Abstract
An infant who receives a placental transfusion at birth, either from cord milking or delayed cord clamping, obtains about 30% more blood volume than the infant whose cord is cut immediately. Receiving an adequate blood volume from placental transfusion at birth may be protective for the distressed neonate as it prevents hypovolemia and can support optimal perfusion to all organs. New research shows that ventilating before clamping the umbilical cord can reduce large swings in cardiovascular function and help to stabilize the infant. Hypovolemia, often associated with nuchal cord or shoulder dystocia, may lead to an inflammatory cascade and subsequent ischemic injury. A sudden unexpected neonatal asystole at birth may occur from severe hypovolemia. The restoration of blood volume is an important action to protect the hearts and brains of these neonates. Current protocols for resuscitation imply immediate cord clamping and the care of the infant away from the mother's bedside. We suggest that an obstetrical provider can achieve placental transfusion for the distressed neonate by milking the cord several times or resuscitating the infant at the perineum with an intact cord. Milking the cord can be done quickly within the current Neonatal Resuscitation Program guidelines. Cord blood gases can be collected with delayed cord clamping. “Bringing the resuscitation” to the mother's bedside is a novel concept and supports an intact cord. Adopting a policy for resuscitation with an intact cord in a hospital setting will take concentrated effort and team work by obstetrics, pediatrics, midwifery, and nursing.
Keywords: placental transfusion, delayed cord clamping, cord milking, hypovolemia, asystole, perfusion, resuscitation, blood volume, shock
INTRODUCTION
Delayed umbilical cord clamping (DCC) or umbilical cord milking offers a neonate approximately 30% more blood volume at birth.1 This transfusion results in higher ferritin levels and iron stores out to 4 to 6 months of age in term infants. 2, 3 The most recent meta-analysis informs us that preterm infants who receive a placental transfusion at birth, either from DCC or umbilical cord milking, are less likely to need a transfusion in the early weeks of life and are offered some protection against intraventricular hemorrhage.4 In 2012, the American College of Obstetricians and Gynecologists published a statement recommending DCC for preterm infants and for term infants in low resource areas. 5 This statement was endorsed by the American Academy of Pediatrics in 2013.6 A recent review offers background information on the mechanisms, benefits, risks, and techniques for delaying umbilical cord clamping at birth.7
But, what about the neonate who needs resuscitation? Most are limp, pale, and without tone, suggesting that hypovolemia plays a role in their condition. This article presents a coherent set of ideas and evidence from physiologic studies, clinical trials, and clinical practice showing how placental transfusion at birth allows the newborn to receive more protective blood volume. For the infant needing resuscitation, the blood volume gained from DCC or umbilical cord milking has the potential to stabilize the cardiovascular system, reduce the severity of an inflammatory response, reduce or prevent damage from hypoxia/ischemia, and help to keep the infant from harm. A discussion of the role of blood volume in neonatal transition and the effects of hypovolemia on the neonate are reviewed. An explanation is offered for what causes low heart rate or cardiac asystole immediately after birth. The process of inflammation and a possible etiology of seizures are described along with techniques to achieve a placental transfusion when the infant needs resuscitation. Our hypothesis is that an adequate blood volume, supported by delaying umbilical cord clamping, plays a major and positive role in the neonatal transition.8
THE EFFECT OF PLACENTAL TRANSFUSION IN FETAL-TO-NEONATAL TRANSITION
Blood volume cannot be measured in human infants without invasive procedures,9 Therefore, the figures most frequently quoted are from the 1960s when infant blood volume was studied with radioactive tracers. The inability to quantitatively measure blood volume has hampered current study of how DCC and blood volume may affect neonatal transition. However, results from an innovative study on neonatal transition using a lamb model support the idea that cardiovascular changes are smoother in fetal-to-neonatal transition with DCC.10 We offer a brief review of transitional physiology to help the reader understand how DCC can benefit an infant needing resuscitation at birth. The interested reader is additionally referred to recent review articles.7, 11
The Physiology of Fetal-to-Neonatal Circulation
Throughout pregnancy, the fetal blood volume is approximately 115 mL/kg.12 In a preterm fetus, about 1/2 is in the placenta at any point in time. At term, approximately two thirds of the blood is circulating in the fetus and one third is circulating through the placenta at any moment in time.1 In the fetal circulation, approximately 10% of the fetal cardiac output goes to the lungs while 30% to 50% goes to the placenta where gas exchange takes place. In order to change from placental gas exchange to lung air exchange at birth, 50% of the newborn cardiac output must rapidly go to the lungs.
Three anatomic shunts allow fetal circulation, which includes the placenta where gas exchange takes place. The three shunts are the ductus venosus, the foramen ovale, and the ductus arteriosus. Deoxygenated blood travels to the placenta by way of the umbilical arteries and oxygenated blood returns to the fetus via the umbilical vein. Approximately 60% of the oxygenated blood bypasses the liver by way of the ductus venosus and travels to the right atrium via the inferior vena cava. In the right atrium, the oxygenated blood streams preferentially across the atrium through the foramen ovale and into the left atrium where it is pumped to the left ventricle and into the ascending aorta. The majority of this better oxygenated blood is delivered to the heart and brain. Desaturated blood also arrives at the right atrium from the superior and inferior venae cavae. This blood travels with the oxygenated blood but in the right atrium it is streamed towards the right ventricle instead of through the foramen ovale. The right ventricle empties into the pulmonary artery but, because of the high pulmonary vascular resistance in the fetus, only 10% of the blood that goes to the pulmonary artery flows into the lungs.13, 14 The remaining 90% of this blood flows through the ductus arteriosus and into the descending aorta.
At birth, the three shunts must close quickly so that the lungs can assume the function of oxygenation and receive the blood that was previously going to the placenta. In order to initiate respiration and for these shunts to close, a dramatic fall in pulmonary vascular resistance and an 8 to 10-fold increase in pulmonary blood flow must occur.
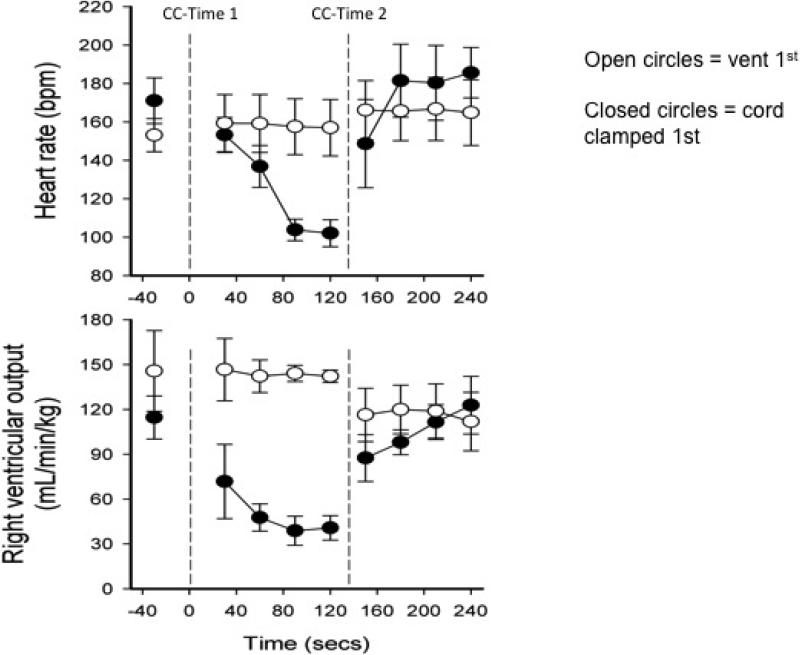
When the umbilical cord is clamped immediately at birth, there is cessation of flow to the umbilical vein leading to the ductus venosus. This abrupt halt in the umbilical circulation causes a dramatic, albeit transient, drop in heart rate and over a 50% fall in right ventricular output.10 Bhatt et al randomized 12 preterm lambs to either Clamp 1st (and then ventilate) or Vent 1st (followed by umbilical cord clamping). Ventilation was controlled so it could be manipulated to occur before or after clamping. Upon umbilical cord clamping, lambs in the Clamp 1st group experienced a large drop in heart rate and right ventricular output as well as a rise in carotid artery pressure and flow and higher pulmonary artery pressures (Figure 1). Lambs in the Vent 1st group (vent followed by clamping) did not have the large swings in pressures and flows. They had higher pulmonary blood flow levels and right ventricular output up to 30 minutes after birth representing higher blood volume circulating through their lungs and body. These results show improved cardiovascular function and stability during neonatal transition when ventilation (or respiration) occurs before umbilical cord clamping. This study suggests that delaying umbilical cord clamping until after ventilation could have beneficial effects for infants requiring respiratory assistance.
Figure 1. Ventilation before cord clamping stabilizes the cardiovascular transition at birth.
Abbreviations: CC = cord clamped.
Lambs whose cords were clamped before ventilation (CC-Time 1 and closed circles) experienced a drop in pulse and right ventricular output (RVO) of over 50%. Lambs who were ventilated before the cord was clamped (CC-Time 2 and open circles) had stable pulse and RVO. Adapted from Bhatt 2013 (with permission). CC = cord clamped.
In summary, new evidence shows us that ventilation (or respiration) before umbilical cord clamping results in a smoother, more stable cardiovascular transition for the neonate and results in higher blood flow in the heart and lungs. Placental transfusion allows increased blood in the circulatory beds in the lungs while the lung changes from an organ of fluid excretion to an organ of gas exchange.
Neonatal Perfusion Following Placental Transfusion
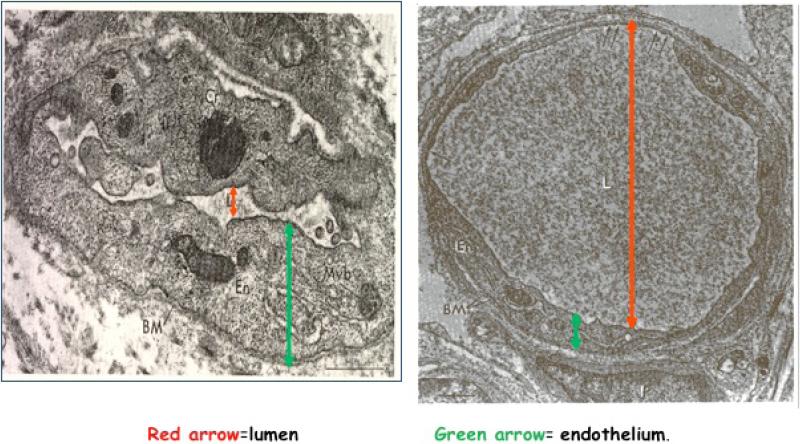
Classic physiologic studies, completed over the past 60 years, document that placental transfusion results in improved perfusion in the newborn's hematologic, urinary, gastrointestinal, neurological and respiratory systems as shown in Table 1.15-17 This improved perfusion is demonstrated in an earlier study by Petra, Oh et al.18 Heel capillaries were biopsied at 2 to 5 hours after birth from 12 infants who had either ICC or DCC (when pulsations ceased). The capillaries in Figure 2 are about the width of one red blood cell. In Capillary A, the lumen is very small and irregular while the endothelium is thick. In Capillary B, the lumen is large and full while the endothelium is thin allowing for maximal exchange of nutrients and waste products. There were significantly more capillaries found resembling Capillary A in the infants who received ICC and more of Capillary B in the infants who experienced DCC, suggesting better blood volume and perfusion with DCC. The heels are usually the last area of the body to be well perfused, so one can assume that other more vital areas such as brain and heart are also better perfused when the infant receives a placental transfusion.
Table 1.
Characteristics of Neonates with Euvolemia versus Hypovolemia.
| Euvolemia (Placental Transfusion) | Hypovolemia (Immediate Clamping) |
|---|---|
| ↑Blood Pressurea,b | ↓Blood Pressure |
| ↓Vascular resistanceb | ↑Vascular resistance |
| ↑RBC flow to brain(18%) and to gut (20%)b | ↓RBC flow to all organs |
| ↑Renal blood flowc | ↓Renal blood flow |
| ↑Urine outputc | ↓Urine output |
| ↓Sodium excretionc | ↑Sodium excretion |
| ↑Pulmonary vasodilatationa | ↓Pulmonary vasodilatation |
| ↑RCV, Hct, Hba,b | ↓(RCV, Hct, Hb) |
Figure 2. Heel Capillaries from Infants with Early and Late Cord Clamping.
Capillaries shown are the width of one red blood cell. The capillary on the left shows a thick endothelium with a collapsed lumen. On the right, one can see the thin endothelium and the full lumen. Small fenestrations can be seen at the top of the capillary, which would allow for rapid transfer of nutrients and waste products. Adapted from: Pietra G, D'Amodio M, Leventhal M, Oh W, Braudo J. Electron microscopy of cutaneous capillaries of newborn infants: effects of placental transfusion. Pediatr 1968;42(4):678-683 (with permission).
THE EFFECTS OF INDIVIDUAL COMPONENTS OF PLACENTAL TRANSFUSION
The 30% more blood volume an infant receives from a placental transfusion contains iron-rich red blood cells, stem cells, and plasma volume and other substances which all play a role in the infant's adaptation to life outside the womb. For a 3 kg infant, this represents between 50 and 85 mL of fresh whole blood which contains a large number of red blood cells. These help to prevent anemia of infancy and may have a positive impact on brain development. Placental transfusion also delivers several million to 1 billion stem cells resulting in an autologous stem cell transplant for the infant.19 Approximately 20 to 30 mL/kg or 60 to 120 mL of plasma is available for volume expansion and circulatory support.1 Other substances in cord blood such as hormones and cytokines are not discussed here.
Red Blood Cell Transfusion
The infant who receives a placental transfusion obtains about 15 mL/kg of red blood cells or 50% more than an infant who has ICC. Since 80% of the iron in the human body resides in the red blood cells, this amount provides the infant with enough iron to meet the infant's needs for three to six months.2, 20 Prior concerns that the increase in red cell volume provided by placental transfusion would lead to increased symptomatic polycythemia or jaundice have not been substantiated in any of the randomized trials conducted since 2000. 7
Clamping the umbilical cord immediately can result in low red cell volume potentially leading to iron deficiency in infancy.2 Iron deficiency from low iron stores can have negative effects even when the hemoglobin level is within normal range.21 Shafir et al found that, at one year of age, iron deficiency without anemia negatively influenced motor development.22 Lozoff found that iinfants with iron deficiency anemia scored lower in cognitive, motor, social-emotional, and neurophysiologic development even 20 years after successful treatment of anemia in infancy.23, 24 She reported an average drop in IQ of 25 points at age 19 years if the individual experienced the double burden of iron deficiency anemia and low socioeconomic status.23 No studies have looked at developmental indices beyond six months in infants with DCC versus those who have ICC although one is underway.25
Iron deficiency's negative effect on the developing brain may be related to iron's essential role in the maturation of oligodendrocytes and subsequently low iron availability may impede their development.26 Oligodendrocytes are the brain cells that make myelin by forming sheaths around axons thereby creating the brain's white matter. Myelination is essential for the development and proper functioning of the nervous system and forms an electrical insulation around the brain's axons, which allows for rapid transmission of signals through the nerve cells. Hypomyelination is a noted feature found on the brain scans of children with autism and other developmental impairments which is why this issue is a high priority.27, 28
Stem Cell Transfusion
Placental transfusion offers an autologous stem cell transplant containing a high volume of stem cells.19 Animal studies show that human umbilical cord blood stem cells will selectively repair damaged areas in the body such as the heart and brain.29 Cytokines (signaling proteins that are released from damaged tissues) summon stem cells to damaged areas for repair.30 The role of these cells in vivo in human neonates is difficult to study and remains unclear. However, animal studies indicate that cerebral damage can be prevented by infusion of human umbilical cord blood within 24 hours after induced brain injury in rats 29. Preliminary studies are underway to examine the effect of stem cell infusions for human neonates with brain damage at birth.31 Some scientists suggest that the stem cells received at birth from DCC or umbilical cord milking may play an important role throughout a person's life time.19
FACTORS AFFECTING THE AMOUNT AND SPEED OF PLACENTAL TRANSFUSION
Factors that affect the amount and speed of the placental transfusion include the timing of umbilical cord clamping, the level the infant is held in relation to the placenta, uterine contractions and milking the umbilical cord.32, 33,34 Holding the infant above the placenta slows transfusion while lowering the infant hastens it.1, 32 Uterine contractions aid in emptying the placenta as does the use of oxytocics. The umbilical cord can be milked to speed placental transfusion in situations when a provider feels that a wait is unwise (Table 2). When the umbilical cord is milked, infants receive almost as much of their placental blood as when umbilical cord clamping is delayed.35 Some providers are hesitant to milk the umbilical cord out of fear that it may be harmful. However, the available literature shows the same early benefits as DCC and an absence of harm when milking is used in both term and preterm infants.4, 35-38
Table 2.
Cord Management Procedures
| Delayed Umbilical Cord Clamping |
|---|
| Vaginal Birth |
| Infant held below level of perineum |
| • Hold the healthy, dried newborn below the level of the perineum or place on a clean dry underpad on the bed. |
| • Wait at least 2 minutes before clamping and cutting the umbilical cord. |
| Infant held Skin-to-Skin |
| • Place the healthy, dried newborn on the mother's bare abdomen and cover with a warm blanket. |
| • Clamp and cut the umbilical cord after it is flat and lifeless (approximately 5 minutes). |
| Cesarean Birth |
| • Place the healthy, dried newborn on the sterile drape between the mother's thighs (below level of placenta). |
| • Wait at least 2 minutes before clamping and cutting the umbilical cord. |
| Milking the Umbilical Cord |
|---|
| Vaginal Birth |
| • Hold the newborn below the level of the perineum or place on a clean dry underpad on the bed. |
| • Compress the umbilical cord between thumb and forefinger near the perineum. |
| • Move down the entire down length of the umbilical cord towards the newborn's umbilicus. |
| • Repeat five times and then clamp and cut the umbilical cord. |
| Cesarean Birth |
| • Place newborn on the sterile drape between the mother's thighs (below level of placenta). |
| • Compress the umbilical cord between thumb and forefinger near the umbilical cord insertion site on the placenta. |
| • Move down the entire down length of the umbilical cord towards the newborn's umbilicus. |
| • Repeat five times and then clamp and cut the umbilical cord. |
HYPOVOLEMIA IN THE NEWBORN
The authors' interest in the subject of placental transfusion began with the birth of an infant born with a double nuchal cord who was extremely pale and lifeless and was resuscitated with an intact cord during a home birth. This case represents our first experience of resuscitation with an intact umbilical cord and a description of the case can be found along with other relevant case reports in another publication.39
In hospital settings, this infant would have been managed with ICC to accommodate resuscitation by neonatal staff at the infant warmer. The Neonatal Resuscitation Program (NRP) guidelines presume ICC.40 This case demonstrated the value of the placenta as a source of blood transfusion for a volume-depleted (hypovolemic) infant. Immediate clamping of the umbilical cord may have inflicted harm by preventing the reperfusion of this pale, lifeless infant. In an out-of-hospital setting, cutting the umbilical cord immediately might have resulted in an unsuccessful resuscitation. In a survey of American College of Nurse-Midwives members on umbilical cord clamping practices, midwives have described positive experiences with resuscitation of an infant with an intact umbilical cord in home birth and birth center settings.41
Nuchal Cord And Shoulder Dystocia
The literature demonstrates that cutting a tight nuchal cord before birth can result in hypovolemia and neonatal anemia. Cashore and Usher (1973) used radioactive tracers to measure blood volume in infants with tight nuchal cords who were anemic, pale, hypotensive, tachycardiac and had low Apgar Scores at birth. They found significant reductions (20%) in red cell volume. 42 In a large Canadian cohort study (more than 10,000 term liveborn infants), infants born with a nuchal cord cut before birth weighed approximately 67 grams less than infants without a nuchal cord (mean of 3481 ± 467 vs. 3548 ±475 grams, (P<0.001). It is probable that more than an average of 60 mL of blood remained in the detached placenta due to pre-birth umbilical cord clamping.43
A review of case reports shows that shoulder dystocia and the presence of a nuchal cord can be especially dangerous for a newborn. In 9 reported cases of shoulder dystocia where the nuchal cord was cut before the birth, Iffy found that all infants had poor Apgar scores and developed signs of hypoxic-ischemic-encephalopathy.44, 45 All births occurred within 3 to 7 minutes after the birth of the head. Seven out of 9 infants developed cerebral palsy. Iffy, an obstetrican, recommended not to cut the nuchal cord before delivery of the shoulders. In another case report of a shoulder dystocia, the obstetrician almost cut a nuchal cord before he was assured that the infant would be born vaginally.46 He was able to slip the umbilical cord over the head. However, he could not deliver the shoulders. The Zavanelli maneuver was used and the infant was born by cesarean section with Apgar scores of 31, 75 and 910. In summary, the practice of cutting the nuchal cord before birth of the shoulders is harmful because it puts the infant at risk for hypoxic-ischemic-encephalopathy or death and the provider at medical-legal risk.47
Clinical Picture of Infants with Severe Hypovolemia
Infants with severe hypovolemia present with a classic presentation. They have white “drained” bodies (or mottled blue and white), lack tone and reflexes, and have no respiratory effort. Usually the heart rate is above 100 bpm but may be lower. Experience as expert witnesses with several cases that involved infants with hypoxic-ischemic-encephalopathy led the authors to develop and publish The Cardiac Asystole Theory.47 This theory offers a hypothesis for why infants who had a good heart rate shortly before birth were born with no heart rate and developed hypoxic-ischemic-encephalopathy.
Cardiac Asystole Theory
The Cardiac Asystole Theory suggests that as the large infant is squeezed tightly in the birth canal, and the umbilical cord becomes constricted, blood is sequestered in the placenta leading to a state of hypovolemia. The pressure on the fetus in the birth canal works like an anti-shock garment and helps to maintain central perfusion keeping the pulse and blood pressure normal even when the blood volume is low.48 At birth, the sudden release of pressure acts like a fast removal of the anti-shock garments and the central blood volume flows rapidly into the peripheral circulation. The infant's heart stops due to sudden severe lack of central perfusion resulting in extreme hypovolemic shock. If one cuts the umbilical cord immediately, the infant can be left with a very low blood volume. The theory suggests that ICC in these cases can result in severe hypovolemic shock leading to an inflammatory response. It is believed that it is the inflammatory response that leads to seizures, hypoxic-ischemic-encephalopathy, brain damage or death.49
Inflammation Can Be Caused by Blood Volume Loss Alone
Infants who develop hypoxic-ischemic-encephalopathy invariably experience brain inflammation during the first week of life.50 There is evidence that severe blood loss may lead to hypovolemic shock and subsequent ischemia and inflammation.51 Rajnik (2002) demonstrated in an animal model that loss of blood volume alone, without infection or reperfusion, can lead to cytokine gene expression - a precursor to inflammation.51 Rajnik's work corroborates earlier studies showing that a severe reduction in blood volume can stimulate a cytokine cascade leading to inflammation in a living organism.49, 52, 53
The Inflammatory Cascade
Hypovolemia reduces perfusion to the various organs and creates subtle to overt hypoxia/ischemia. Hypoxia stresses living cells, which leads to an initiation of inflammatory processes. The damage begins in the endothelial cells that line the infant's blood vessels and alters their normal microvascular function leading to increased permeability of the vessels. With an ischemic insult such as hypovolemic shock, the injury begins immediately with significant upregulation of pro-inflammatory cytokines. Cytokines are signaling proteins produced by many different kinds of cells in distress. They send messages between cells and attach to cell walls causing cells to change behavior. The intracellular junctions in the endothelium move apart allowing leakage between intravascular and interstitial tissues, which causes edema to form. Damaged endothelial cells lose their ability to regulate vascular tone, perfusion, permeability, inflammation and adhesion.54 The microcirculatory injury continues and within 24 to 48 hours there is loss of autoregulation.55 Within three days there is evidence of interstitial invasion of leukocytes, edema, hemorrhage, and fibrin.56 Ischemic damage is progressive and can continue over several hours, days and weeks.57 Loss of these functions has a deleterious effect on the functioning of the target organ whether it is the lung, liver, brain, or kidney and can lead to dysfunction and organ failure. The resulting insult in the target organ is manifested by less blood flow, edema formation, vascular congestion, and infiltration of inflammatory cells.54 Many infants with hypoxic-ischemic-encephalopathy have multiple organ injury and/or failure. Hankins reported 70% to 80% of infants with hypoxic-ischemic-encephalopathy had overlapping organ system injury including cardiac, renal, hepatic and central nervous system damage.58 No other mechanism – other than hypoperfusion and ischemia of these organs from blood loss – could cause such devastating damage so quickly. Evidence of long-term effects after resuscitation suggests that even when an infant is not ill in the newborn period, long lasting subtle damage may occur.
Results from animal studies show that a transfusion with fresh whole blood ameliorates the inflammatory response and organ injury after hemorrhagic shock.52 The authors developed the term “damage control resuscitation” to refer to use of fresh whole blood after hemorrhagic shock. We suggest that DCC provides fresh whole blood to the infant in distress. ICC as used in traditional resuscitation practices may put the infant at a higher risk of damage due to reduced blood volume.
Outcomes of Children after Resuscitation
Odd et al (2009) conducted a cohort study (n=5887) to determine whether infants who were resuscitated at birth had a reduced IQ (less than 80) at 8 years of age.59 There were 3 groups of infants: infants resuscitated at birth but with no evidence of encephalopathy, infants resuscitated at birth with evidence of encephalopathy and infants not resuscitated at birth and no evidence of encephalopathy. For infants resuscitated at birth but without symptoms of encephalopathy, the adjusted odds ratio was 1.65 (95% CI 1.13-2.43) for a reduced IQ and for those with symptoms of hypoxic-ischemic-encephalopathy, the adjusted odds ratio was 6.22 (95% CI 1.57-24.65) when compared to the control group of children who were not resuscitated. This study demonstrates that infants who were resuscitated at birth had an increased risk of a low IQ score even if they were healthy in the neonatal period.59
Lindstrom et al (2008) investigated cognitive function and behavioral problems in adolescents (n = 684) who were born at term in 1985 and had Apgar scores of less than 7 at 5 min.60 Of the total, 56 were identified who developed moderate neonatal encephalopathy and survived without cerebral palsy. At age 15-19 years, 43 children whose parents gave permission were evaluated. Only 8 children were without impairments. In 71% of subjects, they found cognitive dysfunction and 18% had hearing impairments. Children and parents reported that most of these dysfunctions interfered with daily life.
Both of these studies suggest that conditions leading to the need for neonatal resuscitation and the resuscitation itself may impact developmental outcomes. Currently, when resuscitation is needed, ICC at birth is the routine practice. It is theorized that the additional blood volume received with DCC with an intact umbilical cord or umbilical cord milking during resuscitation may improve long-term outcomes by reducing the severity or preventing neonatal encephalopathy.
Lessons from Cardiocerebral Resuscitation in Adults
Recent changes to the adult CPR techniques support the hypothesis that adequate blood volume is a critical first step in human resuscitation. The recommended technique for adult CPR has recently changed to adopt Cardiocerebral resuscitation with emphasis on blood volume to maintain blood pressure and perfusion to the heart and brain.61 Research using animal models has shown that cardiac arrest survival increased up to 80% when perfusion was supported by continuous chest compressions compared to 13% survival rates with standard cardiopulmonary resuscitation (chest compressions and interruptions for breathing).62 The critical factor in improving survival is brain and heart perfusion secondary to stable blood flow. The new adult CPR guidelines for unwitnessed cardiac arrest support continuous chest compressions to circulate blood without interruption for ventilations.61 In adults, the goal is to move the existing blood to the heart and brain. In infants, adequate blood volume is also critical to perfuse and protect the heart and brain. Milking the umbilical cord of an infant needing resuscitation or resuscitating an infant with an intact umbilical cord, allows the infant access to at least 30% more blood volume –a step that may be essential for recovery.
CLINICAL PROCEDURES FOR ACHIEVING PLACENTAL TRANSFUSION
There are at least two ways to get placental transfusion to the neonate who needs resuscitation: umbilical cord milking or DCC. Refer to Table 2 for procedures. Umbilical cord milking is a simple technique that can be instituted quickly by any obstetric provider at any birth.35 This technique supports the NRP guidelines as one can milk the umbilical cord and assess the infant within 30 seconds after birth. Is milking “unnatural?” Yes, but it accelerates the transfer of blood into a pale limp infant which is especially important in a hospital setting. No adverse events from umbilical cord milking have been reported.4, 35-37
Delayed clamping of the umbilical cord is the standard of care in birth center and home birth settings.41 If one is conducting the birth in a setting where there is not a demand to “pass the infant off quickly”, a full resuscitation can be conducted without severing the umbilical cord. If the umbilical cord is left intact or milked during resuscitation, reperfusion will occur as the blood remaining in the placenta returns to the infant's body. If the heart rate is not above 100 bpm, vigorous drying (stimulation) and lowering the infant below the level of the perineum or milking the umbilical cord can support resuscitative efforts by increasing blood volume. Bag and mask ventilation or even intubation can be done at the perineum (on a dry underpad) without clamping the umbilical cord. Once the infant has regained tone and color (reperfused) and breathing stabilizes, the infant can be put skin-to-skin on the mother's abdomen.
Keeping the Umbilical Cord Intact when there is a Nuchal Cord
Many providers have been taught to double clamp and then immediately cut a tight nuchal cord. We recommend that providers learn to use the Somersault Maneuver to keep the umbilical cord intact.63 It is used when a nuchal cord is not easily reducible over the head and is too tight to push down over the shoulders. As the infant is born, the provider keeps the infant's head close to the perineum (or thigh), “folding” the infant up towards the symphysis as it is born. The umbilical cord is gently unwound from around the neck. If the infant has poor tone or is very pale, the infant should be placed on the bed, dried and stimulated while s/he receives placental transfusion (via umbilical cord milking or DCC) and until tone returns and the infant is breathing. Resuscitation can be done at the perineum as described above.
Bringing the Resuscitation to the Infant
In order to provide resuscitation with an intact cord, Hutchon et al devised a way to bring the resuscitation equipment to the infant.64 They have developed a small stand (trolley) that provides a stable, warmed platform for resuscitation of the newly born infant with an intact umbilical cord. This allows for the additional support of placental transfusion providing volume and red blood cells for improved oxygenation. The trolley is compact and allows hands-free height adjustment. Suction, blender and a continuous positive airway pressure (CPAP) device can all be easily mounted on the unit. A timer allows staff to keep track of the post-delivery period. Other equipment such as pulse oximeter can be added to suit local practice. This trolley enables the infant to have the support of the placenta and the available blood while resuscitation with the intact umbilical cord is underway. The trolley can be positioned very close to a labor bed or an operating table. The layout ensures that access for the clinical team is not compromised and the newborn's airway can be easily established. 65 The trolley is currently being tested at several sites in England (personal communication, David Hutchon, 2013). All steps of NRP can be followed when the umbilical cord is still attached to the placenta. There is no substitute for the infant's own blood.
Blood Gas Sampling During Resuscitation
Umbilical cord gas collection is often required at the birth of an infant needing resuscitation. Blood can easily be collected from the intact umbilical cord in the same manner used for detached umbilical cords. Andersson et al, in a recently published randomized controlled trial of 382 healthy term infants, successfully collected umbilical cord blood gas samples from infants with DCC of 180 seconds (n= 130) and infants with ICC (n=139).66 In the DCC group, arterial and venous umbilical cord gas samples were collected within 30 seconds after birth and in the ICC group, similar samples were drawn from a segment of the umbilical cord by 10 minutes after delivery. A 25 or 27 gauge needle was used and leakage from the punctured umbilical cord was minimal. Each sample was analyzed within 20 minutes and no significant differences between the 2 groups were reported with the exception of a lower arterial oxygen tension level noted in the ICC group.
MAKING CHANGES IN CLINICAL PRACTICE
Most midwives and other providers who practice in out of hospital settings already resuscitate infants with an intact umbilical cord.41 They believe that clamping or cutting the umbilical cord on a non-breathing infant is an unsafe practice. However, in most hospitals the policy is to immediately “cut the cord and run” to a warmer with a distressed infant. Umbilical cord milking can be done by any OB provider at any time. But one cannot adopt DCC for infants needing resuscitation without a major policy change as it requires the neonatal team to resuscitate at the bedside. Teamwork is essential and it is important that midwives and other providers start these discussions long before the need arises. Changing policy in a hospital setting is discussed at length in a recent review article by Mercer and Erickson-Owens.7
Currently little research is being done on resuscitation with an intact umbilical cord. In California, a proposed study involving preterm infants will have the attending neonatologist provide two sustained inflations and CPAP before the obstetrician clamps the umbilical cord (personal communication, Anup Katheria, M.D. 2013). According to the study protocol, the obstetric provider will hold the infant and the umbilical cord clamping will be delayed for 60 seconds. In England, Hutchon and colleagues are field testing use of the resuscitation trolley in a hospital setting.64 More research on resuscitation with an intact umbilical cord is needed.
SUMMARY
This article proposes that receiving an adequate blood volume from placental transfusion, at birth, is protective for the neonate, especially when distressed. We suggest that neonates who need resuscitation also need their placental transfusion as much or more than do healthy newborns. Placental transfusion plays a major role in neonatal transition by preventing hypovolemia and providing better perfusion to all organs. Umbilical cord milking can be done quickly by any provider within the NRP resuscitation guidelines. Adopting resuscitation with an intact umbilical cord in a hospital setting will take concentrated effort and teamwork by midwifery, obstetrics, pediatrics, and nursing.
QUICK POINTS.
Placental transfusion at birth results in approximately 30% more blood volume for most neonates improving perfusion to all organs.
Infants who need resuscitation need a placental transfusion as much or more than healthy infants.
Hypovolemia can cause low heart rate, asystole, inflammation, and hypoxia/ischemia in the neonate.
Umbilical cord milking can be done quickly by the provider within the current recommended resuscitation timeline before clamping the umbilical cord.
Resuscitation with an intact umbilical cord, although routine in out-of-hospital settings, will take a concentrated effort and teamwork in a hospital setting.
Acknowledgments
This work was supported by funding from the NIH (NINR RO1NR010015, NICHD RO1HD076589-01), The Thrasher Foundation, and the Bill and Melinda Gates Foundation.
We would like to dedicate this article to the memory of Maureen O'Connor, NNP, MS who was always supportive of the concept of neonatal resuscitation with an intact cord and would have been a wonderful contributing co-author.
This material was presented in part at: European Association of Pediatric Societies, October 7, 2012, Istanbul, Turkey; International Conference on Cord Clamping, April 19, 2013, Birmingham, UK; and Perinatal Rounds, Women & Infants Hospital, Providence, RI, September 11, 2013.
Footnotes
The authors have no conflict of interests
Contributor Information
Judith S. Mercer, University of Rhode Island, Research Scientist at Women & Infants Hospital, and Adjunct Professor of Pediatrics at Brown University..
Debra A. Erickson-Owens, University of Rhode Island, Research Scientist at Women & Infants Hospital, and a Clinical Teaching Associate at Brown University..
REFERENCES
- 1.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2(7626):871–3. doi: 10.1016/s0140-6736(69)92328-9. [DOI] [PubMed] [Google Scholar]
- 2.Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297(11):1241–52. doi: 10.1001/jama.297.11.1241. [DOI] [PubMed] [Google Scholar]
- 3.McDonald S, Middleton P, Dowswell T, Morris P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. status and date: New search for studies and content updated. (7) conclusions changed), published in 2013. [Google Scholar]
- 4.Rabe H, Jewison A, Alvarez RF, Crook D, Stilton D, Bradley R, et al. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol. 2011;117(2 Pt 1):205–11. doi: 10.1097/AOG.0b013e3181fe46ff. [DOI] [PubMed] [Google Scholar]
- 5.ACOG. Committee Opinion No. 543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120(6):1522–6. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 6.AAP. Timing of Umbilical Cord Clamping After Birth. 2013;131(4):1323. [Google Scholar]
- 7.Mercer JS, Erickson-Owens DA. Rethinking placental transfusion and cord clamping issues. J Perinatol Neo Nur. 2012;26(3):202–17. doi: 10.1097/JPN.0b013e31825d2d9a. [DOI] [PubMed] [Google Scholar]
- 8.Mercer J, Skovgaard R. Neonatal transitional physiology: a new paradigm. J Perinat Neonatal Nurs. 2002;15(4):56–75. doi: 10.1097/00005237-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Nalbant D, Bhandary P, Matthews NI, Schmidt RL, Bogusiewicz A, Cress GA, et al. Comparison of multiple red cell volume methods performed concurrently in premature infants following allogeneic transfusion. Pediatric research. 2013;74(5):592–600. doi: 10.1038/pr.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. The Journal of Physiology. 2013;591(8):2113–26. doi: 10.1113/jphysiol.2012.250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graves BW, Haley MM. Newborn Transition. Journal of Midwifery & Women's Health. 2013;58(6):662–70. doi: 10.1111/jmwh.12097. [DOI] [PubMed] [Google Scholar]
- 12.Linderkamp OL. Placental transfusion: determinants and effects. Clin Perinatol. 1982;9:599. [PubMed] [Google Scholar]
- 13.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound in Obstetrics & Gynecology. 2006;28(2):126–36. doi: 10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- 14.Mielke G, Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation. 2001;103(12):1662–8. doi: 10.1161/01.cir.103.12.1662. [DOI] [PubMed] [Google Scholar]
- 15.Nelle M, Zilow EP, Bastert G, Linderkamp O. Effect of Leboyer childbirth on cardiac output, cerebral and gastrointestinal blood flow velocities in full-term neonates. Am J Perinatol. 1995;12(3):212–6. doi: 10.1055/s-2007-994455. [DOI] [PubMed] [Google Scholar]
- 16.Oh W, Oh MA, Lind J. Renal function and blood volume in newborn infant related to placental transfusion. Acta Paediatr Scand. 1966;55:197–210. [Google Scholar]
- 17.Oh W, Wallgren G, Hanson JS, Lind J. The effects of placental transfusion on respiratory mechanics of normal term newborn infants. Pediatrics. 1967;40(1):6–12. [PubMed] [Google Scholar]
- 18.Pietra GG, D'Amodio MD, Leventhal MM, Oh W, Braudo JL. Electron microscopy of cutaneous capillaries of newborn infants: effects of placental transfusion. Pediatr. 1968;42(4):678–83. [PubMed] [Google Scholar]
- 19.Tolosa JN, Park DH, Eve DJ, Klasko SK, Borlongan CV, Sanberg PR. Mankind's first natural stem cell transplant. Journal of cellular and molecular medicine. 2010;14(3):488–95. doi: 10.1111/j.1582-4934.2010.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson O, Hellstrom-Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomized controlled trial. BMJ. 2011;343:d7157. doi: 10.1136/bmj.d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(Suppl 1):S43–8. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafir T, Angulo-Barroso R, Jing Y, Angelilli ML, Jacobson SW, Lozoff B. Iron deficiency and infant motor development. Early Hum Dev. 2008 doi: 10.1016/j.earlhumdev.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160(11):1108–13. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozoff B, Smith JB, Clark KM, Perales CG, Rivera F, Castillo M. Home intervention improves cognitive and social-emotional scores in iron-deficient anemic infants. Pediatrics. 2010;126(4):e884–94. doi: 10.1542/peds.2009-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercer J. Infant Brain Study. Bethesda, MD: [February 13 2014]. p. 2014. Available from: http://clinicaltrials.gov/ct2/show/NCT01620008. [Google Scholar]
- 26.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467–78. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 27.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17(2):83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30(44):14595–609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M, et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res. 2006;59(2):244–9. doi: 10.1203/01.pdr.0000197309.08852.f5. [DOI] [PubMed] [Google Scholar]
- 30.Imitola J, Raddassi K, In Park K, Mueller F, Nieto M, Teng Y, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-dervived factor 1a/CXC chemokine receptor 4 pathway. Proceedings of the National Academy of Sciences. 2004;101(52):18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotten M. [February 20, 2014];Cord Blood- Neonatal Hypoxic Ischemic Encephalopathy. 2014 http://clinicaltrials.gov/ct2/show/NCT00593242.
- 32.Erickson-Owens DA. Placental Transfustion at birth in full-term infants.. 6th Annual Normal Labour and Birth Conference.; Grange-Over-Sands, England, United Kingdom. 2011. [Google Scholar]
- 33.Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG. 2011;118(1):70–5. doi: 10.1111/j.1471-0528.2010.02781.x. [DOI] [PubMed] [Google Scholar]
- 34.Yao AC, Hirvensalo M, Lind J. Placental transfusion-rate and uterine contraction. Lancet. 1968;1(7539):380–3. doi: 10.1016/s0140-6736(68)91352-4. [DOI] [PubMed] [Google Scholar]
- 35.Erickson-Owens DA, Mercer JS, Oh W. Umbilical Cord Milking in Term Infants Delivered by Cesarean Section: a Randomized Controlled Trial. J Perinatol. 2012;32:580–4. doi: 10.1038/jp.2011.159. [DOI] [PubMed] [Google Scholar]
- 36.Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks' gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F14–9. doi: 10.1136/adc.2006.108902. [DOI] [PubMed] [Google Scholar]
- 37.March M, Hacker M, Parson A, Modest A, de Veciana M. The Effects of Umbilical Cord Milking in Extremely Preterm Infants: A Randomized Controlled Trial. Journal of Perinatology. 2013;33:763–7. doi: 10.1038/jp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minami H. Cord milking reduces the need for blood transfusions and the incidence of severe intraventricular hemorrhage in infants born before 26 weeks of gestation. PAS. 2008 Abstract 4450. [Google Scholar]
- 39.Mercer JS, Skovgaard R, Erickson-Owens DA. Fetal to Neonatal Transition: First Do No Harm. In: Downe S, editor. Normal Childbirth Evidence and Debate. 2nd ed. Churchill Livingstone Elsevier; London: 2008. [Google Scholar]
- 40.Kattwinkel J. Neonatal Resuscitation (NRP) 6th edition AAP and Am Heart Association; 2011. [Google Scholar]
- 41.Mercer JS, Nelson CC, Skovgaard RL. Umbilical cord clamping: beliefs and practices of American nurse-midwives. J Midwifery Women Health. 2000;45(1):58–66. doi: 10.1016/s1526-9523(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 42.Cashore WJ, Usher R. Hypovolemia resulting from a tight nuchal cord at birth. Pediatric Research. 1973;7:399. [Google Scholar]
- 43.Osak R, Webster KM, Bocking AD, Campbell MK, Richardson BS. Nuchal cord evident at birth impacts on fetal size relative to that of the placenta. Early Human Development. 1997;49(3):193–202. doi: 10.1016/s0378-3782(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 44.Iffy L, Varadi V. Cerebral palsy following cutting of the nuchal cord before delivery. Med Law. 1994;13:323–30. [PubMed] [Google Scholar]
- 45.Iffy L, Varadi V, Papp E. Untoward neonatal sequelae deriving from cutting of the umbilical cord before delivery. Med Law. 2001;20(4):627–34. [PubMed] [Google Scholar]
- 46.Flamm BL. Tight nuchal cord and shoulder dystocia: a potentially catastrophic combination. Obstet Gynecol. 1999;94(5 Pt 2):853. doi: 10.1016/s0029-7844(99)00392-0. [DOI] [PubMed] [Google Scholar]
- 47.Mercer J, Erickson-Owens D, Skovgaard R. Cardiac asystole at birth: Is hypovolemic shock the cause? Medical hypotheses. 2009;72(4):458–63. doi: 10.1016/j.mehy.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Miller S, Turan JM, Ojengbede A, Ojengbede O, Fathalla M, Morhason-Bello IO, et al. The pilot study of the non-pneumatic anti-shock garment (NASG) in women with severe obstetric hemorrhage: Combined results from Egypt and Nigeria. Int J Gynaecol Obstet. 2006;94(Suppl 2):S154–6. doi: 10.1016/S0020-7292(06)60022-2. [DOI] [PubMed] [Google Scholar]
- 49.Pfeifer R, Litche P, Schreiber H, Sellei RM, Deinstknect T, Sadeghi C, et al. Models of Hemorrhagic Shock: Differences in the Physiological and Inflammatory Response. 2012 doi: 10.1016/j.cyto.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Cowan F, Rutherford M, Groenendaal F. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 51.Rajnik M, Salkowski CA, Thomas KE, Li YY, Rollwagen FM, Vogel SN. Induction of early inflammatory gene expression in a murine model of nonresuscitated, fixed-volume hemorrhage. Shock. 2002;17(4):322–8. doi: 10.1097/00024382-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Makley AT, Goodman MD, Belizaire RM, Friend LAW, Johannigman JA, Dorlac WC, et al. Damage control resuscitation decreases systemic inflammation after hemorrhage. Journal of Surgical Research. 2012;175(2):e75–e82. doi: 10.1016/j.jss.2011.11.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makley AT, Goodman MD, Friend LAW, Deters JS, Johannigman JA, Dorlac WC, et al. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. Journal of Trauma and Acute Care Surgery. 2010;68(2):305–11. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66(2):496–9. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 55.Conger JD, Robinette JB, Schrier RW. Smooth muscle calcium and endothelium-derived relaxing factor in the abnormal vascular responses of acute renal failure. J Clin Invest. 1988;82(2):532–7. doi: 10.1172/JCI113628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leviton A, Dammann O. Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr Res. 2004;55(4):541–5. doi: 10.1203/01.PDR.0000121197.24154.82. [DOI] [PubMed] [Google Scholar]
- 57.Volpe JJ. Neurology of the Newborn. 4th ed. Saunders; Philadelphia: 2001. [Google Scholar]
- 58.Hankins GD, Loen S, Fei AFea. Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol. 2000;99(5):668–91. doi: 10.1016/s0029-7844(02)01959-2. [DOI] [PubMed] [Google Scholar]
- 59.Odd D, Lewis G, Whitelaw A, Gunnel D. Resuscitation at Birth and Cognition at 8 Years of Age: A Cohort Study. The Lancet. 2009 doi: 10.1016/S0140-6736(09)60244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindstrom K, Hallberg B, Blennow M, Wolff K, Fernell E, Westgreen M. Moderate Neonatal Encephalopathy: Pre- and Perinatal Risk Factors and Long-term Outcome. Acta Obstetricia et Gynecologica. 2008;87:7. doi: 10.1080/00016340801996622. [DOI] [PubMed] [Google Scholar]
- 61.Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, et al. Part 1: executive summary 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 suppl 3):S640–S56. doi: 10.1161/CIRCULATIONAHA.110.970889. [DOI] [PubMed] [Google Scholar]
- 62.Ewy GA, Kern KB. Recent advances in cardiopulmonary resuscitation: cardiocerebral resuscitation. Journal of the American College of Cardiology. 2009;53(2):149–57. doi: 10.1016/j.jacc.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 63.Mercer JS, Skovgaard RL, Peareara-Eaves J, Bowman TA. Nuchal cord management and nurse-midwifery practice. Journal of midwifery & women's health. 2005;50(5):373–9. doi: 10.1016/j.jmwh.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Hutchon D, Bewley S. Support Transition by Keeping the Placental Circulation Intact- Even in Newborns Apparently Requiring Resuscitation. Arch Dis Child Fetal Neonatal Ed. 2008;93:3. [Google Scholar]
- 65. [October 3 2013]; Available from: www.inditherm.co.uk/medical/neonatal-resuscitation-lifestart/
- 66.Andersson O, Hellstrom-Westas L, Andersson D, Clausen J, Domellof M. Effects of Delayed Compared With Early Umbilical Cord Clamping on Maternal Postpartum Hemorrhage and Cord Blood Gas Sampling: a Randomized Trial. ACTA Obstetricia et Gynecologica Scandinavica. 2012;92:567–74. doi: 10.1111/j.1600-0412.2012.01530.x. [DOI] [PubMed] [Google Scholar]