Abstract
AIM: To assess the diverse immediate and long-term clinical outcomes, a retrospective comparison between laparoscopic and conventional operation was performed.
METHODS: A total number of 916 clinical cases, from January 2006 to December 2013 in our hospital, were analyzed which covered 492 patients underwent the laparoscopy in radical resection (LRR) and 424 cases in open radical resection (ORR). A retrospective analysis was proceeded by comparing the general information, surgery performance, pathologic data, postoperative recovery and complications as well as long-term survival to investigate the diversity of immediate and long-term clinical outcomes of laparoscopic radical operation.
RESULTS: There were no statistically significance differences between gender, age, height, weight, body mass index (BMI), tumor loci, tumor node metastasis stages, cell differentiation degree or American Society of Anesthesiologists scores of the patients (P > 0.05). In contrast to the ORR group, the LRR group experienced less operating time (P < 0.001), a lower blood loss (P < 0.001), and had a 2.44% probability of conversion to open surgery. Postoperative bowel function recovered more quickly, analgesic usage and the average hospital stay (P < 0.001) were reduced after LRR. Lymph node dissection during LRR appeared to be slightly more than in ORR (P = 0.338). There were no obvious differences in the lengths and margins (P = 0.182). And the occurrence rate in the two groups was similar (P = 0.081). Overall survival rate of ORR and LRR for 1, 3 and 5 years were 94.0% and 93.6% (P = 0.534), 78.1% and 80.9% (P = 0.284) and 75.2% and 77.0% (P = 0.416), respectively.
CONCLUSION: Laparoscopy as a radical operation for rectal cancer was safe, produced better immediate outcomes. Long-term survival of laparoscopy revealed that it was similar to the open operation.
Keywords: Laparoscopic, Open surgery, Short-term outcomes, Long-term outcomes, Rectal cancer
Core tip: This is a retrospective case-control study between laparoscopic surgery and open surgery in rectal cancer. There are 916 clinical cases, collecting from January 2006 to December 2013 in our hospital, which covered 492 cases in laparascopic group and 424 cases in open group. We compared the general information, surgery performance, pathologic data, postoperative recovery and complication as well as the long-term survival of the patients. And than we concluded that laparoscopy can produce better immediate outcomes in rectal cancers. And the long-term survival of laparoscopy was similar to the open operation.
INTRODUCTION
It has been estimated that about 1.4 million new cases of colorectal cancer are diagnosed worldwide and nearly 0.7 million colorectal cancer-related deaths occur, of which about 33% are due to rectal cancer[1]. Owing to the restriction of surgery and medicine the prognoses of rectal cancer patients are critically poor, with up to 40% locoregional recurrence and less than a 50% 5-year survival rate before the 1980s[2]. With improvement of surgery and treatment with neoadjuvant chemoradiotherapy, radiotherapy and immune therapy, the recurrence rates have been reduced with survival rates now greater than 70%[3].
It is more than 20 years since laparoscopy was applied to the treatment of malignant colorectal cancer by Jacobs and others[4]. With improved techniques and updated surgical instruments, laparoscopy is more widely used to treat rectal cancer. Its principal advantages are its clear view and reduced wounding, less interference to the immune system, quick postoperative recovery and a lower overall operating expense[5-7]. Yet many controversies remain whether laparoscopy, although minimally invasive, is inferior to conventional open surgery for the long-term treatment of this serious cancer. For example, it has been suggested that laparoscopy may stimulate tumor invasion and metastasis. Most large scale randomized controlled trials (RCT) worldwide have conducted research [conventional vs laparoscopic-assisted surgery in colorectal cancer (CLASICC), colorectal cancer laparoscopic or open resection (COLOR), etc.] on colon rather than rectal cancer. In 2013, National Comprehensive Cancer Network tended to recommend laparoscopy for rectal cancer patients with clinical research trials encouraged to compare laparoscopic and conventional operations.
A large body of research exists, which involved a comparison between laparoscopic and open radical resection for rectal cancer[8]. The research included resection margin length of both sides, mesentery length and the number of lymph nodes retrieved, which suggested the same effectiveness of radical laparoscopy to an open operation[9,10]. However, laparoscopic surgery also has the advantage of less trauma, less pain postoperatively and a reduced hospital stay for patients. In previous years, laparoscopy took longer than open section surgery but now laparoscopy can be completed in about the same time as open section, mainly due to the accumulation of practical surgical experience using this technique; one additional benefit is the reduced occurrence of complications[11]. Research on the long-term curative effect has revealed closely similar 1-, 3- and 5-year survival rates of laparoscopic rectal cancer surgery compared to open resection[6,12-14]. Earlier research found a high incidence of postoperative implantation metastasis and peritoneal implantation metastasis after laparoscopic radical surgery for rectal cancer[15]. But recent research has reported a similar implantation metastasis incidence for laparoscopic and open section[16]. It was assumed that earlier outcomes could have been related to the neglect of tumor-free principles during the operations.
RCT research includes that of Milson, Schwandner, CLASICC[9,10,17] and others, who reached the conclusion of the similar long-term benefits of laparoscopic and open resection for rectal cancer. Leung also concluded that there were similar long-term effects of laparoscopic and conventional surgery for recto sigmoid colon cancer[16]. The RCT research focused on laparoscopic and open section for rectal tumors, which was conducted by Gong, Park and the COLOR II stage experiments are still in progress[13,18,19]. From the perspective of evidence-based medicine, a RCT outcome based on large number of cases should prove the long-term efficacy or otherwise of a laparoscopic radical operation for rectal cancer.
MATERIALS AND METHODS
Patients
Between January 2006 and December 2013, 492 patients in group LRR and 424 patients in group ORR in the Shanghai First People’s Hospital Affiliated Shanghai Jiao Tong University were enrolled in our study. Open surgeries (OP) and laparoscopic-assisted surgeries (LAP) were performed by the same surgical teams, respectively. Two groups of patients underwent preoperative colonoscopy and had biopsy-proven adenocarcinoma. The trial received approval from the Shanghai First Peoples’ Hospital Affiliated Shanghai Jiao Tong University research ethics committee and prior written informed consent was obtained from all patients.
Patients diagnosed with rectal cancer without other serious diseases were included in the study. Patients with distant metastasis of the tumor or associated malignant tumor; intestinal obstruction; recurrence of the tumor or other digestive system malignancy; those who had a palliative operation for the tumor which could not be resected or had widespread metastasis in the abdominal cavity, were excluded from the study.
Preoperative preparation and operation procedures
Both groups had preoperative fiber colonoscopy and biopsy for pathology confirmation of a clear diagnosis of rectal cancer. Preoperative staging was evaluated by enhanced CT or MRI scanning of the abdomen and pelvis, as well as chest X-ray films, ultrasonography or other assisted auxiliary examination. Both teams employed preoperative intestinal preparations: twice oral gentamicin 80000 U/d, 3 times 0.4 g oral metronidazole for 3 d, and 1 d fluid food before surgery. Polyethylene glycol-electrolyte powder was given to prepare the bowel.
Both groups were operated on by a fixed team of experienced physicians. Under the tumor-free technique principle, the operation should be performed based on colorectal cancer radical excision and rectal tumor patients should be operated according to TME principles. The preoperative preparation of the laparoscopy group closely mimicked the open resection group. Patients were placed in the bladder lithotomy position, with tracheal intubation anesthesia and a pneumoperitoneum established under a maintained pressure of 10-14 mmHg. The 5-trocar technique was adopted by using an ultrasound knife to incise the sigmoid right-sided mesentery and clear the peritoneum to free up presacral space to the right side of the rectum. A medical grasper was used to retract the superior rectum vessels and was directed upwards in a retrograde direction to facilitate separation of the left-sided Toldt anadesma, reveal the ureter and bare the root of the upper mesentery artery. A part of the upper mesentery artery and the inferior mesenteric aorta in the horizontal position was separated using a titanium clamp. Downward pressure was used to separate the presacral space and remove the peritoneum from the right side of the rectum. The sigmoid was isolated and the peritoneum removed to the left side of the rectum and also the anterior sacral fascia to protect the hypogastric nerve and pots plexus. Next, the antetheca denonvillier fascia was separated as well as the right and left ligament down to the pelvic floor, and a further incision made 3-5 cm below the umbilicus in the center. Through this incision, gradually the rectal tumor was removed in the same way as during an open operation. Subsequently, an intestinal anastomosis was completed or an enterostomy, and the pelvic region rinsed with copious quantities of distilled water to decrease the growth of cancer cells. A regular drainage tube was indwelled close to the anastomotic area and was routed to the lower abdomen or ischiorectal fossa. Open section mainly used a high frequency electric knife and the operative principle and methods were identical to LRR operated group.
Perioperative surveillance, postoperative management and follow-up evaluation
Common data included gender, age, height, weight, body mass index (BMI), tumor location, tumor node metastasis (TNM) staging, cell differentiation grade, American Society of Anesthesiologists (ASA) scores and so on. The operative index included the time to complete the operation, blood loss, sample lengths, average retrieved lymph node numbers and the conversation rates. The postoperative pain score on day 1 was adopted as the basis of a numerical rating scale. The degree of pain was assessed by a number from 0 to 10 in which 0 was pain-free and 10 was the highest pain intensity experienced by patients. Individual patients specified their degree of pain: 0-4 was mild pain; 5-6 medium pain, and 7-10 severe pain. Postoperative data was recorded which included comprised peristalsis recovery time, exsufflation time, time until off-bed, time until the first liquid and semi-liquid intake, duration of hospital stay and the overall hospitalization duration.
FOLFOX plan, XELOX plan and the Capecitabine plan were regularly deployed in postoperative chemotherapy for 6 mo, apart from cancer stage 0 and I. The postoperative follow-up commonly involved a clinical visit or telephone follow-up. As of 28th February 2014, the shortest follow-up duration was 2 mo, the longest 109 mo and the average 55 mo, with a 95.1% follow up success rate.
Statistical analysis
SPSS Statistics for Windows, Version 17.0 (SPSS, Inc., Chicago, IL, United States) was used for analyses. A t-test was used to analyze normally distributed data and a Mann-Whitney U-test for other types of distributions. A χ2 test was used to analyze count data. P < 0.05 was considered to be statistically significant. The survival cures for two group were used Kaplan-Meier method, and the log-rank test was applied to analyze the differences between the results.
RESULTS
Clinical data of patients
Of the 916-recorded cases, 492 cases were LRR. There were 301 males and 191 females, aged 64.5 ± 11.9, BMI index 23.274 ± 3.463, I stage 93 cases, II stage 218 cases and III stage 181 cases in TNM staging, with one-score for 109 cases, two-scores for 321 cases and three-scores for 62 cases in ASA assessments. In 424 cases in the ORR group, there were 243 males and 181 females, aged 63.3 ± 12.3, BMI index 23.438 ± 3.533, I stage 83 cases, II stage 211 and III stage 130 cases in TNM staging, with one-score 108 cases, two-score 264 and three-score 52 cases in ASA scores. The cases of previous abdominal surgery in ORR and LRR group were 75 and 65. There was comparability between the two groups in terms of age, gender, tumor staging, ASA scores and so on (P > 0.05; see Table 1).
Table 1.
Clinical comparison of radical operation data for rectal cancer
| LRR (n = 492) | ORR (n = 424) | P value | |
| Gender | |||
| Male | 301 | 243 | 0.235 |
| Female | 191 | 181 | |
| Age (yr, mean ± SD) | 64.5 ± 11.9 | 63.3 ± 12.3 | 0.290 |
| BMI (kg/m2) | 23.274 ± 3.463 | 23.438 ± 3.533 | 0.999 |
| Preoperative comorbid diseases | 52.8% (260/492) | 58.7% (249/424) | 0.074 |
| Hypertension | 60 | 57 | |
| Coronary heart disease | 37 | 34 | |
| Arrhythmia | 21 | 20 | |
| COPD | 10 | 13 | |
| Pulmonary infection | 3 | 5 | |
| Asthma | 2 | 3 | |
| Diabetes | 42 | 70 | |
| Hepatic cirrhosis | 4 | 3 | |
| Cerebral infarction | 20 | 6 | |
| Renal failure | 17 | 7 | |
| Autoimmune | 5 | 0 | |
| Others | 39 | 31 | |
| ASA score | 0.498 | ||
| I | 109 | 108 | |
| II | 321 | 264 | |
| III | 62 | 52 | |
| Previous abdominal surgery | 75 (15.2%) | 65 (15.3%) | 0.971 |
LRR: Laparoscopy in radical resection; ORR: Open radical resection; BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; ASA: American Society of Anesthesiologists.
Comparison of LRR and ORR operations
The operation time of LRR was shorter than ORR (143.89 ± 50.865 min vs 164.86 ± 67.993 min), with a difference that reached statistical significance (P < 0.001). ORR produced more blood loss than LRR (111.54 ± 97.148 mL vs 154.03 ± 154.545 mL) and the difference was statistically significant (P < 0.001). Proximal and distal margins for both samples were negative. The sample length obtained from LRR and ORR were not significantly different (P = 0.182) but the distal margin of LRR was longer compared to ORR (3.214 ± 1.8727 cm vs 2.873 ± 2.4913 cm) the difference being statistically significant (P = 0.022). The average retrieved lymph node numbers during LRR appeared to be slightly above ORR but it was not statistically different (P = 0.338). In LRR there were 12 cases of conversion (2.4% conversion rate) to open resection comprising 1 case of surgical bleeding, 6 cases of tight adhesion between the tumor and surrounding tissues and 5 cases of serious abdominal and pelvic adhesion. Table 2 summarizes these operative results.
Table 2.
Surgery index
| LRR (n = 492) | ORR (n = 424) | P value | |
| Surgery duration (min) | 143.89 ± 50.865 | 164.86 ± 67.993 | < 0.001 |
| Surgery bleeding (mL) | 111.54 ± 97.148 | 154.03 ± 154.545 | < 0.001 |
| Maximum incision (cm) | 4.14 ± 0.738 | 13.8 ± 2.603 | < 0.001 |
| Conversions to open | 12 (2.4%) | ||
| Tumor diameter (cm) | 4.338 ± 1.6387 | 4.325 ± 1.8274 | 0.914 |
| Sample length(cm) | 18.050 ± 5.1748 | 17.553 ± 5.7995 | 0.182 |
| Proximal margin (cm) | 10.487 ± 4.2906 | 10.673 ± 5.2175 | 0.567 |
| Distal margin (cm) | 3.214 ± 1.8727 | 2.873 ± 2.4913 | 0.022 |
| Lymph nodes retrieved (unit) | 11.09 ± 6.503 | 10.68 ± 6.321 | 0.338 |
LRR: Laparoscopy in radical resection; ORR: Open radical resection.
Pathology findings
After postoperative pathology, 88 cases were well-differentiated, 285 cases moderately-differentiated, 86 cases poorly-differentiation and 33 cases of mucinous cancer in LRR, while 92 cases were well-differentiated, 237 moderately-differentiated, 72 cases poorly-differentiated and 23 cases of mucinous cancer in ORR. The differences were not statistically significantly different (P = 0.476). For TNM staging, LRR contained 93 cases of I stage, 218 cases of II stage and 181 cases of III stage while ORR were 83 cases of I stage, 211 cases of II stage and 130 cases of III stage cancer, but the apparent differences were not statistically significant (P = 0.134). Table 3 shows the results in detail.
Table 3.
Pathology findings
| LRR | ORR | χ2 | P value | |
| Differentiation | 2.495 | 0.476 | ||
| Well-differentiated | 88 | 92 | ||
| Moderate-differentiation | 285 | 237 | ||
| Poorly-differentiated | 86 | 72 | ||
| Mucinous cancer | 33 | 23 | ||
| pT | 7.468 | 0.058 | ||
| T1 | 33 | 29 | ||
| T2 | 164 | 108 | ||
| T3 | 207 | 193 | ||
| T4 | 88 | 94 | ||
| pN | 6.865 | 0.076 | ||
| N0 | 252 | 233 | ||
| N1 | 147 | 96 | ||
| N2 | 75 | 73 | ||
| Nx | 18 | 22 | ||
| Lymph node metastasis | 0.259 | |||
| Yes | 240 | 191 | ||
| No | 252 | 233 | ||
| TNM stage | 0.134 | |||
| I | 93 | 83 | ||
| II | 218 | 211 | ||
| III | 181 | 130 |
pT: Pathology Tumor; pN: Pathology Node; pM: Pathology metastasis; TNM: Tumor node metastasis.
Perioperative complications
There were 95 cases of perioperative complications (19.3% incidence rate) for LRR which included: 3 cases of ureter injury, 2 cases of massive hemorrhage, 7 cases of postoperative bleeding, 3 cases of anastomotic hemorrhage, 24 cases of anastomotic leakage, 7 cases of incisional infection, 8 cases of ileus, 1 case of pelvic abscess, 8 cases of pulmonary infection, 2 cases of acute cardiac failure, 1 case of deep vein thrombosis, and 1 case of lymphatic fistula. For ORR, with (a 24.1% occurrence rate) there were 102 cases of perioperative complications in the ORR group, which were: 1 case of ureter injury, 15 cases of anastomotic leakage, 27 cases of incisional infection, 1 case of postoperative pelvic abscess, 9 cases of inflammatory ileus, 10 cases of acute cardiac failure and 2 cases of sudden death. The incidence rate of perioperative complications in the LRR and ORR groups were not significantly different (P = 0.081). Table 4 shows the results in detail.
Table 4.
Perioperative complications for the 2 groups n (%)
| LRR (n = 492) | ORR (n = 424) | χ2 | P value | |
| Occurrence rate of perioperative complications | 19.3% (95/492) | 24.1% (102/424) | 3.041 | 0.081 |
| Intraoperative complications | 10 (2) | 4 (0.9) | 1.795 | 0.180 |
| Massive hemorrhage (> 1000 mL) | 2 (0.41) | 1 (0.24) | ||
| Organ injury | 3 (0.61) | 2 (0.47) | ||
| Equipment disorders | 4 (0.81) | 0 | ||
| Other | 3 (0.61) | 1 (0.24) | ||
| Post-operative complications | 85 (17.3) | 98 (23.31) | 4.853 | 0.028 |
| Anastomotic leakage | 24 (4.88) | 15 (3.54) | ||
| Wound infection | 7 (1.42) | 27 (6.37) | ||
| Ileus | 8 (1.63) | 9 (2.12) | ||
| Anastomotic hemorrhage | 3 (0.61) | 2 (0.47) | ||
| Pelvic abscess | 1 (0.20) | 1 (0.24) | ||
| Abdominal hemorrhage | 7 (1.42) | 4 (0.94) | ||
| Peritonitis/septic shock | 9 (1.83) | 7 (1.65) | ||
| Incisional/port herniation | 4 (0.81) | 10 (2.36) | ||
| Deep vein thrombosis | 1 (0.20) | 0 | ||
| Lymphatic fistula | 1 (0.20) | 0 | ||
| Pulmonary infection | 8 (1.63) | 2 (0.47) | ||
| Acute cardiac failure | 2 (0.41) | 10 (2.36) | ||
| Urinary infection | 4 (0.81) | 4 (0.94) | ||
| Incision split | 4 (0.81) | 5 (1.18) | ||
| Sudden death | 2 (0.41) | 2 (0.47) |
LRR: Laparoscopy in radical resection; ORR: Open radical resection.
Postoperative recovery
The duration of LRR was distinctly shorter than ORR in terms of peristalsis recovery (P < 0.001), off-bed time (P = 0.017), gastrointestinal decompression (P < 0.001), retention catheterization (P < 0.001), oral food time (P < 0.001), abdominal drainage (P = 0.008), hospital stay (P < 0.001) and various other aspects. The difference in duration for the two groups was statistically different. Table 5 shows the detailed results.
Table 5.
Postoperative recovery
| LRR (n = 407) | ORR (n = 326) | P value | |
| Peristalsis recovery (d) | 1.91 ± 0.89 | 2.41 ± 1.13 | < 0.001 |
| Exsufflation recovery (d) | 3.03 ± 1.25 | 3.96 ± 1.53 | < 0.001 |
| Off-bed (d) | 2.80 ± 1.26 | 3.38 ± 1.07 | < 0.017 |
| Liquid intake (d) | 3.90 ± 1.446 | 5.08 ± 1.763 | < 0.001 |
| Semi-liquid intake (d) | 6.55 ± 1.910 | 7.59 ± 2.065 | < 0.001 |
| Abdominal drainage (d) | 9.48 ± 7.386 | 11.07 ± 10.484 | < 0.008 |
| Retention catheterization (d) | 5.63 ± 3.613 | 6.67 ± 4.043 | < 0.001 |
| Post-op hospital stay (d) | 12.27 ± 3.156 | 18.32 ± 5.406 | < 0.001 |
| Total hospital stay (d) | 21.50 ± 4.991 | 25.81 ± 7.868 | < 0.001 |
LRR: Laparoscopy in radical resection; ORR: Open radical resection.
Postoperative analgesics and pain scores
LRR was significantly less than ORR in terms of the requirement for postoperative analgesic administration (P < 0.001). The difference in the NRS postoperative pain score between the 2 groups was statistically significant showing that the degree of postoperative pain for LRR was evidently less than that after ORR (Table 6).
Table 6.
Postoperative analgesics and pain scores
| LRR (n = 492) | ORR (n = 424) | χ2 | P value | |
| Analgesic usage | 95.31 | < 0.001 | ||
| No | 299 | 121 | ||
| PCIA | 152 | 273 | ||
| Short-acting drug | 41 | 30 | ||
| Post-op pain degree | 21.43 | < 0.001 | ||
| Mild | 263 | 193 | ||
| Medium | 213 | 186 | ||
| Severe | 16 | 45 |
LRR: Laparoscopy in radical resection; ORR: Open radical resection; PCIA: Patient controlled intravenous analgesia.
Economic cost
The overall hospitalization cost for LRR and ORR was little different because although the operation costs of LRR were more than ORR, the cost of medication for LRR was lower than ORR. Thus, overall there was no statistically significantly difference between the costs of the operation as detailed in Table 7.
Table 7.
Economic costs of the operations
| LRR (n = 492) | ORR (n = 424) | P value | |
| Operation fee (RMB) | 13628 ± 6771 | 10761 ± 5056 | < 0.001 |
| Medication (RMB) | 15859 ± 10203 | 17562 ± 7006 | 0.06 |
| Total hospitalization (RMB) | 40889 ± 19356 | 42381 ± 17915 | 0.234 |
LRR: Laparoscopy in radical resection; ORR: Open radical resection.
Post-operation survival
A 95.1% patient follow-up was achieved with an average duration time of 55 mo. For the total survival rate, the 1-year overall survival rate for ORR and LRR was 94.0% and 93.6% (P = 0.534), 3-year overall survival rate 78.1% and 80.9% (P = 0.284) and the 5-year overall survival rate 75.2% and 77.0% (P = 0.416), respectively. In stage I, the 1-year overall survival rate for ORR and LRR was 93.8% and 99.2% (P = 0.402), 3-year survival rate 91.6% and 90.3% (P = 0.774) and the 5-year survival rate 89.2% and 88.2% (P = 0.837), respectively. In stageII, the 1-year overall survival rate for ORR and LRR was 92.8% and 94.7% (P = 0.489), 3-year survival rate 79.1% and 86.2% (P = 0.052) and the 5-year survival rate 76.8% and 81.7% (P = 0.140), respectively. For Stage III, the 1 year overall survival rate for ORR and LRR was 87.8% and 91.9% (P = 0.178), the 3-year survival rate 69.2% and 70.7% (P = 0.777) and the 5-year survival rate 65.4% and 66.9% (P = 0.787), respectively. Refer to Table 8 and the survival curves in Figures 1A-D for the relevant data.
Table 8.
Postoperative survival rates
| LRR | ORR | P value | |
| Post-op 1-yr | |||
| I | 99.2% | 93.8% | 0.402 |
| II | 94.7% | 92.8% | 0.489 |
| III | 91.9% | 87.8% | 0.178 |
| Total survival rate | 93.6% | 94.0% | 0.534 |
| Post-op 3-yr | |||
| I | 90.3% | 91.6% | 0.774 |
| II | 86.2% | 79.1% | 0.052 |
| III | 70.7% | 69.2% | 0.777 |
| Total survival rate | 80.9% | 78.1% | 0.284 |
| Post-op 5-yr | |||
| I | 88.2% | 89.2% | 0.837 |
| II | 81.7% | 76.8% | 0.140 |
| III | 66.9% | 65.4% | 0.787 |
| Total survival rate | 77.0% | 75.2% | 0.416 |
LRR: Laparoscopy in radical resection; ORR: Open radical resection.
Figure 1.
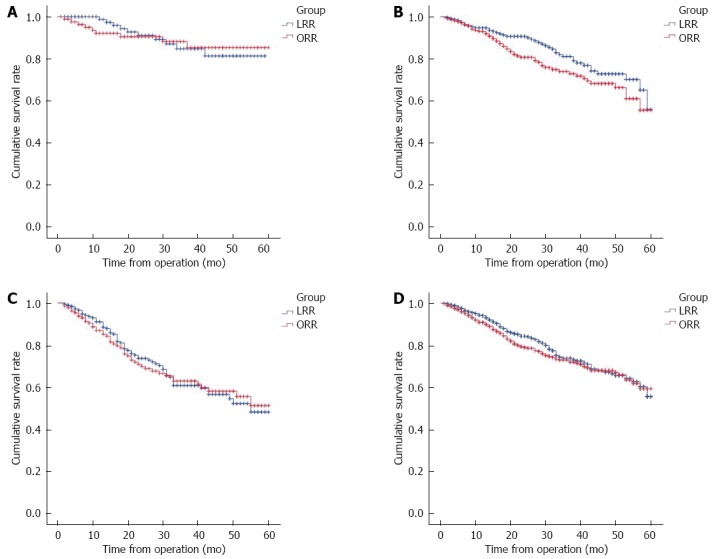
Different stages of postoperative survival status. A: Stage I postoperative survival status; B: Stage II postoperative survival status; C: Stage III postoperative survival status; D: Overall postoperative survival status for both groups. LRR: Laparoscopy in radical resection; ORR: Open radical resection.
DISCUSSION
Due to advances in surgical techniques and medication, the outcomes of rectal cancer treatment have considerably improved[20]. In spite of various published studies[3,13] concerning the similar long-term outcomes of rectal cancer produced by laparoscopic surgery, the argument for choosing laparoscopy or open surgery is still heated. In the latest randomized trial for rectal cancer, it was reported that the disease-free survival (DFS) and overall survival rates for LRR and ORR group were similar[20]. In our current trial, we studied the advantages and disadvantages of laparoscopic vs an open operation, and have retrospectively compared perioperative complications, postoperative recovery, economic costs, survival and so on, of rectal cancer treated in our hospital from January 2006 to December 2013.
Based on published literature[5,7,21], we understand that the short-term outcomes of the LRR group are superior to the ORR group. In early stage laparoscopic radical rectal tumor surgery, much more time is required than for an open operation[9]. With the improvement of medical devices, sufficient training and accumulated experience, the operating time between the two groups was shown to be similar with no statistical significance[22]. In the present study, we found that the operating time of the LRR group was shorter than that of the ORR group, with statistical significance being achieved. It was reported in COLOR II[18] that the blood loss volume was much less than that of open surgery proving definitively that laparoscopic surgery is capable of decreasing the hemorrhage volume. In the present study, the volume of intraoperative bleeding in the LRR group was clearly less than that in the ORR group. This finding can probably be attributed to the fact that operators benefit from the magnified view of laparoscopy, and also improved equipment that can effectively stop bleeding. The clearance and sufficient length of the resected sample was markedly key for the success of each operation. CLASICC showed that the resected intestinal segment in laparoscopic and in open surgery were similar in length[9], and our research verified further that the lengths of the resected sample in the LRR and ORR groups were similar, with no statistical difference being found. However, we established that the distal margin of tumor in the LRR group was longer than in the ORR group and we hypothesized that the difference between the two groups may be caused by preponderant visualization of laparoscopy in the lower pelvis. It was illustrated in detailed by Schwandner et al[10] that there was no difference in the lymph nodes harvested in the two groups, findings consistent with our data. The average lymph nodes retrieved from LRR and ORR were 11.09 ± 6.503 and 10.68 ± 6.321 (P = 0.338) in our study, which suggested a small disparity in the number of lymph nodes retrieved in LRR compared to ORR.
The NRS pain score of the ORR group was much higher than the scores of the LRR group (P < 0.001), and the average postoperative analgesics usage for LRR patients was much less than ORR patients (P < 0.001). We conclude that the postoperative pain degree of the LRR group was obviously milder than in the ORR group, findings consistent with previous reports[6,12,23]. Additionally, the recovery time of intestinal function, off-bed time, regular food intake time, hospital stay and other aspects were evidently less than after ORR, which is in accordance with Schwandner[10], COLOR II[18] and other reports.
It is well documented that the leading reasons for conversion to open surgery included extensive abdominal metastasis, over-sized tumors and a confused anatomic relationship due to serious pelvic adhesion, etc.[6,12,24-26]. The conversion rate was 2.44% in the current research, which is lower than the well-documented 5% of other studies. The potential cause of this discrepancy could be due to the full assessment of partial status for rectal tumor, the high admittance standard for studied cases and the elimination of advanced tumor patients from the study. In addition, a large amount of laparoscopic radical surgery for rectal cancer had been undertaken in our institute prior to this study. Thus, we had had completed the study curve and obtained extensive experience for a lower conversion rate between the two surgical techniques. Moreover, the incidence of perioperative complication rates in the LRR group was similar to that in the ORR group as previously reported[27], findings parallel to our results, with no statistical significance between the LRR (19.3%) and ORR (24.1%) groups.
Economic efficiency is also a vital appraisal index for technology. It has been well documented that the surgical expenses for laparoscopic are well above those for open surgery due to its use of disposable surgical instruments and higher anesthesia costs, and technology equipment standards[28,29]. However, Choi assumed that the surgical expenses only consisted of partial expenses which should have included anesthesia, inspection, medication, medical care agents and other consumption goods[28]. In our research, LRR surgical expenses were a slightly more costly than conventional procedures (P < 0.001) while the medication required for LRR was less than that of ORR, which possessed no difference of statistical importance (P = 0.06). In general, there was no obvious difference between LRR and ORR in terms of total hospitalization costs (P = 0.234). Considering a briefer hospital stay and quick recovery after LRR, the nursing labor costs will be significantly lower, which suggests that the laparoscopic approach is the most cost effective.
Concerning the survival outcomes, various studies have shown that the short-term survival and long-term survival between the LRR and ORR groups is similar. The survival outcomes of the COREAN trial revealed that the 3-year DFS rate was 72.5% in the ORR group and therefore similar to that in the LRR group (79.2%)[30]. Based on published comparative retrospective studies, we have found that the 5-year DFS between the LRR and ORR groups was separately 82% and 79% with no statistical difference[31]. Importantly, a randomized multicenter study revealed that the DFS rates for LRR and ORR group were 74.8% and 70.8% respectively, and that the overall survival rates for the LRR and ORR group were 86.7% and 83.6%, with no statistical difference being detected[20]. In the present study, the follow-up duration of the 2 groups was 55 mo in total, with a 95.1% follow-up rate being achieved. We have illustrated that the 1-, 3- and 5-year survival rate between the ORR and LRR groups were not statistically significant (94.0% vs 93.6%, P = 0.534; 78.1% vs 80.9%, P = 0.284; 75.2% vs 77.0%, P = 0.416), findings similar to the published literature. The results here suggest that the laparoscopic radical operation is analogous to open resection and has prior immediate and long-term clinical effects. It may be related to the proficiency of laparoscopic techniques as well as the relatively protective functions of laparoscopy for its less interference in cell immunity functions. Thus, the long-term survival of laparoscopic patients for rectal cancer proved equal to those given open operations.
In this original clinical research, we conclude that a laparoscopic-assisted operation has more benefits due to reduced trauma, less postoperative pain and a reduced stay in hospital, as well as faster recovery from radical rectal malignancy therapy. It is clear that the surgical scope and radical effects of tumors were similar in the laparoscopic and open resection techniques. In addition, overall hospitalization expenses, immediate perioperative complications and the long-term survival rates of patients who experienced laparoscopic surgery closely resembled the conventional operation. Thus, laparoscopic surgery may become the most effective therapy for rectal malignancy in the future.
COMMENTS
Background
As we all know, the argument for choosing laparoscopy or open surgery in rectal cancer is heated due to the inconsistent outcomes reported by different research centers. In the latest randomized trial for rectal cancer, it was reported that the disease-free survival (DFS) and overall survival rates for laparoscopy in radical resection (LRR) and open radical resection (ORR) group were similar. In this study, they compared the short-term outcomes and long-term outcomes between the LRR group and ORR group.
Research frontiers
The outcomes between LRR group and ORR group in rectal cancer was inconsistent. Their study compared the short-term and long-term outcomes between LRR group and ORR group, and concluded the similar results reported in the lasted randomized trial for rectal cancer.
Innovations and breakthroughs
It is a retrospective case-control study performed in rectal cancer with different operations, and the authors compared the short-term and long-term outcomes between the LRR group and ORR group.
Applications
The outcomes between LRR group and ORR group in rectal cancer verified that the laparascopic surgery could have a better result than open surgery. It may affect the choice of surgery in rectal cancer.
Terminology
Conventional vs laparoscopic-assisted surgery in colorectal cancer and colorectal cancer laparoscopic or open resection: Two trials mainly compared the survival of overall survival, disease-free survival, progression-free survival between the LRR and ORR groups in colorectal cancer.
Peer-review
The authors conducted a clinical retrospective study to compare the short and long-term outcomes between laparoscopic and open surgery for rectal cancer in the Chinese population. They concluded that the laparoscopic resection as a radical operation was safe and effective, while the long-term survival of patients treated with laparoscopic surgery was similar to those with open surgery.
Footnotes
Supported by Grants from the Shanghai Municipal Human Resources and Social Security Bureau, No. 2012040 and No. 13PJD024 to Huang C; grant from the Shanghai Health and Family Planning Commission, No. XYQ2013092 to Huang C; and grant from Shanghai Municipal Science and Technology Commission, No. 14411966800 to Huang C.
Institutional review board statement: Our study was a retrospective case-control study between laparoscopic surgery and open surgery in rectal cancer. And the study was and approved by the Ethics Committee of Shanghai First People’s Hospital Affiliated Shanghai Jiao Tong University.
Conflict-of-interest statement: The authors declare that they have no conflict-of-interest and we have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 8, 2015
First decision: August 26, 2015
Article in press: November 9, 2015
P- Reviewer: Murata A S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang DN
References
- 1.Liu S, Zheng R, Zhang M, Zhang S, Sun X, Chen W. Incidence and mortality of colorectal cancer in China, 2011. Chin J Cancer Res. 2015;27:22–28. doi: 10.3978/j.issn.1000-9604.2015.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaney G. Results of treatment of carcinoma of the colon and rectum. Mod Trends Surg. 1971;3:69–89. [PubMed] [Google Scholar]
- 3.Kusano T, Inomata M, Hiratsuka T, Akagi T, Ueda Y, Tojigamori M, Shiroshita H, Etoh T, Shiraishi N, Kitano S. A comparison of laparoscopic and open surgery following pre-operative chemoradiation therapy for locally advanced lower rectal cancer. Jpn J Clin Oncol. 2014;44:305–310. doi: 10.1093/jjco/hyu013. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 5.Zeng WG, Zhou ZX, Hou HR, Liang JW, Zhou HT, Wang Z, Zhang XM, Hu JJ. Outcome of laparoscopic versus open resection for rectal cancer in elderly patients. J Surg Res. 2015;193:613–618. doi: 10.1016/j.jss.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Huang R, Jiang T, Huang K, Cao J, Qiu Z. Laparoscopic and open resection for colorectal cancer: an evaluation of cellular immunity. BMC Gastroenterol. 2010;10:127. doi: 10.1186/1471-230X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu JJ, Liang JW, Wang Z, Zhang XM, Zhou HT, Hou HR, Zhou ZX. Short-term outcomes of laparoscopically assisted surgery for rectal cancer following neoadjuvant chemoradiotherapy: a single-center experience. J Surg Res. 2014;187:438–444. doi: 10.1016/j.jss.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Feng B, Zheng MH, Mao ZH, Li JW, Lu AG, Wang ML, Hu WG, Dong F, Hu YY, Zang L, et al. Clinical advantages of laparoscopic colorectal cancer surgery in the elderly. Aging Clin Exp Res. 2006;18:191–195. doi: 10.1007/BF03324648. [DOI] [PubMed] [Google Scholar]
- 9.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 10.Schwandner O, Schiedeck TH, Killaitis C, Bruch HP. A case-control-study comparing laparoscopic versus open surgery for rectosigmoidal and rectal cancer. Int J Colorectal Dis. 1999;14:158–163. doi: 10.1007/s003840050203. [DOI] [PubMed] [Google Scholar]
- 11.Lezoche E, Feliciotti F, Paganini AM, Guerrieri M, De Sanctis A, Minervini S, Campagnacci R. Laparoscopic vs open hemicolectomy for colon cancer. Surg Endosc. 2002;16:596–602. doi: 10.1007/s00464-001-9053-2. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Jiang T, Qiu Z, Cen G, Cao J, Huang K, Pu Y, Liang H, Huang R, Chen S. Short-term and medium-term clinical outcomes of laparoscopic-assisted and open surgery for colorectal cancer: a single center retrospective case-control study. BMC Gastroenterol. 2011;11:85. doi: 10.1186/1471-230X-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Laparoscopic resection of extraperitoneal rectal cancer: a comparative analysis with open resection. Surg Endosc. 2009;23:1818–1824. doi: 10.1007/s00464-008-0265-6. [DOI] [PubMed] [Google Scholar]
- 14.Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 15.Wexner SD, Cohen SM. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg. 1995;82:295–298. doi: 10.1002/bjs.1800820305. [DOI] [PubMed] [Google Scholar]
- 16.Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 17.Milsom JW, Böhm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998;187:46–54; discussion 54-55. doi: 10.1016/s1072-7515(98)00132-x. [DOI] [PubMed] [Google Scholar]
- 18.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 19.Gong J, Shi DB, Li XX, Cai SJ, Guan ZQ, Xu Y. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol. 2012;18:7308–7313. doi: 10.3748/wjg.v18.i48.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 21.Lee SD, Park SC, Park JW, Kim DY, Choi HS, Oh JH. Laparoscopic versus open surgery for stage I rectal cancer: long-term oncologic outcomes. World J Surg. 2013;37:646–651. doi: 10.1007/s00268-012-1846-z. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Zhang XM, Liang JW, Hu JJ, Zeng WG, Zhou ZX. Evaluation of short-term outcomes after laparoscopically assisted abdominoperineal resection for low rectal cancer. ANZ J Surg. 2014;84:842–846. doi: 10.1111/ans.12518. [DOI] [PubMed] [Google Scholar]
- 23.Braga M, Vignali A, Zuliani W, Frasson M, Di Serio C, Di Carlo V. Laparoscopic versus open colorectal surgery: cost-benefit analysis in a single-center randomized trial. Ann Surg. 2005;242:890–85, discussion 890-85. doi: 10.1097/01.sla.0000189573.23744.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 25.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 26.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Fujita S, Akasu T, Inada R, Takawa M, Moriya Y. Short-term outcomes of laparoscopic intersphincteric resection for lower rectal cancer and comparison with open approach. Dig Surg. 2011;28:404–409. doi: 10.1159/000332007. [DOI] [PubMed] [Google Scholar]
- 28.Choi YS, Lee SI, Lee TG, Kim SW, Cheon G, Kang SB. Economic outcomes of laparoscopic versus open surgery for colorectal cancer in Korea. Surg Today. 2007;37:127–132. doi: 10.1007/s00595-006-3356-9. [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Kang SB, Kim SW, Cheon GN. Economics and the laparoscopic surgery learning curve: comparison with open surgery for rectosigmoid cancer. World J Surg. 2007;31:1827–1834. doi: 10.1007/s00268-007-9154-8. [DOI] [PubMed] [Google Scholar]
- 30.Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 31.Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250:54–61. doi: 10.1097/SLA.0b013e3181ad6511. [DOI] [PubMed] [Google Scholar]


