Abstract
Background
Absolute neutrophil counts (ANCs) are lower in East African adults. To assess the impact of lower ANCs, we reviewed data from HIV-infected Kenyan women receiving antiretroviral therapy antepartum and postpartum.
Methods
The Kisumu Breastfeeding Study (KiBS) participants received an antiretroviral regimen from 34 weeks’ gestation through 6 months postpartum. Measured ANCs and subsequent illnesses were reviewed. Adverse events (AEs) potentially attributable to neutropenia were identified, and ANCs were graded using the 2004 Division of AIDS table for Grading the Severity of AEs.
Results
Among 478 women with ≥ 1 postpartum ANC measured, 298 (62.1%) women met criteria for an AE (<1.3 × 109 cells/L). Of those, 38 (12.5%) women experienced a nonlife-threatening illness potentially attributable to neutropenia.
Conclusion
More than half of KiBS women met criteria for neutropenia. The mild clinical experience of most participants with low ANCs supports that these values might be typical for this population and may not result in adverse clinical sequelae.
Keywords: absolute neutrophil counts, African, HIV infected, pregnant, adverse events
Introduction
The National Institutes of Health (NIH), Division of AIDS (DAIDS), has produced criteria for grading the severity of adult and pediatric adverse events in clinical trials related to HIV disease, which includes criteria for grading neutrophil and lymphocyte counts.1 These criteria are based on data from North American populations, but they are often used to identify reportable adverse events for research with populations in other regions of the world and as the basis for exclusion criterion from clinical trials. Normal values for white blood cells may vary due to methodological differences by laboratories or due to ethnicity, sex, environmental, and nutritional factors.2–4 Neutrophil counts have been reported to be lower in East African adults than in North American adults,5–11 and studies in South and West African adults have reported neutrophil counts with values similar to those observed among East African adults (Table 1).12–16 In a population of healthy adults in the United States, mean white blood cell counts were higher for white men (4.3 cells × 109/L) and women (4.5 cells × 109/L) than that of black men (3.5 cells × 109/L) and women (3.8 cells × 109/L).17 White blood cell counts are also altered significantly during pregnancy, for example, among North American women, white blood cell counts in the third trimester typically range from 5 to 12 × 109 cells/L, peak at delivery ranging from 20 to 30 × 109 cells/L, then fall soon thereafter to prepregnancy (nonpregnant) levels.18,19
Table 1.
Absolute Neutrophil Counts Reported for Various Populations of East, West, and Southern Africans.
| Population | Year | Number of Patients | Absolute Neutrophil Count, Median (109/L) |
|---|---|---|---|
| Eastern Africa | |||
| Rural Tanzania and Kenya, healthy adult outpatients living at5 | |||
| Sea level | 1958 | 60 | 2.61 (SE: 0.12) |
| 3700 feet elevation | 221 | 2.18 (SE: 0.07) | |
| 6800 feet elevation | 97 | 2.39 (SE: 0.10) | |
| Urban Uganda, healthy adult students6 | 1961 | 160 | 2.10 (SD: 0.55–3.7) |
| Urban Kenya, healthy adult blood donors7 | 1969 | 152 | 2.11 (R: 2.00–10.7) |
| Rural Kenya, adult outpatients living at8 | |||
| Sea level | 1969 | 103 | 2.60 (95% CI: 1.05–5.40) |
| 1800 feet elevation | 96 | 2.41 (95% CI: 0.86–5.65) | |
| 6800 feet elevation | 89 | 2.90 (95% CI: 0.99–6.65) | |
| Urban Uganda, healthy adult blood donors9 | 1971 | 250 | 1.96 (95% CI: 0.32–3.60) |
| Rural Uganda, healthy HIV-negative adults10 | |||
| Age 19–24 years | 2002 | 235 | 1.80 (90% RI: 1.00–3.50) |
| Age >24 years | 845 | 1.80 (90% RI: 0.84–3.37) | |
| Rural Western Kenya, population-based adults (13–34 years)11 | |||
| Female, 13–17 years | 2003–2005 | 57 | 2.0 (95% CI: 1.0–6.2) |
| Male, 13–17 years | 76 | 1.9 (95% CI: 0.8–5.0) | |
| Female, 18–34 years | 83 | 2.3 (95% CI: 1.3–5.4) | |
| Male, 18–34 years | 77 | 2.0 (95% CI: 0.8–3.9) | |
| Western Africa | |||
| Zaria, Nigeria, HIV-infected adults screening visit for HAART11 | 2004 | 400 | 3.20 (R: 6.90–15.60) |
| Nigeria, previously antiretroviral naive HIV-infected adults12 | 2005 | 100 | 2.32 (R: 0.00–5.48) |
| Abidjan, Côte d’Ivoire, healthy HIV-infected taking zidovudine-containing HAART15 | 2005 | 504 | 1.65 (IQR: 1.22–2.26) |
| Southern Africa | |||
| Zambia, healthy adult blood donors13 | 1972 | 255 | 1.91 (R: 0.71–3.11) |
| Zimbabwe, HIV-negative pregnant women14 | 2002 | 998 | 5.30 (R: 0.30–21.30) |
Abbreviations: HAART, highly active antiretroviral therapy; SE, standard error; SD, standard deviation; R, range; IQR, interquartile range; 95% CI, confidence interval; 90% RI, 90% reference interval.
As many as 50% of HIV-infected patients may experience neutropenia over the course of their disease.20 As CD4 counts decrease, the risk of opportunistic infections and complications of neutropenia can increase and patients may have a dysfunctional neutrophil response.21,22 Neutropenia is also a risk factor for bacterial infections, such as pneumonia or sepsis for HIV-infected individuals.23,24 During the early part of the AIDS epidemic in North America, neutropenia was often associated with zidovudine (ZDV) treatment at doses higher than those used today.25–28 Zidovudine is still commonly used in many resource-limited settings for pregnant women because of its widespread availability in a fixed-dose combination tablet, favorable cost, and effectiveness for prevention of mother-to-child transmission (PMTCT) of HIV.28
The Kisumu Breastfeeding Study (KiBS) was a phase IIb open-label single-arm PMTCT trial of maternal triple antiretro-viral (ARV) prophylaxis started at 34 to 36 weeks of gestation and continued until 24 weeks postpartum conducted in western Kenya.29 As part of this study, participants’ neutrophil counts were measured at each scheduled study visit. The unexpectedly large number of low absolute neutrophil counts (ANC) that met Division of AIDS (DAIDS) criteria for an adverse event prompted us to review ANCs measured in this trial and to assess for complications related to low ANCs. Our objectives were to examine the frequency of low ANCs and adverse events potentially caused by low neutrophil counts in HIV-infected women treated with maternal triple ARV prophylaxis.
Methods
The Kisumu Breastfeeding Study enrolled HIV-infected ARV-naive pregnant women attending a public health clinic in Kisumu, Kenya, between July 2003 and November 2006; follow-up was completed in February 2009.29 Women meeting the inclusion criteria received ZDV 300 mg/lamivudine (3TC) 150 mg as a fixed-dose combination tablet (Combivir, GlaxoS-mithKline, Brentford, United Kingdom) taken twice daily (BID), and nevirapine (NVP) 200 mg (Viramune, Boehringer Ingelheim, Ingelheim, Germany) taken BID (after a 2-week initial dose of 200 mg once daily) or nelfinavir (NFV) 1250 mg BID (Viracept, Hoffmann-La Roche Ltd, Basel, Switzerland) from 34 to 36 weeks’ gestation through postpartum week 24. Most women discontinued antiretroviral therapy (ART) at post-partum week 24, though women who met World Health Organization (WHO) treatment criteria (CD4 count < 200 cells/mm3 or WHO stage 3 or 4) at enrollment or before postpartum week 24 remained on triple ART throughout the follow-up period of the study. Follow-up for study participants continued for up to 2 years after delivery. The Kisumu Breastfeeding Study was approved by the ethical review committees of Kenya Medical Research Institute (protocol 691) and US CDC (protocol 3677).
We collected whole blood samples in EDTA-treated anticoagulant Vacutainer tubes (Becton-Dickinson, San Jose, California) at each scheduled visit. Complete blood counts with automated white blood cell differentials were measured (Coulter Ac·T 5diff ACT 5diff Counter, Beckman Coulter, California). All abnormal values were validated by manual count. Abnormal laboratory findings were progressively followed up by recalling the affected participant within 72 hours for repeat confirmatory testing by manual differential. All critical values were reported within 1 hour for clinic management. Management of neutropenia was on a case-by-case basis. The Kisumu Breastfeeding Study mothers were followed closely within 72 hours to confirm neutropenia and then followed weekly depending on the clinical condition of the woman to see whether the low ANC remained stable or continued to decline. If the ANC level continued to decline, study clinicians considered switching ARV drugs.
We selected participants who had at least 1 postpartum ANC measured. We analyzed these participants’ ANCs at scheduled visits both antepartum and postpartum and calculated median, interquartile range (IQR), and range. The US 2004 DAIDS criteria were used to grade neutrophil counts, with grade 0 indicating a normal value and grades 1 to 4 considered adverse neutropenia events (see Table 2 for values).1 Grade 3 or 4 on 2 or more consecutive measurements indicated serious adverse events. We described ANC measures and DAIDS toxicity grades from scheduled antepartum and postpartum visits. Further, we examined postpartum adverse neutropenia events and infectious illnesses potentially attributable to neutropenia at postpartum weeks 2, 6, 14, 24, 36, 52, 72, and 104 and at unscheduled visits between these time points. Where neutropenia first occurred at the same time as an acute infection, we characterized the illness as simultaneous, and not the result of neutropenia, since we could not determine whether the illness was a result of the neutropenia or whether the illness caused the neutropenia. We also assessed whether a women experienced an illness possibly related to neutropenia by examining diagnoses from the subsequent scheduled or unscheduled visit (or visits, if neutropenia persisted) following an initial neutropenia event. We looked for diagnoses potentially related to neutropenia, such as pneumonia, fever, gastroenteritis, boils, abscess, malaria, cellulitis, sepsis, or herpes zoster. We also reviewed the cases of women who died during the study for evidence of infectious causes of death near the time of an event of neutropenia. Statistical calculations were performed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Table 2.
Number of Adverse Events Due to Neutropeniaa in HIV-Infected Kenyan Women Antepartum until Postpartum Week 104 Who Received Triple Antiretroviral Prophylaxis for Prevention of Mother-to-Child Transmission of HIV, Kisumu, Kenya, 2003–2009.b
| Weeks’ Gestation
|
Delivery (n = 450) |
Weeks’ Postpartum
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32–34 weeks (n = 472) |
35–36 weeks (n = 423) |
37–38 weeks (n = 361) |
39–41 weeks (n = 267) |
2 weeks (n = 440) |
6 weeks (n = 411) |
14 weeks (n = 414) |
24 weeks (n = 393) |
36 weeks (n = 349) |
52 weeks (n = 345) |
72 weeks (n = 319) |
104 weeks (n = 332) |
||
| ANC, cells × 109/L | |||||||||||||
| Median Interquartile range (25%–75%) | 3.27 (2.54–4.13) | 2.96 (2.34–3.87) | 2.65 (2.03–3.35) | 2.55 (1.92–3.31) | 5.12 (3.59–7.08) | 1.80 (1.32–2.28) | 1.45 (1.06–1.92) | 1.45 (1.08–1.91) | 1.71 (1.25–2.22) | 1.84 (1.39–2.39) | 1.87 (1.47–2.34) | 1.78 (1.33–2.39) | 1.77 (1.35–2.29) |
| Toxicity grade, ANC, μL | |||||||||||||
| Grade 0: (ANC > 1300) | 466 (98.7) | 417 (98.6) | 339 (93.9) | 249 (93.3) | 439 (97.6) | 336 (76.4) | 244 (59.4) | 257 (62.1) | 285 (72.5) | 280 (80.2) | 293 (84.9) | 244 (76.5) | 259 (78.1) |
| Grade 1: (ANC 1000–1300) | 3 (0.6) | 4 (1.0) | 14 (3.9) | 7 (2.6) | 3 (0.7) | 61 (13.9) | 82 (20.0) | 80 (19.3) | 62 (15.8) | 44 (12.6) | 26 (7.5) | 42 (13.2) | 48 (14.5) |
| Grade 2: (ANC 750–999) | 1 (0.2) | 0 (0) | 7 (1.9) | 7 (2.6) | 1 (0.2) | 27 (6.1) | 51 (12.4) | 51 (12.3) | 31 (7.9) | 16 (4.6) | 19 (5.5) | 20 (6.3) | 21 (6.3) |
| Grade 3: (ANC 500–749) | 2 (0.4) | 2 (0.5) | 0 (0) | 1 (0.4) | 4 (0.9) | 10 (2.3) | 22 (5.4) | 19 (4.6) | 11 (2.8) | 9 (2.6) | 2 (0.6) | 10 (3.1) | 3 (0.9) |
| Grade 4: (ANC < 500) | 0 (0) | 0 (0) | 1 (0.3) | 3 (1.1) | 3 (0.7) | 6 (1.4) | 12 (2.9) | 7 (1.7) | 4 (1.0) | 0 (0) | 5 (1.5) | 3 (0.9) | 1 (0.3) |
Abbreviations: ANC, absolute neutrophil count; WHO, World Health Organization.
Graded according to reference 1.
Women started triple antiretroviral prophylaxis at 34 to 36 weeks’ gestation and continued through postpartum week 24. After postpartum week 24, women who met WHO treatment criteria (CD4 count < 200 cells/mm3 or WHO stage 3 or 4) at enrollment or before 24 weeks’ postpartum remained on triple antiretroviral therapy. An analysis that included an additional 126 ANCs from prepartum and delivery visits from women without a postpartum ANC did not change the median values of prepartum or delivery ANCs in an appreciable manner.
A comparable study population of postpartum HIV-infected women on ZDV was not available for comparison. However, we were able to compare the frequency and distribution of ANC values measured in postpartum mothers participating in KiBS with ANCs measured among nonpregnant women enrolled in the HIV Outpatient Study, a longitudinal prospective cohort study of patients followed at 9 HIV specialty clinics throughout the United States since 1993.30 Women who were taking ZDV were included in the analyses, and data were examined for up to 2 years after initiation of ZDV therapy.
Results
Between July 2003 and November 2006, we screened 602 HIV-positive women, recruited from antenatal PMTCT clinics; 522 (87%) met eligibility criteria and were enrolled into KiBS. Median age of KiBS participants was 23 years (range 15–43 years) and the median CD4 count prior to ART initiation was 398 cells/mm3 (range 32–1340 cells/mm3) with 86% of pre-ART CD4 counts greater than 200 cells/mm3.
We had 4976 ANC observations from 478 women available for these analyses. Median ANCs from 32 weeks of gestation until delivery ranged from 2.55 to 3.27 cells × 109/L, median ANC at the first postpartum visit was 5.12 cells × 109/L, and from postpartum week 2 to 104 ranged from 1.45 to 1.87 cells × 109/L (Table 2). Adverse neutropenia events (DAIDS grade 1–4 events) occurred most commonly in the first few postpartum study visits; the most frequent occurrence was observed in 41% of participants at postpartum week 6. Of the 478 women, 298 (62.3%) had at least 1 ANC value that met criteria for neutropenia. The baseline CD4 count in the 298 women who experienced at least 1 neutropenia event was 385 cells/mm3 (IQR: 232–534 cells/mm3) and for the 180 women who did not experience a neutropenia event was 433 cells/mm3 (IQR: 302–602; P = .004). For women whose highest grade of neutropenia was 1 or 2, the median baseline CD4 count was 399 cells/mm3 (IQR: 246–546); and for women whose highest grade of neutropenia was 3 or 4, the baseline CD4 count was 325 cells/mm3 (IQR: 217–461). Baseline CD4 count was <250 cells/mm3 for 80 (26.8%) of 298 women who experienced neutropenia and for 31 (17.2%) of 180 women who did not experience neutropenia (P = .01).
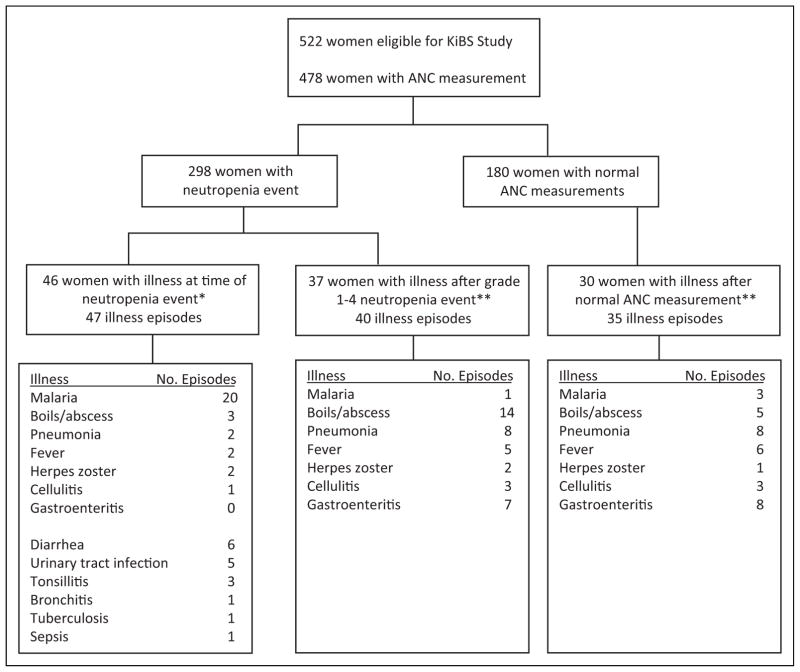
Among the 298 women with an ANC that met the criteria for neutropenia in KiBS, the highest grade of neutropenia was grade 1 or 2 for 230 (77.4%) and grade 3 or 4 for 68 (22.8%) women. The 298 women contributed to a total of 805 episodes of neutropenia, including 444 episodes of grade 1 for 252 women, 236 episodes of grade 2 for 149 women, 87 episodes of grade 3 for 68 women, and 38 episodes of grade 4 for 13 women. Of those women, 207 (69.7%) had more than 1 episode of neutropenia. In all, 46 women (15.4%) had 47 episodes where they had an acute illness at the same time as neutropenia (Table 3 and Figure 1). Malaria (n = 20, 42.6%) and diarrhea (n = 6, 12.8%) accounted for more than half of all illnesses at the same time as a neutropenia event.
Table 3.
Number and Percentage of Illnesses Potentially Attributable to Neutropenia among Women with a Grade 1 to 4 ANC Who Received Triple Antiretroviral Prophylaxis for Prevention of Mother-to-Child Transmission of HIV, Kisumu, Kenya, 2003–2009.a
| Neutropenia Grade
|
||||
|---|---|---|---|---|
| 1 (n = 444) | 2 (n = 236) | 3 (n = 86) | 4 (n = 38) | |
| Illness after low ANC, n (%) | 16 (3.6) | 14 (5.9) | 5 (5.8) | 5 (15.2) |
| Days from low ANC until illness, n | ||||
| 1–30 | 3 | 8 | 5 | 1 |
| 31–60 | 6 | 3 | 0 | 2 |
| ≥61 | 7 | 3 | 0 | 2 |
| Diagnoses for illness following a neutropenia event, n | ||||
| Gastroenteritis | 1 | 5 | 0 | 1 |
| Pneumonia | 4 | 0 | 3 | 1 |
| Boils/abscess | 7 | 4 | 1 | 2 |
| Herpes zoster | 1 | 1 | 0 | 0 |
| Cellulitis | 1 | 1 | 0 | 1 |
| Fever | 2 | 3 | 0 | 0 |
| Malaria | 0 | 0 | 1 | 0 |
| Illness at same visit as neutropenia event, n (%) | 23 (5.2) | 12 (2.1) | 10 (11.6) | 2 (5.3) |
| Diagnoses for simultaneous illness and neutropenia event, n | ||||
| Diarrhea | 3 | 3 | 0 | 0 |
| Pneumonia, bronchitis, or tuberculosis | 2 | 0 | 2 | 0 |
| Boils/abscess | 1 | 2 | 0 | 0 |
| Herpes zoster | 0 | 2 | 0 | 0 |
| Tonsillitis | 3 | 0 | 0 | 0 |
| Sepsis | 1 | 0 | 0 | 0 |
| Urinary tract infection | 3 | 0 | 2 | 0 |
| Cellulitis | 1 | 0 | 0 | 0 |
| Fever | 1 | 0 | 1 | 0 |
| Malaria | 8 | 5 | 5 | 2 |
Abbreviation: ANC, absolute neutrophil count.
See methods for definition of grade of neutropenia.
Figure 1.
Number of mothers enrolled in Kisumu Breastfeeding Study according to neutropenia status during study and the frequencies of illness potentially associated with neutropenia. *For this analysis we considered the following infections occurring at the same time as a neutropenia event as potentially causative of neutropenia: cholera, diarrhea, dysentery, esophageal candidiasis, salmonellosis, typhoid, enteric fever, schistosomiasis, persistent diarrhea, loose stools, aspiration pneumonitis, bronchiolitis, bronchitis, pneumonia, pneumocystis pneumonia, tuberculosis, sepsis, neonatal sepsis, boils/abscess, cellulitis, mumps, tonsillitis, pelvic inflammatory disease, urinary tract infection, genital abscess, cervicitis, infected episiotomy, puerperal sepsis, cerebral malaria, meningitis, pyomyositis, infected laparotomy scar, sexually transmitted diseases, rabies, malaria, measles, and fever septic shock, chills. **Illnesses potentially related to a neutropenia event included pneumonia, fever, gastroenteritis, boils, abscess, malaria, cellulitis, sepsis, sudden death, septicemia, or herpes zoster.
Among the 298 women who experienced neutropenia, 37 (12.5%) women experienced 40 illnesses potentially related to neutropenia at a subsequent visit. In all, 1 woman experienced 2 illnesses and 1 woman experienced 3 illnesses following separate neutropenia events. Seventeen (42.5%) of the illnesses occurred within 30 days of experiencing a low ANC (Table 3 and Figure 1). Of the 40 illnesses, 16 occurred after grade 1 neutropenia event, 14 after grade 2 neutropenia event, 5 after grade 3 neutropenia event, and 5 after grade 4 neutropenia event. The most common diagnostic codes related to illness were boils or abscess (n = 14), pneumonia (n = 8), and gastroenteritis (n = 7; Table 3 and Figure 1).
Among the 180 women who did not experience neutropenia, there were 28 (15.5%) occurrences of illnesses that could potentially cause neutropenia at the same time as an ANC measurement. Of these 180 women, 32 (17.8%; P = .14; compared with 37 of 298 women who experienced neutropenia) experienced 35 illnesses that could have been classified as potentially related to neutropenia, even though they never experienced neutropenia. Of these 35 illnesses, 15 (42.9%) occurred within 30 days of the closest previous ANC measurement, 8 (22.9%) within 31 and 60 days of this date, and 12 (34.3%) more than 60 days after this date. Pneumonia (n = 8), gastroenteritis (n = 8), fever (n = 6), and boils/abscess (n = 5) were the most common illnesses experienced by these women (Figure 1).
There were 12 women who died during KiBS. The highest grade neutropenia was grade 0 (normal) for 1 woman, grade 1 for 2 women, grade 2 for 3 women, grade 3 for 4 women, and grade 4 for 2 women. Of the 4 women with grade 3 neutropenia, 3 had 1 single episode of grade 3 neutropenia at 178, 361, and 646 days prior to death. One woman with a CD4 count of 125 cells/mm3 who had been diagnosed with Pneumocystis pneumonia developed grade 3 neutropenia and died 19 days later but before delivery; the death was attributed to eclampsia and not infection. The other 2 women (CD4 counts = 39 and 38 cells/mm3) with previously normal neutrophil counts were diagnosed with a grade 4 neutropenia simultaneously with an acute pulmonary infection (Pneumocystis pneumonia, pneumonia not otherwise specified) and died 12 and 13 days later (41 and 9 days postpartum), respectively.
Comparison of Neutropenia of KiBS Participants to Women from the US-Based HIV Outpatient Study Taking ZDV
We selected 276 HIV-infected nonpregnant women (173 African American, 77 white, and 26 other/mixed race) enrolled in the HIV Outpatient Study who were taking an ARV regimen that included ZDV. The median CD4 count was 362 cells/ mm3 (IQR: 203–539 cells/mm3) and age was 39 years (IQR: 34–43 years). We evaluated 1963 ANCs measured at scheduled study visits approximately 3 months apart. Absolute neutrophil counts from 28 (10%) women met criteria for an adverse neutropenia event according to DAIDS criteria: grade 1 neutropenia for 10 women, grade 2 for 6 women, grade 3 for 10 women, and grade 4 for 2 women. An adverse neutropenia event was found for 23 (13.3%) of 173 African American women and 4 (5.2%) of 77 white women (P = .08 by Fishers exact test). Of the 26 women of other/mixed race, 1 (3.8%) had an adverse neutropenia event. Of the 28 women with an adverse neutropenia event, 2 (7%) experienced an illness potentially attributable to neutropenia.
Discussion
Kenyan women treated with triple ARV prophylaxis, which included ZDV, for PMTCT of HIV had frequent grade 1 to 4 neutropenia events; however, despite the high frequency of low neutrophil counts, there were few illnesses potentially attributable to neutropenia. The frequency of neutropenia adverse events among Kenyan women appears to be higher than that found in a comparative cohort study (the HIV Outpatient Study) of nonpregnant women taking ZDV in the United States. When compared with other reports in the literature, the neutrophil counts in HIV-infected Kenyan women in KiBS were comparable to neutrophil counts in other African populations (Table 1), though lower neutrophil counts were observed in a US population.17 These results support the findings of other studies that have reported lower neutrophil counts in African populations than the US populations and that these observed lower neutrophil counts may be normal for African populations. A recent study attempted to address this issue by developing local reference ranges from healthy populations in Kenya.11
During KiBS, women’s neutrophil counts were higher when pregnant, peaked at delivery, and decreased to likely normal levels during the postpartum period, which is consistent with conventional observations in pregnant women.18 Adverse neutropenia events occurred commonly at postpartum weeks 2, 6, and 14. Although neutropenia occurred frequently, few illnesses potentially attributable to neutropenia were identified among KiBS participants. Further, the women who did not experience a neutropenia event experienced illnesses potentially attributable to neutropenia at a similar frequency as women who experienced neutropenia events. For the 2 women who died from an infectious cause near the time of grade 4 neutropenia, we are unable to distinguish whether neutropenia was the cause of the acute illness or a consequence of an overwhelming infection in the context of advanced HIV disease. The other death near the time of grade 3 neutropenia in a prepartum woman with Pneumocystis pneumonia was attributed to eclampsia and was not part of our analysis designed to detect postpartum events.
Some of the neutropenia adverse events may have been the result of drug toxicity. The low number of women who experienced illnesses related to neutropenia suggests that what is characterized as abnormal neutrophil counts by US-based standards could be considered “normal” neutrophil counts for this population. Further, the occurrence of subsequent illnesses we documented among women who did not experience neutropenia indicates that all women experience illnesses and it may not be solely attributable to neutropenia.
A limitation of this analysis is that when an illness was diagnosed at the same time as an episode of neutropenia, we could not discern whether the illness contributed to the neutropenic event or whether the episode of neutropenia predisposed to the illness; however, only 46 simultaneous events were observed. Second, the time between postpartum visits increased over the course of the study and our likelihood of identifying an event related to a previous neutropenia event that would not bring a participant to medical attention decreased. There were 12 illnesses that occurred more than 60 days following the neutropenia event; it was not clear whether these illnesses were related to a previous neutropenia event. Data from ideal comparison populations, such as HIV-infected women from the same country who were not taking ARV drugs, HIV-negative postpartum women from the same country, or postpartum African-American HIV-infected women taking an ART regimen that included ZDV, were not readily available for comparison. Nevertheless, the HIV outpatient study (HOPS) data were suggestive that African American women on ZDV may have more frequent occurrence of neutropenia than white women on ZDV, though we were unable to ascertain the precise cause of neutropenia among women in the HOPS cohort.
Conclusion
In summary, more than half of KiBS mothers met criteria for neutropenia during at least 1 postpartum visit according to the US-based toxicity tables. Although some events may have represented drug toxicity, similar postpartum incidences and severity of infections potentially related to neutropenia support limited published data that these values might be typical, rather than pathologic, for this population of postpartum HIV-infected women in Kenya. Using the US standards to define neutropenia in Kenya may overestimate ARV-related adverse events and may exclude potential participants from studies. Locally derived clinical laboratory reference ranges developed for different populations (including pregnant women and infants) in African settings should be the basis for determining toxicity tables for use in clinical trials in these countries or within a region.11
Acknowledgments
The authors are grateful to KiBS participating mothers and infants, KiBS staff for their diligent work in the field, GlaxoSmithKline for donation of Combivir, Boehringer Ingelheim for donation of Viramune, staff of New Nyanza Provincial General Hospital and Kisumu District Hospital for their assistance in recruitment and for caring for study participants, and KEMRI/CDC Kisumu Community Advisory Board for their patience and interest in the study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the study was provided by the Kenya Medical Research Institute (KEMRI) through a cooperative agreement with the US Centers for Disease Control and Prevention (CDC) (Award Number-5U19C1000323-05). Study drugs were provided by GlaxoSmithKline and Boehringer Ingelheim. The study design, data collection instruments, data collection, data analysis, decision to publish, and preparation of the manuscript were led by CDC and KEMRI staff based in Atlanta and at the KEMRI/CDC Field Station in Kisumu, Kenya. The companies that donated study drugs did not have any role in the conduct of the study. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC. Use of trade names is for identification purposes only and does not constitute endorsement by the CDC or the Department of Health and Human Services. This publication has been approved by the Director of the Kenya Medical Research Institute. Funding for the HIV Outpatient Study was supported by CDC (Contract no. 200-2001-00133).
Footnotes
Kisumu Breastfeeding Study was a phase IIB open-label single-arm clinical/intervention trial approved by the ethical review committees of the Kenya Medical Research Institute (KEMRI; protocol 691) and US CDC (protocol 3677). The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC. This publication has been approved by the Director of the Kenya Medical Research Institute. Preliminary findings from this analysis were presented at the 12th Conference on Retroviruses and Opportunistic Infections in Boston, Massachusetts, February 2005, and at the International AIDS Society Conference, Cape Town, South Africa, July 2009.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Division of AIDS table for grading the severity of adult and pediatric adverse events. National Institute for Allergy and Infectious Disease; 2004. [Google Scholar]
- 2.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49(8):664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beisel WR. Single nutrients and immunity. Am J Clin Nutr. 1982;35(2 suppl):417–468. doi: 10.1093/ajcn/35.2.417. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Stevens W, Scharpe S, et al. Seasonal variation in peripheral blood leukocyte subsets and in serum interleukin-6, and soluble interleukin-2 and -6 receptor concentrations in normal volunteers. Experientia. 1994;50(9):821–829. doi: 10.1007/BF01956463. [DOI] [PubMed] [Google Scholar]
- 5.Moore RD. The white blood cell count in the indigenous people of East Africa. J Trop Med Hyg. 1958;61(3):70–72. [PubMed] [Google Scholar]
- 6.Shaper AG, Kyobe J, Stansfield D. Haematological observations in an East African student population. East Afr Med J. 1962;39:1–4. [PubMed] [Google Scholar]
- 7.Kasili EG, Cardwell CL, Taylor JR. Leucocyte counts on blood donors in Nairobi. East Afr Med J. 1969;46(12):679–679. [PubMed] [Google Scholar]
- 8.Hawgood BC. Leucocyte levels in East Africa. East Afr Med J. 1969;46(12):680–682. [PubMed] [Google Scholar]
- 9.Shaper AG, Lewis P. Genetic neutropenia in people of African origin. Lancet. 1971;2(7732):1021–1023. doi: 10.1016/s0140-6736(71)90335-7. [DOI] [PubMed] [Google Scholar]
- 10.Lugada ES, Mermin J, Kaharuza F, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11(1):29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeh C, Amornkul PN, Inzaule S, et al. Population-based biochemistry, immunologic and hematological reference values for adolescents and young adults in a rural population in Western Kenya. PLoS One. 2011;6(6):e21040. doi: 10.1371/journal.pone.0021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babadoko AA, Aminu SM, Suleiman AN. Neutropenia and human immunodeficiency virus-1 infection: analysis of 43 cases. Niger J Med. 2008;17(1):57–60. doi: 10.4314/njm.v17i1.37357. [DOI] [PubMed] [Google Scholar]
- 13.Erhabor O, Ejele OA, Nwauche CA, Buseri FI. Some haematological parameters in human immunodeficiency virus (HIV) infected Africans: the Nigerian perspective. Niger J Med. 2005;14(1):33–38. doi: 10.4314/njm.v14i1.37132. [DOI] [PubMed] [Google Scholar]
- 14.Ezeilo GC. Normal haematological values in adult Zambians. East Afr Med J. 1972;49(2):94–100. [PubMed] [Google Scholar]
- 15.Gomo E, Vennervald BJ, Ndhlovu PD, Kaestel P, Nyazema NZ, Friis H. Reference values and predictors of white blood cell subset counts: a cross-sectional study among HIV seronegative pregnant women in Zimbabwe. Eur J Obstet Gynecol Reprod Biol. 2003;107(2):156–162. doi: 10.1016/s0301-2115(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 16.Moh R, Danel C, Sorho S, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d’Ivoire. Antivir Ther. 2005;10(5):615–624. doi: 10.1177/135965350501000510. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146(7):486–492. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 18.Pitkin RM, Witte DL. Platelet and leukocyte counts in pregnancy. JAMA. 1979;242(24):2696–2698. [PubMed] [Google Scholar]
- 19.Koos BJ, Moore PJ. In: Current obstetrics and gynecologic diagnosis and treatment. 9. DeCherney AH, Nathan L, editors. New York: McGraw-Hill; 2003. [Google Scholar]
- 20.Harbol AW, Liesveld JL, Simpson-Haidaris PJ, Abboud CN. Mechanisms of cytopenia in human immunodeficiency virus infection. Blood Rev. 1994;8(4):241–251. doi: 10.1016/0268-960x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin GC, Fuller ND, Roberts RL, Ho DD, Golde DW. Granulocyte- and granulocyte-macrophage colony-stimulating factors enhance neutrophil cytotoxicity toward HIV-infected cells. Blood. 1989;74(5):1673–1677. [PubMed] [Google Scholar]
- 22.Roilides E, Walsh TJ, Pizzo PA, Rubin M. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991;163(3):579–583. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- 23.Keiser P, Higgs E, Smith J. Neutropenia is associated with bacteremia in patients infected with the human immunodeficiency virus. Am J Med Sci. 1996;312(3):118–122. doi: 10.1097/00000441-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Moore RD, Keruly JC, Chaisson RE. Neutropenia and bacterial infection in acquired immunodeficiency syndrome. Arch Intern Med. 1995;155(18):1965–1970. [PubMed] [Google Scholar]
- 25.Richman DD, Andrews J. Results of continued monitoring of participants in the placebo-controlled trial of zidovudine for serious human immunodeficiency virus infection. Am J Med. 1988;85(2A):208–213. [PubMed] [Google Scholar]
- 26.Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 27.Shaunak S, Bartlett JA. Zidovudine-induced neutropenia: are we too cautious? Lancet. 1989;2(8654):91–92. doi: 10.1016/s0140-6736(89)90325-5. [DOI] [PubMed] [Google Scholar]
- 28.Williams I, Gabriel G, Cohen H, et al. Zidovudine–the first year of experience. J Infect. 1989;18(suppl 1):23–31. doi: 10.1016/s0163-4453(89)80077-5. [DOI] [PubMed] [Google Scholar]
- 29.Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8(3):e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moorman AC, Holmberg SD, Marlowe SI, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: the HIV outpatient study (HOPS) Ann Epidemiol. 1999;9(6):349–357. doi: 10.1016/s1047-2797(99)00005-8. [DOI] [PubMed] [Google Scholar]