergo spinal nerve ligation to model nerve injury, NaV1.1 levels are downregulated. Hence it is unclear as to what role NaV1.1 may play in nociception [8,17]. Although NaV1.2 is found in many regions of the central nervous system (CNS), its level remains low in adult DRG neurons. Therefore, NaV1.2 channels are unlikely to play a major role in nociception [8,17].
NaV1.3 is extensively expressed in neonatal DRG neurons. However, only a little of it can be detected in adult naïve DRG neurons [18]. Following peripheral nerve injury, 37 percent of L5 DRG neurons were NaV1.3 positive [19]. Furthermore, 40–47 percent of medium to large DRG neurons were NaV1.3 positive after SNL [20]. In spite of these findings, the role of NaV1.3 in neuropathic pain remains controversial, as neuropathic pain develops in conditional and conventional NaV1.3 knockout mice [8,21]. Moreover, ectopic discharges from injured nerves were unaltered in NaV1.3 conventional knockout mice [21]. Thus, it is less likely that NaV1.3 plays a major role in the induction of abnormal spontaneous activity in injured neurons. Alternatively, it is possible that the loss of NaV1.3 is compensated by other NaV channel subtypes. It therefore remains uncertain whether or not conotoxins that target NaV1.3 could be developed as pain therapeutics.
NaV1.4 is primarily found in skeletal muscles and NaV1.5 is mainly located in cardiac muscles [14]. Both these channels also do not show a large presence within DRG neurons. Low or complete absence in DRG neurons coupled with localisation outside the DRG, makes NaV1.4 and 1.5 unlikely candidates to play a role in nociception. In terms of analgesic potential, activity of conotoxins at NaV1.4 and 1.5 channels may lead to severe adverse effects.
In contrast, NaV1.6 co-localised with the neurofilament-200 (NF200) marker, which binds to sensory neurons that gives rise to myelinated axons [20]. Therefore, it can be assumed that NaV1.6 is an A-fibre specific channel, which is not surprising because it is associated with nodes of Ranvier. Following SNL, NaV1.6 levels are down-regulated [22]. However, infraorbital nerve injury was found to increase NaV1.6 levels proximal to the lesion site [23]. Despite this elevation, it remains to be determined whether NaV1.6 plays a role in the abnormal spontaneous activity of injured DRG neurons [8].
NaV1.7 shows a wide distribution across different DRG neurons but can be predominantly found in small DRG neurons [8,24]. Since most small DRG neurons are linked to unmyelinated axons, NaV1.7 was primarily co-localised with NF-200 negative neurons [20]. Due to a slow closed-state inactivation, NaV1.7 is unable to respond during high frequency stimulation [25]. However, small depolarizing stimuli close to resting membrane potential have been shown to activate NaV1.7 [8,25]. It may be that NaV1.7 is coupled to NaV1.8 to boost subthreshold stimuli that activates NaV1.8 which recovers from inactivation at a rapid rate and produces high frequency action potentials [6]. Following peripheral inflammation, NaV1.7 mRNA and protein levels are upregulated [26,27]. Furthermore, NaV1.7 knockout mice do not develop hyperalgesia in pain models [28]. In humans, the loss of function mutation of the SCN9A gene that codes for NaV1.7 is linked to channelopathy-associated insensitivity to pain (CIP), while SCN9A gain of function mutations cause paroxysmal extreme pain disorder and primary erythromelalgia [8,29,30]. This evidence suggests that NaV1.7 is important for the development of acute and inflammatory pain.
The role of NaV1.7 in neuropathic pain seems more complex. In a rat diabetic neuropathy model it was found that both NaV1.7 protein and current was elevated [8,31]. However, SNL and spared nerve injury decreased NaV1.7 protein levels [8,22,32]. Furthermore, there was a decline in NaV1.7 protein levels following peripheral axotomy or traumatic central axotomy in the injured DRG neurons of humans [33]. In mice, SNL induced mechanical allodynia despite conditional knockout of NaV1.7 [34]. In contrast, painful neuromas of amputees with phantom limb pain have been found to accumulate NaV1.7 protein [8,35,36]. Nonetheless, conotoxins or other peptide toxins that selectively block NaV1.7 are likely to have therapeutic benefits in at least some pain states. Additionally, as the only recognised deficit in CIP patients is a loss of smell (anosmia), NaV1.7 remains a promising analgesic target with a minimal side effect profile [37].
NaV1.8 is primarily found in small diameter nociceptive DRG neurons [8,38]. Carrageenan injection into the hind paw of rodents to induce inflammation up-regulated NaV1.8 mRNA and protein [26,39]. Moreover, NaV1.8 knockout mice displayed impaired thermal and mechanical pain hypersensitivity following carrageenan induced inflammation [40]. Hence, NaV1.8 is widely accepted to play role in inflammatory pain. However, peripheral nerve injury decreased NaV1.8 mRNA and protein levels in small diameter injured DRG neurons [8,41]. Additionally, SNL at the L5 level up-regulated NaV1.8 in uninjured C-fibres of sciatic nerves [8,42]. It is believed that TNFα may be responsible for these elevated NaV1.8 levels as inhibition of synthesis and knockout of TNFα eliminated the up-regulation of NaV1.8 following nerve injury [43]. The possibility that NaV1.8 plays a role in the development in allodynia was reinforced by a study which revealed that MuO-conotoxin MrVIB selectively blocked Nav1.8 sensory neuron specific sodium channels and chronic pain behaviour without motor deficits [7]. Another report showed that mechanical allodynia was attenuated in a dose dependent manner with NaV1.8 selective blockade in rats with SNL induced neuropathic pain [44]. Additionally, it was found that two NaV1.8 mutations isolated in patients with painful neuropathies enhanced the response of DRG neurons to depolarisation [45]. More intriguing evidence for the role of NaV1.8 in neuropathic pain immerged in an investigation of saphenous nerve neuromas [46]. Results revealed that after 22 days from induced axonal damage, 19 percent of fibres in wild type mice neuromas had spontaneous activity compared to almost no spontaneous activity in the NaV1.8 null mice. Furthermore, 10 days post-surgery, a significantly higher proportion of fibres were mechanosensitive in wild type mice in comparison to NaV1.8 null mice. Thus, it was concluded that NaV1.8 is critical for spontaneous activity and that it may induce ectopic mechanosensitivity in sensory axons that are damaged [46]. These results collated together suggests that NaV1.8 plays a role in painful neuropathies by promoting neuronal hyperexcitability. However, NaV1.8 knockout mice develop neuropathic pain suggesting a possible difference in the role of NaV1.8 in neuropathic pain between rats and mice [8,47]. Moreover, NaV1.8 polymorphisms have been linked to atrial fibrillation. Atrial fibrillation is an electrical disturbance in the heart leading to inefficiencies in cardiac activity [48]. Therefore, a further complication that needs to be considered is the potential for atrial fibrillation due to NaV1.8 block. Overall, NaV1.8 is a promising target for inflammatory analgesia. However, its usefulness for neuropathic pain relief is less clear due to differences between rats and mice as well as potential side effects linked to atrial fibrillation.
NaV1.9 is selectively found in small-diameter DRG neurons [8]. These channels are known to have slow kinetics and currents that are persistent near resting membrane potential [49]. In the complete Freund’s adjuvant (CFA) model to induce inflammation, level of NaV1.9 was significantly elevated [50]. Moreover, NaV1.9 knockout mice show decreased pain behaviours to induced inflammation [51]. Therefore, NaV1.9 may also play an important role in inflammatory pain. However, immunohistochemical analysis after SNL in rats show decreased NaV1.9 levels in the injured DRG neurons [41]. Moreover, mechanical and thermal hypersensitivities developed in NaV1.9 knockout mice after spared nerve injury to the same extent as wild type mice [52]. However, when two NaV1.9 mutations isolated from patients with painful neuropathies were expressed in NaV1.9 knockout mouse DRG neurons, the resting membrane potential was depolarised [53]. Thus, the neurons were more prone to hyperexcitability. Clinically, a heterozygous mutation in NaV1.9 was found in two unrelated individuals with congenital inability to sense pain [54]. However, a different NaV1.9 mutation was isolated in patients with autosomal dominant episodic pain [55]. Therefore, the nature of the NaV1.9 mutation appears to lead to contrasting clinical outcomes. Overall these results indicate that NaV1.9 may play a role in neuropathic pain.
5. Toxin Interaction with NaV Channels
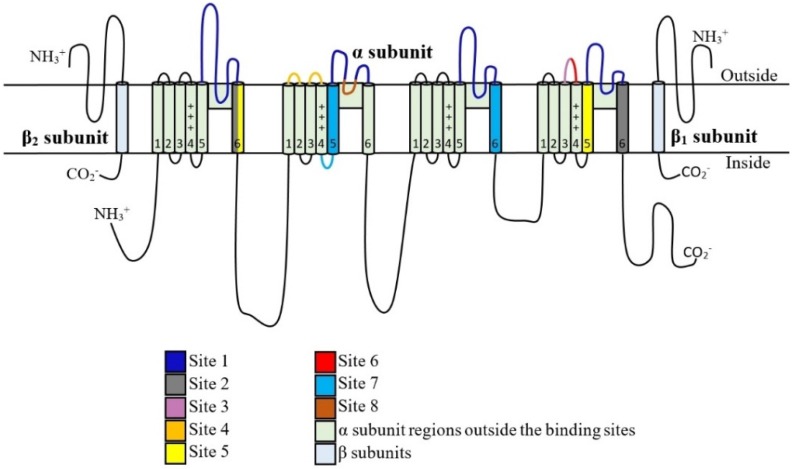
Most pharmacological agents that interact with NaV channels bind to the α subunit [14]. At least eight different binding sites have been isolated for neurotoxins along with a site for local anesthetic binding. There are a range of animal toxins that block NaV channels. These toxins have been identified in venoms from centipedes, spiders and sea dwelling cone snails. Due to the difficulties of isolating native toxins in large quantities, synthetic forms of the native toxins have been usually developed. The specific binding characteristics of these toxins have made them great tools to identify channel structures.
Conotoxins are potent ion channel inhibitors. While there are many different types of conotoxins, four families that target NaV channels with a potential fifth family have been discovered recently. These are identified as µ, μO, δ, ι and µO§. µ and µO-Conotoxins inhibit NaV channels through targeting the channel pore and the voltage sensor, respectively. δ-Conotoxins cause over excitation by slowing inactivation. ι-Conotoxins induce excitotoxicity through shifting the voltage dependence of activation to more hyperpolarised voltages. µO§-Conotoxins cause NaV channel inhibition by mechanisms that are yet to be elucidated. As reviewed elsewhere, it is evident that conotoxins have very diverse mechanisms of action correlated with great structural variability [56]. Therefore, each family of conotoxins need to be studied individually. Identified binding sites on Nav channels are summarized in Figure 1 and conotoxin interactions with these sites are discussed in detail below.
Figure 1.
Site 1 binds μ-conotoxins, GIIIA, GIIIB, GIIIC, PIIIA, TIIIA, BuIIIA, BuIIIB, BuIIIC, SmIIIA, SIIIA, KIIIA. Site 2 binds vetridine and shows no major conotoxin interactions. Site 3 interacts with α-scorpion toxins and does not bind conotoxins. Site 4 binds β-scorpion toxins, μO-contoxins, MrVIA, MrVIB and MfVIA as well as ι-conotoxins RXIA, r11b, r11c, r11d and r11e. Site 5 interacts with brevetoxin and ciguatoxin while no conotoxin interactions have been reported. Site 6 binds δ-conotoxins TxVIA, PVIA, SVIE, GmVIA, EVIA, TsVIA and SuVIA. Insecticide binding site 7 does not interact with conotoxins. Site 8 binds the conotoxin, µO§-GVIIJ.
5.1. μ-Conotoxins that Interact with Binding Site 1
Site 1 binds tetrodotoxin (TTX), saxitoxin (STX) and μ-conotoxins. This site is largely comprised of the P loops between S5 and S6 in each domain that form part of the outer vestibule of the channel. In general, μ-conotoxins bind more superficially to the channel pore than TTX and STX due to their larger size [57]. However, all three toxins inhibit NaV channel current as these P loops play a critical role in controlling permeation and selectivity [58]. Whilst tetrodotoxin and saxitoxin bind to site 1 on most Nav channel types, µ-conotoxins exhibit selectivity for more restricted subsets of channels as summarized in Table 1.
Table 1.
μ-conotoxin activity at NaV channels.
| μ-Conotoxin Type | Conus Species | Primary NaV Channel Targets |
|---|---|---|
| GIIIA | geographus | NaV1.4 [59,60] |
| GIIIB | geographus | NaV1.4 [61] |
| GIIIC | geographus | NaV1.4 [3,62] |
| PIIIA | purpurascens | NaV1.4, 1.2, 1.7 [63,64] |
| TIIIA | tulipa | NaV1.4, 1.2 [65,66] |
| BuIIIA | bullatus | NaV1.4 [67] |
| BuIIIB | bullatus | NaV1.3, NaV1.4 [67,68,69] |
| BuIIIC | bullatus | NaV1.4 [67] |
| SmIIIA | stercusmuscarum | NaV1.8 [70,71] |
| SIIIA | striatus | NaV1.2, 1.3, 1.4 [72,73] |
| KIIIA | kinoshitai | NaV1.2, 1.3, 1.4, 1.5, 1.6 [74,75] |
μ-conotoxins were first isolated from the venom of the fish-hunting snail C. geographus. They are a family of 22-residue peptide amides that inhibit sodium channels [62]. μ-contoxins are only one class of paralytic toxins found in this species of cone snails which also produce ω-conotoxins and α-conotoxins that target CaV channels and acetylcholine receptors, respectively. The first extensively studied μ-conotoxin was GIIIA. It had three hydroxyproline residues and three disulphide bridges. It was shown to compete with TTX and STX for binding at NaV1.4 channels but showed no affinity for NaV neuronal subtypes [76]. Nevertheless, the potency of GIIIA varies between the rat and human NaV1.4 subtypes increasing from an IC50 value of 50 nM in rats to 1500 nM in humans [59]. Moreover, GIIIA shows no inhibition of NaV current in rat DRG neurons [65].
In 1989, GIIIA was synthetically manufactured [60]. The peptide nature of this toxin allowed it to be modified significantly without the loss of biological activity. The three dimensional structure of this toxin revealed that it had an overall disk shape for the backbone with seven Arg and Lys side chains projecting radially. The essential residues for activity were located on one side of the molecule to associate with the receptor site. These key residues important for activity were at positions 13 (Arg13), 19 (Arg19), 16 (Lys16), and 17 (Hyp17 [hydroxyproline]). From these residues, Arg13 was found to be the most significant. The substitution of Arg with Gln at position 13 led to an absence of toxin binding to eel electroplax membranes in a competitive binding assay with STX up to 1 μM [59,77]. Furthermore, Hyp17 in GIIIA is believed to interact with the same NaV channel site as TTX and STX hydroxyl groups [78]. However, GIIIA has some level of redundancy in its residues that interact with NaV1.4 as just the presence of R13 and K16 alone can cause channel block [79]. Hence, GIIIA’s primary purpose is to serve as a histochemical marker for NaV1.4 channels.
GIIIB has a similar global fold to GIIIA along with very similar backbone confirmations and NaV1.4 channel selective activity [61]. In fact, previously mentioned Arg13, Lys16 and Hyp17 residues of GIIIA are also conserved in GIIIB. However, the sequence of GIIIB differs from GIIIA at four positions leading to one more positive charge on GIIIB. These differences include a change to Arg at position 8 and 14 from Lys and Gln respectively, as well as a substitution of Met for Gln at position 18 and Lys for Arg at position 19 [61]. The change at residue 14 has been suggested as the possible reason behind increased toxicity of GIIIB over GIIIA [61]. Nevertheless, not all of these changes in amino acids modify the activity of the toxin. Moreover, when Arg19 was replaced with a Lys on GIIIA, activity was not significantly impaired. This suggested that the conservation of a positive charge was adequate to maintain the original activity [61,77].
μ-conotoxin GIIIC is also another peptide from Conus geographus that selectively targets NaV1.4 channels [3]. It also has an Arg at position 14 similar to GIIIB but differs from GIIIB at position 18 where it has a Leu instead of Met [62]. However, due to similarity in its activity profile to GIIIA and GIIIB, this toxin does not provide any new insights to NaV channel activity.
μ-conotoxin PIIIA was isolated from Conus purpurascens, an Eastern Pacific fish hunting species of cone snails [63]. Similar to GIIIA, it also binds site 1 on NaV channels [63,80]. When rat NaV (rNaV) α subunits were expressed in Xenopus laevis (Xenopus) oocytes, it reversibly inhibited rNaV1.4 channels with an IC50 value of 41 nM. Unlike GIIIA, it also blocked rNaV1.2 channels at an IC50 value of 690 nM and rNaV1.7 channels at an IC50 value of 6.2 μM [64]. Interestingly, PIIIA was more potent at human NaV1.7 channel as seen from an IC50 value of 3.1 μM [64]. Hence it is possible to utilise the varied potency of PIIIA towards TTX sensitive NaV channels to distinguish different subtypes. This was illustrated in a nerve growth factor (NGF) treated PC12 cell line which had a transient upregulation of NaV1.7 in the first 24 h followed by an increase in NaV1.2 beyond 24 h. As a result, it was shown that within the first 24 h, only 13% of the observed NaV current was inhibited by PIIIA followed by 39% at 48 h and 63% at 72 h [64]. Thus, PIIIA serves as a valuable probe to distinguish NaV channel subtypes when used in conjunction with other selective toxins.
A µ-conotoxin with a similar single conformation in solution to PIIIA is TIIIA. It was isolated from the venom duct of Conus tulipa [65]. At the point of discovery, it had less than 35% sequence homology to other µ-conotoxins. Several residues found in µ-conotoxins that inhibit TTX sensitive NaV channels including Arg14 was conserved in TIIIA. TIIIA potently displaces STX from NaV1.2 channels in the rat brain and NaV1.4 channels in skeletal muscle. Consistent with low levels of NaV1.2 and the absence of NaV1.4 channels, 3 µM TIIIA showed no inhibition of NaV current in DRG neurons [65,66]. Therefore, the primary function of TIIIA is to inhibit rat NaV1.2 and 1.4 channels outside of DRG neurons.
Another set of μ-conotoxins that target TTX-sensitive NaV currents are BuIIIA, BuIIIB and BuIIIC from the cone snail species Conus bullatus [67]. More specifically, BuIIIA inhibited NaV1.4 channels expressed in Xenopus oocytes by about 87 percent while BuIIIB and BuIIIC inhibited about 96 percent of the current at a concentration of 1 μM. However, BuIIIA block of NaV1.4 was reversible with a koff value of approximately 0.021 min−1, while BuIIIB and BuIIIC showed little to no recovery in 50 min [67]. Similar to other µ-conotoxins, BuIIIB C-terminal residues such as Trp16, Arg18 and His20 are crucial for NaV blockade [68]. However, a unique feature of these toxins that differentiates them from other μ-conotoxins is an N-terminal extension forming part of a short α-helix that include Glu3 to Asn8. In fact, mutation of residues two and three in this N-terminal extension caused a 40 fold increase in BuIIIB potency from a KD of about 0.2 μM to 0.0048 μM at NaV1.3 channels [69]. Furthermore, a negative charge mutation at the N-terminus of BuIIIB with a γ-carboxyglutamate decreased potency while a positively charged 2,4-diaminobutyric acid substitution increased potency towards NaV1.3 channels [68]. Therefore, it is evident that the N-terminus region is critical to determine toxin potency. In terms of analgesic potential, these BuIIIA, B and C conotoxins do not show great promise due to their activity at NaV1.4 channels that may cause many side effects. As discussed above, the potent NaV1.3 block by mutated BuIIIB need to be further explored in pain research. But this may not produce analgesia since NaV1.3 knockout mice still developed neuropathic pain [8,21].
A µ-conotoxin that is significantly different from the previously described toxins is SmIIIA. Unlike the other TTX sensitive NaV channel inhibitors, SmIIIA blocks TTX resistant NaV channel currents in amphibian DRG and sympathetic neurons [70]. µ-Conotoxin, SmIIIA was isolated from Conus stercusmuscarum. Despite having a different target, SmIIIA shares similar characteristics with other µ-conotoxins, such as the arrangement of cysteine residues in the primary sequence and the conserved Arg13. Nonetheless, structural differences in SmIIIA from other µ-conotoxins include the lack of a Hyp residue and the presence of a Trp15 side chain [70,71]. As TTX resistant NaV1.8 and NaV1.9 channels play a role in pain pathways, it was of interest to further explore this toxin. Unfortunately, later results revealed that 3 µM SmIIIA did not inhibit human NaV1.8 channels [65]. This finding suggested that this toxin was unlikely to have therapeutic potential as it was ineffective at human NaV channels.
Other µ-conotoxins that inhibit TTX resistant NaV channels include SIIIA and KIIIA from the fish-hunting cone snails Conus striatus and Conus Kinoshitai, respectively. Both toxins block TTX resistant NaV currents in neurons of frog sympathetic and dorsal root ganglia (but not in mammals; see below). However, these peptides show poor inhibition of action potentials in frog skeletal muscles. The primary reason for this weak inhibition is most likely due to these action potentials being mediated by TTX sensitive NaV channel currents [72].
Similar to the conotoxin SmIIIA, μ-SIIIA does not have a Hyp residue and adopts a trans conformation [81]. SIIIA shows about 20% inhibition at 5 µM and about 60% percent inhibition at 25 µM of TTX sensitive NaV current in mouse DRG neurons [73]. Furthermore, in a formalin mediated inflammatory mouse pain model, intraperitoneally administered SIIIA showed analgesia at a dose of 10 nmol per animal [73]. However, SIIIA irreversibly blocked NaV1.2, 1.3 and 1.4 channels expressed in Xenopus oocytes [82]. Additionally, no inhibition was observed at NaV1.5, 1.7 or 1.8 channels. This irreversible activity and non-selective profile, limits the analgesic potential of µ-SIIIA. But activity at multiple TTX sensitive channels may be utilized to treat some forms of pain. For example, in an open label trial, intramuscular TTX provided analgesia in 17 out of 31 treatments in 24 patients with cancer related somatic, visceral or neuropathic pain. The achieved analgesia also lasted beyond two or more weeks [83]. In a subsequent double blind, randomised trial, subcutaneous TTX caused analgesia in some patients with cancer pain that did not respond to opioids or other analgesics [84]. Moreover, repeated subcutaneous TTX treatment in a multicentre, open labelled trial proved analgesic and relatively safe in some patients with unrelieved, cancer related pain up to 400 days [85]. Therefore, conotoxins such as µ-SIIIA that inhibit multiple TTX sensitive NaV channels may provide analgesia to some patients. However, the ability of µ-SIIIA to provide pain relief needs to be evaluated alongside the above mentioned, irreversible pharmacological profile at certain NaV channel subtypes.
µ-KIIIA is a 16 residue peptide with three disulphide bridges. It inhibits NaV1.2 channels in Xenopus oocytes [74]. Previously identified residues important in NaV interactions including Lys7, Trp8, Arg10, Asp11, His12 and Arg 14 were all conserved on KIIIA [86]. In mouse DRG neurons, KIIIA inhibited 80% of the TTX sensitive and 20% of TTX resistant current [75]. When KIIIA was tested on mammalian NaV isoforms expressed in Xenopus oocytes, it inhibited NaV1.2 channels irreversibly and NaV1.6 with partial reversal. Moreover, NaV1.5, 1.3 and 1.4 channels in oocytes were also inhibited by KIIIA with progressively increasing potency [75]. Despite this extensive inhibition on multiple NaV channels, KIIIA also showed analgesia in a mouse model of formalin-induced pain without motor impairment. Results revealed that systemic administration of the toxin, decreased the paw-licking frequency of these mice without impairment in motor performance on a rotarod test up to a concentration of 10 nmol [75]. Overall, it appears that both SIIIA and KIIIA have a complex pharmacological profile with possible inflammatory analgesic potential. However, interaction with NaV1.5 may preclude further development due to a high likelihood of cardiac side effects.
An interesting aside to µ-KIIIA activity is its ability to bind site 1 and act alongside TTX. When high concentrations of KIIIA was applied to NaV1.2 channels expressed in Xenopus oocytes there was a residual current that can be abolished by TTX application [87]. However, TTX inhibited the residual current after KIIIA application at a much slower rate than its usual NaV inhibition rate. Additionally, co-application of TTX alongside KIIIA in any order (KIIIA first or TTX first) accelerated the peptide dissociation rate following washout [87]. Therefore, it has been suggested that TTX could move past the bound conotoxin and attach to a deeper site in the outer vestibule of the channel. Hence it is believed that TTX may form a complex with KIIIA bound to NaV channels [88].
5.2. Binding Site 2 and 3 with No Major Conotoxin Interactions
Site 2 binds lipid soluble toxins such as vertridine which amplifies channel activation. This site is primarily located around the S6 segments of domain 1 and 4 [89,90]. More specifically vertidine has been found to bind the S6 segment of domain 1 [89,91]. Site 3 binds α-scorpion toxins and sea anemone toxins, which results in slowed coupling of channel activation and inactivation [14]. This site is partially formed by the domain 4 loop that connects S3 to S4 [90,92].
5.3. µO- and Possibly ι-Conotoxin Binding Site 4
Receptor site 4 binds β-scorpion toxins that also enhance channel activation similar to site 2 [14]. This site consists of the S3–S4 loop towards the extremities of the S4 segment and the linker between S1-S3 in domain 2 [90,93]. Additionally, due to β toxin Tz1 from Venezuelan scorpion tityus zulianus binding, the C terminal loop of domain III has also been included as a β-scorpion toxin interaction site [94]. Outside of β-scorpion toxins, site 4 at least partially binds μO-contoxins. Notably, the structure of the pore loop in domain 3 has been identified as an integral component for μO-contoxin binding [95]. Due to the resemblance of cysteine frameworks between µO, δ and ω conotoxins, µO conotoxins could be regarded as an intermediate between δ and ω conotoxins [96].
The most extensively studied µO-contoxins are MrVIA and MrVIB, isolated from the snail hunting species Conus marmoreus [97]. The µ component of their name stems from their similarity to the biological activity of µ-conotoxins. The relation to the “O” super family is due to their cysteine knot residue structure [98]. It was found that MrVIA functionally competed with β-scorpion toxin Ts1 while showing no competition with site 1 binding µ-conotoxin, GIIIA [99]. This result was consistent with µO-contoxins having no interactions at site 1 and at least some binding at site 4. But β-scorpion toxins trap the S4 of domain II in an outward position, while µO-conotoxins bind the S4 voltage sensor in an inward position preventing it from swinging open upon depolarisation [99,100]. Further evidence for µO-conotoxin interaction with the voltage sensor emerged in experiments which showed that a depolarisation to +40 mV for 300 ms reversed the NaV inhibition mediated by MrVIA in mammalian cells [99]. In contrast, MrVIB mediated inhibition of NaV current in Xenopus oocytes was reversed back to baseline after repeated pulses to +120 mV during washout [101]. Despite the requirement for repeated depolarising steps, these results also suggested that µO-conotoxins associate with the voltage sensor.
µO-conotoxins might also inhibit mammalian CaV channels at high concentrations. In caudodorsal neurons of freshwater snail Lymnaea stagnalis both MrVIA and MrVIB inhibited NaV channels at an IC50 of 0.1–0.2 µM while they also blocked fast inactivating CaV currents above concentrations of 1 µM [96]. Furthermore, the NaV channel current inhibition required extensive washing to restore baseline levels while CaV inhibition was rapidly reversed [96]. A difference in activity between the two µO-conotoxins discussed here arose when the inhibition of the sustained CaV current in the snail neurons was investigated. Results showed that MrVIA inhibited some of the sustained CaV current while MrVIB displayed no inhibition. In mice, MrVIA CNS injection led to extreme reactions such as ataxia and coma at a very low dose of 0.1 nmol. However, intraperitoneal injection showed no distinct change even at doses 100 times greater [97].
An intriguing aspect of MrVIA is its ability to inhibit TTX resistant currents at an IC50 value of 82.8 nM in rat DRG neurons [102]. As this TTX resistant component included NaV1.8 and 1.9 channels that play an important role in pain pathways, this finding was of great interest. However, it is important to note that µO-conotoxins also inhibited TTX sensitive currents in rat DRG neurons at an IC50 value of 1.02 µM [102]. When TTX sensitive, rat NaV1.4 channels were expressed in HEK 293 cells, MrVIA and MrVIB inhibited the NaV current at an IC50 value of around 265 nm and 222 nm, respectively. Thus both toxins block NaV1.4 channels to a similar extent. In comparison, TTX sensitive NaV1.2 channels expressed in HEK 293 cells only showed very weak levels of inhibition. Hence it is evident that µO-conotoxins distinguish between NaV1.2 and NaV1.4 channels.
Another distinction in µO-conotoxins activity arises when different NaV β subunits are expressed alongside the α subunit. In fact, the dissociation constant of MrVIB decreased from 44 nm for the NaV1.8 α subunit alone to 1 nM when the β2 subunit was co-expressed in Xenopus oocytes. This dissociation constant was also different depending on the co-expressed β subunit type where β1, β3 and β4 had dissociation constants of 8.5 nm, 6.5 nm and 3.5 nm, respectively [101]. In contrast, it was seen that the effect of STX at NaV1.8 α subunits expressed in Xenopus oocytes was minimally altered by the co-expressed β subunit type. Therefore, two alternative explanations are that MrVIB interacts with some component of the β subunit or that the β subunit type allosterically modulates the MrVIB binding site on the α subunit to different extents [101]. This increased effect of MrVIB in the presence of β2 subunits is particularly interesting as the β2 subunit is upregulated in rat neuropathic pain models [103]. A further testament to the analgesic potential of MrVIB was its ability to inhibit skin flinch sensitivity in a rat local anaesthetic assay to a much greater extent than lidocaine [104]. Moreover, subcutaneous MrVIB infusion also showed analgesia following foot incision in von Frey testing [104]. As discussed above, MrVIB also decreased allodynia and hyperalgesia in neuropathic and chronic inflammatory pain models without motor side effects at sub micromolar concentrations [7]. Thus, MrVIB shows analgesic potential for further development and study [2].
It is important to note that it was difficult to chemically synthesise a sufficient yield of µO-conotoxins. In fact, the previously implemented two-step oxidative folding strategy only produced a very low yield of MrVIB. Moreover, MrVIB did not fold into the native peptide from its linear, reduced form. Therefore, selenocysteine, an uncommon amino acid was introduced as an isoteric replacement for cysteine. This chemical replacement based on the regioselective formation of a diselenide bond in place of a disulfide bond allowed efficient folding with a greater yield [105]. More importantly, the biological activity of the peptide remained unaltered after the selenocysteine substitution.
Outside of MrVIA and MrVIB, a µO-conotoxin that show some analgesic promise is MfVIA [106]. It is a 32 residue peptide isolated from Conus magnificus, synthetically manufactured using a novel regioselective approach described elsewhere [106]. Subsequently, to characterize the electrophysiological profile of the peptide, the currents from NaV1.4, 1.5, 1.6 and 1.7 channels expressed in HEK 293 and CHO cell lines were recorded in the presences of MfVIA. These results revealed that this toxin inhibited, NaV1.4, 1.5, 1.6 and 1.7 at IC50 values of 81 nM, 431 nM, 1.2 μM and 2.3 μM, respectively. Additionally, NaV1.2 channels had an IC50 values around 5.1 μM MfVIA while NaV1.8 channels had an approximate IC50 value of 158 nM, when expressed in Xenopus oocytes [106]. Therefore, it is evident that MfVIA potently inhibits NaV1.4 and 1.8 channels. Since NaV1.8 is abundant in DRG neurons and show promise as an analgesic target, MfVIA mediated inhibition may provide pain relief. However, the presence of NaV1.4 channels in skeletal muscles make it an undesirable target limiting the analgesic potential of MfVIA.
Besides μO-conotoxins, site 4 is also suggested to bind ι-conotoxins due to functional similarities with β-scorpion toxins [107]. This family of peptides have a new pattern of eight cysteine residues and a four disulphide scaffold. First isolated from Conus radiatus venom, five different toxins labelled r11a (ι-RXIA), r11b, r11c, r11d and r11e were identified [108]. With the exception of r11e, the other four peptides isolated from this venom had many similarities in their primary sequence. However, there were also differences in the peptide structure. For example, r11c had 8 amino acids between the last intercysteine loop compared to the 10 amino acids found on ι-RXIA, r11b and r11d. Furthermore, within this 10 amino acid stretch, there were 8 sequence variants [108]. The functional activity of these peptides were characterised in a frog cutaneous, pectoris preparation. Results showed that nerve stimulation led to repetitive firing of action potentials in the nerve and muscle following 1 μM r11b application. Moreover, there were spontaneous action potentials in the nerve after r11b exposure.
These results found in the presence of r11b were replicated for ι-RXIA, r11c and r11e. However, ι-RXIA was the most potent and least reversible toxin from this group. ι-RXIA caused many more spontaneous action potentials and consistent action potential trains in comparison to the other toxins in this group [108]. The increased toxic activity of ι-RXIA is attributed to a D-Phe44 positioned third to last on the toxin. These results were further supported by in vivo experiments that demonstrated intracranial injections of ι-RXIA caused seizures in mice [107].
Subsequent experiments set out to elucidate the mechanisms behind this excitotoxic profile. It was found that ι-RXIA shifted mouse NaV1.6 voltage dependence of activation to more hyperpolarised potentials with minimal effects on inactivation [107,109]. Additionally, when ι-RXIA was applied to rat NaV α subunits expressed along with β1 in Xenopus oocytes, NaV1.7, 1.2 and 1.6 channels were sensitive while NaV1.1, 1.3, 1.4 and 1.5 channels were insensitive [107]. To assess ι-RXIA effect on NaV1.8, the TTX resistant component of small mouse DRG NaV current was isolated. As this TTX resistant current is primarily composed of NaV1.8, any effect would be mediated through it. However, results indicated that NaV1.8 was fairly insensitive to ι-RXIA [107].
In terms of the ι-conotoxin binding site, several lines of evidence need to be explored further. Firstly, ι-RXIA has a high affinity for NaV1.6, similar to β-scorpion toxin Cn2 [110]. Moreover, when the critical ι-RXIA, D-Phe44 residue was modified to L-Phe44, there was an uncoupling between binding and modulation efficacy. In NaV1.2 channels, the G845N mutation of the S3–S4 loop in domain 2, decreased the affinity of β-scorpion toxin Css IV by almost 10 fold. This decrease in affinity was coupled with a loss of toxin modulation on channel activation despite activation remaining intact due to the mutation [111]. Thus, channel modification yielded similar results for Css IV as the toxin mutation did for ι-RXIA. For these reasons, it is possible that ι-conotoxins also interact at NaV binding site 4 similar to β-scorpion toxins [107]. Overall, ι-conotoxins provide a diverse set of tools to study excitability in neurons.
5.4. Site 5 Does Not Show Major Conotoxin Interactions
Site 5 binds complex polyether toxins such as brevetoxin and ciguatoxin produced by dinoflagellates. In particular, brevetoxin was found to bind S6 of domain 1 and S5 of domain 4 [90]. However, no interactions of conotoxins with site 5 have been reported to date.
5.5. δ-Conotoxin Binding Site 6
Neurotoxin binding site 6 is linked to δ-conotoxins that slow the NaV channel inactivation rate. Although the exact location of binding site 6 is unknown, δ-SVIE conotoxin was found to interact with a conserved residue in the S3-S4 linker of domain 4 [100,112]. In contrast to neurotoxins, local anaesthetics and antiarrhythmic drugs interact with an overlapping receptor site within the inner cavity of the channel pore. Three of the four S6 segments in α subunit domains appear to help form this binding site with the S6 segment in domain four playing a prominent role [14,15]. Selectivity of δ-conotoxins for different Nav channel types is shown in Table 2.
Table 2.
δ-Conotoxin activity at NaV channels.
| δ-Conotoxin Type | Conus Species | Primary NaV Channel Targets |
|---|---|---|
| TxVIA | textile | Undetermined (no activity in mammals) [113,114,115] |
| PVIA | purpurascens | NaV1.2, 1.4, 1.7 [64,116] |
| SVIE | striatus | NaV1.4 [112] |
| GmVIA | gloriamaris | NaV1.2, 1.4 [117,118] |
| EVIA | ermineus | NaV1.2, 1.3, 1.6 [119] |
| TsVIA | tessulatus | NaV1.6 [120] |
| SuVIA | suturatus | NaV1.3, 1.4, 1.6 and 1.7 [121] |
Similar to other conotoxins, δ-conotoxins are small peptides that are rich in disulphides. As mentioned earlier they increase the time course of NaV channel inactivation [122]. King Kong peptide (TxVIA) from Conus textile was the first δ-conotoxin to be isolated from a snail hunting species of cone snails [113]. The unique name “King Kong” peptide stems from the dominant posture that lobsters take following injection of this toxin. It is made up of 27 amino acids with multiple cysteine residues. In fact, this cysteine structure was very similar to that of ω-conotoxin MVIIA. Yet, there was no indication that the King Kong peptide inhibited CaV channels [113]. This functional difference may be due to modified channel interactions mediated by the charge difference between these two toxins and the significantly more hydrophobic nature of the King Kong peptide. In terms of it biological activity, TXVIA has a complex profile showing activity against some molluscs (molluscicidal), some insects (insecticidal) but no activity towards mammals [113,114,115]. Despite the lack of activity in mammals, TXVIA binds rat brain membranes. [115]. Therefore, TXVIA played a protective role and acted as an antagonist in rat brains when administered in vivo with the Conus striatus toxin [115]. However, TXVIA is very unlikely to produce pain relief on its own due to lack of activity in mammals.
Two more intriguing δ-conotoxins are PVIA and SVIE isolated from the fish hunting, Conus purpurascens and Conus striatus, respectively [116,122]. Both toxins slowed the NaV time course of inactivation. However, they differed in the reversal of inhibition where δ-SVIE mediated activity was mostly irreversible while δ-PVIA effects were reversed with washout. An additional difference between these two conotoxins was the level of toxin activity on inactivation as a result of an 80 mV conditioning depolarisation for 300 ms. These results showed that δ-PVIA activity on NaV inactivation was significantly decreased by the conditioning depolarisation in comparison to δ-SVIE activity which was largely unaffected [122]. In Xenopus oocyes, δ-PVIA inhibited rat NaV1.2, 1.4 and 1.7 channels at progressively higher concentrations [64].
When δ-SVIE was tested on NaV1.4 channels expressed in HEK 293 cells, it was found to be a potent gating modifier [112]. More specifically, rapid channel inactivation was slowed by the toxin yielding a KD value of around 500 nM. The specific interaction of the toxin with NaV1.4 channels was believed to be dependent on 3 hydrophobic residues identified as Y1433, F1434 and V1435 on the S3/S4 linker of domain 4. However, the analgesic potential of δ-SVIE is very limited due to its activity at NaV1.4 channels.
δ-GmVIA is a conotoxin from the mollusk-hunting snail Conus gloriamaris that induces convulsions in land snails [117,118]. In sea hare Aplysia abdominal ganglia cells, δ-GmVIA shifted the NaV voltage dependence of steady state inactivation to more depolarised potentials and the steady state activation curve to more hyperpolarised potentials. When the toxin was tested on NaV channels expressed in Xenopus oocytes it inhibited NaV1.2 and 1.4 channels. Upon structural analysis, a similar cysteine framework to the King Kong peptide and CaV inhibiting ω-conotoxins was identified. Overall this toxin was very unlikely to have any analgesic potential due to its activity on NaV1.4 channels [117].
δ-EVIA from Conus ermineus is a 32 amino acid with a six cysteine and four-loop framework similar to omega conotoxins [119]. In amphibian myelinated axons and spinal neurons, δ-EVIA increased the duration of the action potentials by inhibiting NaV channel inactivation. Upon application, δ-EVIA was also found to act on rat NaV1.2, 1.3 and 1.6 channels while showing no activity at NaV1.4 or NaV1.5 channels expressed in Xenopus oocytes. Moreover, when injected intracerebroventricularly, low doses (40 pmol) of δ-EVIA produced hyperactivity and seizures within 1–3 min. High doses (1 nmol) of δ-EVIA were found to be lethal [119]. These results show potential for δ-EVIA to be used as a tool for differentiating NaV channel subtypes.
Recently isolated δ-TsVIA conotoxin was sourced from the venom of Conus tessulatus [120]. It is a 27 amino acid peptide that inhibits TTX sensitive NaV current. Relative to TTX, δ-TsVIA demonstrated slow dissociation kinetics in calcium imaging experiments of mouse DRG neurons. Furthermore, when mouse NaV1.6 α subunits were co-expressed with rat NaVβ1 subunits in Xenopus oocytes, the rapid channel inactivation was inhibited by δ-TsVIA [120]. Future studies to evaluate the analgesic potential of this venom peptide, need to determine the NaV channel subtype specificity and reversibility of inhibition.
δ-Conotoxin SuVIA was isolated from the worm hunting Conus suturatus [121]. It was found to activate human NaV1.3, 1.4, 1.6 and 1.7 channels expressed in HEK 293 cells. Further electrophysiological analysis revealed that δ-SuVIA increased peak NaV1.7 channel current by about 75 percent and shifted the voltage dependence of activation to −15 mV from −25 mV. Half maximal activation voltage was also shifted in a hyperpolarising direction by about 9.5 mV [121]. These changes in activation coupled with a depolarising increase of about 2.8 mV in half maximal inactivation voltage would underlie the toxin mediated enhancement of NaV1.7 current. Overall, δ- SuVIA does not show any analgesic potential due to the absence of inhibitory activity.
5.6. Site 7 Does Not Show Major Conotoxin Interactions
This site is positioned in a hydrophobic cavity surrounded by the domain II S4–S5 linker and S5-helix as well as domain III, S6 helix. Site 7 is associated with binding insecticides such as pyrethroids and DDT [123]. However, there are no identified conotoxin interactions with this binding site.
5.7. µO§-GVIIJ Conotoxin Binding Site 8
Binding site 8 is located around the pore loop of domain II [124]. This site was identified from analogues of µO§-GVIIJ conotoxin that required Cys910 residue near the NaV pore loop to be disulphide tethered to Cys24 on the toxin. As µO§-GVIIJ conotoxin causes NaV channel inhibition, it should be noted that site 8 differs from the binding sites for µ-conotoxins and µO-conotoxin MrVIB which also cause NaV channel inhibition. To functionally verify different binding sites, it was shown that the residual current following µ-conotoxin KIIIA application on to NaV1.2 channels was blocked by 3 µM GVIIJSSG (µO§-GVIIJ conotoxin analogue). In the simultaneous presence of GVIIJSSG and µO-MrVIB, depolarising pulses caused µO-MrVIB to dissociate from the NaV1.2 at an accelerated rate. The remaining component following depolarisation was identical to the residual current that was detected after the application of GVIIJSSG alone. Therefore, it was evident that GVIIJSSG was bound at a separate site to µO-MrVIB [124].
µO§-GVIIJ is a conotoxin extracted from Conus geographus [124]. Activity of this conotoxin is determined by two derivatives known as GVIIJSSG and GVIIJSH due to the limited availability of the native peptide. A primary difference between these two analogues is based around Cys24 which is in a disulphide linkage on GVIIJSSG in comparison to a free thiol form on GVIIJSH. Therefore, GVIIJSH served as a reference to discover the role of the disulphide linkage on Cys24. GVIIJSSG inhibited all TTX sensitive rat NaV channel subtypes expressed in Xenopus oocytes at KD values that ranged from 5 to 360 nm. More interestingly, the IC50 value at NaV1.5 channels was over 200 µM while no NaV1.8 block was detected even at concentrations of 100 µM [124]. Upon analysis of toxin dissociation in oocytes expressing NaV1.2, GVIIJSSG block reversed slowly with washout while GVIIJSH had a two phase recovery which included a rapid phase followed by a slower one. In terms of recovery from inhibition, the native µO§-GVIIJ had a similar slow reversal of inhibition as seen in the presence of GVIIJSSG.
When human NaV channel subtypes were expressed in HEK 293 and CHO cell lines, both GVIIJSSG and GVIIJSH caused inhibition of NaV 1.2, 1.4, 1.6 and 1.7 channels at nm ranges. At a concentration of 10 µM, GVIIJSSG inhibited NaV1.5 by 19% while GVIIJSH only showed 0.3% inhibition. In contrast, NaV1.1 and 1.3 channels were only inhibited by GVIIJSH. Overall, GVIIJSH was more potent at all the channels tested in comparison to GVIIJSSG [124].
Subsequent experiments set out to identify the effect of β subunits on GVIIJSSG activity [124]. These results revealed that when rat NaV1.7 α subunit was expressed with rat β1 or β3 subunit, it was inhibited by GVIIJSSG while the presence of β2 or β4 subunit, led to toxin resistance. Additionally, chimeras of β1 and β2 subunits were made by joining the extracellular and transmembrane domains of one β subtype with the intracellular domain of the other β subtype. When co-expressed with NaV1.7, these chimeras provided the means to identify the β2 region that induced resistance. Thus, results showed that the extracellular domain of β2 was critical to induce toxin resistance [124]. The authors suggested that the primary difference between the β2 and β4 subunits that induce resistance compared to β1 and β3, is the presence of a disulphide bond with NaV1 in the resistant subunits. Therefore, the Cys910 identified earlier may be disulphide linked to its β subunits. Overall, µO§-GVIIJ conotoxin provides another pharmacological tool to study NaV activity.
5.8. Local Anesthetic Binding Site
The final NaV binding site is designated for local anesthetics. It is located in the α helix of domain IV segment 6 [125]. Local anaesthetics such as lignocaine do not show great NaV subtype selectivity and yet produce analgesia without a severe side effect profile [126]. In automated patch clamp experiments that measure use dependent inhibition, lignocaine showed no selectivity between NaV1.1 to 1.7 channels [127]. Despite the lack of selectivity, multiple studies have reported the use of lignocaine for pain relief. In a randomized, double blind, placebo controlled trial of neuropathic pain sufferers due to peripheral nerve injury, intravenous lignocaine showed significant decreases in pain scores compared to placebo [128]. Side effects, such as lightheadedness, were observed in about half of the 11 tested patients. One patient suffered from nausea. Outside of pain relief, there was also a significant reduction in allodynia. In fibromyalgia, where patients suffer from widespread chronic pain and tenderness, three out of eleven tested women reported 50% pain relief that lasted 4–7 days following local anesthetic application [129].
Multiple factors are attributed to the absence of major side effects following lignocaine treatment. For example, channel block is enhanced at more depolarized voltages. This suggests that the local anesthetic would only be primarily active during pain transmission. Moreover, channel block accrues following a train of brief depolarizing pulses. Therefore, repeated firing neurons would be inhibited to a greater extent in comparison to regular firing neurons. Additionally, channel block is dependent on the frequency of stimulation [127]. Overall, these properties of local anesthetic mediated inhibition hinge on state dependence where the channel is preferentially inhibited in its inactivated state [127]. Therefore, local anesthetics can be utilized as analgesics for certain pain conditions at a tightly regulated dose. Although local anesthetics show promise as analgesics, their activity at NaV1.4 and 1.5 channels create significant risks.
6. Future of Conotoxins As Potential Analgesics
Outside of NaV channels, conotoxins bind voltage gated calcium (CaV) channels, voltage gated potassium (KV) channels and many other targets [3]. Therefore, it is important to highlight that the analgesic targets for conotoxins spread beyond NaV channels. In fact, the only conotoxin approved for clinical use is ω-conotoxin MVIIA (Prialt) which mediates its activity through CaV2.2 channels [130]. Unfortunately, Prialt had many side effects. These effects ranged from whole body shakes driven by possible cerebellar motor defects in rats to neurological impairments that included hallucinations in humans [131]. Since Prialt cannot cross the blood brain barrier, it needs to be administered intrathecally [131]. As a result of complex side effects and a difficult route of administration, Prialt has remained a last resort analgesic for chronic pain sufferers. Hence future conotoxin derived analgesics would need to address the shortcomings of Prialt. Because NaV channels are involved in nociceptive neurotransmission throughout sensory nerves, conotoxins appropriately targeting subtypes of these channels should have the advantage of activity via systemic administration or targeted administration to peripheral tissues.
As discussed here, there is evidence to suggest that NaV channels are intimately involved in all different forms of pain. From the NaV targeting conotoxins, it is evident that μ, μO and µO§-conotoxins are more likely to produce analgesia through inhibition of NaV channels involved in nociceptive transduction and transmission. However, within each conotoxin family there is a great number of NaV targets that will need to be refined through structural modification before therapeutically useful agents can be developed. In general, combinations of NaV1.1, 1.3, 1.7, 1.8 and 1.9 channels should be targeted for pain treatments. It may eventuate that the optimal analgesic NaV inhibitors target multiple NaV subtypes among this group. However, different NaV channels play a more substantial role based on the location and type of pain as highlighted in section four. So pain type specific agents may be needed. Perhaps one pitfall of conotoxins that are significantly more selective for one NaV channel subtype is the possibility of redundancy within the system that allows other NaV channel subtypes to compensate for the loss of activity. The studies on pain relief mediated by TTX and local anesthetics such as lignocaine which affect a broad range of NaV channels suggest that inhibiting multiple NaV channels may be an effective approach. In fact, non-selective NaV channel inhibitors such as mexiletine have been shown to be effective in the treatment of neuropathic pain due to peripheral nerve damage and diabetic neuropathies [126,132,133]. However, compounds that target multiple NaV channels have a significant risk associated with them, as they affect skeletal muscle related NaV1.4, cardiac contraction associated NaV1.5 and nerve conduction linked NaV1.6 channels. Therefore, a potential analgesic conotoxin should avoid NaV1.4, 1.5 and possibly NaV1.6 channels to not to minimize the possibility of adverse side effects. In general, future studies should explore conotoxins that target combinations of NaV channels to attain effective pain relief with a minimal side effect profile.
Acknowledgments
NRM is supported by an Australian Postgraduate Award. MJC is supported by a National Health and Medical Research Council of Australia, Senior Principal Research Fellowship.
Author Contributions
N.R.M. and M.J.C. conceived the review. N.R.M. drafted and revised the manuscript with reviews and suggestions by M.J.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gaskin D.J., Richard P. The economic costs of pain in the united states. J. Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Knapp O., McArthur J.R., Adams D.J. Conotoxins targeting neuronal voltage-gated sodium channel subtypes: Potential analgesics? Toxins. 2012;4:1236–1260. doi: 10.3390/toxins4111236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis R.J., Dutertre S., Vetter I., Christie M.J. Conus venom peptide pharmacology. Pharmacol. Rev. 2012;64:259–298. doi: 10.1124/pr.111.005322. [DOI] [PubMed] [Google Scholar]
- 4.Almeida T.F., Roizenblatt S., Tufik S. Afferent pain pathways: A neuroanatomical review. Brain Res. 2004;1000:40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 5.Von Hehn C.A., Baron R., Woolf C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rush A.M., Cummins T.R., Waxman S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekberg J., Jayamanne A., Vaughan C.W., Aslan S., Thomas L., Mould J., Drinkwater R., Baker M.D., Abrahamsen B., Wood J.N., et al. μO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc. Natl. Acad. Sci. USA. 2006;103:17030–17035. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Gu J., Li Y.Q., Tao Y.X. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol. Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damir S., Sandra K., Adriana B., Livia P. Dorsal root ganglion—A potential new therapeutic target for neuropathic pain. J. Pain Res. 2012;5:31–38. doi: 10.2147/JPR.S26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scroggs R.S., Fox A.P. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J. Physiol. 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catterall W.A. Structure and function of voltage-gated sodium channels at atomic resolution. Exp. Physiol. 2014;99:35–51. doi: 10.1113/expphysiol.2013.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beneski D.A., Catterall W.A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc. Natl. Acad. Sci. USA. 1980;77:639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payandeh J., Scheuer T., Zheng N., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catterall W.A., Goldin A.L., Waxman S.G. International union of pharmacology. XlVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 15.Catterall W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 16.Dichgans M., Freilinger T., Eckstein G., Babini E., Lorez-Depiereux B., Biskup S. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim C.H., Oh Y., Chung J.M., Chung K. The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Res. Mol. Brain Res. 2001;95:153–161. doi: 10.1016/S0169-328X(01)00226-1. [DOI] [PubMed] [Google Scholar]
- 18.Waxman S.G., Kocsis J.D., Black J.A. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J. Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindia J.A., Kohler M.G., Martin W.J., Abbadie C. Relationship between sodium channel Nav1.3 expression and neuropathic pain behavior in rats. Pain. 2005;117:145–153. doi: 10.1016/j.pain.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Fukuoka T., Kobayashi K., Yamanaka H., Obata K., Dai Y., Noguchi K. Comparative study of the distribution of the α-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J. Comp. Neurol. 2008;510:188–206. doi: 10.1002/cne.21786. [DOI] [PubMed] [Google Scholar]
- 21.Nassar M.A., Baker M.D., Levato A., Ingram R., Mallucci G., McMahon S.B., Wood J.N. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol. Pain. 2006;2 doi: 10.1186/1744-8069-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berta T., Poirot O., Pertin M., Ji R.R., Kellenberger S., Decosterd I. Transcriptional and functional profiles of voltage-gated Na+ channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol. Cell. Neurosci. 2008;37:196–208. doi: 10.1016/j.mcn.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Henry M.A., Freking A.R., Johnson L.R., Levinson S.R. Sodium channel Nav1.6 accumulates at the site of infraorbital nerve injury. BMC Neurosci. 2007;8 doi: 10.1186/1471-2202-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black J.A., Dib-Hajj S., McNabola K., Jeste S., Rizzo M.A., Kocsis J.D., Waxman S.G. Spinal sensory neurons express multiple sodium channel α-subunit mrnas. Brain Res. Mol. Brain Res. 1996;43:117–131. doi: 10.1016/S0169-328X(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 25.Cummins T.R., Howe J.R., Waxman S.G. Slow closed-state inactivation: A novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J. Neurosci. Off. J. Soc. Neurosci. 1998;18:9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black J.A., Liu S., Tanaka M., Cummins T.R., Waxman S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Strickland I.T., Martindale J.C., Woodhams P.L., Reeve A.J., Chessell I.P., McQueen D.S. Changes in the expression of Nav1.7, Nav1.8 and Nav1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur. J. Pain (Lond. Engl.) 2008;12:564–572. doi: 10.1016/j.ejpain.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Nassar M.A., Stirling L.C., Forlani G., Baker M.D., Matthews E.A., Dickenson A.H., Wood J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dib-Hajj S.D., Rush A.M., Cummins T.R., Hisama F.M., Novella S., Tyrrell L., Marshall L., Waxman S.G. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain A J. Neurol. 2005;128:1847–1854. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- 30.Lee M.J., Yu H.S., Hsieh S.T., Stephenson D.A., Lu C.J., Yang C.C. Characterization of a familial case with primary erythromelalgia from taiwan. J. Neurol. 2007;254:210–214. doi: 10.1007/s00415-006-0328-3. [DOI] [PubMed] [Google Scholar]
- 31.Hong S., Morrow T.J., Paulson P.E., Isom L.L., Wiley J.W. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J. Biol. Chem. 2004;279:29341–29350. doi: 10.1074/jbc.M404167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim C.H., Oh Y., Chung J.M., Chung K. Changes in three subtypes of tetrodotoxin sensitive sodium channel expression in the axotomized dorsal root ganglion in the rat. Neurosci. Lett. 2002;323:125–128. doi: 10.1016/S0304-3940(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 33.Coward K., Aitken A., Powell A., Plumpton C., Birch R., Tate S., Bountra C., Anand P. Plasticity of TTX-sensitive sodium channels PN1 and brain III in injured human nerves. Neuroreport. 2001;12:495–500. doi: 10.1097/00001756-200103050-00014. [DOI] [PubMed] [Google Scholar]
- 34.Nassar M.A., Levato A., Stirling L.C., Wood J.N. Neuropathic pain develops normally in mice lacking both Nav1.7 and Nav1.8. Mol. Pain. 2005;1 doi: 10.1186/1744-8069-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird E.V., Robinson P.P., Boissonade F.M. Nav1.7 sodium channel expression in human lingual nerve neuromas. Arch. Oral Biol. 2007;52:494–502. doi: 10.1016/j.archoralbio.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Black J.A., Nikolajsen L., Kroner K., Jensen T.S., Waxman S.G. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann. Neurol. 2008;64:644–653. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- 37.King G.F., Vetter I. No gain, no pain: Nav1.7 as an analgesic target. ACS Chem. Neurosci. 2014;5:749–751. doi: 10.1021/cn500171p. [DOI] [PubMed] [Google Scholar]
- 38.Djouhri L., Fang X., Okuse K., Wood J.N., Berry C.M., Lawson S.N. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): Expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J. Physiol. 2003;550:739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coggeshall R.E., Tate S., Carlton S.M. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci. Lett. 2004;355:45–48. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Akopian A.N., Souslova V., England S., Okuse K., Ogata N., Ure J., Smith A., Kerr B.J., McMahon S.B., Boyce S., et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 41.Decosterd I., Ji R.R., Abdi S., Tate S., Woolf C.J. The pattern of expression of the voltage-gated sodium channels Nav1.8 and Nav1.9 does not change in uninjured primary sensory neurons in experimental neuropathic pain models. Pain. 2002;96:269–277. doi: 10.1016/S0304-3959(01)00456-0. [DOI] [PubMed] [Google Scholar]
- 42.Gold M.S., Weinreich D., Kim C.S., Wang R., Treanor J., Porreca F., Lai J. Redistribution of Nav1.8 in uninjured axons enables neuropathic pain. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X.H., Zang Y., Chen X., Pang R.P., Xu J.T., Zhou X., Wei X.H., Li Y.Y., Xin W.J., Qin Z.H., et al. TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in drg neurons following motor fiber injury. Pain. 2010;151:266–279. doi: 10.1016/j.pain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Jarvis M.F., Honore P., Shieh C.C., Chapman M., Joshi S., Zhang X.F., Kort M., Carroll W., Marron B., Atkinson R., et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faber C.G., Lauria G., Merkies I.S., Cheng X., Han C., Ahn H.S., Persson A.K., Hoeijmakers J.G., Gerrits M.M., Pierro T., et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl. Acad. Sci. USA. 2012;109:19444–19449. doi: 10.1073/pnas.1216080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roza C., Laird J.M.A., Souslova V., Wood J.N., Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J. Physiol. 2003;550:921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerr B.J., Souslova V., McMahon S.B., Wood J.N. A role for the TTX-resistant sodium channel Nav1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12:3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 48.Savio-Galimberti E., Weeke P., Muhammad R., Blair M., Ansari S., Short L., Atack T.C., Kor K., Vanoye C.G., Olesen M.S., et al. SCN10A/Nav1.8 modulation of peak and late sodium currents in patients with early onset atrial fibrillation. Cardiovasc. Res. 2014;104:355–363. doi: 10.1093/cvr/cvu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett D.L. Voltage-gated sodium channel mutations and painful neuropathy: Nav1.9 joins the family. Brain A J. Neurol. 2014;137:1574–1576. doi: 10.1093/brain/awu105. [DOI] [PubMed] [Google Scholar]
- 50.Tate S., Benn S., Hick C., Trezise D., John V., Mannion R.J., Costigan M., Plumpton C., Grose D., Gladwell Z., et al. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat. Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 51.Priest B.T., Murphy B.A., Lindia J.A., Diaz C., Abbadie C., Ritter A.M., Liberator P., Iyer L.M., Kash S.F., Kohler M.G., et al. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel Nav1.9 to sensory transmission and nociceptive behavior. Proc. Natl. Acad. Sci. USA. 2005;102:9382–9387. doi: 10.1073/pnas.0501549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amaya F., Wang H., Costigan M., Allchorne A.J., Hatcher J.P., Egerton J., Stean T., Morisset V., Grose D., Gunthorpe M.J., et al. The voltage-gated sodium channel Nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 2006;26:12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J., Han C., Estacion M., Vasylyev D., Hoeijmakers J.G., Gerrits M.M., Tyrrell L., Lauria G., Faber C.G., Dib-Hajj S.D., et al. Gain-of-function mutations in sodium channel Nav1.9 in painful neuropathy. Brain A J. Neurol. 2014;137:1627–1642. doi: 10.1093/brain/awu079. [DOI] [PubMed] [Google Scholar]
- 54.Leipold E., Liebmann L., Korenke G.C., Heinrich T., Giesselmann S., Baets J., Ebbinghaus M., Goral R.O., Stodberg T., Hennings J.C., et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat. Genet. 2013;45:1399–1404. doi: 10.1038/ng.2767. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X.Y., Wen J., Yang W., Wang C., Gao L., Zheng L.H., Wang T., Ran K., Li Y., Li X., et al. Gain-of-function mutations in SCN11A cause familial episodic pain. Am. J. Hum. Genet. 2013;93:957–966. doi: 10.1016/j.ajhg.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akondi K.B., Muttenthaler M., Dutertre S., Kaas Q., Craik D.J., Lewis R.J., Alewood P.F. Discovery, synthesis, and structure-activity relationships of conotoxins. Chem. Rev. 2014;114:5815–5847. doi: 10.1021/cr400401e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li R.A., Tomaselli G.F. Using the deadly μ-conotoxins as probes of voltage-gated sodium channels. Toxic. Off. J. Int. Soc. Toxinol. 2004;44:117–122. doi: 10.1016/j.toxicon.2004.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fozzard H.A., Lipkind G.M. The tetrodotoxin binding site is within the outer vestibule of the sodium channel. Mar. Drugs. 2010;8:219–234. doi: 10.3390/md8020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cummins T.R., Aglieco F., Dib-Hajj S.D. Critical molecular determinants of voltage-gated sodium channel sensitivity to μ-conotoxins GIIIA/B. Mol. Pharmacol. 2002;61:1192–1201. doi: 10.1124/mol.61.5.1192. [DOI] [PubMed] [Google Scholar]
- 60.Cruz L.J., Kupryszewski G., LeCheminant G.W., Gray W.R., Olivera B.M., Rivier J. μ-conotoxin GIIIA, a peptide ligand for muscle sodium channels: Chemical synthesis, radiolabeling and receptor characterization. Biochemistry. 1989;28:3437–3442. doi: 10.1021/bi00434a043. [DOI] [PubMed] [Google Scholar]
- 61..Hill J.M., Alewood P.F., Craik D.J. Three-dimensional solution structure of μ-conotoxin GIIIB, a specific blocker of skeletal muscle sodium channels. Biochemistry. 1996;35:8824–8835. doi: 10.1021/bi960073o. [DOI] [PubMed] [Google Scholar]
- 62.Cruz L.J., Gray W.R., Olivera B.M., Zeikus R.D., Kerr L., Yoshikami D., Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J. Biol. Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- 63.Shon K.J., Olivera B.M., Watkins M., Jacobsen R.B., Gray W.R., Floresca C.Z., Cruz L.J., Hillyard D.R., Brink A., Terlau H., et al. μ-conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J. Neurosci. Off. J. Soc. Neurosci. 1998;18:4473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Safo P., Rosenbaum T., Shcherbatko A., Choi D.-Y., Han E., Toledo-Aral J.J., Olivera B.M., Brehm P., Mandel G. Distinction among neuronal subtypes of voltage-activated sodium channels by μ-conotoxin PIIIA. J. Neurosci. 2000;20:76–80. doi: 10.1523/JNEUROSCI.20-01-00076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis R.J., Schroeder C.I., Ekberg J., Nielsen K.J., Loughnan M., Thomas L., Adams D.A., Drinkwater R., Adams D.J., Alewood P.F. Isolation and structure-activity of μ-conotoxin TIIIA, a potent inhibitor of tetrodotoxin-sensitive voltage-gated sodium channels. Mol. Pharmacol. 2007;71:676–685. doi: 10.1124/mol.106.028225. [DOI] [PubMed] [Google Scholar]
- 66.Ekberg J., Adams D.J. Neuronal voltage-gated sodium channel subtypes: Key roles in inflammatory and neuropathic pain. Int. J. Biochem. Cell Biol. 2006;38:2005–2010. doi: 10.1016/j.biocel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Holford M., Zhang M.M., Gowd K.H., Azam L., Green B.R., Watkins M., Ownby J.P., Yoshikami D., Bulaj G., Olivera B.M. Pruning nature: Biodiversity-derived discovery of novel sodium channel blocking conotoxins from conus bullatus. Toxic. Off. J. Int. Soc. Toxinol. 2009;53:90–98. doi: 10.1016/j.toxicon.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green B.R., Zhang M.M., Chhabra S., Robinson S.D., Wilson M.J., Redding A., Olivera B.M., Yoshikami D., Bulaj G., Norton R.S. Interactions of disulfide-deficient selenocysteine analogs of μ-conotoxin BUIIIB with the α-subunit of the voltage-gated sodium channel subtype 1.3. FEBS J. 2014;281:2885–2898. doi: 10.1111/febs.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuang Z., Zhang M.-M., Gupta K., Gajewiak J., Gulyas J., Balaram P., Rivier J.E., Olivera B.M., Yoshikami D., Bulaj G., et al. The mammalian neuronal sodium channel blocker μ-conotoxin BuIIIB has a structured N-terminus that influences potency. ACS Chem. Biol. 2013;8:1344–1351. doi: 10.1021/cb300674x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.West P.J., Bulaj G., Garrett J.E., Olivera B.M., Yoshikami D. μ-conotoxin smiiia, a potent inhibitor of tetrodotoxin-resistant sodium channels in amphibian sympathetic and sensory neurons. Biochemistry. 2002;41:15388–15393. doi: 10.1021/bi0265628. [DOI] [PubMed] [Google Scholar]
- 71.Keizer D.W., West P.J., Lee E.F., Yoshikami D., Olivera B.M., Bulaj G., Norton R.S. Structural basis for tetrodotoxin-resistant sodium channel binding by μ-conotoxin SmIIIA. J. Biol. Chem. 2003;278:46805–46813. doi: 10.1074/jbc.M309222200. [DOI] [PubMed] [Google Scholar]
- 72.Bulaj G., West P.J., Garrett J.E., Watkins M., Zhang M.M., Norton R.S., Smith B.J., Yoshikami D., Olivera B.M. Novel conotoxins from conus striatus and conus kinoshitai selectively block TTX-resistant sodium channels. Biochemistry. 2005;44:7259–7265. doi: 10.1021/bi0473408. [DOI] [PubMed] [Google Scholar]
- 73.Green B.R., Catlin P., Zhang M.M., Fiedler B., Bayudan W., Morrison A., Norton R.S., Smith B.J., Yoshikami D., Olivera B.M., et al. Conotoxins containing nonnatural backbone spacers: Cladistic-based design, chemical synthesis, and improved analgesic activity. Chem. Biol. 2007;14:399–407. doi: 10.1016/j.chembiol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhang M.-M., Han T.S., Olivera B.M., Bulaj G., Yoshikami D. µ-conotoxin KIIIA derivatives with divergent affinities versus efficacies in blocking voltage-gated sodium channels. Biochemistry. 2010;49:4804–4812. doi: 10.1021/bi100207k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang M.M., Green B.R., Catlin P., Fiedler B., Azam L., Chadwick A., Terlau H., McArthur J.R., French R.J., Gulyas J., et al. Structure/function characterization of μ-conotoxin KIIIA, an analgesic, nearly irreversible blocker of mammalian neuronal sodium channels. J. Biol. Chem. 2007;282:30699–30706. doi: 10.1074/jbc.M704616200. [DOI] [PubMed] [Google Scholar]
- 76.Moczydlowski E., Olivera B.M., Gray W.R., Strichartz G.R. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and mu-conotoxins. Proc. Natl. Acad. Sci. USA. 1986;83:5321–5325. doi: 10.1073/pnas.83.14.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Becker S., Prusak-Sochaczewski E., Zamponi G., Beck-Sickinger A.G., Gordon R.D., French R.J. Action of derivatives of μ-conotoxin GIIIA on sodium channels. Single amino acid substitutions in the toxin separately affect association and dissociation rates. Biochemistry. 1992;31:8229–8238. doi: 10.1021/bi00150a016. [DOI] [PubMed] [Google Scholar]
- 78.Wakamatsu K., Kohda D., Hatanaka H., Lancelin J.M., Ishida Y., Oya M., Nakamura H., Inagaki F., Sato K. Structure-activity relationships of μ-conotoxin GIIIA: Structure determination of active and inactive sodium channel blocker peptides by nmr and simulated annealing calculations. Biochemistry. 1992;31:12577–12584. doi: 10.1021/bi00165a006. [DOI] [PubMed] [Google Scholar]
- 79.Mahdavi S., Kuyucak S. Molecular dynamics study of binding of µ-conotoxin GIIIA to the voltage-gated sodium channel Nav1.4. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0105300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McArthur J.R., Singh G., O'Mara M.L., McMaster D., Ostroumov V., Tieleman D.P., French R.J. Orientation of μ-conotoxin PIIIA in a sodium channel vestibule, based on voltage dependence of its binding. Mol. Pharmacol. 2011;80:219–227. doi: 10.1124/mol.111.071779. [DOI] [PubMed] [Google Scholar]
- 81.Yao S., Zhang M.M., Yoshikami D., Azam L., Olivera B.M., Bulaj G., Norton R.S. Structure, dynamics, and selectivity of the sodium channel blocker μ-conotoxin SIIIA. Biochemistry. 2008;47:10940–10949. doi: 10.1021/bi801010u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schroeder C.I., Ekberg J., Nielsen K.J., Adams D., Loughnan M.L., Thomas L., Adams D.J., Alewood P.F., Lewis R.J. Neuronally selective μ-conotoxins from conus striatus utilize an α-helical motif to target mammalian sodium channels. J. Biol. Chem. 2008;283:21621–21628. doi: 10.1074/jbc.M802852200. [DOI] [PubMed] [Google Scholar]
- 83.Hagen N.A., Fisher K.M., Lapointe B., du Souich P., Chary S., Moulin D., Sellers E., Ngoc A.H. An open-label, multi-dose efficacy and safety study of intramuscular tetrodotoxin in patients with severe cancer-related pain. J. Pain Symptom Manag. 2007;34:171–182. doi: 10.1016/j.jpainsymman.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 84.Hagen N.A., du Souich P., Lapointe B., Ong-Lam M., Dubuc B., Walde D., Love R., Ngoc A.H. Tetrodotoxin for moderate to severe cancer pain: A randomized, double blind, parallel design multicenter study. J. Pain Symptom Manag. 2008;35:420–429. doi: 10.1016/j.jpainsymman.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Hagen N.A., Lapointe B., Ong–Lam M., Dubuc B., Walde D., Gagnon B., Love R., Goel R., Hawley P., Ngoc A.H., et al. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr. Oncol. 2011;18:e109–e116. doi: 10.3747/co.v18i3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khoo K.K., Feng Z.P., Smith B.J., Zhang M.M., Yoshikami D., Olivera B.M., Bulaj G., Norton R.S. Structure of the analgesic μ-conotoxin KIIIA and effects on the structure and function of disulfide deletion. Biochemistry. 2009;48:1210–1219. doi: 10.1021/bi801998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang M.M., McArthur J.R., Azam L., Bulaj G., Olivera B.M., French R.J., Yoshikami D. Synergistic and antagonistic interactions between tetrodotoxin and μ-conotoxin in blocking voltage-gated sodium channels. Channels (Austin) 2009;3:32–38. doi: 10.4161/chan.3.1.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.French R.J., Yoshikami D., Sheets M.F., Olivera B.M. The tetrodotoxin receptor of voltage-gated sodium channels—Perspectives from interactions with μ-conotoxins. Mar. Drugs. 2010;8:2153–2161. doi: 10.3390/md8072153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang S.Y., Wang G.K. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 2003;15:151–159. doi: 10.1016/S0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 90.Cestèle S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/S0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 91.Zhu H.-L., Wassall R.D., Takai M., Morinaga H., Nomura M., Cunnane T.C., Teramoto N. Actions of veratridine on tetrodotoxin-sensitive voltage-gated Na+ currents, Nav1.6, in murine vas deferens myocytes. Br. J. Pharmacol. 2009;157:1483–1493. doi: 10.1111/j.1476-5381.2009.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Campos F.V., Chanda B., Beirao P.S., Bezanilla F. α-scorpion toxin impairs a conformational change that leads to fast inactivation of muscle sodium channels. J. Gen. Physiol. 2008;132:251–263. doi: 10.1085/jgp.200809995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gurevitz M. Mapping of scorpion toxin receptor sites at voltage-gated sodium channels. Toxic. Off. J. Int. Soc. Toxinol. 2012;60:502–511. doi: 10.1016/j.toxicon.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 94.Leipold E., Hansel A., Borges A., Heinemann S.H. Subtype specificity of scorpion β-toxin Tz1 interaction with voltage-gated sodium channels is determined by the pore loop of domain 3. Mol. Pharmacol. 2006;70:340–347. doi: 10.1124/mol.106.024034. [DOI] [PubMed] [Google Scholar]
- 95.Zorn S., Leipold E., Hansel A., Bulaj G., Olivera B.M., Terlau H., Heinemann S.H. The μO-conotoxin MrVIA inhibits voltage-gated sodium channels by associating with domain-3. FEBS Lett. 2006;580:1360–1364. doi: 10.1016/j.febslet.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 96.Fainzilber M., van der Schors R., Lodder J.C., Li K.W., Geraerts W.P., Kits K.S. New sodium channel-blocking conotoxins also affect calcium currents in lymnaea neurons. Biochemistry. 1995;34:5364–5371. doi: 10.1021/bi00016a007. [DOI] [PubMed] [Google Scholar]
- 97.McIntosh J.M., Hasson A., Spira M.E., Gray W.R., Li W., Marsh M., Hillyard D.R., Olivera B.M. A new family of conotoxins that blocks voltage-gated sodium channels. J. Biol. Chem. 1995;270:16796–16802. doi: 10.1074/jbc.270.28.16796. [DOI] [PubMed] [Google Scholar]
- 98.Heinemann S.H., Leipold E. Conotoxins of the O-superfamily affecting voltage-gated sodium channels. Cell. Mol. Life Sci. 2007;64:1329–1340. doi: 10.1007/s00018-007-6565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leipold E., DeBie H., Zorn S., Borges A., Olivera B.M., Terlau H., Heinemann S.H. μO-conotoxins inhibit Nav channels by interfering with their voltage sensors in domain-2. Channels (Austin) 2007;1:253–262. doi: 10.4161/chan.4847. [DOI] [PubMed] [Google Scholar]
- 100.Stevens M., Peigneur S., Tytgat J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011;2 doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilson M.J., Zhang M.-M., Azam L., Olivera B.M., Bulaj G., Yoshikami D. Navβ subunits modulate the inhibition of Nav1.8 by the analgesic gating modifier μO-conotoxin MrVIB. J. Pharmacol. Exp. Ther. 2011;338:687–693. doi: 10.1124/jpet.110.178343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daly N.L., Ekberg J.A., Thomas L., Adams D.J., Lewis R.J., Craik D.J. Structures of μO-conotoxins from conus marmoreus. I nhibitors of tetrodotoxin (TTX)-sensitive and TTX-resistant sodium channels in mammalian sensory neurons. J. Biol. Chem. 2004;279:25774–25782. doi: 10.1074/jbc.M313002200. [DOI] [PubMed] [Google Scholar]
- 103.Pertin M., Ji R.R., Berta T., Powell A.J., Karchewski L., Tate S.N., Isom L.L., Woolf C.J., Gilliard N., Spahn D.R., et al. Upregulation of the voltage-gated sodium channel β2 subunit in neuropathic pain models: Characterization of expression in injured and non-injured primary sensory neurons. J. Neurosci. 2005;25:10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bulaj G., Zhang M.-M., Green B.R., Fiedler B., Layer R.T., Wei S., Nielsen J.S., Low S.J., Klein B.D., Wagstaff J.D., et al. Synthetic μO-conotoxin MrVIB blocks TTX-resistant sodium channel Nav1.8 and has a long-lasting analgesic activity. Biochemistry. 2006;45:7404–7414. doi: 10.1021/bi060159+. [DOI] [PubMed] [Google Scholar]
- 105.De Araujo A.D., Callaghan B., Nevin S.T., Daly N.L., Craik D.J., Moretta M., Hopping G., Christie M.J., Adams D.J., Alewood P.F. Total synthesis of the analgesic conotoxin MrVIB through selenocysteine-assisted folding. Angew. Chem. 2011;50:6527–6529. doi: 10.1002/anie.201101642. [DOI] [PubMed] [Google Scholar]
- 106.Vetter I., Dekan Z., Knapp O., Adams D.J., Alewood P.F., Lewis R.J. Isolation, characterization and total regioselective synthesis of the novel μO-conotoxin MfVIA from conus magnificus that targets voltage-gated sodium channels. Biochem. Pharmacol. 2012;84:540–548. doi: 10.1016/j.bcp.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 107.Fiedler B., Zhang M.-M., Buczek O., Azam L., Bulaj G., Norton R.S., Olivera B.M., Yoshikami D. Specificity, affinity and efficacy of iota-conotoxin RXIA, an agonist of voltage-gated sodium channels Nav1.2, 1.6 and 1.7. Biochem. Pharmacol. 2008;75:2334–2344. doi: 10.1016/j.bcp.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jimenez E.C., Shetty R.P., Lirazan M., Rivier J., Walker C., Abogadie F.C., Yoshikami D., Cruz L.J., Olivera B.M. Novel excitatory conus peptides define a new conotoxin superfamily. J. Neurochem. 2003;85:610–621. doi: 10.1046/j.1471-4159.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- 109.Buczek O., Wei D., Babon J.J., Yang X., Fiedler B., Chen P., Yoshikami D., Olivera B.M., Bulaj G., Norton R.S. Structure and sodium channel activity of an excitatory I1-superfamily conotoxin. Biochemistry. 2007;46:9929–9940. doi: 10.1021/bi700797f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schiavon E., Sacco T., Cassulini R.R., Gurrola G., Tempia F., Possani L.D., Wanke E. Resurgent current and voltage sensor trapping enhanced activation by a β-scorpion toxin solely in Nav1.6 channel. Significance in mice purkinje neurons. J. Biol. Chem. 2006;281:20326–20337. doi: 10.1074/jbc.M600565200. [DOI] [PubMed] [Google Scholar]
- 111.Cestele S., Qu Y., Rogers J.C., Rochat H., Scheuer T., Catterall W.A. Voltage sensor-trapping: Enhanced activation of sodium channels by β-scorpion toxin bound to the S3-S4 loop in domain II. Neuron. 1998;21:919–931. doi: 10.1016/S0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 112.Leipold E., Hansel A., Olivera B.M., Terlau H., Heinemann S.H. Molecular interaction of δ-conotoxins with voltage-gated sodium channels. FEBS Lett. 2005;579:3881–3884. doi: 10.1016/j.febslet.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 113.Hillyard D.R., Olivera B.M., Woodward S., Corpuz G.P., Gray W.R., Ramilo C.A., Cruz L.J. A molluscivorous conus toxin: Conserved frameworks in conotoxins. Biochemistry. 1989;28:358–361. doi: 10.1021/bi00427a049. [DOI] [PubMed] [Google Scholar]
- 114.Bruce C., Fitches E.C., Chougule N., Bell H.A., Gatehouse J.A. Recombinant conotoxin, TxVIA, produced in yeast has insecticidal activity. Toxicon. 2011;58:93–100. doi: 10.1016/j.toxicon.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Fainzilber M., Kofman O., Zlotkin E., Gordon D. A new neurotoxin receptor site on sodium channels is identified by a conotoxin that affects sodium channel inactivation in molluscs and acts as an antagonist in rat brain. J. Biol. Chem. 1994;269:2574–2580. [PubMed] [Google Scholar]
- 116.Buczek P., Buczek O., Bulaj G. Total chemical synthesis and oxidative folding of δ-conotoxin PVIA containing an N-terminal propeptide. Biopolymers. 2005;80:50–57. doi: 10.1002/bip.20211. [DOI] [PubMed] [Google Scholar]
- 117.Hasson A., Shon K.J., Olivera B.M., Spira M.E. Alterations of voltage-activated sodium current by a novel conotoxin from the venom of conus gloriamaris. J. Neurophysiol. 1995;73:1295–1301. doi: 10.1152/jn.1995.73.3.1295. [DOI] [PubMed] [Google Scholar]
- 118.Shon K.-J., Hasson A., Spira M.E., Cruz L.J., Gray W.R., Olivera B.M. δ-conotoxin GmVIA, a novel peptide from the venom of conus gloriamaris. Biochemistry. 1994;33:11420–11425. doi: 10.1021/bi00204a003. [DOI] [PubMed] [Google Scholar]
- 119.Barbier J., Lamthanh H., Le Gall F., Favreau P., Benoit E., Chen H., Gilles N., Ilan N., Heinemann S.H., Gordon D., et al. A δ-conotoxin from conus ermineus venom inhibits inactivation in vertebrate neuronal Na+ channels but not in skeletal and cardiac muscles. J. Biol. Chem. 2004;279:4680–4685. doi: 10.1074/jbc.M309576200. [DOI] [PubMed] [Google Scholar]
- 120.Aman J.W., Imperial J.S., Ueberheide B., Zhang M.-M., Aguilar M., Taylor D., Watkins M., Yoshikami D., Showers-Corneli P., Safavi-Hemami H., et al. Insights into the origins of fish hunting in venomous cone snails from studies of conus tessulatus. Proc. Natl. Acad. Sci. USA. 2015;112:5087–5092. doi: 10.1073/pnas.1424435112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin A.-H., Israel M.R., Inserra M.C., Smith J.J., Lewis R.J., Alewood P.F., Vetter I., Dutertre S. δ-conotoxin SuVIA suggests an evolutionary link between ancestral predator defence and the origin of fish-hunting behaviour in carnivorous cone snails. Proc. R. Soc. Lond. B Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.West P.J., Bulaj G., Yoshikami D. Effects of δ-conotoxins PVIA and SVIE on sodium channels in the amphibian sympathetic nervous system. J. Neurophysiol. 2005;94:3916–3924. doi: 10.1152/jn.01304.2004. [DOI] [PubMed] [Google Scholar]
- 123.O'Reilly A.O., Khambay B.P.S., Williamson M.S., Field L.M., Wallace B.A., Davies T.G.E. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem. J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gajewiak J., Azam L., Imperial J., Walewska A., Green B.R., Bandyopadhyay P.K., Raghuraman S., Ueberheide B., Bern M., Zhou H.M., et al. A disulfide tether stabilizes the block of sodium channels by the conotoxin μO§-GVIIJ. Proc. Natl. Acad. Sci. USA. 2014;111:2758–2763. doi: 10.1073/pnas.1324189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ragsdale D.S., McPhee J.C., Scheuer T., Catterall W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science (New York N.Y.) 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 126.Kalso E., Tramer M.R., McQuay H.J., Moore R.A. Systemic local-anaesthetic-type drugs in chronic pain: A systematic review. Eur. J. Pain (Lond. Engl.) 1998;2:3–14. doi: 10.1016/S1090-3801(98)90041-6. [DOI] [PubMed] [Google Scholar]
- 127.Clare J.J. Targeting voltage-gated sodium channels for pain therapy. Expert Opin. Investig. Drugs. 2010;19:45–62. doi: 10.1517/13543780903435340. [DOI] [PubMed] [Google Scholar]
- 128.Wallace M.S., Dyck J.B., Rossi S.S., Yaksh T.L. Computer-controlled lidocaine infusion for the evaluation of neuropathic pain after peripheral nerve injury. Pain. 1996;66:69–77. doi: 10.1016/0304-3959(96)02980-6. [DOI] [PubMed] [Google Scholar]
- 129.Sorensen J., Bengtsson A., Backman E., Henriksson K.G., Bengtsson M. Pain analysis in patients with fibromyalgia. Effects of intravenous morphine, lidocaine, and ketamine. Scand. J. Rheumatol. 1995;24:360–365. doi: 10.3109/03009749509095181. [DOI] [PubMed] [Google Scholar]
- 130.Olivera B.M., Cruz L.J., de Santos V., LeCheminant G., Griffin D., Zeikus R., McIntosh J.M., Galyean R., Varga J. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using ω-conotoxin from conus magus venom. Biochemistry. 1987;26:2086–2090. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- 131.Snutch T. Targeting chronic and neuropathic pain: The N-type calcium channel comes of age. Neurotherapeutics. 2005;2:662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chabal C., Jacobson L., Mariano A., Chaney E., Britell C.W. The use of oral mexiletine for the treatment of pain after peripheral nerve injury. Anesthesiology. 1992;76:513–517. doi: 10.1097/00000542-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 133.Dejgard A., Petersen P., Kastrup J. Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet (Lond. Engl.) 1988;1:9–11. doi: 10.1016/S0140-6736(88)90999-3. [DOI] [PubMed] [Google Scholar]