Abstract
Background. Hepatitis C virus (HCV) infection may increase the risk of cardiovascular disease (CVD). We evaluated the association of chronic HCV infection and coronary atherosclerosis among participants in the Multicenter AIDS Cohort Study.
Methods. We assessed 994 men with or without human immunodeficiency virus (HIV) infection (87 of whom had chronic HCV infection) for coronary plaque, using noncontrast coronary computed tomography (CT); 755 also underwent CT angiography. We then evaluated the associations of chronic HCV infection and HIV infection with measures of plaque prevalence, extent, and stenosis.
Results. After adjustment for demographic characteristics, HIV serostatus, behaviors, and CVD risk factors, chronic HCV infection was significantly associated with a higher prevalence of coronary artery calcium (prevalence ratio, 1.29; 95% confidence interval [CI], 1.02–1.63), any plaque (prevalence ratio, 1.26; 95% CI, 1.09–1.45), and noncalcified plaque (prevalence ratio, 1.42; 95% CI, 1.16–1.75). Chronic HCV infection and HIV infection were independently associated with the prevalence of any plaque and of noncalcified plaque, but there was no evidence of a synergistic effect due to HIV/HCV coinfection. The prevalences of coronary artery calcium, any plaque, noncalcified plaque, a mixture of noncalcified and calcified plaque, and calcified plaque were significantly higher among men with an HCV RNA load of ≥2 × 106 IU/mL, compared with findings among men without chronic HCV infection.
Conclusions. Chronic HCV infection is associated with subclinical CVD, suggesting that vigilant assessments of cardiovascular risk are warranted for HCV-infected individuals. Future research should determine whether HCV infection duration or HCV treatment influence coronary plaque development.
Keywords: hepatitis C virus infection, atherosclerosis, plaque, cardiovascular disease, human immunodeficiency virus type 1
Chronic hepatitis C virus (HCV) infection and human immunodeficiency virus type 1 (HIV) infection contribute to increased immune activation and systemic inflammation [1, 2], which are associated with the development and progression of atherosclerosis [3]. HIV-infected individuals have an increased prevalence of subclinical atherosclerosis [4] and a higher rate of acute coronary events, compared with HIV-uninfected persons [5]. Previously, among participants in the Multicenter AIDS Cohort Study (MACS), we demonstrated that HIV-infected men who have sex with men (MSM) have more-subclinical coronary atherosclerosis than HIV-uninfected MSM [6]. HCV infection has been associated with carotid atherosclerosis [7], heart failure [8], and stroke [9], but studies evaluating links between HCV infection and cardiac events, specifically myocardial infarction, have yielded conflicting results [8, 10].
HIV/HCV coinfection is common, owing to shared modes of transmission [11]. Among HIV-infected populations, HCV coinfection may be associated with a greater risk of carotid atherosclerosis [12] and with cardiovascular and cerebrovascular events [13–15], although associations between HCV and carotid atherosclerosis have not been demonstrated consistently [16, 17]. It remains unclear, however, whether the effects of HIV and HCV infection on atherosclerosis are independent of each another or whether the 2 viruses act synergistically in coinfected individuals.
The purpose of the present study was to determine whether HCV infection was associated with the prevalence and extent of subclinical coronary atherosclerosis among HIV-infected and HIV-uninfected MSM in the MACS and to explore the potential synergistic effect of HIV/HCV coinfection on atherosclerosis.
METHODS
Study Population
The MACS is an ongoing observational cohort study of the natural and treated histories of HIV infection among MSM [18, 19]. Briefly, 6972 men were enrolled between 1984 and 2003 in 4 large metropolitan areas of the United States (Baltimore, Maryland/Washington, DC; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California) Data were collected at study entry and semiannual visits via interviewer-administered and computer-assisted questionnaires and physical examinations. Biological specimens were obtained at each visit for laboratory testing and repository storage. The MACS protocol and data collection forms are available at http://statepi.jhsph.edu/macs/macs.html.
Participants in active follow-up during January 2010 were eligible to undergo noncontrast computed tomography (CT) as part of the MACS cardiovascular ancillary study if they were 40–70 years old, did not have a history of cardiac surgery or percutaneous coronary intervention, and weighed <136 kilograms. Coronary CT angiography (CCTA) was performed on a subset of men without contrast allergy, impaired renal function (estimated glomerular filtration rate, <60 mL/min/1.73 m2 within 30 days of scanning), and atrial fibrillation at the time of CT. The study protocol was approved by institutional review boards at each site. All participants provided written informed consent, and human experimentation guidelines of the US Department of Health and Human Services and each institution were followed in the conduct of this clinical research.
Cardiac CT
Subclinical coronary atherosclerosis was assessed using noncontrast cardiac CT and CCTA [20]. Details of CT procedures for this study have been described previously [6, 21]. Briefly, CT images were transferred to the core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center) and analyzed by trained readers blinded to participant characteristics, including HIV or HCV serostatus. Coronary artery calcium scores were computed using the Agatston method [22]. We used the modified 15-segment model of the American Heart Association [23] to assess CCTA images for the presence, size, and composition of coronary plaque and the degree of stenosis in all assessable coronary segments. Plaque size for each segment was graded as 0, for no plaque; 1, for a mild amount; 2, for a moderate amount; or 3, for a severe amount. Segment stenosis was defined as 0, for no plaque; 1, for 1%–29% (minimal) stenosis; 2, for 30%–49% (mild) stenosis; 3, for 50%–70% (moderate) stenosis; or 4, for >70% (severe) stenosis. The overall extent of coronary plaque (ie, the total plaque score) was calculated by summing the plaque size score for all assessable coronary segments. The segment involvement score was calculated as the sum of coronary artery segments with plaque, regardless of the degree of stenosis.
Each coronary segment was then classified as normal or containing noncalcified, mixed (<50% of plaque area occupied by calcium), or calcified plaque. Calcified plaque was defined as any structure with a density of >130 Hounsfield units that was visualized as separate from the intravascular lumen and identified in at least 2 independent planes. Noncalcified plaque was defined as any discernible structure that could be clearly assignable to the vessel wall, had a CT density less than the contrast-enhanced coronary lumen but greater than the surrounding connective tissue, and was identified in at least 2 independent planes. The noncalcified, mixed, and calcified plaque scores for each participant were calculated by separately summing the scores for noncalcified, mixed, and calcified plaque across all coronary segments [21]. Finally, fatty liver disease was defined as a liver to spleen attenuation ratio of <1 Hounsfield units on noncontrast CT images [24].
HCV Testing
HCV testing in the MACS has been described in detail elsewhere [25]. Briefly, the MACS implemented a prospective HCV testing protocol in 2001 through which active participants in the MACS were screened for HCV antibody (anti-HCV; ADVIA Centaur HCV assay, Siemens Healthcare Diagnostics, Tarrytown, New York) every 2 years while they remained anti-HCV negative, with additional testing of HCV seroconverters to identify the first visit during which anti-HCV was detected. For anti-HCV–positive participants, we quantified HCV RNA first in a blood sample obtained during 2001–2003 or at the visit following HCV seroconversion and then in another sample obtained at the last MACS visit, occurring any time through March 2012, using a quantitative real-time polymerase chain reaction assay (COBAS AmpliPrep COBAS TaqMan HCV assay; Roche Molecular Systems, Pleasanton, CA). Participants with HCV RNA detected at both time points were classified as having chronic HCV infection during the entire interval, whereas men with undetectable RNA at both time points were classified as having cleared HCV infection. For men who cleared HCV RNA during this interval, we repeatedly tested for HCV RNA at interim visits until identification of the last visit during which HCV RNA was detected and first visit during which HCV RNA was not detected. Using these results, we determined the chronic HCV infection status of all participants at the time of CT, with chronic HCV infection confirmed or rejected on the basis of at least 2 positive or negative RNA results, respectively, obtained at least 6 months apart. On the basis of studies demonstrating that HCV RNA levels remain relatively constant over time [26, 27], we further classified men with chronic HCV infection according to the most recent HCV RNA level that was obtained prior to CT.
Covariate Measurement
Serological, clinical, and behavioral data were obtained from the MACS research visit a median of 57 days (interquartile range [IQR], 23–112 days) prior to the date of CT. Education level, race/ethnicity, age, medication use (for treatment of hypertension of diabetes mellitus and for reduction of lipid levels), cigarette use, alcohol consumption, and injection drug use were self-reported. Hepatitis B virus (HBV) infection was defined on the basis of a test positive for HBV surface antigen. Serum glucose and triglyceride levels were measured prospectively from fasting blood samples, while total cholesterol and high-density lipoprotein cholesterol levels were measured regardless of fasting status. Low-density lipoprotein cholesterol levels were either calculated using the Friedewald equation or measured directly when participants were not fasting or had triglyceride levels of >400 mg/dL. An aspartate aminotransferase to platelet ratio of ≥1.5 was interpreted as indicative of significant liver fibrosis or cirrhosis.
HIV-uninfected men were tested for HIV at each MACS semiannual visit, and HIV serostatus was established by a positive result of enzyme-linked immunosorbent assay confirmed by Western blot [18]. Measures of HIV disease activity, including CD4+ T-cell counts and plasma HIV RNA levels (determined by the Roche ultrasensitive assay, with a limit of detection of 50 copies/mL; Roche Diagnostics, Nutley, New Jersey), were evaluated at each visit for HIV-infected men. Duration of highly active antiretroviral therapy (HAART) was calculated based on the longitudinal participant self-report of antiretroviral medications use at each MACS visit, and history of clinically defined AIDS was determined by medical record confirmation of self-reported outcomes.
Statistical Analysis
The distributions of demographic, behavioral, and clinical characteristics of men with and without chronic HCV infection were compared using the χ2 test for categorical variables, a nonparametric test for trend across ordered groups [28], and the Kruskal–Wallis tests for continuous variables. The observed prevalence of each plaque type and distribution of plaque scores were described by chronic HCV infection status and compared using χ2 or Wilcoxon rank sum tests.
To adjust for potential confounding factors, we compared plaque prevalence by HIV and chronic HCV infection status using a Poisson regression model with robust variance [29]. We first fit minimally adjusted models that accounted for age, race, education level, study site, and enrollment period (enrolled 1984–1990 vs 2001–2003) and then fit fully adjusted models to further account for established cardiovascular disease (CVD) risk factors (body mass index [BMI], cumulative smoking exposure [in pack-years], use of hypertension medications, use of diabetes medications, use of lipid level–lowering medications, systolic blood pressure [BP], fasting glucose, and total and high-density lipoprotein cholesterol of men not receiving anti-hypertension, diabetes, or lipid level–lowering medications), history of injection drug use, and heavy alcohol use (average weekly consumption, >21 alcohol-containing drinks). Linear regression was used to assess the association of HIV infection and chronic HCV infection with plaque extent measures among individuals with plaque present (ie, those with a plaque score of >0), after adjustment for the demographic and CVD risk factors described above. Plaque scores were natural-log transformed to account for skewed distributions. We then explored the possibility of a synergistic relationship, defined as a positive interaction, between HIV and HCV infections on coronary plaque outcomes by testing an interaction term in each multivariate model. We also tested for an association between plaque prevalence and HCV RNA level by stratifying men according to the median HCV RNA level (<2 × 106 IU/mL vs ≥2 × 106 IU/mL vs men without chronic HCV infection) obtained at the visit closest to CT and then using a Wald test to compare plaque prevalence between groups.
We used multiple imputation to account for missing data in the analysis; no covariate had data missing from >4% of participants. The imputation model comprised all variables that were included in the fully adjusted models. Missing values were imputed 10 times based on the distribution of covariates, using the data augmentation Markov chain Monte Carlo method [30] and assuming a multivariate normal distribution. Statistical significance was defined as a P value of <.05. All analyses used Stata, version 13.1 (StataCorp, College Station, Texas).
RESULTS
Among 1005 HIV-infected or HIV-uninfected men who underwent noncontrast CT, we excluded 11 whose last HCV test had been performed >2.5 years before CT. Of the remaining 994 men (87 with chronic HCV infection), 755 (76%; 56 with chronic HCV infection) also completed CCTA. The most common reason for not having undergone CCTA was impaired renal function (65% of participants). Seventy-five men (86%) with chronic HCV infection were infected with HCV prior to enrollment in the MACS. The duration of chronic HCV infection is unknown in this subgroup, but the median time between cohort enrollment and CT was 8.2 years among these participants. Men who had previously cleared HCV infection composed <5% of the study population (43 underwent CT and 26 also underwent CCTA). Only 24 men (2%) included in this analysis were infected with HBV.
Compared with men without chronic HCV infection, men with chronic HCV infection were significantly (P < .05) more likely to be infected with HIV (80.5% and 59.9%, respectively), African American, to participate at the Baltimore MACS site, to have lower educational attainment, to be current smokers with greater cumulative smoking pack-years, to be currently abstinent from alcohol, and to have injected drugs (Table 1). In addition, men with chronic HCV infection had a lower BMI, were more likely to be receiving hypertension or diabetes medications, had lower total and low-density lipoprotein cholesterol levels, and were less likely to be receiving lipid level–lowering medications. The median serum HCV RNA level among men with chronic HCV infection was 2.0 × 106 IU/mL (IQR, 4.2 × 105–5.8 × 106 IU/mL). Furthermore, among HIV-infected men, those with chronic HCV coinfection had a shorter duration of HAART exposure and were more likely to have detectable plasma HIV RNA levels (>50 copies/mL).
Table 1.
Demographic, Behavioral, and Clinical Characteristics of Multicenter AIDS Cohort Study (MACS) Participants Assessed by Computed Tomography, by Chronic Hepatitis C Virus (HCV) Infection Status
| Characteristic | Overall (n = 994) | Men Without Chronic HCV Infection (n = 907) |
Men With Chronic HCV Infection (n = 87) |
P Valuea |
|---|---|---|---|---|
| Age, y | 53 (48–59) | 53 (48–59) | 53 (50–58) | .96 |
| Race | <.001 | |||
| White | 58.1 | 62.4 | 13.8 | |
| African American | 30.5 | 26.2 | 74.7 | |
| Hispanic or other | 11.4 | 11.4 | 11.5 | |
| Education level | <.001 | |||
| High school or less | 21.0 | 18.4 | 48.3 | |
| At least 1 y of college | 29.3 | 28.7 | 35.6 | |
| Undergraduate degree | 22.1 | 23.7 | 5.7 | |
| Graduate degree | 27.6 | 29.2 | 10.3 | |
| Study site | <.001 | |||
| Baltimore | 27.5 | 25.6 | 47.1 | |
| Chicago | 25.9 | 26.4 | 20.7 | |
| Pittsburgh | 20.6 | 21.2 | 14.9 | |
| Los Angeles | 26.1 | 26.9 | 17.2 | |
| Enrollment period | <.001 | |||
| 1984–1995 | 54.8 | 58.1 | 20.7 | |
| 2001–2003 | 45.2 | 41.9 | 79.3 | |
| Tobacco use | <.001 | |||
| Never smoker | 25.3 | 26.5 | 12.6 | |
| Former smoker | 47.4 | 49.1 | 29.9 | |
| Current smoker | 27.4 | 24.5 | 57.5 | |
| Smoking history, pack-yearsb | 11.9 (1.9–29.7) | 10.4 (0.9–28.7) | 23.9 (10.2–38.9) | <.001 |
| Alcohol use, drinks/wk | <.001 | |||
| 0 | 21.0 | 18.9 | 43.0 | |
| 1–3 | 51.1 | 51.7 | 44.2 | |
| 4–21 | 20.5 | 22.0 | 4.7 | |
| >21 | 7.4 | 7.3 | 8.1 | |
| Fatty liver disease | 16.2 | 17.1 | 6.8 | .02 |
| Significant liver fibrosisc | 1.9 | 0.7 | 15.3 | <.001 |
| Hepatitis B virus infection | 2.4 | 2.5 | 1.1 | <.01 |
| Ever injected drugs | 14.2 | 9.9 | 58.6 | <.001 |
| Body mass indexd | 25.9 (23.5–28.9) | 26.0 (23.6–29.3) | 25.0 (22.0–27.6) | <.01 |
| Systolic blood pressure, mm Hg | 127 (116–137) | 127 (116–137) | 129 (113–140) | .69 |
| Hypertension medication use | 34.3 | 33.4 | 43.7 | .05 |
| Glucose level, mg/dL | 98 (90–107) | 97 (90–107) | 100 (90–114) | .13 |
| Diabetes medication use | 8.1 | 7.5 | 14.9 | .02 |
| Total cholesterol level, mg/dL | 187 (161–213) | 190 (163–215) | 166 (146–193) | <.001 |
| HDL cholesterol level, mg/dL | 48 (40–58) | 48 (40–58) | 46 (37–55) | .13 |
| LDL cholesterol level, mg/dL | 108 (85–133) | 109 (87–134) | 86 (65–113) | <.001 |
| Triglyceride level, mg/dL | 120 (85–183) | 120 (85–181) | 127 (86–205) | .36 |
| Lipid level–lowering medication use | 33.2 | 35.4 | 10.3 | <.001 |
| HCV RNA load, IU/mL | … | … | 2 030 000 (421 000–5 780 000) | |
| HIV-infected participants | ||||
| No. (%) | 613 (61.7) | 543 (59.9) | 70 (80.5) | <.001 |
| HIV RNA load currently undetectablee | 82.1 | 83.7 | 69.6 | <.01 |
| Current CD4+ T-cell count, cells/mm3 | 599 (422–766) | 609 (431–775) | 498 (383–740) | .05 |
| Nadir CD4+ T-cell count, cells/mm3 | 283 (171–397) | 283 (171–399) | 272 (174–378) | .62 |
| Current HAART use | 88.3 | 89.2 | 80.9 | <.01 |
| Duration of HAART use, y | 9.5 (6.4–12.5) | 9.7 (6.6–12.6) | 8.0 (5.0–11.3) | .01 |
| History of AIDS | 14.8 | 14.2 | 20.0 | .20 |
Data are median values (interquartile ranges) or % of MACS participants, unless otherwise indicated. Results of laboratory analyses of glucose and triglyceride levels represent fasting levels.
Abbreviations: HAART, highly active antiretroviral therapy; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein.
a By the χ2 test or nonparametric test for trend, for categorical variables, or the Kruskal–Wallis test, for continuous variables.
b Among current and former smokers.
c Liver fibrosis defined as an aspartate aminotransferase to platelet ratio index of ≥1.5.
d Defined as the weight in kilograms divided by the height in meters squared.
e Defined as an HIV RNA load of <50 copies/mL.
Associations Between HCV and Coronary Atherosclerosis
The prevalence and extent of plaque and stenosis among MACS participants stratified by chronic HCV infection status are presented in Table 2. Men with chronic HCV infection had a significantly higher unadjusted prevalence of any plaque on CCTA (89.3% vs 75.4%; P = .02) and of noncalcified plaque (76.8% vs 57.5%; P < .01), compared with men without chronic HCV infection. The prevalence did not differ significantly by chronic HCV infection status for coronary artery calcium, mixed plaque, calcified plaque, or coronary artery stenosis of ≥50%, and chronic HCV infection status was not significantly associated with any of the plaque scores (plaque extent) among the men with plaque.
Table 2.
Prevalence and Extent of Observed Coronary Artery Plaque Among Multicenter AIDS Cohort Study (MACS) Participants, by Chronic Hepatitis C Virus (HCV) Infection Status
| Variable | Overall | Men Without Chronic HCV Infection | Men With Chronic HCV Infection | P Valuea |
|---|---|---|---|---|
| Noncontrast CT | ||||
| CAC Agatston score >0 | 525/994 (52.8) | 473/907 (52.2) | 52/87 (59.8) | .17 |
| CAC score | 73 (22–209) | 70 (20–211) | 103 (30–146) | .66 |
| Coronary CT angiography | ||||
| Coronary plaque | ||||
| Any | 577/755 (76.4) | 527/699 (75.4) | 50/56 (89.3) | .02 |
| Total plaque score | 4 (2–7) | 4 (2–7) | 3 (2–5) | .38 |
| Segment involvement score | 3 (2–5) | 3 (2–5) | 3 (2–4) | .22 |
| Noncalcified plaque | ||||
| Any | 445/755 (58.9) | 402/699 (57.5) | 43/56 (76.8) | <.01 |
| Noncalcified plaque score | 2 (1–3) | 2 (1–3) | 2 (1–3) | .38 |
| Mixed plaque | ||||
| Any | 251/755 (33.2) | 232/699 (33.2) | 19/56 (33.9) | .91 |
| Mixed plaque score | 2 (1–3) | 2 (1–4) | 2 (1–2) | .55 |
| Calcified plaque | ||||
| Any | 279/755 (37.0) | 259/699 (37.1) | 20/56 (35.7) | .84 |
| Calcified plaque score | 2 (1–4) | 2 (1–4) | 2 (1–3) | .88 |
| Coronary stenosis ≥50% | 120/755 (15.9) | 114/699 (16.3) | 6/56 (10.7) | .27 |
Data are proportion (%) of MACS participants or median value (interquartile range). Scores are presented for participants with prevalent plaque.
Abbreviation: CT, computed tomography.
a By χ2 or Wilcoxon rank sum tests, as appropriate.
Next, we adjusted for participant characteristics and CVD risk factors to better characterize the associations of coronary atherosclerosis with chronic HCV infection and HIV serostatus (Table 3). Both chronic HCV infection and HIV infection were independently and positively associated with the prevalence of any plaque on CCTA (adjusted prevalence ratio [aPR], 1.26 [95% confidence interval {CI}, 1.09–1.45] and 1.12 [95% CI, 1.03–1.22], respectively) and noncalcified plaque (aPR, 1.42 [95% CI, 1.16–1.75] and 1.27 [95% CI, 1.11–1.45], respectively). Chronic HCV infection, but not HIV infection, was associated with a 29% higher coronary artery calcium prevalence (aPR, 1.29; 95% CI, 1.02–1.63). Among men with plaque, chronic HCV infection was not associated with the extent of any coronary plaque type and did not alter the previously reported [6] association of HIV infection with a higher noncalcified plaque score (mean difference in log score, 0.15 [95% CI, 0.01–0.28]). We also tested for an interaction between HIV infection and chronic HCV infection in each model and found no evidence of HIV/HCV coinfection synergy on any plaque outcome (data not shown).
Table 3.
Adjusted Prevalence Ratio of Coronary Artery Plaque and Mean Difference of Plaque Extent, by Chronic Hepatitis C Virus (HCV) and HIV Serostatus
| Plaque Outcome or Measure of Extent, Predictor |
Adjusted Prevalence Ratio (95% CI) |
|---|---|
| Coronary artery calcium | |
| HCV serostatus | 1.29a (1.02 to 1.63) |
| HIV serostatus | 1.10 (.97 to 1.24) |
| Any plaque | |
| HCV serostatus | 1.26b (1.09 to 1.45) |
| HIV serostatus | 1.12b (1.03 to 1.22) |
| Noncalcified plaque | |
| HCV serostatus | 1.42c (1.16 to 1.75) |
| HIV serostatus | 1.27c (1.11 to 1.45) |
| Mixed plaque | |
| HCV serostatus | 1.07 (.67 to 1.71) |
| HIV serostatus | 1.19 (.95 to 1.49) |
| Calcified plaque | |
| HCV serostatus | 1.41 (.93 to 2.14) |
| HIV serostatus | 0.99 (.81 to 1.20) |
| Stenosis ≥50% | |
| HCV serostatus | 0.58 (.24 to 1.38) |
| HIV serostatus | 1.14 (.79 to 1.65) |
| Mean Difference(95% CI) | |
| Agatston score (n = 525) | |
| HCV serostatus | 0.34 (−.25 to .93) |
| HIV serostatus | −0.03 (−.35 to .30) |
| Segment involvement score (n = 577) | |
| HCV serostatus | 0.04 (−.19 to .27) |
| HIV serostatus | 0.10 (−.02 to .22) |
| Total coronary plaque score (n = 577) | |
| HCV serostatus | 0.03 (−.24 to .30) |
| HIV serostatus | 0.12 (−.03 to .26) |
| Noncalcified plaque score (n = 445) | |
| HCV serostatus | −0.04 (−.30 to .21) |
| HIV serostatus | 0.15a (.01 to .28) |
| Mixed plaque score (n = 251) | |
| HCV serostatus | −0.33 (−.77 to .10) |
| HIV serostatus | 0.13 (−.08 to .34) |
| Calcified plaque score (n = 279) | |
| HCV serostatus | 0.21 (−.19 to .61) |
| HIV serostatus | −0.08 (−.28 to .12) |
The HCV- and HIV-adjusted prevalence ratios are from the same models and, thus, represent independent effects. The results for each plaque outcome are adjusted for age, race, education level, study site, enrollment period, body mass index, cumulative smoking pack-years, use of hypertension medications, use of diabetes medications, use of lipid level–lowering medications, systolic blood pressure, fasting glucose level, total and high-density lipoprotein cholesterol levels, history of injection drug use, and heavy alcohol use (average weekly consumption, >21 alcohol-containing drinks per week).
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
a P < .05.
b P < .01.
c P < .001.
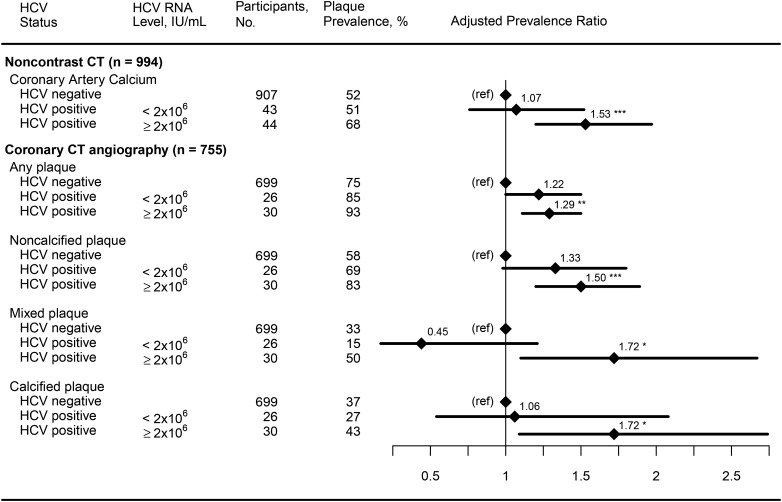
Among the 3 coronary plaque outcomes significantly associated with chronic HCV infection (coronary artery calcium, any plaque, and noncalcified plaque), we found a significant relationship between HCV RNA level and the presence of coronary artery calcium (Figure 1) after adjustment for HIV serostatus and demographic, behavioral, and CVD risk factors. The prevalence of coronary artery calcium was significantly elevated among men with chronic HCV infection who had an HCV RNA load of ≥2 × 106 IU/mL (aPR, 1.53 [95% CI, 1.20–1.97] vs men without chronic HCV infection) but not among men with an HCV RNA load of <2 × 106 IU/mL (aPR, 1.07; 95% CI, 0.76–1.52), and the difference between these 2 aPRs was borderline significant (P = .06). Similarly, the prevalence of any plaque and noncalcified plaque was significantly elevated in men with chronic HCV infection who had an HCV RNA level of ≥2 × 106 IU/mL, compared with men without chronic HCV infection, and men with chronic HCV infection and lower HCV RNA levels also had an elevated prevalence of these 2 outcomes that was of borderline statistical significance (P = .05 and P = .07, respectively), compared with men without chronic HCV infection. The prevalences of both mixed plaque and calcified plaque were also significantly elevated among the men with chronic HCV infection, but only among those who had an HCV RNA level of ≥2 × 106 IU/mL.
Figure 1.
Association of chronic hepatitis C virus (HCV) infection with coronary artery calcium, any plaque, noncalcified plaque, mixed plaque, and calcified plaque, by HCV RNA level. Regression analyses adjusted for age, race, education level, center, enrollment period, injection drug use history, alcohol, human immunodeficiency virus serostatus, and cardiovascular disease risk factors. Abbreviations: CT, computed tomography; ref, reference group. *P < .05, **P < .01, and ***P < .001.
Finally, we performed a series of sensitivity analyses to examine the robustness of the results from the multiple regression analyses. In separate analyses, we found that adjustment for current injection drug use (substituted for any history of injection drug use), the presence of fatty liver disease, fibrosis, CD4+ T-cell levels, and HIV RNA suppression, as well as the exclusion of men with HBV infection, did not change the inferences regarding the associations of plaque type and extent with HIV infection and chronic HCV infection (data not shown). Furthermore, stratification of men without chronic HCV infection into those who had never been infected with HCV and those with cleared HCV revealed that the aPRs for these 2 groups were statistically indistinguishable from one another for each plaque outcome (data not shown).
DISCUSSION
This is the largest study to date to demonstrate that chronic HCV infection is associated with subclinical coronary atherosclerosis, an important predictor of future cardiovascular events [31], in a large, well-characterized group of HIV-infected and HIV-uninfected MSM. In the subgroup of men who also underwent CCTA, chronic HCV infection was associated with a higher prevalence of any plaque and of noncalcified plaque. While men with chronic HCV infection in our cohort were more likely to be African American, less educated, and current smokers and to have a history of injection drug use, the association of chronic HCV infection with plaque remained significant after adjustment for these and other recognized CVD risk factors and was independent of HIV infection. Our findings are consistent with prior reports linking HCV infection with cardiovascular and cerebrovascular events among HIV-uninfected persons [10, 32], and they support the hypothesis that chronic HCV infection is independently associated with the development of CVD.
Previous studies demonstrated that HIV/HCV-coinfected individuals had a greater prevalence of CVD and cerebrovascular disease events [13, 14], compared with HIV-monoinfected individuals, but conflicting reports existed regarding the association between HIV/HCV coinfection and subclinical carotid atherosclerosis [12, 16]. Our study is the first to show that HIV and chronic HCV infection are independently associated with increased subclinical coronary atherosclerosis in a large cohort. Although we found no evidence of a synergistic effect for HIV/HCV coinfection, our study had insufficient numbers to formally examine the interaction between infection with these 2 viruses. Nevertheless, our study adds to the literature by demonstrating that a greater burden of subclinical coronary atherosclerosis exists among men with chronic HCV infection, whether or not they are coinfected with HIV.
The mechanism by which HCV infection may increase CVD remains unclear, but systemic inflammation and immune activation resulting from chronic HCV infection may provide a mechanistic link. HCV activates the inflammasome leading to interleukin 18 (IL-18) production [33], and elevated levels of IL-18 have been demonstrated in patients with acute coronary syndrome [34] and are associated with unstable coronary atherosclerotic plaque [35]. Chattergoon et al reported that HIV infection leads to IL-18 production through inflammasome activation and postulated that this may be an explanation for the increased risk of CVD associated with HIV infection [33]. In addition, we found that the prevalence of coronary artery calcium was significantly elevated among men with high HCV RNA levels, which is consistent with a prior study demonstrating an association between higher HCV RNA levels and greater risk of death due to cerebrovascular conditions, compared with HCV-uninfected controls [36]. We also observed that the prevalence of any, noncalcified, calcified, and mixed plaque outcomes measured by CCTA were each significantly elevated among men with chronic HCV infection and an HCV RNA load of ≥2 × 106 IU/mL. Collectively, these findings suggest that HCV infection is associated with plaque initiation and that a high HCV RNA level promotes calcification. Further longitudinal studies are needed to improve our understanding of the mechanisms that link chronic HCV infection and CVD.
An important strength of our analysis is that our study population comprised a well-characterized cohort of MSM enrolled in a CVD study. All participants underwent a comprehensive CVD evaluation, including CCTA for most participants, which allowed for detailed characterization of coronary atherosclerosis. This study also benefited from comprehensive HCV testing of all participants. Some limitations of this study include the cross-sectional study design, which limits our ability to establish causal relationships, and potential lack of generalizability to individuals other than MSM. While the high level of cohort characterization allowed adjustment for possible confounding factors, the possibility of unmeasured confounding remains. Notably, men with renal dysfunction were excluded from participation, to minimize the risks associated with intravenous contrast injection, but kidney disease is a known risk factor for CVD among HCV-infected and HIV-infected individuals [37–39], and chronic kidney disease is more prevalent among HCV-infected persons [37]. Our findings, therefore, may represent a more conservative estimate of the excess CVD risk associated with chronic HCV infection than has been demonstrated in the general HCV-infected population. Another important limitation to our study was the relatively small number of men with chronic HCV infection, which reduced the power of this study to examine fully the plaque extent outcomes. Last, we could not assess CVD clinical events, such as angina or myocardial infarction, although the presence of coronary atherosclerotic plaque is known to increase the likelihood of these events [31].
In conclusion, the elevated prevalence of subclinical coronary atherosclerosis among men with chronic HCV infection, especially men with the highest HCV RNA levels, provides further evidence supporting a link between chronic HCV infection and CVD. Future research is needed to confirm our novel finding of an association between higher HCV RNA levels and atherosclerosis and to determine whether duration of HCV infection or the use of curative HCV treatment impacts the formation or progression of coronary plaque. Nevertheless, the presence of chronic HCV infection may warrant vigilant cardiovascular risk assessment in these patients.
Notes
Acknowledgments. Data in this article were collected by the MACS, with centers (principal investigators) at the Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick), the Northwestern University Feinberg School of Medicine and the Cook County Bureau of Health Services (Steven M. Wolinsky), the University of California–Los Angeles (Roger Detels), and the University of Pittsburgh (Charles R. Rinaldo) and the data center at the Johns Hopkins Bloomberg School of Public Health (Lisa P. Jacobson). The MACS Web site is available at: http://www.statepi.jhsph.edu/macs/macs.html. Accessed 14 August 2015.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Heart Lung and Blood Institute (RO1 HL095129 to W. S. P.); the National Center for Advancing Translational Sciences (UL1 TR001079), a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research; the University of Washington's CVD and Metabolic Complications of HIV/AIDS Data Coordinating Center (5R01 HL095126); the American Heart Association (predoctoral fellowship award 13PRE16970094 to R. M.); and Johns Hopkins University (predoctoral research program award to R. M.). The MACS is funded by the National Center for Research Resources, the National Institute of Allergy and Infectious Diseases, and the National Cancer Institute (UO1-AI-35042, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041).
Potential conflicts of interest. M. D. W. has served on the Hepatitis C Advisory Board for Gilead Sciences. F. J. P. is on the speaker's bureaus for Gilead Sciences, Janssen Pharmaceuticals, Merck, and Bristol Myers Squibb. T. T. B. is a consultant for Gilead Sciences, Bristol Myers Squibb, Merck, Abbvie, EMD-Serono, and ViiV Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology 2009; 49:676–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis 2006; 185:1–11. [DOI] [PubMed] [Google Scholar]
- 3.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 2009; 27:165–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo J, Abbara S, Shturman L et al. . Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010; 24:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH et al. . HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post WS, Budoff M, Kingsley L et al. . Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizaka Y, Ishizaka N, Takahashi E et al. . Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J 2003; 67:26–30. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther 2013; 37:647–52. [DOI] [PubMed] [Google Scholar]
- 9.Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One 2012; 7:e31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009; 49:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on HIV/HCV coinfection. Current HIV/AIDS Rep 2013; 10:226–34. [DOI] [PubMed] [Google Scholar]
- 12.Sosner P, Wangermez M, Chagneau-Derrode C, Le Moal G, Silvain C. Atherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infection. Atherosclerosis 2012; 222:274–7. [DOI] [PubMed] [Google Scholar]
- 13.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med 2010; 11:462–8. [DOI] [PubMed] [Google Scholar]
- 14.Freiberg MS, Cheng DM, Kraemer KL, Saitz R, Kuller LH, Samet JH. The association between hepatitis C infection and prevalent cardiovascular disease among HIV-infected individuals. AIDS 2007; 21:193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillis J, Smieja M, Cescon A et al. . Risk of cardiovascular disease associated with HCV and HBV coinfection among antiretroviral-treated HIV-infected individuals. Antivir Ther 2014; 19:309–17. [DOI] [PubMed] [Google Scholar]
- 16.Tien PC, Schneider MF, Cole SR et al. . Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the women's interagency HIV study. AIDS 2009; 23:1781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masia M, Padilla S, Robledano C, Ramos JM, Gutierrez F. Evaluation of endothelial function and subclinical atherosclerosis in association with hepatitis C virus in HIV-infected patients: a cross-sectional study. BMC Infect Dis 2011; 11:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 19.Silvestre AJ, Hylton JB, Johnson LM et al. . Recruiting minority men who have sex with men for HIV research: results from a 4-city campaign. Am J Public Health 2006; 96:1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voros S, Rinehart S, Qian Z et al. . Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011; 4:537–48. [DOI] [PubMed] [Google Scholar]
- 21.Hacioglu Y, Gupta M, Choi TY et al. . Use of cardiac CT angiography imaging in an epidemiology study - the Methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu Kardiyol Derg 2013; 13:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15:827–32. [DOI] [PubMed] [Google Scholar]
- 23.Austen WG, Edwards JE, Frye RL et al. . A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975; 51:5–40. [DOI] [PubMed] [Google Scholar]
- 24.Price JC, Seaberg EC, Latanich R et al. . Risk factors for Fatty liver in the multicenter AIDS cohort study. Am J Gastroenterol 2014; 109:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaberg EC, Witt MD, Jacobson LP et al. . Differences in hepatitis C virus prevalence and clearance by mode of acquisition among men who have sex with men. J Viral Hepat 2014; 21:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo AE, Ghany M, Conry-Cantilena C et al. . Stability of HCV-RNA level and its lack of correlation with disease severity in asymptomatic chronic hepatitis C virus carriers. J Viral Hepat 2001; 8:256–63. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DL, Shih JW, Alter HJ et al. . Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis 1996; 174:690–5. [DOI] [PubMed] [Google Scholar]
- 28.Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985; 4:87–90. [DOI] [PubMed] [Google Scholar]
- 29.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 30.Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall, 1997. [Google Scholar]
- 31.Ahmadi N, Nabavi V, Hajsadeghi F et al. . Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol 2011; 107:10–6. [DOI] [PubMed] [Google Scholar]
- 32.Adinolfi LE, Restivo L, Guerrera B et al. . Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis 2013; 231:22–6. [DOI] [PubMed] [Google Scholar]
- 33.Chattergoon MA, Latanich R, Quinn J et al. . HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal toll-like receptors without induction of type 1 interferon. PLoS Pathog 2014; 10:e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Q, Zeng Q, Huang Y et al. . Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm 2014; doi:10.1155/2014/165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallat Z, Corbaz A, Scoazec A et al. . Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 2001; 104:1598–603. [DOI] [PubMed] [Google Scholar]
- 36.Lee MH, Yang HI, Wang CH et al. . Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke 2010; 41:2894–900. [DOI] [PubMed] [Google Scholar]
- 37.Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat 2012; 19:601–7. [DOI] [PubMed] [Google Scholar]
- 38.George E, Lucas GM, Nadkarni GN, Fine DM, Moore R, Atta MG. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS 2010; 24:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell LJ, Desai M, Hegazi A et al. . Renal impairment is associated with coronary heart disease in HIV-positive men. HIV Clin Trials 2012; 13:343–9. [DOI] [PubMed] [Google Scholar]