Abstract
The rise in influenza-specific neutralizing antibody levels is proceeded by a burst of antigen-specific antibody secreting cells (ASC) or plasmablasts identified in peripheral blood approximately 5–10 days post immunization. Blood antigen-specific ASC may function as an immune marker of vaccine responses in comparison to the pre- and post- neutralizing titers; however, some have questioned whether there is adequate survival of ASC isolated from peripheral blood after freezing, making multi-center vaccine trials difficult. Here, we demonstrate similar frequencies of influenza-specific ASC from fresh and frozen peripheral blood mononuclear cells (PBMC). Influenza Hemagglutinin (HA) H1, H3, and H7-specific ASC IgG ELISpots frequencies were compared from the same fresh and frozen PBMC 7 days after 2006 Trivalent Influenza Vaccine (TIV) in 10 young healthy subjects. H1-, H3-, and H7-specific IgG ASC spots/106 from fresh PBMC on day 7 were 229 ± 341, 98 ± 90, and 6 ± 11 respectively. Total IgG ASC spots/million PBMC pre- & 7-day post-vaccination were 290 ± 188 (0.029% PBMC) and 1691 ± 836 (0.17% PBMC) respectively. There was no difference in the H1 -H3-, and total specific ASC IgG ELISpot frequencies from the fresh versus frozen PBMC on day 7 (p=0.43, 0.28, 0.28 respectively). These results demonstrate feasibility of testing whether antigen-specific ASC from frozen PBMC are an early biomarker of long-term antibody responses in multi-center vaccine trials.
Keywords: Antibody secreting cells, plasmablasts, vaccine, influenza
Introduction
Early biomarkers of influenza vaccine responses are needed to judge vaccine efficacy during clinical trials especially during influenza pandemics particularly in highly vulnerable populations such as those who are elderly, pregnant or immunocompromised. Identifying poor vaccine responders rapidly (within days) would be important during each routine influenza season especially of the immunocompromised patients in order to re-boost. However, during an influenza pandemic when vaccine supplies are limited and time is of the essence, identifying responders rapidly would become essential. The transient antigen-specific ASC in the blood at 5–9 days could function as an early biomarker of vaccine response, and there is a high potential that this early biomarker will correlate with traditional 4-fold rise in Hemagglutination Inhibition (HAI) titers in the serum at 4 weeks.
Trivalent influenza vaccination results in a transient burst of ASC in the peripheral blood. These frequencies peak 5 to 9 days after vaccination and immediately disappear and are highly enriched for antigen specificity (unpublished results) (Cox et al., 1994; Sasaki et al., 2007). Recently, generation of monoclonal antibodies from isolation of single cell clones of ASC after vaccination have been demonstrated (Wrammert et al., 2008). These cells are likely responsible for the 28-day rise in vaccine-specific antibody titers; however, correlates of influenza-specific ASC in the blood with 28 day rises in vaccine titers have not been definitively shown. While most of these ASC undergo apoptosis, some are believed to migrate to the bone marrow to become long-lived plasma cells (Slifka and Ahmed, 1998; Radbruch et al., 2006).
The transient burst of ASC may be quite heterogeneous. They may consist of the following different subsets: cells (1) that undergo apotosis, (2) home to inflamed tissue sites, or (3) migrate to the bone marrow (Radbruch et al., 2006). Many vaccines induce immunological memory and establish long-term humoral protection against infectious agents. (Amanna et al., 2007) A good vaccine response induces long-term protection; however, identifying long-term responders is difficult without the tincture of time or sampling human bone marrow. A subset of ASC with bone marrow homing markers such as CXCR4 is a likely candidate to differentiate into long-lived plasma cells. Thus, it is possible that this small specific subset of transient blood influenza-specific ASC will correlate with long-term antibody responses. Appearance of this potentially long-lived plasma cell in the blood could be used as an early biomarker for long-term protective vaccine responses.
The adequacy of survival and function of antibody secreting cells after cryogenic preservation has been questioned. To address this issue most vaccine studies currently require ASC assays to be performed only on fresh cells making multi-center vaccine trials with the use of a single central lab with standardized analysis techniques very difficult. In this paper, we demonstrate similar frequencies of influenza H1- and H3-specific ASC ex vivo by ELISpot assays from the same fresh and frozen PBMC collected from subjects 7 days post influenza vaccination.
METHODS
Subjects
Ten young healthy human subjects, between the ages of 19 to 32 years (mean ± SD: 26 ± 4), without concurrent illnesses and who had not received influenza vaccination for the current year were recruited at the University of Rochester Medical Center during winter/spring 2007. Five were men, and 5 were women. Six subjects reported no previous influenza vaccination, but 4 of these subjects reported a history of influenza infection. Three subjects reported multiple annual influenza vaccinations but could not recall a history of influenza infection. Subjects received the 2006–07 trivalent influenza vaccine (TIV). Blood was collected pre and 7 days post TIV. This study was approved by the Research Subjects Review Boards at the University of Rochester Medical Center.
PBMC Isolation, freezing, and thawing
Peripheral Blood Mononuclear Cells were isolated within 2 hours after the blood was drawn using BD Vacutainer® CPT™ tubes. Tubes were immediately inverted 8 – 10 times and centrifuged at 1500×g for 30 minutes at 20°C with brake. A 5mL sterile serological pipette was used to isolate the buffy coat layer in a 50mL conical tube. The sides of the CPT tube and the gel surface were washed with Hank’s balanced salt solution (HBSS) to collect any remaining cells. The cells were washed with HBSS 3 times (300×g, 10 min, 4°C) and re-suspended in approximately 1 mL of RPMI with 8% FBS (R8) per tube of blood collected. The cells were counted using 0.4% Trypan Blue exclusion. An aliquot of the cells was used in fresh ASC ELISpot assays, and the remaining cells were frozen at 10–15 × 106 per mL per cryovial in Fetal Bovine Serum (FBS) with 10% DMSO chilled at 4°C. Cryovials containing cells were placed in an ice bath for 15 minutes and were subsequently placed in a “Mr. Frosty”, (Nalgene, Rochester, NY) cryocontainer before transfer to −80°C for 24 hours and then transferred to liquid nitrogen tanks for 4–8 weeks.
To thaw cells, the cryovials were removed from liquid nitrogen tanks and brought on dry ice to the lab. Vials were thawed immediately with shaking in a 37° C water bath until a few ice crystals remained. No more than 2 cryovials were thawed at the same time. The cell suspension was transferred to a 4° C cooled 15 mL conical tube, and 4–5 mL of cold RPMI added drop-wise, mixing gently after each drop. The total volume was then brought up to 12 mL per tube with additional RPMI and the tubes were centrifuged at 300×g for 8–10 minutes. After one additional wash in RPMI, the cells were re-suspended in R8 at appropriate cell concentrations. Cells were counted and viability assessed using 0.4% Trypan Blue exclusion. Viability ranged from 70–85% of the fresh cells.
Influenza-specific antibody secreting cell (ASC) ELISpot
The frequency of Influenza antigen-specific ASC was measured by ELISpot. Briefly, 96-well ELISpot plates (MAIPS4510 96 well) were coated overnight at 4°C in a humidified chamber with 1ug/mL of either purified Hemagglutinin A (HA) proteins from H1N1 (New Caledonia), H3N2 (Wisconsin) and H7N2 (Netherlands) strains (Protein Sciences Corp, Meriden, CT) or 5ug/mL of anti-human IgG (Jackson Immunoresearch, West Grove, PA). 2% Bovine Serum Albumin (BSA, MP Biomedicals, Solon, OH) in sterile PBS was used as an irrelevant antigen for coating. The plates were blocked with R8 media for 2 hours and incubated at 37°C for 18–20 hours with 300,000, 100,000, or 33,333 PBMC for the HA antigens and BSA, either freshly isolated from the blood, or thawed immediately before each experiment as described above. For total IgG 100,000, 33,000, and 11,000 PBMC per well were added. After incubation, cells were aspirated and plates were washed with PBS with 0.1% Tween (PBST) 6 times. Antigen specific antibodies bound to the plate were detected with alkaline phosphatase-conjugated anti-human IgG antibody (Jackson Immunoreseach) for 2 hours and developed with VECTOR Blue, Alkaline Phosphatase Substrate Kit III (Vector Laboratories, Burlingame, CA). The spots per well were counted using the CTL immunospot reader (Cellular Technologies Ltd). For analysis, all samples had background spots from wells without any capture antigen subtracted.
Polychromatic flow cytometry
The following antibody panel against human markers was used to identify total plasmablasts or ASC from fresh or frozen PBMC: IgD-FITC, CD3-Cy5.5PE, CD19-Cy7PE, CD38- Pacific Blue, CD27-APC, CD14-Alexa 700 (BD Biosciences). Cells were collected on an LSRII instrument (BD Biosciences) configured to detect 11 fluorochromes. One to 2 million events were collected per sample. Analysis was performed using FlowJo softwar (Treestar, Inc version 8.7.1). Total PBMC were gated on lymphocytes and monocytes using FSC and SSC. To exclude non-specific staining and non-B cells, CD14 and CD3 were used. Plasmablasts were identified as both CD27hi and CD38hi of the CD19+ B cell population.
Statistics
A two-tailed Wilcoxian Signed Rank test was used to compare influenza-specific H1, H3, H7 and total IgG ASC frequencies pre and day 7 post-vaccination and between the same day 7 fresh and frozen samples (Prism GraphPad software, La Jolla, CA). Two-tailed Mann-Whitney (non-parametric) test was used to compare the antigen-specific and total IgG ASC frequencies between the first time and repeat vaccinees. Comparison of the frequencies of antigen-specific fresh and frozen ASC was calculated using the one-tailed Spearman Coefficient Correlation.
RESULTS
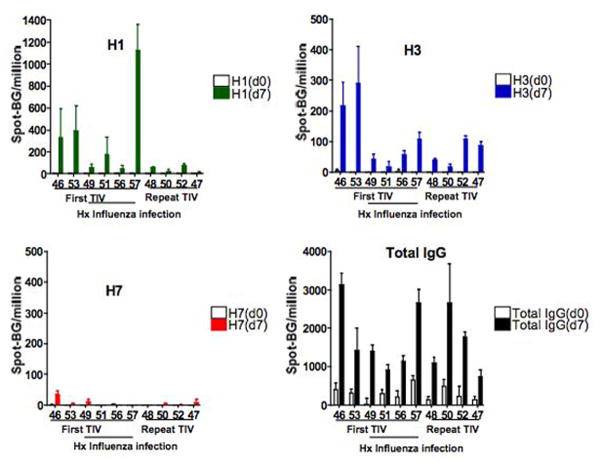
Blood from 10 healthy human subjects were collected pre- and post- 7 days TIV (2006). An aliquot of fresh PBMC were isolated and were used for the following assays while the remaining PBMC were frozen for several weeks. The influenza viral strains included in the 2006 TIV vaccine were two Influenza A, H1N1 A/New Caledonia/20/99 and H3N2 A/Wisconsin/67/2005, and one Influenza B B/Malysia/2506/2004. Influenza-specific human IgG ASC were performed from the blood directly ex vivo for H1 A/New Caledonia/20/99 and H3 A/Wisconsin/67/2005 and an irrelevant avian H7N2, BSA, and RPMI +8% FBS alone (no antigen) were used as negative controls. Mean spots in the background (no antigen) wells were 0.6 ± 1 and the mean spots in the BSA coated wells were 1.8 ± 3.9. Since the background from the wells coated with BSA or “no antigen” were similar, all sample wells had counts from the no antigen control wells subtracted from them. Total IgG ASC frequencies in fresh blood were found at a basal frequency of 290 ± 188 (0.029% PBMC) and increased significantly to 1691 ± 836 (0.17% PBMC) spots/106 PBMC (p=0.002) on day 7 after immunization (fig. 1). The H1- and H3-specific ASC showed a statistically significant increase on day 7 compared to day 0 to 229 ± 341 and 98 ± 90 spots/106 PBMC (p=0.002 and p=0.006) respectively. The detection limit was 1 spot per 3×105 PBMC. The H7-specific ASC was 6 ± 11 and remained less than 10 spot/106 PBMC in 9/10 subjects on day 7. H1-, H3-, and H7- specific of total IgG ASC spots percentages were 11.9 ± 13.7, 6.3 ± 5.8, and 0.3 ± 0.5 respectively. One subject had a small response 34/106 PBMC but had childhood exposure to chickens and wild fowl and could have had some potential cross-reactive epitopes with an HA in the 2006 TIV.
Figure 1.
Comparison of influenza-specific and total IgG ASC from fresh PBMC of 10 subjects pre and 7 day-post TIV immunization. All spots shown had background wells subtracted. No H1- or H3-specific ASC spots/106 PBMC are noted on day 0. Increased H1- (green, top left) and H3-specific (blue, top right) ASC spots/106 PBMC were seen on day 7 compared to day 0 (Wilcoxian Rank Test, p= 0.002 and p= 0.006 respectively). Minimal avian H7 responses (red, bottom right) were noted on 7-day post vaccination (p= 0.06) and functioned as a negative control. A rise in total IgG (black, bottom right) ASC were seen in the blood from day 0 to 7 (p=0.002)
There was a trend toward higher H1-specific ASC frequencies in peripheral blood in subjects receiving TIV for the first time compared to those with repeat TIV administration. However, comparing the ASC frequencies between the six first time TIV vaccine subjects to the four repeat vaccinees, the H1-, H3-specific or total IgG ASC frequencies did not reach statistical significance (p= 0.067, p=0.476, and p=0.610 respectively). H3N2 viruses have been circulating since 1968, and it is likely subclinical infections by natural exposures to H3N2 in our study population occurred since all were born after 1968. Therefore, despite a first time exposure to the influenza antigens via vaccination, the H3N2 antigen may not have been a novel antigen to these subjects. In addition, some HA components can be identical from year to year in the influenza vaccine. In 2005, influenza vaccine consisted of H1N1 A/New Caledonia/99, H3N2 A/California/2004, and B/Shanghai/2002 while the 2006 influenza vaccine consisted of the same H1N1 A/New Caledonia/99, but different H3N2 A/Wisconsin/2005, and B/Malaysia/2004. Interestingly, the H1N1 component of the vaccine remained the same from 2000–2006. All subjects with repeat immunization had repeat exposures to the same H1N1 strains whereas the H1N1 antigens may have been new for the first time vaccinees. Therefore, despite a lack of statistical significance, we note a trend of increased H1-specific ASC frequencies in the blood with first time- compared to repeat-antigen exposures at 7 days post-vaccination.
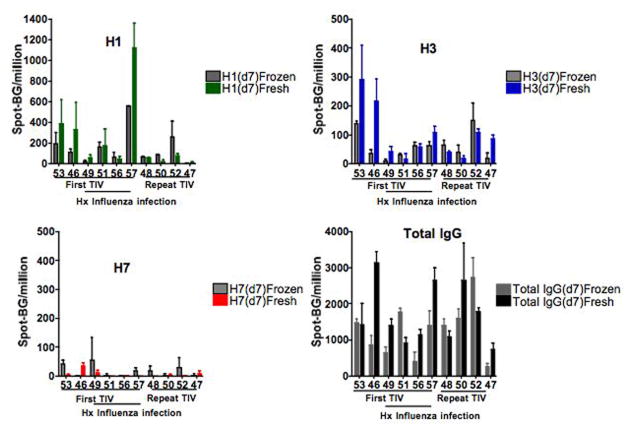
The H1- and H3- specific ASC ELISpot frequencies were not different from the same fresh and frozen PBMC samples (fig. 2). H1- and H3-specific ASC frequencies from the 7-day post vaccination of fresh and frozen PBMC were 229 ± 341 and 152 ± 163/106 PBMC (p=0.43) and 98 ± 90 and 61 ± 48/106 PBMC (p=0.28) respectively. The total IgG ASC responses were also similar in the same fresh and frozen samples 1691 ± 836 and 1252 ± 737 (p=0.28) respectively. Although the fresh and frozen ASC responses were similar, the correlation coefficient was the strongest for H1-specific ASC as compared to H3–specific and total IgG ASC respectively (Spearman Coefficient N=10, R=0.806 p=0.004, R=.40 p=0.124, R= 0.26 p= 0.235). The responses with H7 were less correlative likely due to the very low frequencies. It appears that some correlation may have improved with increased titrations of total PBMC.
Figure 2.
Comparison of influenza-specific and total IgG ASC frequencies 7-days post TIV vaccine from the same fresh and frozen PBMC sample. All spots shown had background wells subtracted. There was no difference in the fresh and frozen samples for H1- (top left) and H3-(top right) specific ASC spots/106 PBMC from fresh (H1-green, H3-blue) and frozen (corresponding gray) PBMC (Wilcoxian Rank Test, p= 0.43, p=0.28, respectively). Fresh and frozen H7 (bottom right) ASC spots/106 PBMC were minimal and similar. The total IgG (bottom right) ASC spots/106 PBMC were also not different from the fresh and frozen samples (p=0.28).
Despite similar antigen-specific and total IgG ASC ELISpot frequencies from fresh and frozen samples, cell surface markers to identify plasmablasts or ASC were not as stable with freezing. Flow cytometry can identify all subclasses (IgG, IgA, IgE, and IgM) of antibody secreting cells whereas our current ASC ELISpot assay only measured total IgG ASC. Plasmablasts or ASC were analyzed by flow markers CD19+, CD27hi, CD38hi, from the same fresh and frozen PBMC samples. The same fresh and frozen samples from 3 subjects 7 days post-vaccination were analyzed by cell surface markers (figure 3). By flow cytometry, the frozen samples demonstrated only 20–30% of the plasamblasts or ASC as compared to the same fresh samples. Although there were only 3 subjects, generally, plasamblasts or ASC by flow cytometry were decreased in the frozen samples compared to total IgG ASC by ELISpots which measures total cellular IgG secretion. Interestingly, from the same frozen samples, one (subject 46) had similar ASC by flow cytometry and total IgG ELISpots. One explanation for the similar numbers could be due to an underestimate of the ASC by ELISpot due to increased IgA secreting cells that were not measured by the ELISpot assay.
Figure 3.
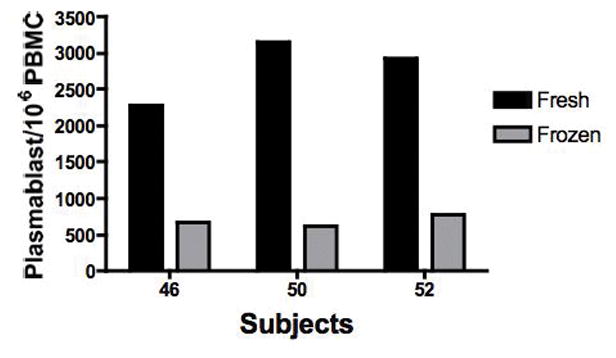
Comparison of plasmablasts or ASC by flow cytometry from the same fresh and frozen PBMC samples of 3 subjects are shown. Plasmablasts were identified as CD19+ CD27hi and CD28hi. Total PBMC numbers were identified by FSC and SSC. Numbers of ASC by flow cytometry are presented per 106 PBMC.
DISCUSSION
This paper clearly demonstrates that ASC from frozen PBMC can survive freezing with similar function and adequate numbers to make large multi-center trials possible. Our data demonstrate similar influenza-specific ASC frequencies from both fresh and frozen cells, and the central implication of this data demonstrates the functional identity of ASC that lends itself to high throughput antigen-specific ASC ELISpot assays from frozen samples. These similar ASC frequencies suggest equivalent losses of total PBMC and ASC to yield similar numbers. Contrary to previous belief, recovery of ASC function is preserved in such frozen cells.
Total IgG ELISpot numbers may not have correlated as well in fresh and frozen PBMC due to several factors. We suspect a multi-titration of total IgG ASC frequencies for the higher numbers on 7 days post vaccination would have yielded more accurate numbers and tighter correlations. Other interpretations may include heterogeneous survival of antigen-specific and non-antigen-specific ASC during an immune response. It is possible that non-antigen specific-ASC ascribed to by-stander proliferation (Bernasconi et al., 2002) may be more susceptible to death after freezing and thawing.
Although we demonstrate that influenza-specific ASC may survive freezing, it is unclear whether this phenomenon is transferable to all antigens or all classes of IgA, IgM, and IgE ASC that are found transiently in the blood. We have tested other respiratory viral antigens from Respiratory Syncytial Virus (RSV), and we are able to detect RSV-specific F and Ga ASC in frozen PBMC of subjects during acute RSV infection (unpublished data). However, controlled side by side fresh and frozen comparisons of the RSV antigens were not performed.
The antibody contribution of the H1 and H3 components are only a portion of the total vaccine response which includes antibody responses to H1, H3, and B as well as NA, NP M and potentially other internal influenza proteins (Wrammert et al., 2008). Although not demonstrated in this paper, we have analyzed the contributions of individual HA-specific IgG ASC from young healthy subjects. Frequencies of H1 and H3 ASC can range from 8–60% and 10–65% respectively of the TIV-specific ASC numbers on day 7 and these percentages can also vary dramatically from day to day post-vaccination (unpublished data).
Interestingly, the flow markers of the CD19+ ASC or plasmablasts are not readily retained on frozen cells. CD38hi and CD27hi are the hallmark of blood CD19+ human plasmablasts and are important markers of total (non-antigen and antigen-specific ASC of all antibody classes IgM, IgG, IgA, and IgE) in the peripheral blood. This data demonstrates a recovery of 20–30% of the plasmablasts by flow cytometry from frozen samples. After influenza vaccination, antigen-specific ASC can range from 20–90% of the entire IgG ASC response measured by ELISpot assays (unpublished data). Therefore, we believe that identification of antigen-specific plasmablasts by flow cytometry in the cryo-preserved sample is possible; however, the antigen-specific ASC may only constitute 4–27% of the total frozen plasmablasts. Thus, ASC may be rescued and cloned for the generation of monoclonal antibodies from frozen samples; however, representation of the entire antigen-specific repertoire may have many limitations.
This is the first paper to demonstrate that the human influenza-specific antibody (IgG) secreting function of ASC is preserved with freezing. Stringent methods of freezing and rapid thawing of frozen human PBMC increase cell viability and maintain function of ASC. ASC function by the ELISpot assay is maintained for several weeks and up to one year after freezing (unpublished data). However, the effects of long-term storage of these cells are yet to be determined, and whether all ASC survive freezing will need further evaluation.
Acknowledgments
Supported by:
Rochester Center for the Biodefense of Immunocompromised Populations HHSN2662005500029C (N01-AI50029), K23 AI67501-01A1 N01-AI-50020, R24 AO054953
We would like to thank Deanna Maffet, RN for her assistance in recruiting subjects for this study, Hulin Wu, PhD for his valuable biostatistical consultation, and Shelley Secor-Socha for isolation and cryopreservation of specimens in the Rochester Human Immunology Center Core Laboratory.
Abbreviations
- ASC
antibody secreting cell
- PBMC
peripheral blood mononuclear cells
- TIV
trivalent influenza vaccine
References
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–9. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–28. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–8. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]