Abstract
The present study evaluates the synergistic effect of sulbactam/tazobactam in combination with meropenem or colistin against multidrug resistant (MDR) Acinetobacter baumannii isolated from hospitalized patients from a tertiary care hospital in Saudi Arabia. During the study period, 54 multidrug and carbapenem-resistant isolates of A. baumannii isolates were collected from blood and respiratory samples of patients with ventilator-associated pneumonia or bacteremia. Microbroth checkerboard assay (CBA) and E-test were performed to look for synergistic interface of sulbactam and tazobactam with meropenem or colistin. All 54 MDR isolates of A. baumannii were resistant to carbapenem. Minimum inhibitory concentration [50/90] value against sulbactam, tazobactam, meropenem, colistin was found to be 64/128, 64/128, 64/256, and 0.5/1.0 respectively. Synergy was detected in more isolates with CBA compared to E-test. All four combinations showed significant synergistic bactericidal activity. However, the combination with colistin showed greater synergistic effect than combination with meropenem. Antagonism was not detected with any of the combinations and any method, but indifference was seen in tazobactam and colistin combination alone. A significant bactericidal effect was seen with sulbactam combination with meropenem or colistin in both methods. A combination therapy can be a choice of treatment. As colistin is known to exhibit nephrotoxicity, the combination of sulbactam and meropenem might be considered as an alternative antibiotic treatment for such multi- and extremely resistant bacteria. Yet, sample size is small in our study, so further well-designed in vitro and clinical studies on large scale should confirm our findings.
KEY WORDS: Acinetobacter baumannii, additive, meropenem, synergy, sulbactam, tazobactam
INTRODUCTION
Acinetobacter baumannii persists in hospital environments and cause severe, life-threatening infections in immunocompromised patients [1]. The spectrum of antibiotic resistances of these organisms together with their survival capabilities makes them a threat - in hospital healthcare [2]. A. baumanni is the second-most-commonly-isolated, non-fermenting bacteria in human specimens. It is frequently isolated in nosocomial infections and is especially prevalent in intensive care units [2-8]. A. baumannii displays mechanisms of resistance to all existing antibiotic classes, as well as a prodigious capacity to acquire new determinants of resistance [6,9].
A. baumannii was susceptible to most of the antibiotics till the 1970s. It has now become a major cause of hospital-acquired infections worldwide due to its remarkable propensity to rapidly acquire resistance to a wide range of antibacterial agents [5,9-11]. Sequence similarity and phylogenetic analyses confirm that most of the resistance genes found in the A. baumannii have been recently acquired from bacteria of the genera Pseudomonas, Salmonella, or Escherichia [10]. A. baumannii exhibits a remarkable ability to rapidly develop antibiotic resistance that led to multidrug resistance (MDR) within a few decades [2,9]. To date, some strains of A. baumannii have become resistant to almost all currently available antibacterial agents, mostly through the acquisition of plasmids, transposons, or integrons carrying clusters of genes encoding resistance to several antibiotic families at once [2,5,9,11].
At present, colistin and tigecycline are being used as drug of choice for the treatment of infection caused by Acinetobacter spp. [12,13]. However, Food and Drug Administration allowed the use of tigecycline only for complicated and soft tissue infections, including intra-abdominal infection and community acquired pneumonia, but did not approve its use for ventilator-associated pneumonia. On another hand, the use of colistin is associated with difficulties with optimization of dosage during the course of treatment [14]. Colistin is a decades-old drug that floor out of indulgence due to its nephrotoxicity [2], however, it remains a last-resort antibiotic for multidrug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter [15].
In recent days the use of two different antibiotics in combination has been explored as a successful therapeutic option [16-18]. Sulbactam and tazobactam are an irreversible inhibitors of β-lactamase and they are given in combination with β-lactam antibiotics to inhibit β-lactamase [19]. Sulbactam and tazobactam are considered having intrinsic activity against Acinetobacter spp. and can be explored as a therapeutic option in combination with the drug of choice, which are being limited day by day. Sulbactam has been used in combination with ampicillin and cefoperazone for the treatment of Acinetobactor spp. while tazobactam used with ceftolozane and piperacillin against S. pneumonaie and Pseudomoans respectively [20-22]. Previous reports suggest that sulbactam are active against Acinetobactor in combination with ampicillin, as well as alone however breakpoints are not yet decided for sulbactam.
In our study, we determine the minimum inhibitory concentration (MIC) for sulbactam/tazobactam individually and in vitro synergistic activity of both in combination with meropenem and colistin against multidrug-resistant A. baumanii.
MATERIALS AND METHODS
Ethics statement
The present study was designed and approved by the Institutional Review Board and Ethics Committee of King Saud University, Riyadh. The patients were informed and consent was obtained for the use of the clinical isolates originated from them.
Study design and bacterial isolates
The study was conducted at Clinical laboratory department, College of applied Medical sciences, King Saud University, Riyadh for a period of one year during July 2013 to June 2014. Non duplicate clinical strain of A. baumannii resistant to more than two families of antibiotics including carbapenem was collected from sputum and blood sources of hospitalized patients.
Standard microbiological lab protocol was used to identify clinical isolates. A. baumannii species were further confirmed by Vitex 2 system and sequence analysis of 16S rRNA region of DNA from each isolate. All the isolates were preserved in 5% nutrient agar deeps and stored at 4°C further future use [23,24].
Antibiotic susceptibility testing
Susceptibility to different antibiotics such as amikacin, netilmicin, levofloxacin, piperacillin-tazobactam, cefotaxime, ceftazidime, cefepime, tobramycin, imipenem, meropenem, and colistin was determined by disk diffusion testing according to Clinical and Laboratory Standards Institute (CLSI) guidelines (M100-S23 2013) [25].
MIC of sulbactam, tazobactam, meropenem, and colistin were determined by agar dilution method as per CLSI guidelines. The MIC50 and MIC90 were determined for all four antibiotics tested for MIC.
In vitro synergy testing using E-test and checkerboard assay (CBA)
To evaluating the efficacy of the drug combination as an option for therapy all the isolates were tested for the bactericidal synergistic effect by using sulbactam and tazobactam incorporated in agar with meropenem or colistin E-test strip (Biomeurix, France) on plate. Different sets of MHA plates incorporated with Sulbactam or tazobactam were prepared for respective combination depending upon the MICs of the organisms to be tested. For example the isolates with MIC of 8, 16, 32, 64, 128, 256, and 512 µg/mL were plated on MHA agar media plates incorporated with 4, 8, 16, 32, 64, 128, and 256 µg/mL of sulbactam or tazobactam respectively followed by E-test strip.
Also, checkerboard synergy testing was performed in triplicate with all isolates. The test was performed and interpretation of the results was determined as described earlier in literature [16,17]. In brief, Fractional Inhibitory Concentration Index (FIC Index) was used to assess synergistic activity, which was determined by the addition of fractional inhibitory concentrations of the antibiotic combination used in the test. FIC of each agent was calculated as a ratio of MIC when used in combination and MIC when used alone. Sulbactam or tazobactam was identified to act synergistically to meropenem or colistin when there was a ≥3 dilution reduction in the MIC of the combination compared to the MIC of individual meropenem or colistin. However, FIC Index was used to assess synergistic activity. The FIC index of all was calculated as follows: ΣFIC = FICA + FICB = (CA/MICA) + (CB/MICB), where MICA and MICB represent are MICs of antibiotic A and B alone, respectively, while CA and CB are the MICs of the antibiotics in combination. According to accepted criteria results were recorded as follows: ≤0.5, synergy; >0.5-≤1, additivity; >1-≤4, indifference; and >4, antagonism.
Statistical analysis
The two-tailed Student’s t-test was used to interpret statistical significance of the results.
RESULTS
During a study period of one year total of 54 MDR including resistant to carbapenem-resistant A. baumannii isolates were tested. Twenty-four isolates were from sputum and the rest were from the blood samples.
Antimicrobial testing and MIC
Disc diffusion testing showed all isolates to be MDR defined as non-susceptible to at least one agent in all but two or fewer antimicrobial categories. All isolates were susceptible to colistin. Results showed high MIC value for sulbactam, tazobactam and meropenem with MIC ≥16, MIC ≥32 and MIC ≥16 µg/mL respectively (Figure 1). MIC for colistin range between 0.125 and 4.0 µg/mL.
FIGURE 1.
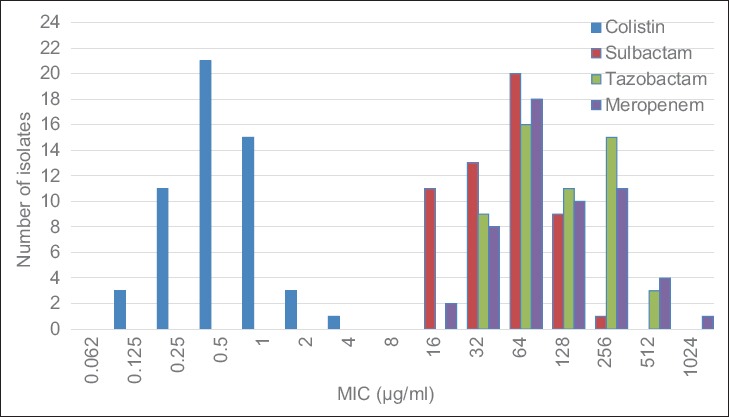
MIC for multidrug resistant Acinetobacter baumannii (n = 54) sulbactam, tazobatam, meropenem and colistin. MIC: minimum inhibitory concentration
In vitro synergy testing using E-test and CBA
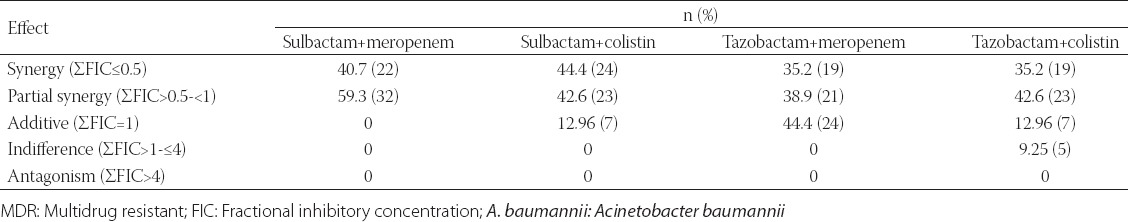
The FIC index for all four antibiotics combination is presented in Tables 1 and 2 (CBA and E-test). FIC index 0.5-1 in CBA for all combination whereas it was in the range of 0.5-4 when tested with E-test strip. Synergy in CBA was seen in 24 (44.4%) isolates for sulbactam plus meropenem, 29 (53.7%) isolates for sulbactam plus colistin which was comparatively more than tazobactam combination with meropenem (22 [40.7%]) or colistin (23 [42.6%]), Synergy by E testing was seen in 22 (40.7%) isolates for sulbactam plus meropenem, 24 (44.4%) isolates for sulbactam plus colistin which was comparatively more than tazobactam combination with meropenem (19 [35.2%]) or colistin (19 [35.2%]). In all combination synergy was detected in more isolates by checkerboard method than E-test method. Interestingly partial synergy was detected in more isolates by checkerboard method than E-test method. Additive case was detected significantly more in tazobactam combination with meropenem or colistin irrespective of testing method. No cases of antagonism were detected in any isolates and 5 (9.25%) indifference was seen in tazobactam and colistin combination alone (Tables 1 and 2).
TABLE 1.
Combination effect of sulbactam/tazobactum with meropenem and colistin against MDR A. baumannii by CBA
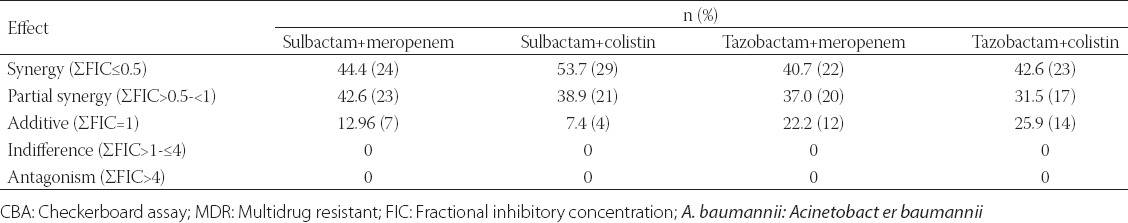
TABLE 2.
Combination effect of sulbactam/tazobactum with meropenem and colistin against MDR A. baumannii by E-test
DISCUSSION
There is rapidly increase bacterial resistant and it is alarming that we may face the end of the “antibiotic era.” The preliminary and apparently overwhelming success of antibiotics has been countered by an increase of resistance mechanisms and emergence of many genera of bacteria that are resistant to almost all antibiotics exiting [26-28]. In respect of Gram-negative particular to A. baumannii, Pseudomonas and K. pneumonaie, colistin and tigecyclin are the last drugs of choice which are known for nephrotoxicity [2,15,29]. In recent past combine drug therapy is getting more attention in clinics for treatment of such MDR bacterial infection. Coistin and tigecyclin are last line of drug choice and they can be used as monotherapies against MDR bacteria, such as colistin, tigecycline and b-lactams. However, irrational continuous use of monotherapies tends to induced resistant in bacterial community, therefore, combine therapy can be best alternative [30].
We performed the study which evaluates the synergistic effect of sulbactam/tazobactam with a combination of meropenem or colistin. However, sulbactam and tazobactam has been used in combination with ampicillin and piperacillin already. This study might be first from our region to show in vitro synergy between sulbactam/tazobactam plus meropenem or colistin against multidrug isolates originating from clinical samples.
The detection level of synergy or indifference was concordant between the two methods in approximately half of the isolates for all drug combinations. With regard to sulbactam plus meropenem or sulbactam plus colistin combination, this synergistic or partial synergistic effect was found in more number of isolates than tazobactam plus meropenem or tazobactam plus colistin respectively in both assay. Almost 87% of isolates were showing synergy and partial synergy in sulbactam plus meropenem combination by both assay with no significant differences. Additive was detected only in 7 (12.96%) and 4 (7.4%) isolates in sulbactam plus meropenem and sulbactam plus colistin combination respectively in checkerboard assay, but in E-test assay additive was detected only in sulbactam plus colistin combination (7 [12.96%]). Isolates showing additive were significantly more in tazobactam plus meropenem (in checkerboard assay 12 [22.2%]), (E-test 24 [44.4%]) and in tazobactam plus colistin (in checkerboard assay 14 [25.9%]), (E-test 7 [12.96%]). Even though isolates were highly resistant to meropenem, sulbactam and tazobactam with high MIC value the combination of the drugs seems to be highly synergistic in vitro. In a couple of study done previously have reported synergy between meropenem, colistin, sulbactam and tigecycline against imipenem-resistant A. baumannii [31] MDR and Pan drug resistant [9,32,33]. Earlier studies investigated potential synergy between meropenem and sulbactam only by CBA showing synergy and/or partial synergy in more than 50% of carbapenem-resistant isolates of A. baumannii [34,35].
All the antibiotic combinations that showed synergy in the E-test assay for tested isolates, also showed synergy in CBA (Tables 1 and 2). On the other hand, rest of these isolates showed additivity (no synergy) or partial synergy in the CBA. Our results are quite `contradictory with one published earlier [9] however antibiotics combination were different in our study.
In our testing, we have used CBA which is considered to be standard method to evaluate synergistic effect of the drug in combination whereas E-test can be alternative technique. An expertise is a need while performing CBA, E-test was also easier to perform, less time-consuming, and less expensive [9]. Time-kill assay is another choice of technique, but limited drug concentrations and ratios can be tested whereas advantage of using checkerboard over time-kill assay is the use of multiple combinations of the agents at varying concentrations and ratios. There is a degree of dissimilarity of the results between two tested methods for the two drug combinations. Another limitation of our study include that these combinations were not tested by time-kill analysis and do not know results if tested by time-kill analysis. The aptitude of in vitro combination testing to forecast clinical outcome is unidentified as a previous study showed contrast in vitro results to clinical benefits that compared colistin versus colistin plus rifampin [36]. Further, clinical studies determining the relevance of these data are warranted. The clinical benefits of these antibiotic combinations in vivo can only be determined by assessing synergies through carefully designed pharmacokinetic studies and through multicenter randomized clinical trials [34].
With each day passing, there is a rapid increase in bacterial resistance and a chance of ending the drug of choice for treatment [13,27]. Due to the lack of more options and effective antimicrobial agents against multidrug and extremely drug resistant strains, the use of combined drug therapy is increased; in such scenario our findings are even more important. A useless antibiotic as a single therapy might be effective in a drug combination therapy.
CONCLUSION
Our results indicate significant synergistic effect between sulbactam and meropenem or colistin against MDR A. baumannii isolates. A large-scale study should be designed and performed further in vitro or clinical settings to draw a solid conclusion.
ACKNOWLEDGMENTS
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (MED 3127).
DECLARATION OF INTERESTS
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- [1].Al-Anazi KA, Abdalhamid B, Alshibani Z, Awad K, Alzayed A, Hassan H, et al. Acinetobacter baumannii septicemia in a recipient of an allogeneic hematopoietic stem cell transplantation. Case Rep Transplant 2012. 2012. p. 646195. http://www.dx.doi.org/10.1155/2012/646195 . [DOI] [PMC free article] [PubMed]
- [2].Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, et al. Effectiveness of tigecycline-based versus colistin- based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: A matched cohort analysis. BMC Infect Dis. 2014;14:102. doi: 10.1186/1471-2334-14-102. http://www.dx.doi.org/10.1186/1471-2334-14-102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dash M, Padhi S, Pattnaik S, Mohanty I, Misra P. Frequency, risk factors, and antibiogram of Acinetobacter species isolated from various clinical samples in a tertiary care hospital in Odisha, India. Avicenna J Med. 2013;3:97–102. doi: 10.4103/2231-0770.120501. http://www.dx.doi.org/10.4103/2231-0770.120501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dougari HJ, Ndakidemi PA, Human IS, Benade S. Virulence factors and antibiotic susceptibility among verotoxic non O157: H7 Escherichia coli isolates obtained from water and waste water samples in Cape Town, South Africa. Afr J Biotechnol. 2011;10:14160–8. [Google Scholar]
- [5].Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. http://www.dx.doi.org/10.1128/CMR.00058-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu YS, Yang Q, Xu XW, Kong HS, Xu GY, Zhong BY. Typing and characterization of carbapenem-resistant Acinetobacter calcoaceticus-baumannii complex in a Chinese hospital. J Med Microbiol. 2004;53:653–6. doi: 10.1099/jmm.0.05513-0. http://www.dx.doi.org/10.1099/jmm.0.05513-0 . [DOI] [PubMed] [Google Scholar]
- [7].Falagas ME, Karveli EA. The changing global epidemiology of Acinetobacter baumannii infections: A development with major public health implications. Clin Microbiol Infect. 2007;13:117–9. doi: 10.1111/j.1469-0691.2006.01596.x. http://www.dx.doi.org/10.1111/j.1469-0691.2006.01596.x . [DOI] [PubMed] [Google Scholar]
- [8].Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–9. doi: 10.1086/500202. http://www.dx.doi.org/10.1086/500202 . [DOI] [PubMed] [Google Scholar]
- [9].Sopirala MM, Mangino JE, Gebreyes WA, Biller B, Bannerman T, Balada-Llasat JM, et al. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. italic>Antimicrob Agents Chemother. 2010;54:4678–83. doi: 10.1128/AAC.00497-10. http://www.dx.doi.org/10.1128/AAC.00497-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. http://www.dx.doi.org/10.1371/journal.pgen.0020007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maragakis LL, Perl TM. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–63. doi: 10.1086/529198. http://www.dx.doi.org/10.1086/529198 . [DOI] [PubMed] [Google Scholar]
- [12].Manchanda V, Sanchaita S, Singh N. Multidrug resistant Acinetobacter. J Glob Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. http://www.dx.doi.org/10.4103/0974-777X.68538 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lakshmana Gowda K, Marie MA, Al-Sheikh YA, John J, Gopalkrishnan S, Chikkabidare Shashidhar P, et al. A 6-year surveillance of antimicrobial resistance patterns of Acinetobacter baumannii bacteremia isolates from a tertiary care hospital in Saudi Arabia during 2005-2010. Libyan J Med. 2014;9:24039. doi: 10.3402/ljm.v9.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].US Food and Drug Administration. Full prescribing information for Tygacil. 2010. [Last accessed on 2014 Jun 30]. pp. 431–3. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf.
- [15].Falagas ME, Grammatikos AP, Michalopoulos A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev Anti Infect Ther. 2008;6:593–600. doi: 10.1586/14787210.6.5.593. http://www.dx.doi.org/10.1586/14787210.6.5.593 . [DOI] [PubMed] [Google Scholar]
- [16].Cetin ES, Tekeli A, Ozseven AG, Us E, Aridogan BC. Determination of in vitro activities of polymyxin B and rifampin in combination with ampicillin/sulbactam or cefoperazone/sulbactam against multidrug-resistant Acinetobacter baumannii by the E-test and checkerboard methods. Jpn J Infect Dis. 2013;66:463–8. doi: 10.7883/yoken.66.463. http://www.dx.doi.org/10.7883/yoken.66.463 . [DOI] [PubMed] [Google Scholar]
- [17].Berçot B, Poirel L, Dortet L, Nordmann P. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J Antimicrob Chemother. 2011;66:2295–7. doi: 10.1093/jac/dkr296. http://www.dx.doi.org/10.1093/jac/dkr296 . [DOI] [PubMed] [Google Scholar]
- [18].Krishnappa LG, Marie MA, Al Sheikh YA. Characterization of carbapenem resistance mechanisms in Klebsiella pneumoniae and in vitro synergy of the colistin-meropenem combination. J Chemother. 2014 doi: 10.1179/1973947814Y.0000000197. 1973947814Y0000000197. [DOI] [PubMed] [Google Scholar]
- [19].Totir MA, Helfand MS, Carey MP, Sheri A, Buynak JD, Bonomo RA, et al. Sulbactam forms only minimal amounts of irreversible acrylate-enzyme with SHV-1 beta-lactamase. Biochemistry. 2007;46:8980–7. doi: 10.1021/bi7006146. http://www.dx.doi.org/10.1021/bi7006146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. http://www.dx.doi.org/10.1128/CMR.00037-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Corbella X, Ariza J, Ardanuy C, Vuelta M, Tubau F, Sora M, et al. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother. 1998;42:793–802. doi: 10.1093/jac/42.6.793. http://www.dx.doi.org/10.1093/jac/42.6.793 . [DOI] [PubMed] [Google Scholar]
- [22].Choi JY, Kim CO, Park YS, Yoon HJ, Shin SY, Kim YK, et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med J. 2006;47:63–9. doi: 10.3349/ymj.2006.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winn W, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Ch. 7. Baltimore, USA: Lippincott Williams & Wilkins; 2006. The nonfermentative Gram-negative bacilli; pp. 309–55. [Google Scholar]
- [24].Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clinical and Laboratory Standards Institute. CLSI Document M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational supplement. [Google Scholar]
- [26].Ku K, Pogue JM, Moshos J, Bheemreddy S, Wang Y, Bhargava A, et al. Retrospective evaluation of colistin versus tigecycline for the treatment of Acinetobacter baumannii and/or carbapenem-resistant Enterobacteriaceae infections. Am J Infect Control. 2012;40:983–7. doi: 10.1016/j.ajic.2011.12.014. http://www.dx.doi.org/10.1016/j.ajic.2011.12.014 . [DOI] [PubMed] [Google Scholar]
- [27].Al Sheikh YA, Marie MA, John J, Krishnappa LG, Dabwab KH. Prevalence of 16S rRNA methylase genes among ß-lactamase-producing Enterobacteriaceae clinical isolates in Saudi Arabia. Libyan J Med. 2014;9:p24432. doi: 10.3402/ljm.v9.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marie MA, John J, Krishnappa LG, Gopalkrishnan S. Molecular characterization of the ß-lactamases in Escherichia coli and Klebsiella pneumoniae from a tertiary care hospital in Riyadh, Saudi Arabia. Microbiol Immunol. 2013;57:805–10. doi: 10.1111/1348-0421.12104. http://www.dx.doi.org/10.1111/1348-0421.12104 . [DOI] [PubMed] [Google Scholar]
- [29].Spapen H, Jacobs R, Van Gorp V, Troubleyn J, Honoré PM. Renal and neurological side effects of colistin in critically ill patients. Ann Intensive Care. 2011;1:14. doi: 10.1186/2110-5820-1-14. http://www.dx.doi.org/10.1186/2110-5820-1-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dong X, Chen F, Zhang Y, Liu H, Liu Y, Ma L. In vitro activities of rifampin, colistin, sulbactam and tigecycline tested alone and in combination against extensively drug-resistant Acinetobacter baumannii. J Antibiot (Tokyo) 2014;67:677–80. doi: 10.1038/ja.2014.99. http://www.dx.doi.org/10.1038/ja.2014.99 . [DOI] [PubMed] [Google Scholar]
- [31].Peck KR, Kim MJ, Choi JY, Kim HS, Kang CI, Cho YK, et al. In vitro time-kill studies of antimicrobial agents against blood isolates of imipenem-resistant Acinetobacter baumannii including colistin- or tigecycline-resistant isolates. J Med Microbiol. 2012;61:353–60. doi: 10.1099/jmm.0.036939-0. http://www.dx.doi.org/10.1099/jmm.0.036939-0 . [DOI] [PubMed] [Google Scholar]
- [32].Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57:5104–11. doi: 10.1128/AAC.01230-13. http://www.dx.doi.org/10.1128/AAC.01230-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pongpech P, Amornnopparattanakul S, Panapakdee S, Fungwithaya S, Nannha P, Dhiraputra C, et al. Antibacterial activity of carbapenem-based combinations againts multidrug-resistant Acinetobacter baumannii. J Med Assoc Thai. 2010;93:161–71. [PubMed] [Google Scholar]
- [34].Kiffer CR, Sampaio JL, Sinto S, Oplustil CP, Koga PC, Arruda AC, et al. In vitro synergy test of meropenem and sulbactam against clinical isolates of Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2005;52:317–22. doi: 10.1016/j.diagmicrobio.2005.03.003. http://www.dx.doi.org/10.1016/j.diagmicrobio.2005.03.003 . [DOI] [PubMed] [Google Scholar]
- [35].Turk Dagi H, Kus H, Arslan U, Tuncer I. In vitro synergistic activity of sulbactam in combination with imipenem, meropenem and cefoperazone against carbapenem-resistant Acinetobacter baumannii isolates. Mikrobiyol Bul. 2014;48:311–5. doi: 10.5578/mb.7104. http://www.dx.doi.org/10.5578/mb.7104 . [DOI] [PubMed] [Google Scholar]
- [36].Simsek F, Gedik H, Yildirmak MT, Iris NE, Türkmen A, Ersoy A, et al. Colistin against colistin-only-susceptible Acinetobacter baumannii-related infections: Monotherapy or combination therapy? Indian J Med Microbiol. 2012;30:448–52. doi: 10.4103/0255-0857.103767. http://www.dx.doi.org/10.4103/0255-0857.103767 . [DOI] [PubMed] [Google Scholar]


