Abstract
Changes in the interactions among the gut microbiota, intestinal epithelium, and host immune system are associated with many diseases, including cancer. We discuss how environmental factors influence this cross-talk during oncogenesis and tumor progression and how manipulations of the gut microbiota might improve the clinical activity of anticancer agents.
One hundred trillion organisms (mainly bacteria) collectively referred to as the gut microbiota colonize the human intestine. Reflecting a notable degree of coevolution, the gut microbiota thrives in mutually advantageous equilibrium with the host (eubiosis). The intestine offers a protected, warm, and nutrient-rich microenvironment to resident microbes, while the gut microbiota assists humans in the digestion of complex carbohydrates, provides them with non-nutrient essential factors, and occupies ecological niches that might otherwise be colonized by pathogenic microorganisms (1). The immune system tolerates the normal gut microbiota while ensuring immunosurveillance against invading pathogens. Moreover, accumulating evidence indicates that the proper development of both intestinal and extraintestinal components of the immune system requires the gut microbiota (2). In this Perspective, we discuss how disequilibria in the intimate relationship between the host and intestinal bacteria (dysbiosis) affect oncogenesis, tumor progression, and response to cancer therapy and how the gut microbiota may be manipulated for therapeutic purposes. A detailed description of the intestinal immune system is beyond the scope of this article and can be found in (2).
DYSBIOSIS AND CARCINOGENESIS
Dysbiosis can be caused not only by pathogenic organisms and passenger commensals but also by aging and environmental factors such as antibiotics, xenobiotics, smoking, hormones, and dietary cues (1); these are also well-established risk factors for the development of intestinal or extraintestinal neoplasms. In addition, genetic defects that affect epithelial, myeloid, or lymphoid components of the intestinal immune system favor dysbiosis because they promote inflammatory states, such as Crohn’s disease, that increase the host’s risk for neoplastic transformation (3). Thus, several factors that favor carcinogenesis also promote dysbiosis.
Epidemiological studies that link intra-abdominal infections, the use of antibiotics, or both to an increased incidence of colorectal cancer (4) underscore the clinical importance of the association between dysbiosis and intestinal carcinogenesis. In fact, the gut microbiota affects colorectal carcinogenesis by various mechanisms. Abrogating or specifically altering the composition of the gut microbiota influences the incidence and progression of colorectal carcinoma in both genetic and carcinogen-induced models of tumorigenesis (5–7). Moreover, several by-products of the gut microbiota directly target intestinal epithelial cells (IECs) and either mediate oncogenic effects (as reported for hydrogen sulfide and the Bacteroides fragilis toxin) or suppress tumorigenesis (as demonstrated for short-chain fatty acids) (8).
Intestinal bugs participate in more than just colorectal carcinogenesis. Experimental alterations of the gut microbiota also influence the incidence and progression of extraintestinal cancers, including breast and hepatocellular carcinoma, presumably through inflammatory and metabolic circuitries (9, 10). These results are compatible with the findings of epidemiological studies that reveal an association between dysbiosis, its consequences or determinants (in particular the overuse of antibiotics), and an increased incidence of extracolonic neoplasms, including breast carcinoma (11, 12). These findings may reflect the systemic distribution of bacteria and their by-products in the course of inflammatory responses that compromise the integrity of the intestinal barrier (9).
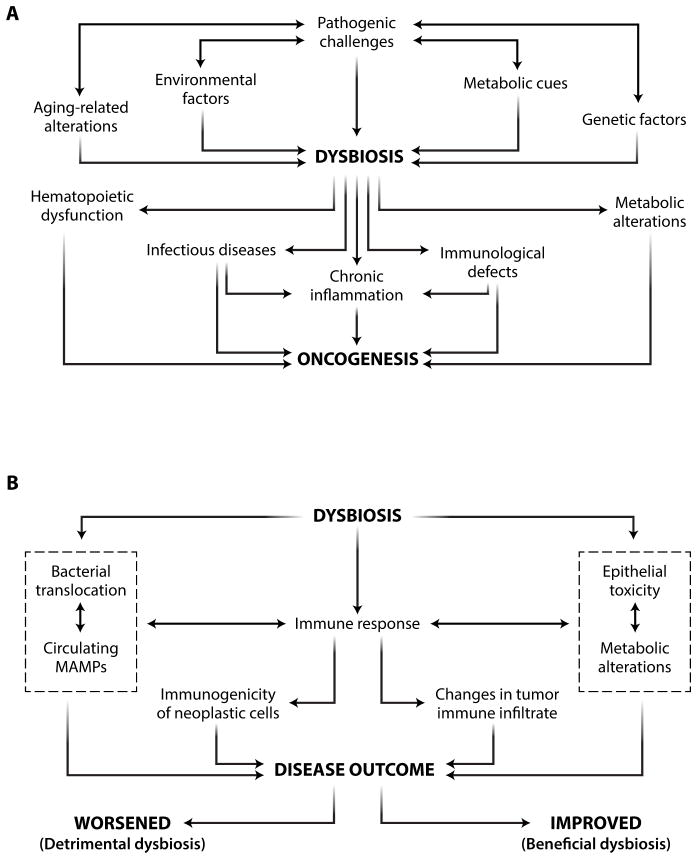
Thus, the gut microbiota influences oncogenesis and tumor progression both locally and systemically. Although inflammatory and metabolic cues support this phenomenon, additional, hitherto uncharacterized mechanisms can contribute to the ability of dysbiosis to promote carcinogenesis (Fig. 1).
Fig. 1. Links between dysbiosis and cancer.
(A) Mechanisms by which dysbiosis affects oncogenesis. (B) Detrimental and beneficial effects of dysbiosis on disease outcome. MAMP, microbe-associated molecular pattern.
RELATIONSHIP STATUS: IT’S COMPLICATED
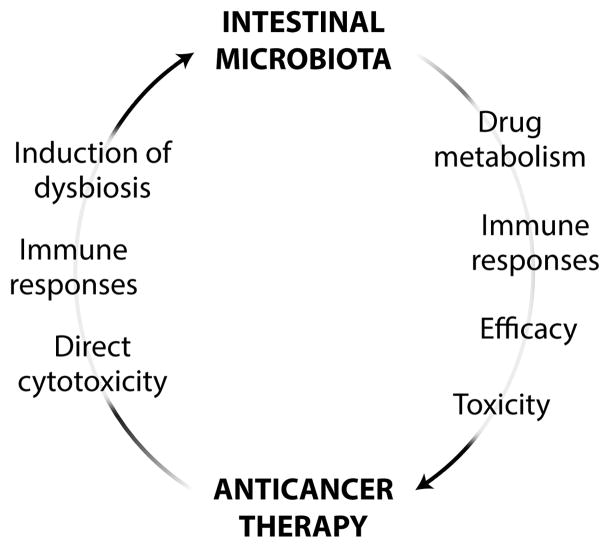
During cancer therapy, the gut microbiota and antineoplastic agents interact in a bidirectional fashion. On the one hand, several interventions currently used for the management of neoplastic diseases exert cytotoxic effects on intestinal bacteria, de facto promoting dysbiosis (13).Thus, radiation therapy, allogeneic stem cell transplantation, and several chemotherapeutic agents such as irinotecan (a topoisomerase I inhibitor licensed for the treatment of colorectal carcinoma) and 5-fluorouracil (a nucleoside analog used against several carcinomas) can be toxic for the gut microbiota—and hence alter its composition—either directly or by activating an immune response (14–16). Moreover, these (and other) therapeutic interventions exert unwarranted side effects on the intestinal barrier (table S1). On the other hand, accumulating evidence indicates that the gut microbiota influences both the therapeutic activity and the side effects of anticancer agents, via pharmacodynamic (17, 18) and immunological mechanisms (19, 20) (Fig. 2).
Fig. 2. Links between the gut microbiota and anticancer therapy.
Intestinal bacteria interact with chemo-, radio-, and immunotherapeutic anticancer agents in a bidirectional manner.
Pharmacodynamic effects
By virtue of their abundance and pronounced metabolic activity, intestinal bacteria can determine the bioavailability and biological effects, be they warranted (efficacy) or not (toxicity), of ingested xenobiotics. This has been demonstrated for several drugs, including irinotecan (17, 18). The dose-limiting diarrhea associated with irinotecan has been attributed to the ability of the gut microbiota to reactivate the drug locally (17). Moreover, the gastrointestinal toxicity of irinotecan is reduced by the administration of a Chinese herbal medicine (PHY906) that acquires the ability to stimulate the regeneration of intestinal progenitor cells only upon transformation by bacterial β-glucuronidase (which is highly expressed by the gut microbiota) (18).
The effects of the gut microbiota on the pharmacodynamics of anticancer agents may not be limited to orally administered molecules (which physically get in contact with intestinal bacteria), but may involve systemic interventions. Indeed, germ-free (GF) mice have been reported to differ from their conventional, pathogen-free counterparts in the expression of a broad panel of hepatic genes involved in xenobiotic metabolism (21). Moreover, the gut microbiota may play a critical role in the elicitation of acute graft-versus-host disease (GVHD), a critical obstacle against the clinical success of allogeneic stem cell transplantation. Several reports link dysbiosis (most often characterized by an enrichment in Enterobacteriaceae spp.) to overt infections and intestinal GVHD, with a major role for Paneth cell destruction and alterations in the TLR9/MYD88 signaling axis (15, 22). Thus, besides influencing the gastrointestinal side effects of some anticancer interventions, dysbiosis may undermine their therapeutic activity. Conversely, a eubiotic gut microbiota may limit the unwarranted side effects of various antineoplastic agents.
Immunological effects
Accumulating evidence indicates that the gut microbiota also modulates the response of several tumor types to cancer therapy via immunological circuitries, at least in mice (19, 20, 23). For example, lymphodepleting total body irradiation reportedly promotes the translocation of the gut microbiota or at least some of its components or products across the intestinal epithelium. This not only correlates with increased dendritic cell activation and elevated levels of blood-borne proinflammatory cytokines but also contributes to the ability of irradiation to maximize the efficacy of adoptively transferred CD8+ T lymphocytes (23). Accordingly, antibiotic-treated mice, mice injected with a lipopolysaccharide (LPS)–neutralizing antibody, as well as Cd14−/− and Tlr4−/− mice (which do not respond to LPS normally) are less sensitive to lymphodepleting irradiation than are their control counterparts (23).
The injection of cyclophosphamide (an immunostimulatory alkylating agent used against multiple carcinomas) into mice maintained in pathogen-free conditions promotes mucosal injury and translocation of specific Gram-positive bacteria across the intestinal epithelium (20). This phenomenon was linked to therapeutically relevant T helper type 1 (TH1) and TH17 immune responses in the spleen (20). GF and antibiotic-treated tumor-bearing mice, which failed to mount such antibacterial T cell–mediated responses, were more resistant than their control counterparts to the therapeutic effects of cyclophosphamide (20). Moreover, the full-blown antineoplastic activity of cyclophosphamide could be restored in antibiotic-treated mice upon the adoptive transfer of TH17 cells established and propagated in vitro (20). However, not all Gram-positive bacteria were able to elicit beneficial TH17 immune responses in this setting. Rather, specific prokaryotes such as Parabacteroides distasonis [which exerts regulatory T (Treg) cell–stimulatory effects] and segmented filamentous bacteria (which trigger conventional TH17 responses) reduced the beneficial effects of anticancer chemotherapy.
Consistent with these data, a healthy gut microbiota has been shown to contribute to the therapeutic activity of a CpG oligodeoxynucleotide-based immunotherapeutic regimen and platinum derivatives (19). The gut microbiota influenced the propensity of CpG oligodeoxynucleotides combined with a monoclonal antibody that neutralizes interleukin-10 receptor a (IL10RA) to elicit a therapeutically relevant, tumor necrosis factor–α (TNF-α)–dependent innate immune response against malignant cells. In addition, a eubiotic gut microbiota was necessary for oxaliplatin (an immunogenic platinum salt approved for use in colorectal cancer patients) to promote tumor infiltration by myeloid cells that mediated antineoplastic effects by producing reactive oxygen species (ROS) (19). In line with this notion, the chemotherapy-impairing effects of antibiotics could be mimicked by the Cybb−/− genotype (corresponding to the lack of a ROS-generating enzyme) as well as by the systemic administration of antioxidants (19). Mice lacking Myd88 or Tlr4 (encoding critical components of the machinery sensing microbe-associated molecular patterns) were also more resistant to oxaliplatin-based chemotherapy than were their wild-type counterparts (19). Thus, the full-blown therapeutic activity of oxaliplatin involves the detection of components of the gut microbiota by the immune system, allowing for the generation of tumor-infiltrating myeloid cells with antineoplastic activity.
Altogether, these observations indicate that anticancer therapy can promote two functionally opposite types of dysbiosis: detrimental dysbiosis, which limits the therapeutic efficacy or increases the toxicity of treatment, and beneficial dysbiosis, which is required for, or at least markedly improves, its clinical activity (Fig. 1). This suggests that the pharmacological manipulation of the gut microbiota holds great promise as an adjuvant to improve the therapeutic index of anticancer therapy.
MANIPULATING THE MICROBIOTA FOR CANCER THERAPY
At least hypothetically, four distinct measures can be used to alter the effects of the gut microbiota on anticancer therapy: (i) antibiotics, chemicals with a preferential cytotoxicity for one or more bacterial species; (ii) probiotics, living bacteria or other microorganisms that, when administered in adequate amounts, confer a health benefit; (iii) prebiotics, nondigestible compounds that stimulate the growth and/or functions of specific components of the gut microbiota; and (iv) postbiotics, nonviable products of the gut microbiota that exert biological activities in the host.
Using common antibiotics (which often target multiple types of Gram-positive or Gram-negative bacteria) to cause a state of dysbiosis that supports, rather than counteracts, the efficacy of chemotherapeutic agents may not be feasible because of specificity issues. However, it may be possible to use antibiotics to reverse a previously established state of detrimental dysbiosis (24). Recent data indicate that bacteriocins, proteinaceous antibiotics produced by some bacterial strains, may be harnessed to specifically deplete one or a few components of the gut microbiota for therapeutic purposes (1). Moreover, specific chemicals may be successfully used to limit the negative impact of the gut microbiota on the pharmacodynamics of specific chemotherapeutics. As a proof of principle, a potent inhibitor of bacterial (but not mammalian) β-glucuronidase has been shown to protect mice from the intestinal side effects of irinotecan, widening its therapeutic window (17).
Probiotics have been extensively tested in animal tumor models for their ability to prevent (mostly intestinal) carcinogenesis, with promising results (25, 26). Moreover, genetically modified probiotics have been successfully used as vectors for the delivery of tumor-associated antigens, immunostimulatory molecules, or enzymes that limit the toxicity of conventional chemotherapy, at least in animal models (27). Some of these approaches, notably anticancer vaccines based on live, attenuated variants of Listeria monocytogenes or Salmonella enterica, are currently being tested for their safety and ability to elicit therapeutically relevant immune responses in cancer patients (28), reflecting a considerable progress in the academic and industrial development of vaccines harnessing mucosal immunity (29).
Thus far, epidemiological studies have been unable to firmly establish whether probiotics can reduce the risk of developing colorectal carcinoma in specific patient populations (26). Similarly, clinical data on the use of probiotics as a means to limit the gastrointestinal toxicity of radiation therapy and some chemotherapeutics are insufficient to draw a firm conclusion on their actual benefits (30). Although prebiotics (such as inulin or oligofructose) and postbiotics (such as butyrate) have attracted attention as potential means of preventing colorectal cancer, the ability of these agents to widen the therapeutic window of chemotherapy remains poorly explored (31).
In view of the recent findings showing that specific alterations in the gut microbiota are instrumental, rather than detrimental, to the efficacy of anticancer chemotherapy, it is tempting to speculate that the clinical profile of at least some chemotherapeutics can be improved by combinatorial interventions relying on one or more antibiotics, prebiotics, probiotics, and/or postbiotics. This hypothesis urgently awaits experimental confirmation.
Accumulating evidence demonstrates that intestinal bacteria influence oncogenesis, tumor progression, and response to therapy. Thus, selectively manipulating the gut microbiota may represent a feasible means to (i) limit the incidence of specific tumors in the general population and/or (ii) improve the activity of various anticancer agents (32). Although the first possibility has been investigated in several models of oncogenesis with promising results, the actual oncopreventive effects of anti-, pre-, pro-, and postbiotics in humans remain to be established. Conversely, selectively manipulating the composition of the gut microbiota as a gateway to optimal responses to chemo-, radio-, or immunotherapy in the clinic is a relatively new concept, and additional studies are required to understand the clinical value of such an approach. In this context, the limited selectivity of most conventional antibiotics and the elevated interindividual heterogeneity of the gut microbiota may constitute major obstacles. Highly specific antimicrobials such as bacteriocins and the development of new technologies allowing for the rapid in-depth characterization of the gut microbiota on a personalized basis may circumvent these issues, at least in part. Modulating the gut microbiota may constitute a viable strategy for improving the clinical efficacy of anticancer chemo-, radio-, and immunotherapy.
Supplementary Material
Acknowledgments
Funding: L.Z. and G.K. are supported by the Ligue contre le Cancer (équipe labelisée); Agence Nationale de la Recherche (ANR); Association pour la Recherche sur le Cancer (ARC); Cancéropôle Ile-de-France; AXA Chair for Longevity Research; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the Site de Recherche Intégrée sur le Cancer (SIRIC) Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). M.M. is supported by R01 CA154947A, R01 CA173861, U01AI095611, R01AI104848, and U19 AI089987.
Footnotes
www.sciencetranslationalmedicine.org/cgi/content/full/7/271/271ps1/DC1
Table S1. Links between the gastrointestinal side effects of common anticancer regimens and the gut microbiota.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 4.Wang JL, Chang CH, Lin JW, Wu LC, Chuang LM, Lai MS. Infection, antibiotic therapy and risk of colorectal cancer: A nationwide nested case-control study in patients with Type 2 diabetes mellitus. Int J Cancer. 2014;135:956–967. doi: 10.1002/ijc.28738. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Déchelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res. 2014;20:859–867. doi: 10.1158/1078-0432.CCR-13-1343. [DOI] [PubMed] [Google Scholar]
- 6.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan Y, Chen PJ, Sadler WD, Wang F, Poe S, Núñez G, Eaton KA, Chen GY. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res. 2013;73:7199–7210. doi: 10.1158/0008-5472.CAN-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 10.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuan C, Shamonki JM, Chung A, Dinome ML, Chung M, Sieling PA, Lee DJ. Microbial dysbiosis is associated with human breast cancer. PLOS One. 2014;9:e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. J Am Med Assoc. 2004;291:827–835. doi: 10.1001/jama.291.7.827. [DOI] [PubMed] [Google Scholar]
- 13.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 14.Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLOS One. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, Gobourne A, Lipuma L, Young LF, Smith OM, Ghosh A, Hanash AM, Goldberg JD, Aoyama K, Blazar BR, Pamer EG, van den Brink MR. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Bültzingslöwen I, Adlerberth I, Wold AE, Dahlén G, Jontell M. Oral and intestinal microflora in 5-fluorouracil treated rats, translocation to cervical and mesenteric lymph nodes and effects of probiotic bacteria. Oral Microbiol Immunol. 2003;18:278–284. doi: 10.1034/j.1399-302x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 17.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S, Redinbo MR. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam W, Bussom S, Guan F, Jiang Z, Zhang W, Gullen EA, Liu SH, Cheng YC. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2:45ra59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- 19.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anti-cancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Björkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLOS One. 2009;4:e6958. doi: 10.1371/journal.pone.0006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriguchi Y, Takashima S, Oka H, Shimoji S, Nakamura K, Uryu H, Shimoda S, Iwasaki H, Shimono N, Ayabe T, Akashi K, Teshima T. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120:223–231. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]
- 23.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD. The gut microbiome modulates colon tumorigenesis. MBiol. 2013;4:e00692–e13. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci. 2008;9:854–863. doi: 10.3390/ijms9050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capurso G, Marignani M, Delle Fave G. Probiotics and the incidence of colorectal cancer: When evidence is not evident. Dig Liver Dis. 2006;38(Suppl 2):S277–S282. doi: 10.1016/S1590-8658(07)60010-3. [DOI] [PubMed] [Google Scholar]
- 27.Bermúdez-Humarán LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, Chatel JM, Langella P. Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol. 2013;16:278–283. doi: 10.1016/j.mib.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Pol J, Bloy N, Obrist F, Eggermont A, Galon J, HervéFridman W, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: DNA vaccines for cancer therapy. OncoImmunology. 2014;3:e28185. doi: 10.4161/onci.28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, Bouguin C, Merillon N, Dransart E, Tran T, Quintin-Colonna F, Autret G, Thiebaud M, Suleman M, Riffault S, Wu TC, Launay O, Danel C, Taieb J, Richardson J, Zitvogel L, Fridman WH, Johannes L, Tartour E. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5:172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamad A, Fragkos KC, Forbes A. A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353–360. doi: 10.1016/j.clnu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Taper HS, Roberfroid MB. Possible adjuvant cancer therapy by two prebiotics—Inulin or oligofructose. In Vivo. 2005;19:201–204. [PubMed] [Google Scholar]
- 32.Holmes E, Kinross J, Gibson GR, Burcelin R, Jia W, Pettersson S, Nicholson JK. Therapeutic modulation of microbiota-host metabolic interactions. Sci Transl Med. 2012;4:137rv6. doi: 10.1126/scitranslmed.3004244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.