Abstract
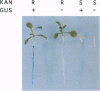
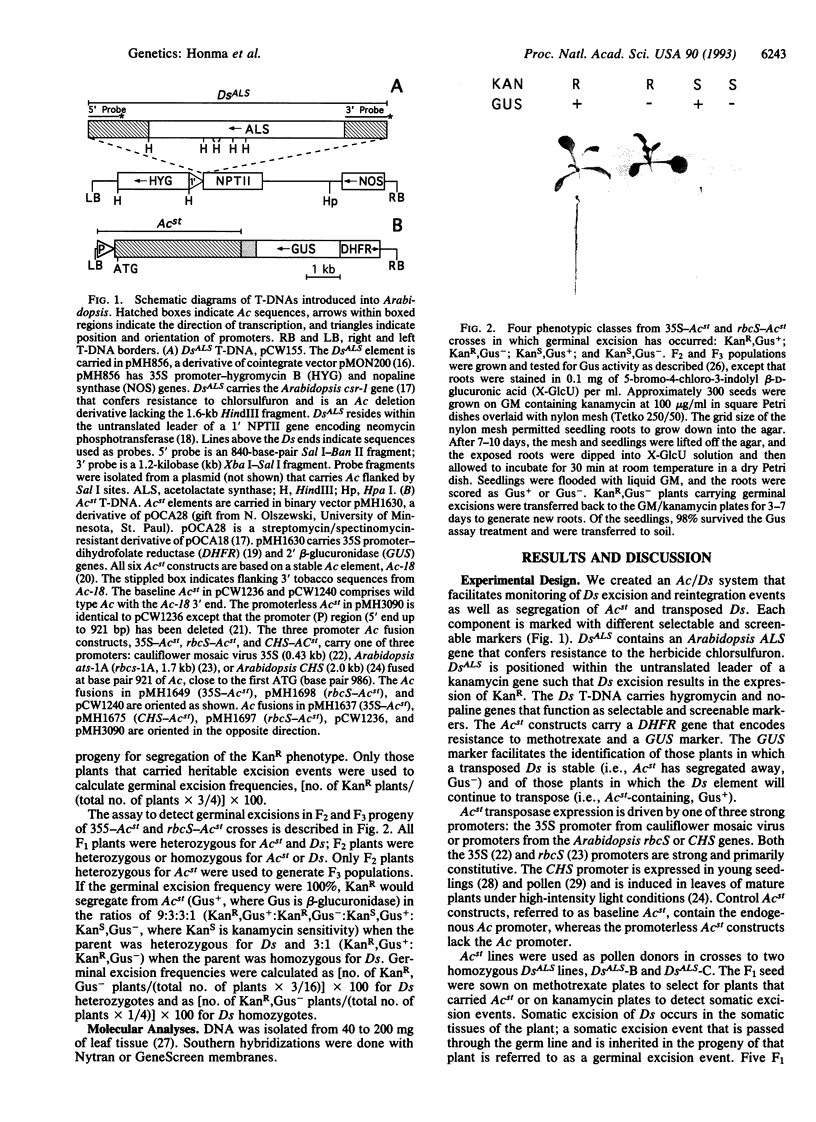
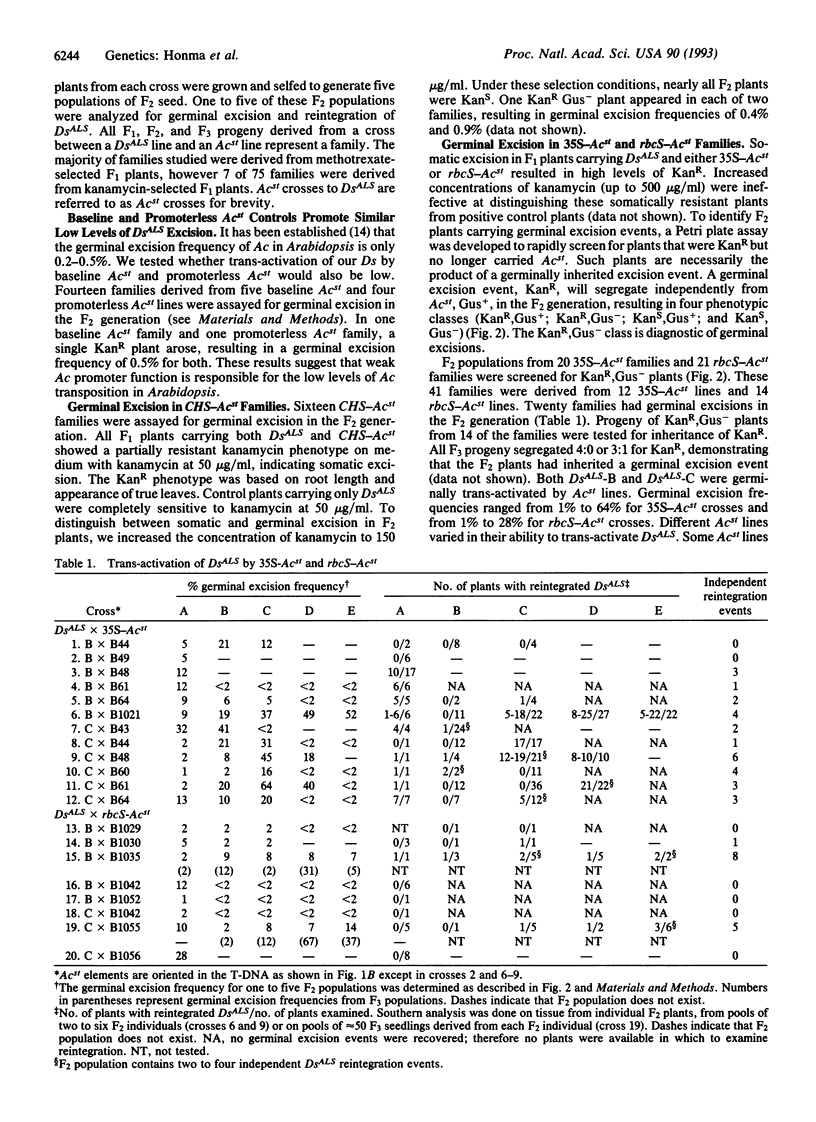
We have established an efficient transposontagging system in Arabidopsis thaliana using the Activator/Dissociation (Ac/Ds) elements from maize. This system consists of two components, a stable trans-activator, Acst, that supplies transposase, and a cis-responsive Ds element. Ds and Acst were constructed with different selectable and screenable markers to facilitate monitoring of Ds excisions and insertions as well as segregation of Ds and Acst. Fusions of the 35S, rbcS, or CHS promoters to Ac transposase were used to trans-activate DsALS, a Ds element carrying an herbicide-resistance gene. The ALS gene encoding acetolactase synthase, which confers resistance to chlorsulfuron, functioned as a versatile marker for selection of plants grown in tissue culture as well as in soil. Thirty-five Acst lines were crossed to two DsALS lines, and the resulting progeny were assayed for germinal transposition of DsALS. Trans-activation of DsALS by Acst resulted in germinal excision frequencies of up to 64% when using 35S promoter-Ac transposase fusions, up to 67% when using rbcS-transposase fusions, and up to 1% when using CHS-transposase fusions. Amongst progeny bearing terminal excisions, Southern analysis revealed that 45% from 35S-Acst crosses and 29% from rbcS-Acst crosses carried reintegrated DsALS elements. The Ac/Ds system we have developed should prove to be an effective tool for stable gene tagging in Arabidopsis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B., Coupland G., Fedoroff N., Starlinger P., Schell J. Phenotypic assay for excision of the maize controlling element Ac in tobacco. EMBO J. 1987 Jun;6(6):1547–1554. doi: 10.1002/j.1460-2075.1987.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B., Schell J., Lörz H., Fedoroff N. Transposition of the maize controlling element "Activator" in tobacco. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4844–4848. doi: 10.1073/pnas.83.13.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft I., Bhatt A. M., Sjodin C., Scofield S., Jones J. D., Dean C. Development of an efficient two-element transposon tagging system in Arabidopsis thaliana. Mol Gen Genet. 1992 Jun;233(3):449–461. doi: 10.1007/BF00265443. [DOI] [PubMed] [Google Scholar]
- Brusslan J. A., Tobin E. M. Light-independent developmental regulation of cab gene expression in Arabidopsis thaliana seedlings. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7791–7795. doi: 10.1073/pnas.89.16.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Peto C. A. Mutations in the DET1 gene affect cell-type-specific expression of light-regulated genes and chloroplast development in Arabidopsis. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8776–8780. doi: 10.1073/pnas.87.22.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland G., Baker B., Schell J., Starlinger P. Characterization of the maize transposable element Ac by internal deletions. EMBO J. 1988 Dec 1;7(12):3653–3659. doi: 10.1002/j.1460-2075.1988.tb03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. G., Cashmore A. R. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J. 1990 Jun;9(6):1717–1726. doi: 10.1002/j.1460-2075.1990.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum R. L., Storz G., Ausubel F. M. High intensity and blue light regulated expression of chimeric chalcone synthase genes in transgenic Arabidopsis thaliana plants. Mol Gen Genet. 1991 May;226(3):449–456. doi: 10.1007/BF00260658. [DOI] [PubMed] [Google Scholar]
- Feldmann K. A., Marks M. D., Christianson M. L., Quatrano R. S. A Dwarf Mutant of Arabidopsis Generated by T-DNA Insertion Mutagenesis. Science. 1989 Mar 10;243(4896):1351–1354. doi: 10.1126/science.243.4896.1351. [DOI] [PubMed] [Google Scholar]
- Grevelding C., Becker D., Kunze R., von Menges A., Fantes V., Schell J., Masterson R. High rates of Ac/Ds germinal transposition in Arabidopsis suitable for gene isolation by insertional mutagenesis. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6085–6089. doi: 10.1073/pnas.89.13.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl R., Baker B. Properties of the maize transposable element Activator in transgenic tobacco plants: a versatile inter-species genetic tool. Plant Cell. 1990 Aug;2(8):709–721. doi: 10.1105/tpc.2.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A., Voytas D. F., Cummings M. P., Ausubel F. M. A superfamily of Arabidopsis thaliana retrotransposons. Genetics. 1991 Apr;127(4):801–809. doi: 10.1093/genetics/127.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasek W. L., Shirley B. W., McKillop A., Goodman H. M., Briggs W., Ausubel F. M. Regulation of Flavonoid Biosynthetic Genes in Germinating Arabidopsis Seedlings. Plant Cell. 1992 Oct;4(10):1229–1236. doi: 10.1105/tpc.4.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M. Arabidopsis thaliana. Annu Rev Genet. 1987;21:93–111. doi: 10.1146/annurev.ge.21.120187.000521. [DOI] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Olszewski N. E., Martin F. B., Ausubel F. M. Specialized binary vector for plant transformation: expression of the Arabidopsis thaliana AHAS gene in Nicotiana tabacum. Nucleic Acids Res. 1988 Nov 25;16(22):10765–10782. doi: 10.1093/nar/16.22.10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleman J., Cottyn B., Van Camp W., Van Montagu M., Inzé D. Transient occurrence of extrachromosomal DNA of an Arabidopsis thaliana transposon-like element, Tat1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3618–3622. doi: 10.1073/pnas.88.9.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Tp., Goodman H. M., Ausubel F. M. Cloning the Arabidopsis GA1 Locus by Genomic Subtraction. Plant Cell. 1992 Feb;4(2):119–128. doi: 10.1105/tpc.4.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburne J., Balcells L., Scofield S. R., Jones J. D., Coupland G. Elevated levels of Activator transposase mRNA are associated with high frequencies of Dissociation excision in Arabidopsis. Plant Cell. 1992 May;4(5):583–595. doi: 10.1105/tpc.4.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sluys M. A., Tempé J., Fedoroff N. Studies on the introduction and mobility of the maize Activator element in Arabidopsis thaliana and Daucus carota. EMBO J. 1987 Dec 20;6(13):3881–3889. doi: 10.1002/j.1460-2075.1987.tb02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas D. F., Ausubel F. M. A copia-like transposable element family in Arabidopsis thaliana. Nature. 1988 Nov 17;336(6196):242–244. doi: 10.1038/336242a0. [DOI] [PubMed] [Google Scholar]
- Wessler S. R., Baran G., Varagona M., Dellaporta S. L. Excision of Ds produces waxy proteins with a range of enzymatic activities. EMBO J. 1986 Oct;5(10):2427–2432. doi: 10.1002/j.1460-2075.1986.tb04517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]