Abstract
Cardiac rehabilitation (CR) following myocardial infarction is vastly underused. As such the aim of this study was to test a digital health intervention (DHI) as an adjunct to CR. Patients undergoing standard Mayo Clinic CR were recruited prior to CR (n=25) or after three months CR (n=17). Changes in risk factors and rehospitalizations plus emergency department (ED) visits were assessed after three months. Patients assigned to DHI during CR had significant reductions in weight (−4.0+5.2 kg, P=.001), blood pressure (−10.8±13.5 mmHg, P=.0009), and the group using DHI after three months of CR had significant reductions in weight (−2.5±3.8 kg, P=.04) and systolic BP (−12.6±12.4 mmHg, P=.001) compared to the control groups. Both DHI groups also displayed significant reductions in rehospitalizations/ED visits (−37.9%, P=0.01 and −28%, P=.04, respectively). This study suggests that a guideline-driven, DHI CR program can augment secondary prevention strategies during usual CR by improving risk factors for repeat events.
Keywords: Digital Health, Mobile Health, Cardiovascular Disease, Cardiac Rehabilitation, Secondary Prevention, Online Health Monitoring
Introduction
Cardiovascular disease (CVD) is the primary cause for morbidity, mortality, and rising health care associated costs in the United States. Recent estimates attribute over one in every three deaths to CVD [1, 2], and over 90% of CVD morbidity and mortality to preventable risk factors [3]. Poor diet, smoking, and lack of physical activity continue to account for an overwhelming majority of CVD and death [4]. Data from 2012 demonstrate that nearly one million people in the US suffered an acute coronary syndrome (ACS) with roughly half of these being a repeat event. What is more, the average hospitalization for ACS costs roughly $20,000 with repeat events costing up to two and three times the original amount [5]. Consequently, the follow up care and subsequent episodes for those with established CVD can be substantially greater than those without CVD [6]. A primary driver of these exorbitant costs to the health system secondary to repeat CVD events is re-hospitalizations. Most medical centers still report an 18-30% 30-day rehospitalization rate among patient populations admitted for ACS [7]. One year rehospitalization rates are less frequently reported but are even more staggering [8, 9]. Clearly, there is a need to reduce the burden of repeat events and their associated costs.
Randomized controlled trials have shown that cardiac rehabilitation (CR) is superior to counseling alone in reducing cardiovascular risk profiles of patients at high risk for CVD [10]. Participation, at least once weekly, in a CR program following percutaneous coronary intervention (PCI) is associated with a decrease in all-cause mortality [11]. The importance of CR as a chief instigator of necessary lifestyle modification in high-risk CVD patients is highlighted by recent reports showing that up to 40% of the premature deaths in the US are brought about by behavioral causes [12]. Although CR has been shown to reduce mortality and is recommended in clinical practice guidelines, CR referral and utilization rates remain unacceptably low secondary to such barriers as low referral, geographic distance, and high cost [13, 14]. Furthermore, compliance within the programs is hindered by difficult logistical and monitoring hurdles such as age, gender, lower socioeconomic status, travel distance, and other comorbidities[15-20].
One of the major challenges for CR programs is to entice patients to access and engage in CR in concert with the reduction of CVD risk [21]. Emerging web-based solutions and social networks in healthcare show promise [22, 23], but are often poorly integrated into standard healthcare resulting in variable efficacies [24]. Few, if any, digital/mobile health interventions have been designed in a comprehensive, evidence-based, and web-based or smart-phone accessible manner and can significantly affect an individual patient’s composite primary prevention CVD risk factor profile in a higher risk population. Additionally, such interventions should be based on behavior change theory which customizes the mobile health application to the CR participant, thus improving the secondary prevention capabilities for the patient [25].
Similar to a recently reported primary prevention mobile health intervention [26], we have developed an online and smartphone based application delivering Mayo Clinic's CR, whereby patients input and monitor their own CVD indices, diet and exercise adherence, and are tasked with accessing educational materials in a personal health assistant (PHA). The intent of this initial study was to extend our previous observations [26] and assess feasibility of such a mobile health intervention in patients during standard Mayo Clinic CR, as well as in the three months following standard CR. We hypothesized that using this online and smartphone-based CR application will improve the risk factor profiles, reduce rehospitalization, and improve lifestyle behaviors of those enrolled in standard CR.
Methods
Patient enrollment and experimental design
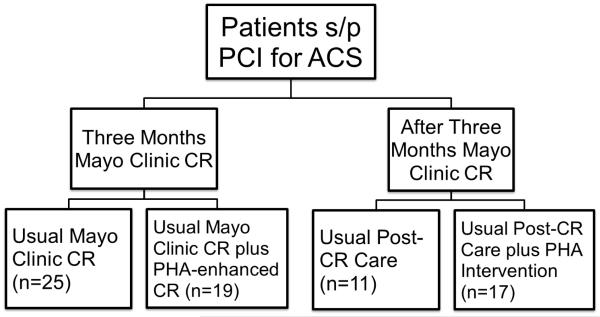
Patients were recruited, consented, and enrolled in a prospective fashion after PCI according to an approved Mayo Clinic IRB protocol. Inclusion criteria included willingness to participate in CR as well as access to the Internet. The four study groups consisted of two groups of patients entering three months of Mayo Clinic CR and two groups completing three months of Mayo Clinic CR, divided into those utilizing the PHA and those not using the PHA (Figure 2). The two groups utilizing the PHA, during and after CR, respectively, were educated on the use of the online CR program within one week following enrollment, and entered their metrics (weight, BMI, blood pressure, glucose, lipids, diet habits, physical activity, quality of life, medication adherence, and smoking status) with the help of a study coordinator. Those with compatible smart phones were assisted in downloading the appropriate application. Patients carried on the standard Mayo Clinic CR program as described previously [11] for 36 sessions (approximately three months). Patients could contact the study team or obtain technical support through the online program, and inquiries were usually answered within 24 hours. Patients who entered values for metrics such as blood pressure, lipids, glucose, or weight which were two standard deviations over than their prior entries or the normal limits for lab values were asked to verify the intended entry. If patients confirmed these values, messages to consult their physician appeared, as these changes in weight, blood pressure, or lab values could represent a potential danger to their health.
Figure 2.
Trial design of the four groups analyzed for the study.
Two similar groups of CR patients, entering CR and completing CR, who were eligible for but declined participation in the feasibility study but did choose to participate in cardiac rehabilitation at Mayo Clinic, Rochester for the same duration and at a similar time point, were used for comparison. Baseline and three month assessments included standard laboratory blood tests for fasting lipid panels and serum glucose values. Most patients in the study group underwent exercise stress testing at baseline and after three months per clinical protocol. The patients’ CR providers assessed blood pressure, height, weight, and the health behavior questionnaires (including diet, physical activity, quality of life (QOL), stress, and smoking status) at baseline and after three months in standard fashion. Weight and blood pressures were taken at every CR visit in standard fashion, with weight being assessed with clothes on and shoes off and blood pressure assessed by BpTRU (Canada). Stress scores were answered on a 1-10 scale [27], with QOL surveys utilizing the Dartmouth format [28]. Diet scores were calculated by the summation of daily servings of fruits, vegetables, whole grains, and lean proteins with points taken away for daily servings of saturated fats and sweets. Follow up assessment at three months consisted of a replication of the baseline parameters in a similar fashion. De-identified data was transmitted through Healarium, Inc. (Dallas, TX) to the investigators for a comprehensive de-identified data analysis at the completion of the program. Patient usage frequency was assessed by number of log in days divided by total number of active days and also assessed by log-ins per week with a higher frequency/percentage indicating higher patient participation. Patient-provided data was confirmed with a thorough chart review, and those not enrolled in the online and smartphone-based group (control patients) had data extracted by chart review at that time as well.
The Personal Health Assistant (PHA)
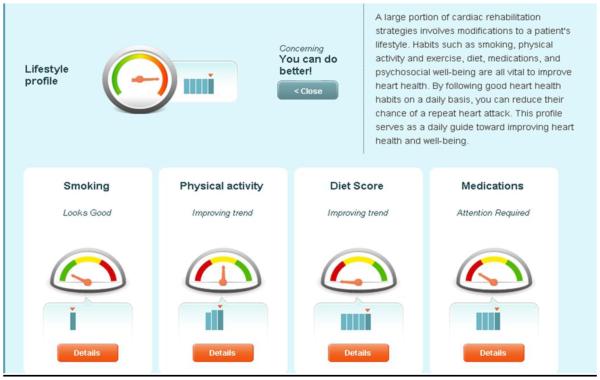
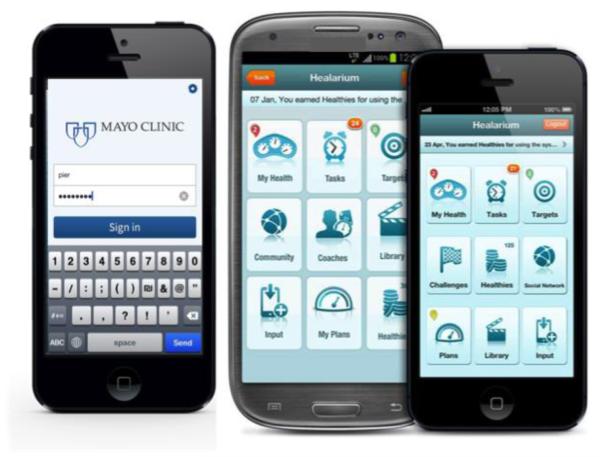
The PHA is an integrated and personalized interface that tracks, logs, educates, and forms actionable tasks for the user seeking to improve their current state of health in online and smart phone-based platforms as previously described [26] (Figure 1). A team of investigators at Mayo Clinic and Healarium, Inc. (Dallas, TX) partnered to construct a set of guideline and evidence-based, user-friendly tasks and educational materials to individually guide patients through their routine CR program and the months that follow. The PHA provides user-friendly and interactive access to health status information, tasks, targets, plans, awards and a social reinforcement network that encourages the adoption and maintenance of a healthier lifestyle for improved wellness [26]. Daily tasks keep patients on track towards achieving health plan recommendations. Reminders to complete tasks were received via email. Graphics that show a patient’s history and trends help visually demonstrate changes over time and the direction in which the patient is progressing. Simple, guideline-based recommendations tell patients what they need to accomplish with each goal in accordance with behavior change theory [29].
Figure 1.
Online (1a) and smartphone (1b) versions of the PHA. Figure 1a demonstrates the multiple risk factors for which patients enter their own information and the progress can be individually tracked and trended. Figure 1b demonstrates the same information that can be utilized on a mobile platform (Apple and Android). Explanations of recommendations and further educational information are available with one click regardless of the platform.
Specifically for this population, patients were taken through the intake process by a study coordinator, and asked to input their own CVD risk factor indices including height, weight, blood pressure, laboratory values, minutes of physical activity per day, and current dietary habits. Patients were asked via daily tasks and reminders to update these indices. Patients only received automated reminders to complete educational or recall tasks once logged into the system, with occasional reminder emails sent if the patient had not logged in recently. No text messages or phone communication was used. Should the patients have questions regarding the program or the content they could submit feedback using the designated tab, and answers were returned within 24 hours. In addition to tasks requesting the patient enter their indices of CVD risk such as weight, blood pressure, diet, and physical activity, patients were also asked to complete educational tasks on a regular basis. Education modules consisted of plans to enhance their physical activity and dietary measures produced by Mayo Clinic investigators. These were also available under a “library” tab within the program that the patients could access at any time.
Statistics
For binary data, percentages were calculated and chi-Square tests used to evaluate data. For normally distributed continuous data, means ± standard deviations or standard errors of the mean were calculated and presented in graphical form. Paired student’s t-tests and one-way ANOVA tests (with Tukey’s post-hoc where necessary) were used to statistically compare means at baseline and 90 days. Wilcoxon-Rank tests were used for non-parametric data. The percentage of patients achieving healthy benchmarks at baseline and 90 days were calculated and compared using Fisher’s and McNemar’s test for association. Significance was considered at an alpha = 0.05.
Results
Baseline demographics
Baseline demographics revealed similar baseline statistics between both groups with the CR+PHA group being significantly younger than the non-PHA group (Table 1). There were no differences in comorbidities between those using the PHA versus non-users regarding type 2 diabetes (27% vs. 36%, P = .72), hypertension (95% vs. 100%, p=0.99) or hyperlipidemia (68% vs. 69%, P = .99). Additionally, all participants were on lipid-lowering medications, dual anti-platelet therapy, as well as anti-hypertensive medications upon dismissal from the hospital, which continued throughout the duration of CR (100% self-reported medication compliance for both groups). There was no difference between those who utilized the PHA versus not at baseline regarding those currently employed (52% vs. 33%, P = .32), marital status (74% vs. 73%, P = .99), total years of education (14.4±0.4 vs. 15.1±0.6, P = .30), or mean number of sessions attended (29.0±3.8 vs. 30.7±4.1, P = .77). All participants deemed to have completed CR attended an average of 36 sessions over a 12-week period. Those who were considered drop-outs were deemed such according to documentation in their medical record according to CR program standards with correspondence sent by mail. Median follow up was 92.5 days (52.75, 100) with an average number of log-ins per week of 2.0±2.2 and average usage frequency of 44%±28%. Five participants utilized the program on their smartphones.
Table 1.
Baseline patient characteristics at the time of enrollment into the program in all four groups with no significant statistical differences across the four groups were found.
| Category | CR+PHA (n=25) |
CR (n=19) |
Post CR+PHA (n=17) |
Post CR (n=15) |
|---|---|---|---|---|
| Age, yrs | 60.2±12.1 | 70.4±9.9 | 66.9±8.3 | 69.4±10.1 |
|
| ||||
| Male | 19/25 (76%) | 17/19 (89%) | 11/17 (65%) | 8/15 (53%) |
|
| ||||
| Ethnicity, Caucasian | 24/25 (96%) | 19/19 (100%) | 15/17 (88%) | 14/15 (93%) |
|
| ||||
| Working Status, working | 12/25 (52%) | 5/15 (33%) | 10/17 (59%) | 7/15 (47%) |
|
| ||||
| Educational Years | 14.4±2.2 | 15.1±1.9 | 13.6±2.3 | |
|
| ||||
| Marital Status | 17/25 (74%) | 11/15 (73%) | 12/17 (71%) | 9/15 (60%) |
|
| ||||
| Hypertension | 21/22 (95%) | 14/15 (93%) | 15/17 (88%) | 12/15 (80%) |
|
| ||||
| Diabetes | 6/22 (27%) | 5/15 (33%) | 4/17 (24%) | 2/15 (13.3%) |
|
| ||||
| Hyperlipidemia | 15/22 (68%) | 10/14 (71%) | 12/17 (71%) | 9/15 (60%) |
|
| ||||
| Mild Cognitive Impairment | 0/25 | 1/19 | 0/17 | 1/15 |
|
| ||||
| Family History of CVD | 20/25 (80%) | 13/19 (68%) | 12/17 (71%) | 10/15 (67%) |
|
| ||||
| Weight, kg | 89.4±17.7 | 90.7±18.8 | 93.2±23.1 | 89.7±20.6 |
|
| ||||
| BMI, kg/m2 | 29.2±4.4 | 30.6±5.6 | 30.4±4.9 | 31.3±7.1 |
|
| ||||
| Systolic Blood Pressure, mmHg | 125.6±15.7 | 123.6±13.8 | 127.5±16.6 | 123.5±16.2 |
|
| ||||
| Diastolic Blood Pressure, mmHg | 69.7±11.3 | 69.5±8.6 | 69.0±13.2 | 65.5±11.1 |
|
| ||||
| Glucose, mg/dL | 122.8±37.7 | 122.7±42.4 | 113.5±29.3 | 117.9±56.4 |
|
| ||||
| Total Cholesterol | 178.5±42.5 | 173.8±51.8 | 167.7±34.3 | 137.1±30.3 |
|
| ||||
| LDL-Cholesterol, mg/dL | 99.9±40.9 | 94.9±41.8 | 90.5±26.1 | 69.9±26.0 |
|
| ||||
| HDL-Cholesterol, mg/dL | 45.4±16.1 | 51.1±18.9 | 54.5±22.8 | 47.5±14.0 |
|
| ||||
| Triglycerides, mg/dL | 154.3±84.1 | 185.5±253.9 | 142.1±135.2 | 114.3±58.1 |
|
| ||||
| Current Smoking | 3/22 (14%) | 2/19 (11%) | 0/17 (0%) | 1/15 (7%) |
CR and PHA
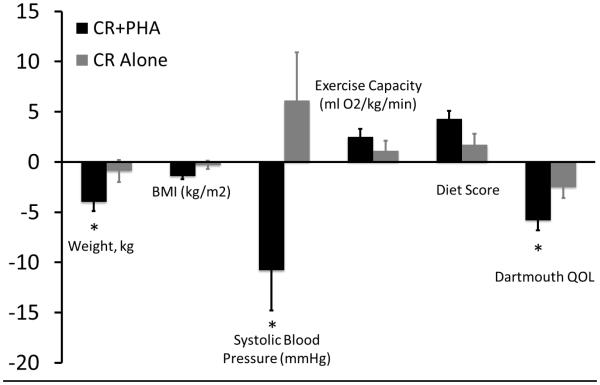
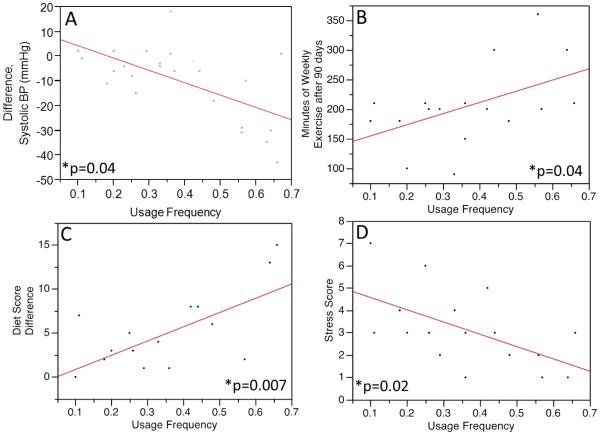
Patients in the CR+PHA group had significant reductions from baseline in systolic blood pressure (−10.8±13.5 mmHg, P = .0009), body weight (−4.0±5.2 kg, P = .001), BMI (−1.2±1.7 kg/m2, P = .006), total cholesterol (−46.9±38.3 mg/dL, P < 0.0001), LDL cholesterol (−36.7±35.7 mg/dL, P = .0004), and triglycerides (−39.3±69.1 mg/dL, P = .03), compared to baseline (Table 2). There was a similarly significant improvement in exercise capacity assessed by Bruce protocol treadmill exercise test (+2.5±2.7 ml O2/min/kg, P = .004); minutes of weekly exercise (+148.1±78.5 min/wk, P < 0.0001), food score (+4.3±4.3, P = .0003), stress scores (−1.3±1.3, P = .008), and Dartmouth QOL scores (−5.8±1.0, P = .009), all as assessed by survey responses. Those in the CR+PHA group noted significantly more weight loss (P = .03), decreased systolic blood pressure (p=0.01), and improved Dartmouth QOL (P = .04) compared to those in the control group with non-significant improvements in exercise time per week (+148.1±15.9 min/wk vs. 117.3±20.1 min/wk, P = .24), self-reported diet scores (+4.21±0.9 vs. 2.1±1.4, P = .33), and exercise capacity (+2.5±0.8 ml O2/kg/min vs. 1.1±1.0 ml/kg/min, P = .28) (Figure 2a). Patient usage frequency was associated with the weekly minutes of exercise at 90 days (r2=0.24, P = .04), reductions in blood pressure (r2=0.38, P = .04) and stress scores (r2=0.32, P = .02), as well as an improvement in self-reported diet score (r2=0.41, P = .007). There were no significant associations between usage frequency and age (r2=0.04, P = .46) or gender (r2=0.0001, P = .97). All patients who were smokers at the time of their PCI were self-reported former smokers at the conclusion of CR regardless of the group.
Table 2.
Change from baseline in patients enrolled in the CR+PHA program and standard CR program alone. P-values listed represent three-month changes from baseline within groups;
| Category | CR+PHA 3 Mo Delta |
P-value | CR 3 Mo Delta |
P-value |
|---|---|---|---|---|
| Systolic Blood Pressure, mmHg | −10.8±13.5, n=23 | 0.0009* | 6.1±25.5, n=16 | 0.36 |
|
| ||||
| Diastolic Blood Pressure, mmHg | −3.5±10.2, n=23 | 0.26 | −0.3±13.2, n=16 |
0.94 |
|
| ||||
| Weight, kg | −4.0±5.2, n=23 | 0.001* | −0.9±2.8, n=16 | 0.20 |
|
| ||||
| BMI, kg/m2 | −1.2±1.7, n=23 | 0.006* | −0.3±1.0, n=16 | 0.21 |
|
| ||||
| Total Cholesterol, mg/dL | −46.9±38.3, n=18 | <0.0001* | −33.9±51.9, n=11 |
0.06 |
|
| ||||
| LDL Cholesterol, mg/dL | −36.7±35.7, n=18 | 0.0004* | −35.9±47.3, n=11 |
0.04* |
|
| ||||
| HDL Cholesterol, mg/dL | 2.8±9.0, n=18 | 0.20 | −0.4±9.8, n=11 | 0.91 |
|
| ||||
| Triglycerides, mg/dL | −39.3±69.1, n=18 | 0.03* | −51.8±128.8, n=11 |
0.22 |
|
| ||||
| Glucose, mg/dL | −6.5±46.5, , n=12 | 0.64 | −9.9±19.6, n=8 | 0.20 |
|
| ||||
| Weekly Exercise, min | 148.1±78.5, n=21 | <0.0001* | 117.3±61.6, n=13 |
<0.0001* |
|
| ||||
| Exercise Capacity, mLO2/min/kg | 2.5±2.7, n=14 | 0.004* | 1.1±3.3, n=8 | 0.37 |
|
| ||||
| Food Score, 1-15 | 4.3±4.3, n=20 | 0.0003* | +1.7±2.6, n=11 | 0.06 |
|
| ||||
| Stress Score, 1-10 | −1.3±1.3, n=20 | 0.008* | −0.6±1.6, n=11 | 0.22 |
|
| ||||
| Dartmouth QOL | −5.8±1.0, n=10 | 0.009* | −2.5±1.1, n=8 | 0.22 |
p<0.05.
Post-CR and PHA
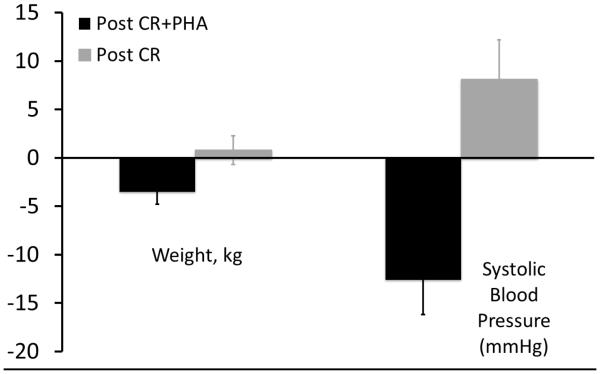
Those in the Post CR+PHA group also had significant reductions in blood pressure (−12.6±12.4 mmHg vs +8.1±4.1 mmHg; P = .0001; Figure 3b) and weight (−2.5±3.8 kg vs +0.7±1.5 kg; P = .04; Figure 3b) compared to those in usual post-CR care.
Figure 3b.
Improved weight loss (−2.5±3.8 kg vs +0.7±1.5 kg; p=0.04) and blood pressure (−12.6±12.4 mmHg vs +8.1±4.1 mmHg; p=0.0001) reduction in those using the PHA in the three months following CR compared to controls.
ED Visits and Rehospitalizations
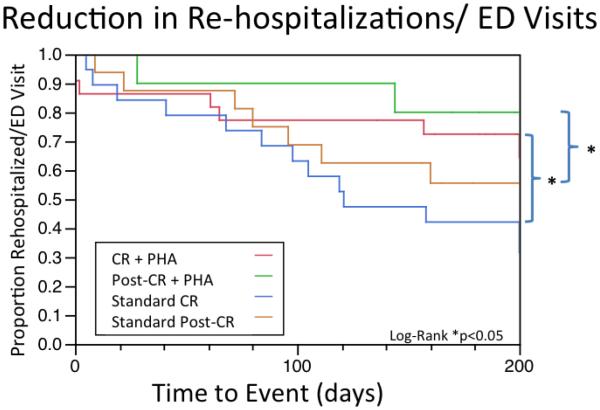
Additionally, there was a significant reduction in re-hospitalization and emergency department visits in those who utilized the PHA during CR (5/25 (20%) vs. 11/19 (58%), P = .01), and post-CR (−28%, P = .04). Those in the CR+PHA group also had a significantly reduced rate of rehospitalizations plus ED visits plus drop-outs over the course of the study (10/25, 40% vs 14/19, 74%; P = .03).
Discussion
The current results demonstrate for the first time that a mobile health-based CR program adjunctive to standard CR is associated with a significantly beneficial impact on risk factor profiles, lifestyle habits, and rehospitalizations plus ED visits in patients during and beyond CR after PCI for ACS. These improvements persist in comparison to a CR group undergoing “usual care” during phase 2 CR, extend to the three months following usual CR, and appear to be ubiquitous regardless of age and gender. Moreover, we show a usage-dependent effect of the mobile health intervention in reducing risk factors and changing lifestyle habits, thus reinforcing behavior change theory with this approach. Thus, the current study suggests that an adjunct mobile health-based tool comprised of patient-centered and evidence based material for patients enrolled in, and after, CR after PCI for ACS can improve the risk factor profile of patients seeking secondary prevention from CVD.
A current emphasis from the AHA Presidential Advisory is on restyling of CR programs to improve access, adherence, and outcomes [30]. Certainly, the contemporary approach to CR falls short, despite proven benefits and enhanced efforts to increase CR enrollment and adherence. There is a need to improve the risk factor profile of patients who attend usual CR following ACS in order to alleviate the burden of repeat events and reduce cost. Professional society guidelines recommend that CR programs should encompass a patient-centered, evidence-based, and technologically innovative approach [31], however, cost/personnel constraints and patient compliance often prohibit this strategy. There is a need for an outreach solution that is personalized and well integrated into the daily lives of patients seeking secondary CVD prevention based on independent, actionable, personalized care plans, and online personalized training materials. A recent systematic review and meta-analysis has effectively defined the current state of mobile health and CR, outlining a rigorous framework to evaluate mobile health interventions for CR [25]. This group eloquently provides a compelling case for expanding the evaluation of these interventions to not only include the common core components of CR, but also encompass behavior change theory, enable individual tailoring of features, demonstrate high usability, and improve patient-centered outcomes such as lifestyle behaviors, risk factors, patient-reported health status, and cardiovascular outcomes.
The current study extends our previous observations [26] and demonstrates that the PHA-based CR intervention is a viable and powerful addition to standard CR, and has the potential to be a long-term instrument capable of improving surrogate markers of CVD secondary prevention and decreasing the healthcare burden of repeat CVD events. Our data show an augmented reduction in risk factors during usual CR such as weight and blood pressure and improvement in quality of life reflected by QOL and stress scores, and demonstrate that behavior change, evidenced by increased PHA usage, is associated with improved diet scores. Furthermore, importantly we show a hard impact on CVD outcomes regarding rehospitalizations and ED visits in the days and months following PCI for ACS. Thus, this comprehensive PHA-based CR program effectively satisfies many of the novel components for mobile health in CVD [25].
The close association between improved lifestyle behaviors translating to improved risk factor profiles is long known; yet, our PHA provides a tangible, technologically-based instrument, potentially ubiquitous in the Western World both online and through smartphone capabilities, with which to implement such a positive effect. It is estimated that over 90% of US adults own a mobile phone with 56% owning a smartphone [32]. Moreover, over 85% of US adults have access and utilize the internet regularly, including 53% of those over 65 years – a number continuing to increase [33]. Clearly, efforts to enhance CR have been lagging in expanding their technological footprint, as only one in five smartphone users have downloaded a health-related application [34], however there is a relative paucity of guideline and evidence-based programs directed at utilizing this technology with even fewer showing positive results [25]. Moreover, smartphone and internet approaches could effectively target minorities and females who have typically been underrepresented in CR [14] yet increasingly utilizing mobile technologies in everyday life and for health information [32, 34].
Despite a vast majority of the risk factors and causes of CVD attributable to preventable lifestyle-related choices, data regarding the efficacy and compliance of CR during and beyond the prescribed three months of usual CR show, overwhelmingly, that compliance and documented changes in positive lifestyle behaviors decrease during and in the unsupervised months following CR [35]. While no hard CVD endpoints such as repeat events or death have been reported, most post-CR studies estimate that adherence to lifestyle changes such as physical activity, diet, and medication adherence, while initially improved during three months of outpatient cardiac rehabilitation, return to near pre-event levels in the nine to 12 months following usual CR [36]. Data from our own institution estimate medication compliance to statins and beta-blockers at 1 year hovers near the 70% mark in patients who have undergone three-months of usual CR following ACS and plummet to 50% at 3 years [37]. Moreover, the functional gains achieved during three months of standard CR regress substantially, irrespective of the dose of physical activity prescribed during the initial three months of usual CR [38]. Despite high readmission and ED visit rates, our data fall in line with national [39] and Mayo Clinic data [40]. Here we show continued improvements in weight loss and systolic blood pressure in the three months of usual CR – with an intervention which could easily be continued in the months beyond CR, providing a potential permanent lifestyle adjunct in the secondary prevention of CVD. Interestingly, new data regarding the psychology of post-CR care seems to indicate that patients responsible for the scheduling and execution of their own post-CR plan appear to show the greatest adherence [41].
CVD preventative measures rooted in behavior change theory have been shown to be more effective than those designed with no specific theoretical foundation [29, 42]. Ideally, mobile health interventions could accomplish this in a more resource-effective manner than the time and cost-intensive traditional CR model; however published studies have yet to properly integrate behavior change theory in mobile health interventions. Our data showing a positive association between risk factor reduction as well as positive lifestyle habits and usage frequency would, indeed, support this notion as there were positive associations between PHA usage and improvements in blood pressure and diet. Non-significant trends toward improvements in minutes of exercise weekly, weight loss, and QOL might also underscore the dose-dependent effect of this intervention as well as the patient-centered component of this intervention. The synergy of evidence-based, patient-centered, and technologically advanced components have been lacking from previous mobile health attempts at CR, and point to the need for a larger, randomized study to confirm and expand upon these results.
The study does possess a few limitations. One of the main limitations of the study is the lack of randomization between the groups. As this was a pilot study, we made a choice to not match the groups for age or gender in favor of ensuring we studied all patients in Mayo CR during that same time period as using an age/gender matched cohort during an earlier period could have been confounded by temporal trends in CR and/or DHI. Thus, individuals who agreed to participate in the study may represent a group of motivated patients who tend to adhere more vehemently to risk factor modifications. Furthermore, as data were collected from medical records and CR databases, some data (such as follow up stress tests, food questionnaires, and exercise minute surveys) were unable to be harvested due to an incomplete medical record. As both post-CR groups were not in CR with measures taken three times weekly, there were fewer data points for these groups which accounts for the lack of data reporting. Moreover, the high rate of drop outs and rehospitalized patients (nearly 75% in the control group) also reduced the final numbers used for statistical comparisons, thus compromising the statistical power to detect significant changes in such parameters as exercise capacity, exercise minutes per week, QOL scores, and diet scores. Despite the inability to closely track the metrics in the control groups throughout the duration of the study, reporting bias did not adversely affect the results as self-reported weight and blood pressure values were actually overestimated compared to the charted/database values. Therefore we believe that the PHA benefits are not overestimated. Certainly, future randomized controlled trials should aim to confirm these findings with larger patient populations over a longer duration which can appropriately assess the potential benefits on lifestyle changes, risk factor reduction, and possibly reduced burden on the healthcare system.
In conclusion, we show an impactful adherence to not only the PHA-based CR program with its beneficial lifestyle improvements in diet and exercise, but also an additional improvement in risk factors such as weight and blood pressure. Additionally, we show significant reductions in ED visits/rehospitalizations among those utilizing the mobile health-based CR program. These observations support the use of this PHA-based CR program to improve lifestyle behaviors, CVD risk factors, and reduce healthcare burden in a secondary prevention, CR-based population.
Figure 3a.
Comparison of the change from baseline to three months in weight (kg), BMI (kg/m2), systolic blood pressure (mmHg), exercise capacity (ml O2/kg/min), diet scores, and Dartmouth QOL scores in those using the PHA during CR and those undergoing usual CR. Significant reductions in weight (−4.0±0.9 kg, p=0.03), systolic blood pressure (−10.8±6.1, p=0.01), and QOL (−5.6±3.9, p=0.009) were observed in those patients utilizing the PHA concomitantly with standard Mayo Clinic CR.
Figure 4.
Association between the frequencies of PHA usage compared to blood pressure (a), weekly minutes of exercise at the end of CR (b), food scores (c), and stress scores (1-10, d). With increasing PHA usage, patients had a more substantial improvement in systolic blood pressure (r2=0.38 p=0.001), dietary habits (r2=0.41 p=0.007), weekly minutes of exercise at 90 days (r2=0.24, p=0.04), and reductions in stress scores (r2=0.32, p=0.02).
Figure 5.
Kaplan-Meyer curve showing reduced time to rehospitalizations/ED visits among those using the PHA during and after CR, as well as controls.
Statement on clinical relevance.
“This study demonstrates feasibility of a digital health initiative in cardiac rehabilitation, not only reducing cardiovascular risk factors but also demonstrating drastic reductions in clinically important outcomes such as rehospitalizations and emergency department visits over and above usual cardiac rehabilitation. This work should be used as a guide for future digital/mobile health studies in secondary CVD risk and event reduction.”
Acknowledgements
The authors thank the Binational Industrial Research and Development (BIRD) Foundation for their financial support. We also extend appreciation to Arturo Weschler, MD and Healarium Inc. an e-Health company, Dallas TX for their assistance in data de-identification and gathering. The sponsors and granting institutions had no impact on the results or preparation of the manuscript.
Funding: This study was funded by Binational Industrial Research and Development (BIRD) Foundation #1303. This publication was also made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: “Informed consent was obtained from all individual participants included in the study.”
References:
- 1.Roger V, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Circulation. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Yusef S, et al. Effects of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case controlled study. Lancet. 2004;342:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Go A, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee., Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 5.Pfuntner A, Wier LM, Steiner C. Costs for Hospital Stays in the United States, 2010. HCUP Statistical Brief #146. Agency for Healthcare Research and Quality, Rockville, MD. 2013 Jan; [PubMed] [Google Scholar]
- 6.Likosky D, Zhou W, Malenka DJ, Borden WB, Nallamothu BK, Skinner JS. JAMA Intern Med. Epub ahead of print; 2013. Growth in Medicare Expenditures for Patients With Acute Myocardial Infarction: A Comparison of 1998 Through 1999 and 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlay S, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11–18. doi: 10.7326/0003-4819-157-1-201207030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddox T, Reid KJ, Rumsfeld JS, Spertus JA. One-year health status outcomes of unstable angina versus myocardial infarction: a prospective, observational cohort study of ACS survivors. BMC Cardiovasc Disord. 2007;7(28) doi: 10.1186/1471-2261-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khumri T, Reid KJ, Kosiborod M, Spertus JA, Main ML. Usefulness of left ventricular diastolic dysfunction as a predictor of one-year rehospitalization in survivors of acute myocardial infarction. Am J Cardiol. 2009;103(1):17–21. doi: 10.1016/j.amjcard.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 10.Balducci S, et al. for the Italian Diabetes Exercise Study, Investigators, Effect of an Intensive Exercise Intervention Strategy on Modifiable Cardiovascular Risk Factors in Subjects With Type 2 Diabetes Mellitus: A Randomized Controlled Trial: The Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170(20):1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 11.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123(21):2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder S. Shattuck Lecture. We can do better--improving the health of the American people. N Engl J Med. 2007;357(12):1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- 13.Suaya J, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 14.Brown T, Hernandez AF, Bittner V, Cannon CP, Ellrodt G, Liang L, Peterson ED, Piña IL, Safford MM, Fonarow GC. Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association's Get With The Guidelines Program. J Am Coll Cardiol. 2009;54(6):515–521. doi: 10.1016/j.jacc.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper A. Factors associated with cardiac rehabilitation attendance: a systematic review of the literature. Clin Rehabil. 2002;16(5):541–552. doi: 10.1191/0269215502cr524oa. [DOI] [PubMed] [Google Scholar]
- 16.Corra U, et al. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training. European Heart Journal. 2010;31:1967–1974. doi: 10.1093/eurheartj/ehq236. %R 10.1093/eurheartj/ehq236. [DOI] [PubMed] [Google Scholar]
- 17.Mattila E, et al. Mobile diary for wellness management - results on usage and usability in two user studies. IEEE T-ITB. 2008;12(4):501–512. doi: 10.1109/TITB.2007.908237. [DOI] [PubMed] [Google Scholar]
- 18.McAlister F, Lawson FM, Teo KK, Armstrong PW. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. British Medical Journal. 2001;323(7319):957–962. doi: 10.1136/bmj.323.7319.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKellar S, et al. Development of the Diet Habits Questionnaire for Use in Cardiac Rehabilitation. Australian Journal of Primary Health. 2008;14(3):43–47. [Google Scholar]
- 20.Scott I, Lindsay KA, Harden HE. Utilisation of outpatient cardiac rehabilitation in Queensland. Med J Aust. 2003;179(7):332–333. doi: 10.5694/j.1326-5377.2003.tb05588.x. [DOI] [PubMed] [Google Scholar]
- 21.Teo K, Lear S, Islam S, Mony P, Dehghan M, Li W, Rosengren A, Lopez-Jaramillo P, Diaz R, Oliveira G, Miskan M, Rangarajan S, Iqbal R, Ilow R, Puone T, Bahonar A, Gulec S, Darwish EA, Lanas F, Vijaykumar K, Rahman O, Chifamba J, Hou Y, Li N, Yusuf S. PURE Investigators, Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: The Prospective Urban Rural Epidemiology (PURE) study. JAMA. 2013;309(15):1613–1621. doi: 10.1001/jama.2013.3519. [DOI] [PubMed] [Google Scholar]
- 22.Hawn C. Take two aspirin and tweet me in the morning: how Twitter, Facebook, and other social media are reshaping health care. Health Aff. 2009;28(2):361–368. doi: 10.1377/hlthaff.28.2.361. [DOI] [PubMed] [Google Scholar]
- 23.Lefebvre R. The new technology: the consumer as participant rather than target audience. Soc Mar Q. 2007;13:31–42. [Google Scholar]
- 24.Freeman B.a.C. Measuring interactivity on tobacco control websites. J Health Commun. 2012;17:857–865. doi: 10.1080/10810730.2011.650827. S. [DOI] [PubMed] [Google Scholar]
- 25.Beatty A, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc. 2013;2(6):e000568. doi: 10.1161/JAHA.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widmer R, Allison TG, Keane B, Dallas A, Lerman LO, Lerman A. Using an online, personalized program reduces cardiovascular risk factor profiles in a motivated, adherent population of participants. Am Heart J. 167(1):93–100. doi: 10.1016/j.ahj.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Clark M, Warren BA, Hagen PT, Johnson BD, Jenkins SM, Werneburg BL, Olsen KD. Stress level, health behaviors, and quality of life in employees joining a wellness center. Am J Health Promot. 2011;26(1):21–25. doi: 10.4278/ajhp.090821-QUAN-272. [DOI] [PubMed] [Google Scholar]
- 28.Van Weel C. Functional status in primary care: COOP/WONCA charts. Disabil Rehabil. 1993;15:96–101. doi: 10.3109/09638289309165878. [DOI] [PubMed] [Google Scholar]
- 29.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- 30.Balady G, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2. [DOI] [PubMed] [Google Scholar]
- 31.Thomas R, King M, Lui K, Oldridge N, Piña IL, Spertus J. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342–1350. doi: 10.1161/CIR.0b013e3181f5185b. [DOI] [PubMed] [Google Scholar]
- 32.Smith A. Smartphone ownership 2013. 2013 http://pewinternet.org/Reports/2013/Smartphone-Ownership-2013.aspx.
- 33.Zickuhr K, Madden M. Older adults and internet use. Pew Internet & American Life Project. 2012 http://www.pewinternet.org/~/media//Files/Reports/2012/PIP_Older_adults_and_internet_use.pdf.
- 34.Fox S, Duggan M. Pew Research Center’s Internet & American Life Project. 2012 http://pewinternet.org/Reports/2012/Mobile-Health.aspx.
- 35.Arrigo I, Brunner-LaRocca H, Lefkovits M, Pfisterer M, Hoffmann A. Comparative outcome one year after formal cardiac rehabilitation: the effects of a randomized intervention to improve exercise adherence. Eur J Cardiovasc Prev Rehabil. 15(3):306–311. doi: 10.1097/HJR.0b013e3282f40e01. [DOI] [PubMed] [Google Scholar]
- 36.Willich S, Müller-Nordhorn J, Kulig M, Binting S, Gohlke H, Hahmann H, Bestehorn K, Krobot K, Völler H. PIN Study Group, Cardiac risk factors, medication, and recurrent clinical events after acute coronary disease; a prospective cohort study. Eur Heart J. 2001;22(4):307–313. doi: 10.1053/euhj.2000.2294. [DOI] [PubMed] [Google Scholar]
- 37.Shah N, Dunlay SM, Ting HH, Montori VM, Thomas RJ, Wagie AE, Roger VL. Long-term medication adherence after myocardial infarction: experience of a community. Am J Med. 2009;122(10):e7–e13. doi: 10.1016/j.amjmed.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen D, Dendale P, Raskin A, Schoonis A, Berger J, Vlassak I, Meeusen R. Long-term effect of rehabilitation in coronary artery disease patients: randomized clinical trial of the impact of exercise volume. Clin Rehabil. 2010;24(4):319–327. doi: 10.1177/0269215509353262. [DOI] [PubMed] [Google Scholar]
- 39.Gore J, Peterson E, Amin A, Anderson FA Jr, Dasta JF, Levy PD, O'Neil BJ, Sung GY, Varon J, Wyman A, Granger CB. STAT Investigators, Predictors of 90-day readmission among patients with acute severe hypertension. The cross-sectional observational Studying the Treatment of Acute hyperTension (STAT) study. Am Heart J. 2010;160(3):521–527. doi: 10.1016/j.ahj.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Dunlay S, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127(6):538–546. doi: 10.1016/j.amjmed.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers W, Murray TC, Selzler AM, Norman P. Development and impact of exercise self-efficacy types during and after cardiac rehabilitation. Rehabil Psychol. 2013;58(2):178–184. doi: 10.1037/a0032018. [DOI] [PubMed] [Google Scholar]
- 42.Webb T, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12:e4. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]