Abstract
Background
Clostridium difficile infection (CDI) is a major cause of antibiotic-associated diarrhoea. Given an increasing CDI incidence and global spread of epidemic ribotypes, a 1-year study was performed to analyse the molecular characteristics of C. difficile isolates and associated clinical outcomes from patients diagnosed with CDI in the Internal Medicine department at University Hospital Motol, Prague from February 2013 to February 2014.
Results
A total of 85 unformed stool samples were analysed and CDI was laboratory confirmed in 30 patients (6.8 CDI cases per 10,000 patient bed days and 50.6 CDI cases per 10,000 admissions). The CDI recurrence rate within 3 months of treatment discontinuation was 13.3% (4/30). Mortality within 3 months after first CDI episode was 26.7% (8/30), with CDI the cause of death in two cases. 51.9% of C. difficile isolates belonged to PCR-ribotype 176. MLVA of ribotype 176 isolates revealed two clonal complexes formed by 10/14 isolates. ATLAS scores and Horn’s index were higher in patients with ribotype 176 infections than with non-ribotype 176 infections.
Conclusion
This study highlights the clinical relevance of C. difficile PCR-ribotype 176 and its capacity to spread within a healthcare facility.
Keywords: Clostridium difficile, PCR-ribotype 176, Horn’s index, ATLAS score, Ribotyping, MLVA
Findings
Background
Clostridium difficile infection (CDI) is a major cause of antibiotic-associated diarrhoea and a significant burden to healthcare services worldwide [1]. Results of a pan-European epidemiological study in 2008 indicated that the Czech Republic has a relatively low CDI incidence (1.1 per 10,000 patient bed-days and 7.0 per 10,000 hospital admissions) [2], although a recent epidemiological study suggested a CDI incidence rate of 4.4 and 6.2 cases per 10,000 patient bed-days in 2011–12 and 2012–13, respectively [3].
In 2013, the high prevalence of PCR-ribotype 176 (n = 251; 40 %) was revealed by ribotyping of 624 C. difficile isolates from 11 Czech healthcare facilities [4] C. difficile ribotype 176 is thought to share many similarities to ribotype 027 [5–7] and it has been suggested that this type may be misdiagnosed as a ribotype 027 infection [8]. The long-term epidemic occurrence of C. difficile PCR-ribotype 176 was also reported in Poland [9, 10].
In response to the reported unfavourable global CDI epidemiological situation, including in Czech Republic, a 1-year study was initiated to monitor the incidence of CDI, clinical features and outcomes and to investigate the molecular characteristics of C. difficile isolates in patients with CDI hospitalised in the Internal Medicine department of University Hospital Motol, Prague, Czech Republic, from February 2013 to February 2014.
Microbiological testing
Stool samples of 85 patients aged ≥18 years with three or more unformed stools per day were investigated at the Department of Medical Microbiology. CDI was laboratory diagnosed using the C. difficile Quik Chek Complete® test (Alere) and C. difficile Alere and simultaneous toxin A/B positivity was detected in 24 samples (80 %). In six samples that were only GDH-positive but where patients had relevant clinical symptoms, the presence of toxigenic C. difficile was confirmed using PCR (GeneXpert®, Cepheid). Positive stool samples (GDH and toxin positive; GDH positive, toxin negative and PCR positive) were cultured anaerobically, after an alcohol shock treatment, on selective media (Oxoid); anaerobic culture was positive for C. difficile in 27/30 samples (90 %). Antibiotic susceptibility of C. difficile isolates to metronidazole and vancomycin was determined by E-test® (BioMérieux) and minimum inhibitory concentrations for all C. difficile isolates ranged from 0.03–2 mg/L for metronidazole and 0.015–1 mg/L for vancomycin (Table 1). No isolates were found to be resistant to either metronidazole or vancomycin.
Table 1.
Microbiological and molecular characteristics of C. difficile isolates
| CDI case no. | Isolate no. | GDH | Toxin A/B | Anaerobic culture |
PCR-ribotype | Toxin gene presence (A/B/bin)a,b | Vancomycin MIC (mg/L) | Metronidazole MIC (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | n/a | + | + | – | n/a | n/a | n/a | n/a |
| 2 | 259 | + | + | + | 176 | A/B/bin | 0.25 | 0.25 |
| 3 | 263 | + | + | + | 176 | A/B/bin | 0.25 | 0.25 |
| 4 | 269 | + | + | + | 176 | A/B/bin | 0.5 | 0.5 |
| 5 | 205 | + | + | – | n/a | n/a | n/a | n/a |
| 6 | 273 | + | + | + | 176 | A/B/bin | 0.6 | 0.25 |
| 7 | 298 | + | – | + | 049 | A/B | 1 | 0.5 |
| 8 | 280 | + | + | – | n/a | n/a | n/a | n/a |
| 9 | 279 | + | + | + | 176 | A/B/bin | 1 | 0.25 |
| 10 | 294 | + | + | + | 176 | A/B/bin | 0.12 | 1 |
| 11 | 303 | + | + | + | 176 | A/B/bin | 0.25 | 0.5 |
| 12 | 277 | + | – | + | 001 | A/B | 0.5 | 0.5 |
| 13 | 304 | + | + | + | 014 | A/B | 0.25 | 0.5 |
| 14 | 44 | + | – | + | 002 | A/B | 0.25 | 0.5 |
| 15 | 307 | + | + | + | 017 | A/B | 0.25 | 0.25 |
| 16 | 308 | + | + | + | 176 | A/B/bin | 0.25 | 0.25 |
| 17 | 316 | + | + | + | 176 | A/B/bin | 0.5 | 0.25 |
| 18 | 320 | + | – | + | 012 | A/B | 0.5 | 0.5 |
| 19 | 319 | + | + | + | 012 | A/B | 0.5 | 0.12 |
| 20 | 322 | + | + | + | 176 | A/B/bin | 0.5 | 2 |
| 21 | 365 | + | – | + | 176 | A/B/bin | 0.5 | 2 |
| 22 | 323 | + | + | + | 020 | A/B | 0.5 | 0.5 |
| 23 | 325 | + | + | + | 015 | A/B | 0.5 | 0.25 |
| 24 | 331 | + | + | + | 078 | A/B/bin | 0.5 | 0.25 |
| 25 | 336 | + | + | + | 176 | A/B/bin | 0.5 | 1 |
| 26 | 351 | + | + | + | 176 | A/B/bin | 0.25 | 1 |
| 27 | 365 | + | – | + | 014 | A/B | 0.5 | 0.5 |
| 28 | 391 | + | + | + | 005 | A/B | 0.25 | 0.5 |
| 29 | 388 | + | + | + | 434 | A/B | 0.25 | 0.12 |
| 30 | 248 | + | + | + | 176 | A/B/bin | 0.12 | 0.5 |
GDH glutamate dehydrogenase, MIC minimum inhibitory concentration
aToxin A/toxin B/binary toxin
bPrimers used to amplify tcdA are located upstream of the repetitive region in the 3′-end. The TcdA-negative strains due to 3′-end deletion revealed positive PCR amplification [13]
C. difficile isolates molecular characterisation
PCR-ribotyping was performed according to the Standard Operating Protocol of ECDIS-net (http://www.ecdisnet.eu) using capillary electrophoresis after PCR amplification with primers previously described by Stubbs et al. [11]. Electrophoreograms were confirmed using the Webribo database [12]. PCR-ribotypes were identified for all 27 C. difficile isolates and 14 (51.9 %) belonged to ribotype 176. Other identified ribotypes were 012 (n = 2; 7.4 %), 014 (n = 2; 7.4 %), 001, 002, 005, 017, 020, 049, 078, 434 and 015 (all n = 1; 3.7 %).
The presence of toxin genes was determined by multiplex PCR with specific primers for tcdA (toxin A), tcdB (toxin B), cdtA and cdtB (binary toxin) [13]. All C. difficile isolates revealed presence of genes for production of toxins A/B, while genes for production of binary toxin (cdtA/cdtB), which has been associated with increased attachment to epithelial cells, increased virulence and higher recurrence rates [14–16] were only found in isolates of ribotypes 176 and 078 (15/27; 55.6 %). Summary of microbiological and molecular characteristics of C. difficile isolates is shown in Table 1.
The tcdC gene was amplified with primers C1 and C2 [17] and the obtained sequence was compared to NCBI reference sequence NC_009089.1. Two deletions (position 117, which introduces a frame-shift mutation leading to protein truncation [17], and 330–347) in the tcdC gene were found in all 14 ribotype 176 isolates. One isolate, ribotype 078, revealed 39-bp deletion from nucleotides 341–379 in the tcdC gene. No deletion in other 12 isolates was found. The precise function of the tcdC gene is not yet clear [18].
Genetic relatedness among C. difficile ribotype 176 isolates was achieved using multi-locus-variable tandem repeats-analysis (MLVA). The number of tandem repeats were determined by Sanger sequencing in five previously published variable tandem repeat (VNTR) loci (A6Cd, B7Cd, C6Cd, G8Cd) [19, 20] and CDR60 [21]. A Minimum Spanning Tree (MST) was created by Bionumerics v5.0 (Applied Maths) using a Manhattan coefficient to calculate the summed tandem repeat difference (STRD). A cluster analysis using the categorical distance and unweighted pair group method with arithmetic mean algorithms was also applied. The number of tandem repeats for each locus is summarised in Fig. 1. MST identified two clonal complexes when STRD ≤1 (Fig. 2). The first clonal complex was formed from eight isolates (55, 269, 351, 263, 273, 279, 294 and 308). The second clonal complex consisted of two isolates (336 and 322). Between clonal complexes one and two, STDR = 6 were found. Isolate 248 revealed STDR = 9 to isolate 294 (CC1), isolate 259 showed STDR = 5 to isolate 336 (CC2), isolate 316 showed STDR = 6 and isolate 303 STDR = 9 to isolate 322 (CC2).
Fig. 1.
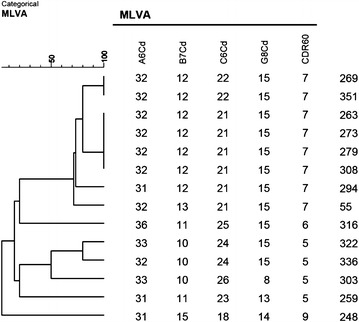
A Categorical MLVA of C. difficile ribotype 176 isolates (Bionumerics v5.0, Applied Maths)
Fig. 2.
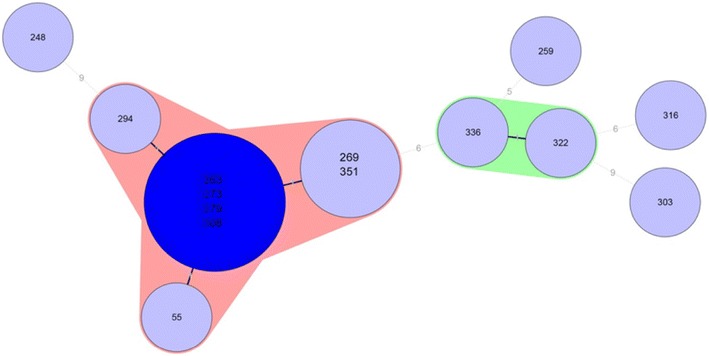
A Minimum Spanning Tree of C. difficile ribotype 176 isolates (Bionumerics v5.0, Applied Maths). The numbers in the circles represent C. difficile PCR-ribotype 176 isolate number. The numbers in the lines represent the sum of tandem repeat differences between isolates
The time intervals of hospitalisation of patients infected by C. difficile ribotype 176 did not overlap except for two patients. This finding suggests that the probable source of infection may have come from the hospital environment and, given the high incidence of this ribotype previously reported in the Czech Republic [4], it is possible that ribotype 176 is endemic in the country and this type has been introduced into the hospital environment on several occasions.
Clinical and epidemiological data analysis
CDI was diagnosed in 30 patients (female n = 13, male n = 17; mean age 69.0 years). The overall CDI incidence in the Internal Medicine ward during the study period was calculated as 6.8 CDI cases per 10,000 patient bed-days and 50.6 CDI cases per 10,000 admissions which indicated a higher CDI incidence compared with recently reported rates [3].
Healthcare-associated CDI (HA-CDI) was diagnosed in 26 CDI cases (86.7 %) and community-associated CDI (CA-CDI) was diagnosed in four CDI cases (13.3 %). Severe CDI was diagnosed in 17 (56.7 %) patients according to the Horn’s index [22, 23] and 18 (60 %) according to the ATLAS score [24]. Antibiotic treatment prior to CDI diagnosis was noted for 83.3 % (25/30) of patients. The most commonly used antibiotics were aminopenicillins with beta-lactamase inhibitors (n = 12), fluoroquinolones (n = 12), broad-spectrum cephalosporins (n = 11), carbapenems (n = 4), piperacillin–tazobactam (n = 3) and aminoglycosides (n = 3). Administered CDI treatments, according to valid guidelines at the time of the study [25], were metronidazole (n = 10; 33.3 %), vancomycin (n = 3; 10.0 %), combined metronidazole and vancomycin (n = 13; 43.3 %), and metronidazole with other therapies (n = 1; 3.3 %). Three patients did not receive treatment for CDI. CDI recurrence within 3 months of treatment discontinuation was observed in 13.3 % (4/30) of patients and two received faecal transplant for recurrent disease. Mortality within 3 months after first CDI episode was 26.7 % (8/30); CDI was the cause of death in two cases 6.7 % (2/30) (Table 2).
Table 2.
Study population and patient demographics (n = 30)
| Patient characteristic | N (%) |
|---|---|
| Male | 17 (56.7) |
| Age ≥ 65 years | 22 (73.3) |
| HA-CDI | 26 (86.7) |
| CA-CDI | 4 (13.3) |
| Recurrent CDI | 4 (13.3) |
| Severe CDI—Horn’s index | 17 (56.7) |
| Severe CDI—Atlas score | 18 (60) |
| Mortality within 3 months | 8 (26.7) |
| CDI cause of death | 2 (6.7) |
| Previous hospitalisation | 13 (59.1) |
| Previous antibiotic use | 25 (83.3) |
| Aminopenicillin/beta-lactamase inhibitors | 12 (40) |
| Cephalosporines | 11 (36.7) |
| Fluoroquinolones | 12 (40) |
| Carbapenems | 4 (13.3) |
| Piperacilin/tazobactam | 3 (10) |
| Aminoglycosides | 3 (10) |
To assess the association between C. difficile ribotype and disease severity, the clinical outcomes of patients with ribotype 176 infections were compared to those with other ribotype infections (Table 3). Analysis of ATLAS scores and the Horn’s index found that 11/14 (78.6 %) patients with ribotype 176 infections had an ATLAS score of 6–9 or a Horn’s index score of 3 or 4 compared with 6/13 (46.2 %) and 7/13 (53.9 %) of patients with non-ribotype 176 infections. Furthermore, the mortality rate appeared to be higher in patients with ribotype 176 infections compared with non-ribotype 176 infections (35.7 versus 15.4 %). No significant ribotype-associated differences were noted in recurrence rates, ICU admission rates or prior antibiotic use (Table 3).
Table 3.
Comparison of clinical outcomes in patients grouped by isolated C. difficile PCR-ribotype
| Clinical outcome | Ribotype 176 (n = 14) | Other ribotypes (n = 13) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Horn’s index | ||||
| 1 | 1 | 7.7 | ||
| 2 | 3 | 21.4 | 6 | 46.1 |
| 3 | 9 | 64.3 | 5 | 38.5 |
| 4 | 2 | 14.3 | 1 | 7.7 |
| Atlas score | ||||
| 1–2 | 2 | 15.4 | ||
| 3–5 | 3 | 21.4 | 4 | 30.8 |
| 6–7 | 9 | 64.3 | 5 | 38.5 |
| 8–9 | 2 | 14.3 | 2 | 15.4 |
| Recurrent CDI within 3 months of first episode (Yes) | 2 | 14.3 | 2 | 15.4 |
| CDI in 8 weeks prior to admission (Yes) | 1 | 7.1 | 1 | 7.7 |
| Admitted to ICU (Yes) | 3 | 21.4 | 3 | 23.1 |
| Antibiotic treatment within 1 month prior to admission (Yes) | 12 | 85.7 | 10 | 76.9 |
| Mortality within 3 months of first CDI episode (Yes) | 5 | 35.7 | 2 | 15.4 |
Clostridium difficile ribotype 027 strains are often thought to be associated with CDI outbreaks of increased disease severity [1, 5], but the clinical severity associated with ribotype 176 infections has not yet been studied in detail with exception of clinical data on ten patients, of whom 50 % had severe form of CDI, reported by Obuch-Woszczatyński et al. [9]. Our finding of a trend towards increased Horn’s index and ATLAS scores in patients with ribotype 176 infections compared with non-ribotype 176 infections provides some evidence to support the clinical importance of this ribotype. However, the small sample size of patients in this study indicates a need for further studies, incorporating a larger number of patients, to better understand the relative virulence of ribotype 176. The high incidence of epidemic C. difficile PCR-ribotype 176 in our study emphasises the importance of implementing continuous surveillance programmes for CDI at national and European level, including PCR ribotyping.
Authors’ contributions
JD analysed and interpreted of data, drafted manuscript. ON co-designed and coordinated the study, supervised the microbiological part of the study, critical revised manuscript. MK carried out molecular analysis of isolates, drafted a part of the manuscript. JS analysed data, drafted the part of the manuscript. JM was responsible for bacteriological investigation of stool samples. RK designed and coordinated the study, supervised the clinical part of the study, critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Medical writing services were provided by David Burns on behalf of Astellas Pharma EMEA to assist with grammar and English translations. Astellas Pharma EMEA had no role in study design, data collection or the decision to publish the manuscript. We would like to thank Bohdana Hola for statistical analysis of data. The authors thank the ESCMID Study Group for Clostridium difficile (ESGCD) for their professional support. Molecular and microbiological analysis of C. difficile isolates was supported by the Ministry of Health, Czech Republic Internal Grant Agency NT/14209-3 and MH CZ-DRO, University Hospital Motol, Prague, Czech Republic 00064203.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Jiri Drabek, Email: jiri.drabek@lfmotol.cuni.cz.
Otakar Nyc, Email: otakar.nyc@lfmotol.cuni.cz.
Marcela Krutova, Email: marcela.krutova@seznam.cz.
Jan Stovicek, Email: jan.stovicek@fnmotol.cz.
Jana Matejkova, Email: jana.matejkova@lfmotol.cuni.cz.
Radan Keil, Phone: +420 224433076, Email: Radan.Keil@fnmotol.cz.
References
- 1.Kuijper EJ, Coignard B, Tüll P, ESCMID Study Group for Clostridium difficile EU Member States; European Centre for Disease Prevention and Control. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 2.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ, ECDIS Study Group Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 3.Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, Delmée M, Fitzpatrick F, Ivanova K, Kuijper E, Macovei IS, Mentula S, Mastrantonio P, von Müller L, Oleastro M, Petinaki E, Pituch H, Norén T, Nováková E, Nyč O, Rupnik M, Schmid D, Wilcox MH. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID) Lancet Infect Dis. 2014;14(12):1208–1219. doi: 10.1016/S1473-3099(14)70991-0. [DOI] [PubMed] [Google Scholar]
- 4.Krutova M, Nyc O, Kuijper EJ, Geigerova L, Matejkova J, Bergerova T, Arvand M. A case of imported Clostridium difficile PCR-ribotype 027 infection within the Czech Republic which has a high prevalence of C. difficile ribotype 176. Anaerobe. 2014;30:153–155. doi: 10.1016/j.anaerobe.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Valiente E, Cairns MD, Wren BW. The Clostridium difficile PCR ribotype 027 lineage: a pathogen on the move. Clin Microbiol Infect. 2014;20(5):396–404. doi: 10.1111/1469-0691.12619. [DOI] [PubMed] [Google Scholar]
- 6.Valiente E, Dawson LF, Cairns MD, Stabler RA, Wren BW. Emergence of new PCR ribotypes from the hypervirulent Clostridium difficile 027 lineage. J Med Microbiol. 2012;61(Pt 1):49–56. doi: 10.1099/jmm.0.036194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knetsch CW, Hensgens MP, Harmanus C, van der Bijl MW, Savelkoul PH, Kuijper EJ, Corver J, van Leeuwen HC. Genetic markers for Clostridium difficile lineages linked to hypervirulence. Microbiology. 2011;157(Pt 11):3113–3123. doi: 10.1099/mic.0.051953-0. [DOI] [PubMed] [Google Scholar]
- 8.Krutova M, Matejkova J, Nyc O. C. difficile ribotype 027 or 176? Folia Microbiol (Praha). 2014;59(6):523–526. doi: 10.1007/s12223-014-0323-5. [DOI] [PubMed] [Google Scholar]
- 9.Obuch-Woszczatyński P, Lachowicz D, Schneider A, Mól A, Pawłowska J, Ożdżeńska-Milke E, Pruszczyk P, Wultańska D, Młynarczyk G, Harmanus C, Kuijper EJ, van Belkum A, Pituch H. Occurrence of Clostridium difficile PCR-ribotype 027 and it’s closely related PCR-ribotype 176 in hospitals in Poland in 2008–2010. Anaerobe. 2014;28:13–17. doi: 10.1016/j.anaerobe.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Pituch H, Obuch-Woszczatyński P, Lachowicz D, Wultańska D, Karpiński P, Młynarczyk G, van Dorp SM, Kuijper EJ. Hospital-based Clostridium difficile infection surveillance reveals high proportions of PCR ribotypes 027 and 176 in different areas of Poland, 2011 to 2013. Euro Surveill. 2015 doi: 10.2807/1560-7917.ES.2015.20.38.30025. [DOI] [PubMed] [Google Scholar]
- 11.Stubbs SL, Brazier JS, O’Neill GL, Duerden BI. PCR targeted to the 16S–23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol. 1999;37(2):461–463. doi: 10.1128/jcm.37.2.461-463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indra A, Schmid D, Huhulescu S, Hell M, Gattringer R, Hasenberger P, Fiedler A, Wewalka G, Allerberger F. Characterization of clinical Clostridium difficile isolates by PCR ribotyping and detection of toxin genes in Austria, 2006–2007. J Med Microbiol. 2008;57(Pt 6):702–708. doi: 10.1099/jmm.0.47476-0. [DOI] [PubMed] [Google Scholar]
- 13.Persson S, Torpdahl M, Olsen KE. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect. 2008;4(11):1057–1064. doi: 10.1111/j.1469-0691.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart DB, Berg A, Hegarty J. Predicting recurrence of C. difficile colitis using bacterial virulence factors: binary toxin is the key. J Gastrointest Surg. 2013;17(1):118–124. doi: 10.1007/s11605-012-2056-6. [DOI] [PubMed] [Google Scholar]
- 15.Bacci S, Mølbak K, Kjeldsen MK, Olsen KE. Binary toxin and death after Clostridium difficile infection. Emerg Infect Dis. 2011;17(6):976–982. doi: 10.3201/eid/1706.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, Wehland J, Aktories K. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5(10):e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spigaglia P, Mastrantonio P. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile clinical isolates. J Clin Microbiol. 2002;40(9):3470–3475. doi: 10.1128/JCM.40.9.3470-3475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker D, Smits WK, Kuijper EJ, Corver J. TcdC does not significantly repress toxin expression in Clostridium difficile 630ΔErm. PLoS One. 2012;7(8):e43247. doi: 10.1371/journal.pone.0043247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg RJ, Schaap I, Templeton KE, Klaassen CHW, Kuijper EJ. Typing and subtyping of Clostridium difficile isolates by using multiple-locus variable-number tandem-repeat analysis. J J Clin Microbiol. 2007;45(3):1024–1028. doi: 10.1128/JCM.02023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goorhuis A, Legaria MC, van den Berg RJ, Harmanus C, Klaassen CH, Brazier JS, Lumelsky G, Kuijper EJ. Application of multiple-locus variable-number tandem-repeat analysis to determine clonal spread of toxin A-negative Clostridium difficile in a general hospital in Buenos Aires. Argent Clin Microbiol Infect. 2009;15(12):1080–1086. doi: 10.1111/j.1469-0691.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- 21.Marsh JW, O’Leary MM, Shutt KA, Pasculle AW, Johnson S, Gerding DN, Muto CA, Harrison LH. Multilocus variable-number tandem-repeat analysis for investigation of Clostridium difficile transmission in hospitals. J Clin Microbiol. 2006;44(7):2558–2566. doi: 10.1128/JCM.02364-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora V, Kachroo S, Ghantoji SS, DuPont HL, Garey KW. High Horn´s index score predicts poor outcomes in patients with Clostridium difficile infection. J Hosp Infect. 2011;79(1):23–26. doi: 10.1016/j.jhin.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Horn SD. Measuring severity of illness: comparisons across institutions. Am J Public Health. 1983;73(1):25–31. doi: 10.2105/AJPH.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MA, Louie T, Mullane K, Weiss K, Lentnek A, Golan Y, Kean Y, Sears P. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect Dis. 2013;13:148. doi: 10.1186/1471-2334-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI) Clin Microbiol Infect. 2009;15(12):1067–1079. doi: 10.1111/j.1469-0691.2009.03099.x. [DOI] [PubMed] [Google Scholar]


