Abstract
AIM: To study the neural mechanism by which electroacupuncture (EA) at RN12 (Zhongwan) and BL21 (Weishu) regulates gastric motility.
METHODS: One hundred and forty-four adult Sprague Dawley rats were studied in four separate experiments. Intragastric pressure was measured using custom-made rubber balloons, and extracellular neuron firing activity, which is sensitive to gastric distention in the dorsal vagal complex (DVC), was recorded by an electrophysiological technique. The expression levels of c-fos, motilin (MTL) and gastrin (GAS) in the paraventricular hypothalamic nucleus (PVN) were assayed by immunohistochemistry, and the expression levels of motilin receptor (MTL-R) and gastrin receptor (GAS-R) in both the PVN and the gastric antrum were assayed by western blotting.
RESULTS: EA at RN12 + BL21 (gastric Shu and Mu points), BL21 (gastric Back-Shu point), RN12 (gastric Front-Mu point), resulted in increased neuron-activating frequency in the DVC (2.08 ± 0.050, 1.17 ± 0.023, 1.55 ± 0.079 vs 0.75 ± 0.046, P < 0.001) compared with a model group. The expression of c-fos (36.24 ± 1.67, 29.41 ± 2.55, 31.79 ± 3.00 vs 5.73 ± 2.18, P < 0.001), MTL (22.48 ± 2.66, 20.76 ± 2.41, 19.17 ± 1.71 vs 11.68 ± 2.52, P < 0.001), GAS (24.99 ± 2.95, 21.69 ± 3.24, 23.03 ± 3.09 vs 12.53 ± 2.15, P < 0.001), MTL-R (1.39 ± 0.05, 1.22 ± 0.05, 1.17 ± 0.12 vs 0.84 ± 0.06, P < 0.001), and GAS-R (1.07 ± 0.07, 0.91 ± 0.06, 0.78 ± 0.05 vs 0.45 ± 0.04, P < 0.001) increased in the PVN after EA compared with the model group. The expression of MTL-R (1.46 ± 0.14, 1.26 ± 0.11, 0.99 ± 0.07 vs 0.65 ± 0.03, P < 0.001), and GAS-R (1.63 ± 0.11, 1.26 ± 0.16, 1.13 ± 0.02 vs 0.80 ± 0.11, P < 0.001) increased in the gastric antrum after EA compared with the model group. Damaging the PVN resulted in reduced intragastric pressure (13.67 ± 3.72 vs 4.27 ± 1.48, P < 0.001). These data demonstrate that the signals induced by EA stimulation of acupoints RN12 and BL21 are detectable in the DVC and the PVN, and increase the levels of gastrointestinal hormones and their receptors in the PVN and gastric antrum to regulate gastric motility.
CONCLUSION: EA at RN12 and BL21 regulates gastric motility, which may be achieved through the PVN-DVC-vagus-gastric neural pathway.
Keywords: Dorsal vagal complex, Gastrin receptor, Motilin receptor, Neuronal firing activity, Paraventricular hypothalamic nucleus, RN12, BL21
Core tip: This study supports the “targeted convergence” hypothesis: Shu-acu point and Mu-acu point signals gather not only in spinal cord but also evince a ‘targeted convergence’ in brain stem and hypothalamus. We determined that the signals induced by electroacupuncture (EA) stimulation of gastric Shu and Mu points gather in the dorsal vagal complex (DVC) and paraventricular hypothalamic nucleus (PVN), increasing levels of gastrointestinal hormones and their receptors in the PVN and gastric antrum to regulate gastric motility. We hypothesize that EA at gastric Shu and Mu points regulates gastric motility, which may be achieved through the PVN-DVC-vagus-gastric pathway.
INTRODUCTION
According to the theory of traditional Chinese medicine (TCM), the application of acupuncture at a combination of Back-shu and Front-mu points has a solid theoretical basis in the theories of Yin and Yang and of Qijie. RN12 and BL21 points belong to the gastric Front-Mu point and gastric Back-Shu point, respectively, and it has been demonstrated that the combination of RN12 and BL21 is effective for the regulation of motility in clinical practice. These points regulate the function of the bowel and viscera via the closely related neuro-endocrine-immuno network regulatory system. Among these components, the functional activity of the hypothalamus and brainstem is the core foundation of acupuncture-modulated effects.
The dorsal vagal complex (DVC) comprises the nucleus tractus solitari (NTS) and the dorsal motor nucleus of the vagus (DMN). The paraventricular hypothalamic nucleus (PVN) located on the top of the hypothalamus, on the side of the third ventricle, is one of the most prominent nuclei in the anterior hypothalamus. DVC and PVN are considered to be the most important nuclei regulating gastrointestinal function in the higher central nervous system (CNS), a regulated effect that is achieved through neuroendocrine and autonomic function. The hypothalamus can affect some independent activities through direct projections to the DVC. The stimulation of the neurons in the PVN activates the DMN neurons projecting into the gastrointestinal system, namely, the locus coeruleus-dorsal motor nucleus of the vagus (LC-DMN) pathway, which may participate in the regulation of vagal preganglionic neurons in the DMN by the PVN[1]. The NTS sends nerve fibers to the hypothalamus, and also receives the most extensive projections of the PVN[2,3]. The PVN-DVC-vagus nerve pathway is involved in the gastric response to noxious stimuli[4]. Our recent study demonstrated that electroacupuncture (EA) at RN12 and BL21 could induce the up-regulation of c-fos in the DVC, which, remarkably, suggests the convergence of acupuncture signals in the medullary DVC, and the participation of gastrointestinal hormones such as motilin and gastrin in modulating the effect of EA as well[5]. The integrative activity that occurs within the DVC nuclei is the result of inputs originating from higher CNS (PVN) areas as well as from neurohormonal signals. All of these signals finely tune the coordinated function of the stomach. As the DVC contains gastric distension-sensitive neurons, the exact acupuncture signals that gather in the DVC remain to be studied by using the electrophysiology method; however, the more important target is to explore whether the higher CNS (PVN) and gastrointestinal hormones play an important role in regulating gastric motility with EA stimulation of RN12 and BL21 (gastric Shu and Mu points).
Therefore, the present study aimed to explore: the exact acupuncture signals that gather in the DVC by the electrophysiology method; the neural mechanisms by which the PVN mediates the regulation of gastric motility by RN12 (Wei Shu) and BL21 (Zhong Wan) stimulation; and the brain-gut peptide receptors, such as motilin-receptor (MTL-R) and gastrin-receptor (GAS-R or CCK-B receptor), through which gastrointestinal hormones can influence gastric functions in the PVN, determined via Western blotting and immunohistochemistry.
MATERIALS AND METHODS
Animals
Adult male Sprague Dawley rats (SD, 220-280 g) were obtained from Anhui Medical University (Hefei, Anhui, China) in the present study. All animals were housed under controlled conditions at a temperature of 22 ± 2 °C and a 12-h light/dark cycle. Commercial chow diet and autoclaved water were available to the animals ad libitum. Animals were fasted for 12 h, and anesthetized with pentobarbital (40 mg/kg, ip) before sacrifice. All experimental procedures were conducted in accordance with Anhui University of Traditional Chinese Medicine guidelines for the care and use of experimental animals and were approved by the Institutional Animal Care and Use Committee at Anhui University of Traditional Chinese Medicine (NO. 201309106).
Experimental design
Four separate experiments were performed, as follows: (1) In experiment A, the intragastric pressure was recorded, while the PVN was damaged. Rats were randomly divided into three groups: the model group (MOD, non-EA while submitted to gastric distention alone), the PVN lesion group (damaged PVN) and the PVN lesion + EA group (damaged PVN + EA at RN12 together with BL21); (2) In experiment B, rats were randomly divided into the following five groups to explore neuron firing activity in the DVC: the model group (MOD), the non-acupoint (the middle points of the line connecting the meridians where RN12 or BL21 belong and their lateral neighbor meridians) group (NA), the EA at RN12 group (RN12), the EA at BL21 group (BL21), and the EA at RN12 plus BL21 (RN12 is located on the abdomen and BL21 is located on the back) group (RN12 + BL21); (3) In experiment C, the grouping was the same as in experiment B, except the hypothalamus was removed to determine the expression of c-fos, MTL and GAS by immunohistochemical staining; and (4) In experiment D, the grouping was the same as in experiment B, except both the hypothalamus and gastric antrum were removed to determine the expression of MTL-R and GAS-R by western blotting.
Measurement of intragastric pressure
The method was very similar to our previous experiments[5]. Briefly, a self-made balloon was used to measure the ingastric pressure. It was inserted into the body of the anesthetized rats’ stomach through a small incision in the duodenum, which we call the gastric distention model (MOD). Intragastric pressures were recorded by the PowerLab 8/30 system (Australia) and analyzed by the LabChart.
PVN lesion
After the rats were anesthetized, they were placed in a stereotaxic frame. Based on the “rat brain in stereotaxic coordinates” of Paxinos and Watson[6], the electrode was advanced into the PVN (coordinates: AP 1.5 mm, R 0.4 mm, H 7.7-7.8 mm), and the bilateral PVN were destroyed using DC current (2 mA, 10 s) via a lesion-making device.
Electroacupuncture procedure
Based on the anatomical localization in rats as compared with that in the human body (Figure 1), EA was performed at RN12 (20 mm above the umbilicus on the midline of the upper abdomen, with a needle inserted at 2 mm), left BL21 (5 mm on side of the twelfth thoracic vertebra, with a needle inserted at 4 mm) and non-points (the middle points of the line connecting the meridians where RN12 or BL21 belonged and their lateral neighbor meridians). These needles were connected to an SDZ-IV type electronic acupuncture instrument (Suzhou, Jiangsu, China), with the frequency set to 20-100 Hz, the current intensity set to 2 mA. In experiments A and B, the EA process was conducted for 20 min each time in each rat when the intragastric pressure measurement instrument in experiment A or the neural data acquisition system in experiment B were ready. The EA process lasted 20 min every day for 7 d to ensure changes in c-fos, gastrointestinal hormones, and their receptors in experiment C and D.
Figure 1.
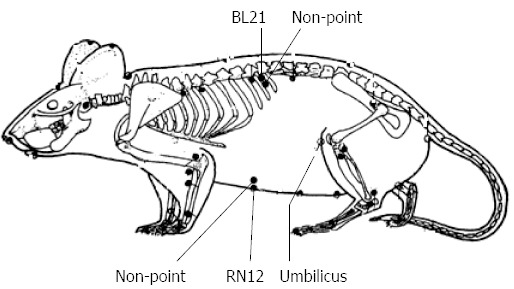
The location of RN12, BL21 and non-points in rat.
Extracellular neuronal recording
Rats were anesthetized with pentobarbital (40 mg/kg, ip) and tightly fixed in a stereotaxic apparatus; the bregma and lambda surface of the brain were exposed and then placed at the same level. An approximately 2 mm × 2 mm hole was drilled into the skull and brain membrane was carefully removed according to the coordinates of Paxinos and Watson: DVC (Ap: 11.3-14.3 mm, L: 0.5-1.7 mm, and H: 7.5-8.7 mm). The electrode was inserted into the coordinates during the experiment, while the wound was covered with a moist cotton ball of 37 °C to protect brain tissues from drying out before recording started.
Each recording of extracellular neuron firing activity was made using a pair of 16-channel stainless steel microwire arrays (reference and recording) as an electrode, the ends of which were covered with polyethylene glycol (PEG) to reinforce its strength. The electrode was connected with an electrically isolated digitizing amplifier into an OmniPlex neural data acquisition system (Plexon, United States). These neural firing activity data were analyzed using an Offline Sorter (Plexon, United States).
Before the experiment began, all the instruments for measuring intragastric pressure were prepared. The electrode was then inserted into the DVC, and after the neuronal firing pattern became stable, the neuron was then tested with 37 °C water inserted into the balloon to determine whether it was responsive to gastric distention. If the neuron was responsive to gastric distention, we began to record its discharge activity for approximately 30 s, then performed the EA procedure for another 30 s before finally removing the EA process and allowing it to recover to its firing pattern before EA.
Immunohistochemistry
To detect the expression of the assayed proteins c-fos, motilin and gastrin in the PVN, immunohistochemistry was performed. The technology was very similar to our previous experiments[5]. Briefly, we prepared the corresponding polyclonal rabbit antibody diluted in PBS (primary antibody), dropping the biotinylated goat anti-rabbit secondary antibody. All the operations were performed according to the immunohistochemical staining procedure (SP kit). c-fos, MTL, and GAS immunoreactivity in the PVN can be seen under an electron microscope as a dark brown nuclear staining. Image-Pro Plus 5.1 software analyzed the integrated optical density (IOD) in the PVN.
Western blotting
To detect the expression of the assayed proteins, gastrin/CCK-B receptor and motilin receptor in both hypothalamus and gastric antrum, western blotting was performed. The receptor proteins were extracted from the hypothalamus and gastric antrum (1.5 cm above the pylorus). Samples were mechanically dissociated and lysed in radioimmunoprecipitation assay 37 (RIPA) buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L Na2-EDTA, 1% NP-40, 0.25% Na-deoxycholate) containing protease and phosphatase inhibitor cocktails (Roche). After brief sonication and heating, the supernatants were subjected to SDS-PAGE and transferred to PVDF membranes. Blots were incubated overnight at 4 °C with primary antibodies (CCK-BR, Beyotime Biotechnology, 1:800; MTL-R, Beyotime Biotechnology, 1:500; β-actin, Beyotime Biotechnology, 1:500) and were then incubated with HRP-conjugated secondary antibodies (GE Healthcare). Blots were visualized with an enhanced chemiluminescence reagent (Millipore) and quantified with Image Lab (Bio-Rad).
Statistical analysis
All experimental values were expressed as the mean ± SD. Statistical analysis was performed with one-way ANOVA followed by Fisher’s Least Significant Difference (LSD) test. A difference with a P value < 0.05 was considered statistically significant.
RESULTS
The recording of extracellular neuron firing activity in the DVC
We tested whether there is a direct neuronal link by which DVC could regulate gastric motility that could be activated by EA to explore the neural pathway of EA at gastric Shu and Mu points (Figure 2A). Compared with the MOD group, the neuron-activating frequency of gastric distension sensitive neurons increased significantly in the RN12 + BL21, RN12 and BL21 groups (P < 0.01). EA had no significant effects on the non-acupoint group. Compared with the RN12 + BL21 group, the neuron-activating frequency decreased in the RN12 and BL21 groups (P < 0.01) (Figure 2B). These results suggest that EA at RN12 and BL21 can activate DVC neurons and that acupuncture signals may gather in the DVC.
Figure 2.
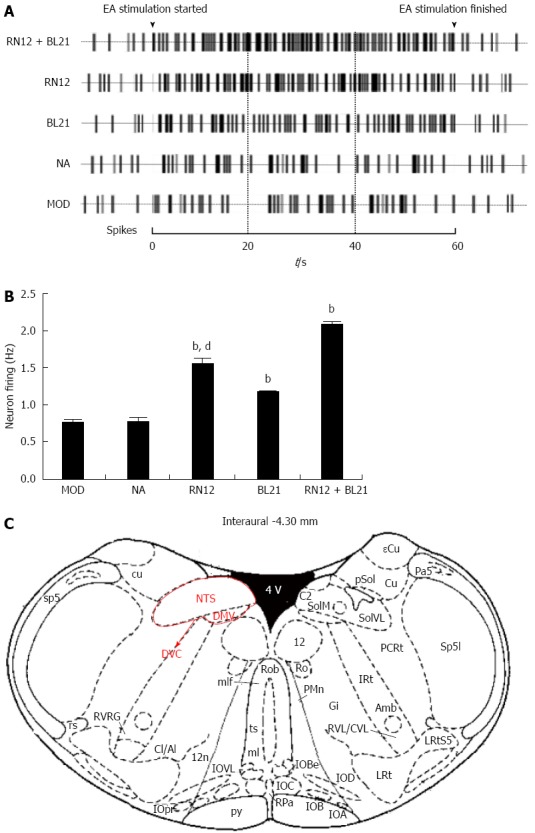
Recording of extracellular neuron firing activity in the dorsal vagal complex. A: Representative rat neuronal firing pattern in the dorsal vagal complex (DVC) induced by stimulating RN12 and BL21; B: Summarized data for neuronal firing activity by electroacupuncture (EA) stimulation at RN12 and BL21 in DVC (bP < 0.01 vs the MOD group; dP < 0.01 vs the RN12 + BL21 group); C: DVC coordinates: the anatomic location of the DVC and the rat brain in stereotaxic coordinates were adapted from the atlas of Paxinos and Watson.
c-fos expression in the PVN
To establish whether the RN12 and BL21 acupuncture signals gather in the PVN, we used immunohistochemistry to determine c-fos expression in the PVN (Figure 3A). Compared with the MOD group, Fos-positive neurons increased significantly in the RN12+BL21, RN12 and BL21 groups (P < 0.01). They were significantly increased in the RN12+BL21 group compared with the RN12 and BL21 groups (P < 0.05). EA had no significant effects on the non-acupoint group (Figure 3B). These results suggest that RN12 and BL21 acupuncture signals gather in the PVN.
Figure 3.
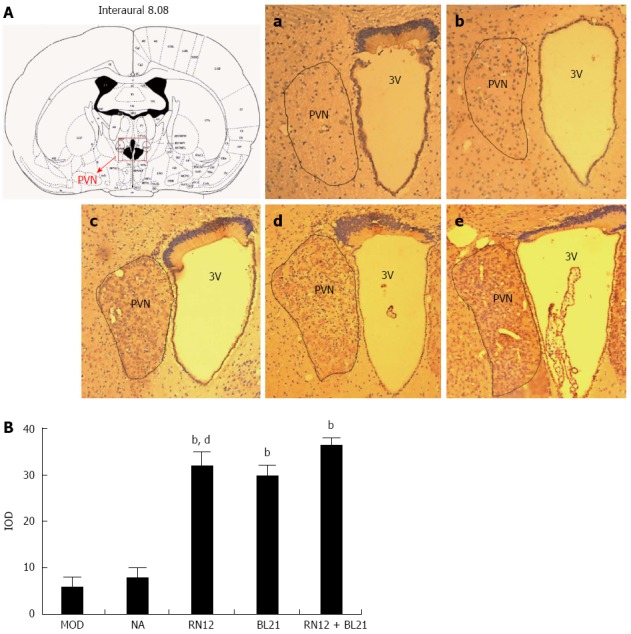
c-fos expression in the paraventricular hypothalamic nucleus. A: Photomicrographs of the hypothalamus sections showing Fos immunoreactivity in the paraventricular hypothalamic nucleus (PVN). Lane a: Model group; Lane b: Non-acupoint group; Lane c: BL21 group; Lane d: RN12 group; Lane e: RN12 + BL21 group. The anatomic locations of the photomicrographs are indicated at the top, adapted from the atlas of Paxinos and Watson. Fos-positive neurons are presented as dark brown staining in the cell nuclei; B: Integral optical density (IOD) of Fos-positive neurons in the PVN (bP < 0.01 vs the MOD group; dP < 0.01 vs the RN12 + BL21 group). 3 V: Third ventricle.
The recording of intragastric pressure
To demonstrate whether PVN was the central target of EA at RN12 + BL21 that regulated gastric motility, the PVN was damaged stereotaxically with a lesion-making device. The intragastric pressure (IGP) was decreased compared with the MOD group (P < 0.01), and the waves did not become disordered (in the DVC lesion, the waves became disordered, however). The decreased IGP induced by the PVN lesion did not increase after EA at RN12 + BL21 (Figure 4A and B), suggesting that PVN plays critical roles in EA at RN12 + BL21 regulating gastric motility.
Figure 4.
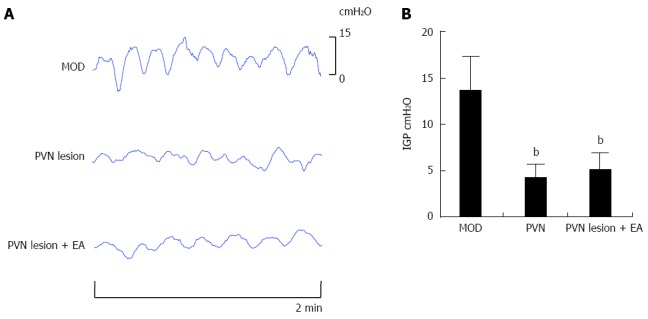
The effects of paraventricular hypothalamic nucleus regulation of intragastric pressure by stimulating RN12 + BL21. A: Representative waves of intragastric pressure (IGP) in rats induced by damaging the paraventricular hypothalamic nucleus (PVN) with or without stimulating RN12 + BL21; B: Summarized data for the effect of stimulation at RN12 + BL21 with PVN lesion on intragastric pressure (bP < 0.01 vs the MOD group).
MTL and GAS expression in the PVN
To establish whether endogenous MTL and GAS expression may be altered in response to EA in PVN, we used immunohistochemistry to determine the expression of these gastrointestinal hormones in the PVN. Compared with the MOD group, the expression of MTL and GAS in the rat PVN significantly increased in the three EA groups (P < 0.01). Additionally, MTL expression levels increased in the RN12 + BL21 group compared with the RN12 group (P < 0.01), and GAS expression levels increased in the RN12 + BL21 group compared with the BL21 group (P < 0.05). EA had no significant effects in the non-acupoint group (Figure 5). Our data suggest that gastrointestinal hormones in the PVN participate in the modulation of gastric motility by the EA stimulation of RN12 and BL21.
Figure 5.
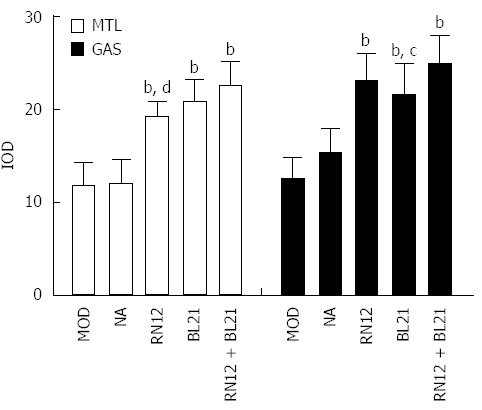
Motilin and gastrin expression in the paraventricular hypothalamic nucleus. Motilin (MTL) and gastrin (GAS) expression in paraventricular hypothalamic nucleus (PVN) is shown by immunohistochemistry. Summarized data for the expression of MTL and GAS by electroacupuncture (EA) stimulation at RN12 and BL21 in the PVN (bP < 0.01 vs the MOD group; cP < 0.05 or dP < 0.01 vs the RN12 + BL21 group).
MTL-R and GAS-R expression in the hypothalamus
To establish whether gastrointestinal hormones in the PVN participate in the modulation of gastric motility by EA stimulation of RN12 and BL21 through their receptors, we used western blotting to determine the expression of MTL-R and GAS-R in the hypothalamus (Figure 6A). Compared with the MOD group, the expression of MTL-R and GAS-R in the hypothalamus increased significantly in the RN12 + BL21, RN12 and BL21 groups (P < 0.01); MTL-R did not significantly increase in the non-acupoint group, but did significantly increase in the RN12 + BL21 group compared with the RN12 and BL21 groups (P < 0.01) (Figure 6B). These results demonstrate that the modulatory effect of EA may be due to the interaction of gastrointestinal hormones and their receptors in the hypothalamus.
Figure 6.
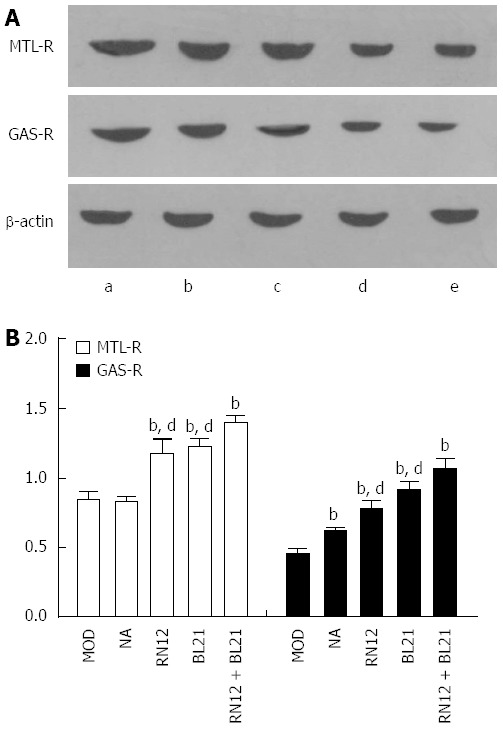
Motilin receptor and gastrin receptor expression in the hypothalamus. A: Motilin receptor (MTL-R) and gastrin receptor (GAS-R) protein expression in the hypothalamus is shown by western blotting. Lane a: RN12 + BL21 group; Lane b: BL21 group; Lane c: RN12 group; Lane d: Non-acupoint group; Lane e: Model group; B: Summarized data for the expression of MTL-R and GAS-R by electroacupuncture (EA) stimulation at RN12 and BL21 in the hypothalamus (bP < 0.01 vs the MOD group; dP < 0.01 vs the RN12 + BL21 group.
MTL-R and GAS-R expression in the gastric antrum
To establish whether endogenous MTL-R and GAS-R expression may be altered in response to EA in the gastric antrum, we used western blotting to determine the expression of these receptors in the gastric antrum (Figure 7A). Compared with the MOD group, the expression of MTL-R and GAS-R in the gastric antrum increased significantly in the RN12 and BL21 groups, as well as in the RN12 + BL21 group (P < 0.01). GAS-R expression significantly increased in the RN12 + BL21 group compared to the RN12 and BL21 groups (P < 0.05 or P < 0.01) but did not significantly increase in the non-acupoint group (Figure 7B). These results, at least in part, demonstrate that the modulatory effect of EA may be due to the increase in some brain-gut-related receptors in the gastric antrum, such as MTL-R and GAS-R.
Figure 7.
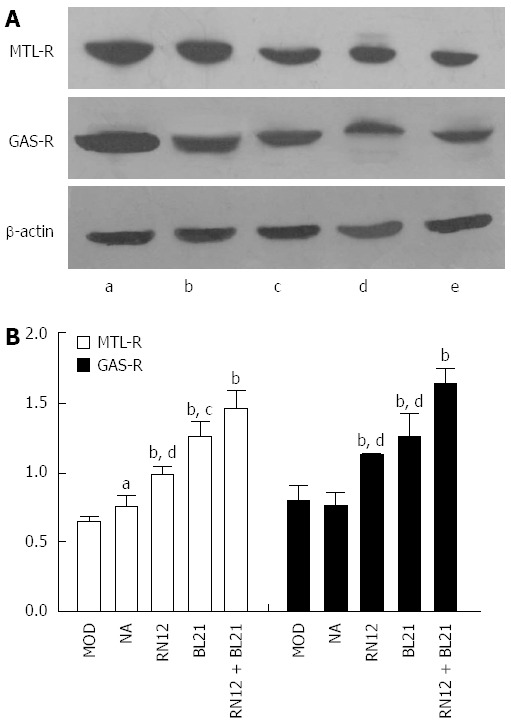
Motilin receptor and gastrin receptor expression in the gastric antrum. A: Motilin receptor (MTL-R) and gastrin receptor (GAS-R) protein expression in the gastric antrum shown by western blotting. Lane a: RN12 + BL21 group; Lane b: BL21 group; Lane c: RN12 group; Lane d: Non-acupoint group; Lane e: Model group; B: Summarized data for the expression of MTL-R and GAS-R by electroacupuncture (EA) stimulation at RN12 and BL21 in the gastric antrum (aP < 0.05 or bP < 0.01 vs the MOD group; cP < 0.05 or dP < 0.01 vs the RN12 + BL21 group).
DISCUSSION
It has been demonstrated that stimulation of the combination of Back-shu points and Front-mu points is effective in clinical practice. However, studies of the mechanisms underlying this phenomenon have been focused at the level of the spinal cord. Advances in the study of Back-Shu and Front-Mu point combinations have focused on whether the superior nerve center involves the regulation of zang-fu organs by a combination of Back-shu and Front-mu points. To address whether this convergent effect could extend to the superior nerve center, we put forward a “targeted convergence” hypothesis: gastric Shu and Mu point acupuncture signals gather not only in the spinal cord but also in a targeted way in the brain stem and hypothalamus in the higher central nervous system, achieving an integrative effect through the neural microcircuitry. Likely, gastric Shu and Mu afferent signals primarily convey acupuncture input signals to different levels of the central nervous system, such as the spinal cord, medulla, brain stem, hypothalamus and subcortex.
The DVC in the brainstem plays an important role in autonomic regulation of gastric motility. Based on our previous studies, we found that gastric motility was blocked by DVC lesions or bilateral sub-diaphragmatic vagotomy, and EA at RN12 + BL21 could not restore this depression. DVC was found to play an important role in the regulation of gastric motility by EA at RN12 + BL21, an effect that depended upon the vagus nerve[5]. Electrophysiological experiments in this study further clarified that DVC participated in the regulation of gastric motility by the stimulation of RN12 and BL21, which, in turn, indicated that there was a direct neuronal link in the DVC by which they could participate in the regulation of gastric motility.
The PVN may modulate a range of functions, including ingestive behavior, sodium intake, and glucose metabolism, as well as cardiovascular, gastrointestinal, and respiratory activities. Neurons in these PVN-innervated subnuclei of the lateral PB may play a critical role in relaying ingestive feedback information involving the mechanical distention of the stomach[7]. Some studies have shown that electrical stimulation of the lateral hypothalamic area (LHA) increased the firing activities of GD-responsive neurons in the PVN and promoted gastric motility[8]. The PVN serves as a central nucleus for modulating gastrointestinal activities. The present study demonstrated that repeated electroacupuncture at the bilateral Zusanli and Yanglingquan points has a cumulative analgesic effect by remodeling the synaptic structure of the PVN[9]. In this study, we found that EA at RN12 and BL21 could induce the expression of c-fos in the PVN and that the even more marked upregulation in the RN12 + BL21 group demonstrates that the acupuncture signals of the gastric Shu and Mu points gather in the PVN as well. In addition, we found that PVN lesions caused a decrease in gastric motility, and that EA at RN12 + BL21 was unable to restore this decreased gastric motility. It was demonstrated that the PVN was another central target of the gastric Shu and Mu points that combined to regulate gastric motility. This finding verifies the “targeted convergence” hypothesis as Shu and Mu point acupuncture signals converge not only in the DVC but also in a targeted manner in the PVN.
Emerging evidence indicates that acupuncture treatment not only activates distinct brain regions in different types of diseases but also modulates adaptive neurotransmitters in related brain regions to alleviate autonomic responses[10,11].
In our previous study, motilin and gastrin in the serum, gastric antrum and DVC increased significantly with EA at gastric Shu and Mu points[5,12]. This showed the gastrointestinal hormones react remarkably to gastric motility with EA. Neurons with motilin mRNA expression, motilin immunoreactivity, and motilin receptors have been found in various regions of the brain in different species[13,14]. Some studies found that PVN was a central nucleus for modulating gastric motility and motilin expression in the thyroid. PVN excitation was shown to prompt gastric motility, which was partly prevented by the motilin receptor antagonist, GM-109[15]. Furthermore, studies have shown that Motilin/FG-labeled neurons were detected in the PVN, and a motilin receptor antagonist (GM109) could abolish the responses of neurons and excitatory effect of gastric motility induced by motilin[16]. The receptor of gastrin is always called the CCK-B receptor. The majority of CCK-B receptors in the brain are abundantly distributed throughout brain regions such as the cerebral cortex, olfactory bulbs, hippocampus, amygdala, nucleus accumbens, and the nucleus tractus solitary(NTS)[17-19]. The roles of CCK-B receptor in numerous physiologic processes in the gastrointestinal tract and central nervous system have been well documented[20]. Some results showing that antagonists for the CCK-B receptor microinfused into the PVN inhibited colonic motility in non-fasted rats[21] suggest a specific function of the CCK-B receptor in integrative satiety controls via the PVN[22]. Furthermore, some studies suggest that the CCK-B receptor might play a role in the tonic inhibition of 100 Hz EA-induced analgesia and in the mediation of chronic tolerance to 100 Hz EA in mice[23]. Results also suggest that the level of CCK-B receptor mRNA expression in the hypothalamus has an important relationship with the individual variations in the response to high frequency EA analgesia in rats[24]. In the present study, we found that EA at RN12 and BL21 could increase the expression of MTL, GAS, MTL receptor and GAS receptor in PVN, as well as MTL receptor and GAS receptor in gastric antrum. This suggests that the increased levels of MTL and GAS (via MTL and GAS receptors) within the PVN participate in EA-regulated gastric motility, and that the MTL and GAS receptors in gastric antrum also participate in EA-regulated gastric motility.
Taking into account the principles of traditional Chinese medicine and all the data on gastric motility, DVC neuronal activity, and the expression of brain-gut peptides in both hypothalamus and gastric antrum, this study demonstrated that EA stimulation of the gastric Shu and Mu points could regulate gastric motility, with combined stimulation of these acupoints eliciting a synergistic effect. The effect was closely connected with the DVC and PVN, as acupuncture signals generated by EA at gastric Shu and Mu points gather in the DVC and PVN, elevating the expression of gastrointestinal hormone receptors in the hypothalamus and gastric antrum, which in turn play a role in regulating gastric motility through the vagus nerve. Thus, we propose that the effects of combined EA stimulation of gastric Shu and Mu points may be achieved through the combined efforts of the PVN-DVC-vagus nerve-gastric channel (Figure 8).
Figure 8.
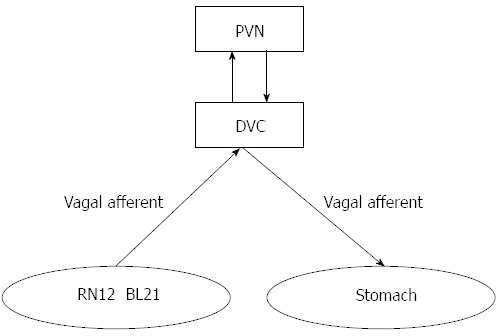
The transmission path of acupuncture signals of the gastric Shu and Mu points.
ACKNOWLEDGMENTS
The authors would like to thank the members of the Neurobiology (acupuncture) Laboratory, and the Xinan Medicine Key Laboratory, Combined Traditional Chinese and Western Medicine Research Institute, Cell and Molecular Biology Laboratory.
COMMENTS
Background
Clinically, electroacupuncture (EA) at BL21 and RN12 has been frequently utilized as a therapy for patients with gastrointestinal dysfunction in Eastern Asia. Previous studies by the authors demonstrated that EA stimulation of the BL21 and RN12 could synergistically regulate gastric motility via elevating gastrointestinal hormones in the dorsal vagal complex (DVC). Similar to the DVC, the paraventricular hypothalamic nucleus (PVN) is also an important nucleus for regulating gastrointestinal function. The present study aims to evaluate if there is a direct neuron link for DVC to regulate gastric motility which can be activated by EA at BL21 and RN12, and further to explore the acupuncture signals of combined BL21 and RN12 not only in the DVC, but also as “targeted convergence” in the PVN.
Research frontiers
Previous experiments have already proved that the acupuncture information of Shu-acu points and Mu-acu points gathers in the spinal cord, while another part gathers at the superior nerve center (brain stem and hypothalamus).
Innovations and breakthroughs
The authors put forward a hypothesis called “targeted convergence”: the acupuncture signal of gastric Shu and Mu points gathers not only in the spinal cord, but also have a phenomenon of targeted convergence in the brain stem and hypothalamus of the higher central nervous system, achieving an integration effect through the neural microcircuitry. This is the first study to show the acupuncture signal of gastric Shu and Mu points gathers in superior nerve centers, especially the PVN.
Applications
The present data demonstrate that the signals induced by EA stimulation of acupoints RN12 and BL21 gather in the DVC and the PVN, increasing the levels of gastrointestinal hormone and the receptors in the PVN and gastric antrum to regulate gastric motility. The study indicates the neural biological basis of combination of Back-shu and Front-mu points, to direct the compatibility of acupoints in clinical acupuncture, improving the clinical efficacy.
Terminology
DVC and PVN are two important nuclei regulating gastrointestinal function in the higher central nervous system.
Peer-review
The subject has a very good foundation, and they forward a hypothesis called “targeted convergence”, it is stimulating and innovative. The authors attempt to shed a light on the matter of specificity of acupuncture stimuli is welcome, but the article needs some work to be better.
Footnotes
Supported by The National Nature Science Foundation Council of China, No. 81473784; the Natural Science Foundation of Anhui Province, No. 1408085MH166; and the Natural Science Foundation of Anhui University of Traditional Chinese Medicine, No. 2013qn002.
Institutional review board statement: The study was reviewed and approved by the Anhui University of Traditional Chinese Medicine Institutional Review Board (No. 201409106).
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Anhui University of Traditional Chinese Medicine (IACUC No. 24564562W).
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at shengm_66@163.com. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 11, 2015
First decision: July 10, 2015
Article in press: October 16, 2015
P- Reviewer: Lin YW, Shen GM, Silva JBG S- Editor: Yu J L- Editor: Logan S E- Editor: Wang CH
References
- 1.Yuan PQ, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am J Physiol Endocrinol Metab. 2002;283:E436–E448. doi: 10.1152/ajpendo.00538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav. 2003;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 3.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi CX, Ru LQ, Hu DS. Transmission of gastric noxious signals to hypothalamus mediated by vagus nerve. Zhongguo Shenjing Kexue Zazhi. 2004;20:353–356. [Google Scholar]
- 5.Wang H, Shen GM, Liu WJ, Huang S, Zhang MT. The Neural Mechanism by Which the Dorsal Vagal Complex Mediates the Regulation of the Gastric Motility by Weishu (RN12) and Zhongwan (BL21) Stimulation. Evid Based Complement Alternat Med. 2013;2013:291764. doi: 10.1155/2013/291764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: People’s Medical Publishing House; 2005. [Google Scholar]
- 7.Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- 8.Guo FF, Xu L, Gao SL, Sun XR, Li ZL, Gong YL. The effects of nesfatin-1 in the paraventricular nucleus on gastric motility and its potential regulation by the lateral hypothalamic area in rats. J Neurochem. 2015;132:266–275. doi: 10.1111/jnc.12973. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Liu T, Chen S, Gao Y, Wang J, Qiao L, Liu J. Correlation between the cumulative analgesic effect of electroacupuncture intervention and synaptic plasticity of hypothalamic paraventricular nucleus neurons in rats with sciatica. Neural Regen Res. 2013;8:218–225. doi: 10.3969/j.issn.1673-5374.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li QQ, Shi GX, Xu Q, Wang J, Liu CZ, Wang LP. Acupuncture effect and central autonomic regulation. Evid Based Complement Alternat Med. 2013;2013:267959. doi: 10.1155/2013/267959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen GM, Zhou MQ, Xu GS, Xu Y, Yin G. Role of vasoactive intestinal peptide and nitric oxide in the modulation of electroacupucture on gastric motility in stressed rats. World J Gastroenterol. 2006;12:6156–6160. doi: 10.3748/wjg.v12.i38.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Shen GM, Wang KM. Effects of electroacupuncture at “Wei shu” (BL 21) and “Zhongwan” (CV 12) on gastric movement and serum levels of motilin and gastrin in rats. Anhui Zhongyixueyuan Xuebao. 2011;30:37–39. [Google Scholar]
- 13.Xu L, Depoortere I, Tang M, Peeters TL. Identification and expression of the motilin precursor in the guinea pig. FEBS Lett. 2001;490:7–10. doi: 10.1016/s0014-5793(01)02125-1. [DOI] [PubMed] [Google Scholar]
- 14.Depoortere I, Van Assche G, Peeters TL. Distribution and subcellular localization of motilin binding sites in the rabbit brain. Brain Res. 1997;777:103–109. doi: 10.1016/s0006-8993(97)01094-9. [DOI] [PubMed] [Google Scholar]
- 15.Guo F, Xu L, Sun X, Gao S, Zhu H. The paraventricular nucleus modulates thyroidal motilin release and rat gastric motility. J Neuroendocrinol. 2011;23:767–777. doi: 10.1111/j.1365-2826.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Gao S, Guo F, Sun X. Effect of motilin on gastric distension sensitive neurons in arcuate nucleus and gastric motility in rat. Neurogastroenterol Motil. 2011;23:265–70, e120-1. doi: 10.1111/j.1365-2982.2010.01661.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill DR, Woodruff GN. Differentiation of central cholecystokinin receptor binding sites using the non-peptide antagonists MK-329 and L-365,260. Brain Res. 1990;526:276–283. doi: 10.1016/0006-8993(90)91232-6. [DOI] [PubMed] [Google Scholar]
- 18.Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Ohta H, Izumi H, Matsuda Y, Seki M, Toda T, Akiyama M, Matsushima Y, Goto Y, Kaga M, et al. Behavioral and cortical EEG evaluations confirm the roles of both CCKA and CCKB receptors in mouse CCK-induced anxiety. Behav Brain Res. 2013;237:325–332. doi: 10.1016/j.bbr.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 20.Willard MD, Lajiness ME, Wulur IH, Feng B, Swearingen ML, Uhlik MT, Kinzler KW, Velculescu VE, Sjöblom T, Markowitz SD, et al. Somatic mutations in CCK2R alter receptor activity that promote oncogenic phenotypes. Mol Cancer Res. 2012;10:739–749. doi: 10.1158/1541-7786.MCR-11-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mönnikes H, Tebbe J, Grote C, Sonntag A, Pluntke K, Sturm K, Arnold R. Involvement of CCK in the paraventricular nucleus of the hypothalamus in the CNS regulation of colonic motility. Digestion. 2000;62:178–184. doi: 10.1159/000007811. [DOI] [PubMed] [Google Scholar]
- 22.Mohammad S, Ozaki T, Takeuchi K, Unno K, Yamoto K, Morioka E, Takiguchi S, Ikeda M. Functional compensation between cholecystokinin-1 and -2 receptors in murine paraventricular nucleus neurons. J Biol Chem. 2012;287:39391–39401. doi: 10.1074/jbc.M112.416214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Hu ZP, Jiang SZ, Li HT, Han JS, Wan Y. CCK(B) receptor antagonist L365,260 potentiates the efficacy to and reverses chronic tolerance to electroacupuncture-induced analgesia in mice. Brain Res Bull. 2007;71:447–451. doi: 10.1016/j.brainresbull.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Ko ES, Kim SK, Kim JT, Lee G, Han JB, Rho SW, Hong MC, Bae H, Min BI. The difference in mRNA expressions of hypothalamic CCK and CCK-A and -B receptors between responder and non-responder rats to high frequency electroacupuncture analgesia. Peptides. 2006;27:1841–1845. doi: 10.1016/j.peptides.2006.01.002. [DOI] [PubMed] [Google Scholar]


