Abstract
It has now become increasingly clear that a complete atomic description of how biomacromolecules recognize each other requires knowledge not only of the structures of the complexes but also of how kinetics and thermodynamics drive the binding process. In particular, such knowledge is lacking for protein–glycosaminoglycan (GAG) complexes. Isothermal titration calorimetry (ITC) is the only technique that can provide various thermodynamic parameters—enthalpy, entropy, free energy (binding constant), and stoichiometry—from a single experiment. Here we describe different factors that must be taken into consideration in carrying out ITC titrations to obtain meaningful thermodynamic data of protein–GAG interactions.
Keywords: Glycosaminoglycan (GAG), Heparin, Free energy, Thermodynamics, Isothermal titration calorimetry (ITC), Enthalpy, Entropy
1 Introduction
Glycosaminoglycans (GAGs), such as heparan sulfate, are highly sulfated polysaccharides that are ubiquitously expressed by most cell types, and bind a wide range of proteins including growth factors, chemokines, and enzymes. Naturally occurring human mutations and animal knockout mice studies have shown that protein–GAG interactions play crucial roles in processes ranging from development to the host immune response [1, 2]. We refer the reader to other chapters in this book for a description of the GAG structures and properties. A complete understanding of biomacromolecular interactions requires knowledge not only of the structures of the complexes but also of how the kinetics and thermodynamics drive the binding process. At this time, very little is known regarding the thermodynamic basis of protein–GAG interactions.
Isothermal titration calorimetry (ITC) is the only technique that can provide various thermodynamic parameters—enthalpy (ΔH), entropy (ΔS), free energy (ΔG) that is also related to equilibrium binding constant (Ka), and stoichiometry—from a single experiment. The enthalpy of binding provides insight into how favorable hydrophobic, H-bonding, and electrostatic interactions mediate binding, whereas the entropy of binding arises due to changes in restriction/freedom of the backbone and side chain atoms and rearrangement or release of solvent water molecules and ions [3]. Such knowledge is essential to understand the relationship between structure, dynamics, thermodynamics, and function that otherwise can be completely missed from considering structures alone, and also not tractable from conventional mutational studies. ITC studies have been routinely used for studying protein binding to other proteins, peptides, and DNA, but has been challenging for characterizing protein–GAG interactions. We will discuss why the complex interrelationship between factors such as binding-induced precipitation, GAG heterogeneity and size, binding affinities, and sample requirements must be taken into consideration in designing the ITC experiments. We also discuss how to interpret and get the most out of the experimental data to gain thermodynamic insights (see Note 1).
We have written this chapter on the basis of our ITC experience in characterizing chemokine–heparin interactions. Heparin and heparan sulfate are linear highly sulfated polysaccharides consisting of repeating disaccharide units. Heparin is generally used as a structural and functional surrogate of heparan sulfate, as it can be easily purified in large amounts and readily available. Sequence analysis and binding studies have shown that chemokines bind GAGs such as heparan sulfate expressed on endothelial and epithelial cells and the extracellular matrix, and in vivo studies have shown that GAG binding functions as directional cues and regulates cellular trafficking [4, 5]. Compared to other macromolecular interactions, ITC studies have been few and far between for GAG–protein complexes. This paucity is evident if we consider that a PubMed search for the word “chemokine” results in >70,000 hits, whereas just 1 hit is obtained for the words “chemokine, isothermal titration calorimetry, and heparin” (16th Oct 2013). Even this ITC study was part of a larger study, and merely describes the binding affinities and stoichiometry of one chemokine binding to various commercially available heparins with no discussion of the enthalpy or entropy of binding [6].
2 Materials
Most commercially available heparins are from porcine intestinal mucosa or bovine lungs. Various size heparins ranging from a disaccharide and various oligosaccharides to high molecular weight polysaccharides are available from popular vendors such as Sigma and Calbiochem, and specialized vendors such as Neoparin and Iduron. Moreover, heparin is structurally more homogeneous than heparan sulfate, and not surprisingly, most structural and biophysical studies reported in literature have used heparin. Our discussion on experimental design will be for heparin but is applicable to heparan sulfate and most likely for all other GAGs.
The concentrations of protein and GAG solutions should be determined as accurately as possible. Weigh the protein and GAG accurately using a microbalance (see Notes 2 and 3). Determine the concentration of the protein solution using UV–vis spectrophotometer and/or bicinchoninic acid (BCA) or similar assay. The extinction coefficients can be calculated from the number of tryptophans, tyrosines, and disulfide bonds [7], and if necessary, precise protein concentrations can be obtained from amino acid analysis from which the experimental extinction coefficient can be obtained. We recommend preparing stock solutions of the protein and GAG and then diluting as required for the ITC experiments.
Make sure that the buffer conditions of the protein and GAG solutions match. Buffer mismatch may not be a problem if the commercially purchased GAGs were dialyzed against water and then lyophilized. We have also observed that titration of commercial heparins into a buffer results in negligible heat release, indicating that heparin dilution does not complicate the titration. If required, dialyze the samples into the required buffer to prevent solvent mismatch. In this case, it is important to measure the concentrations before and after to account for any loss during the dialysis procedure.
3 Methods
3.1 Instrument Setup and Experimental Parameters
In a typical ITC instrument, a reference cell and a measurement cell are placed in an adiabatic jacket. The reference cell is filled with 1 % azide solution whereas the measurement cell is filled with the protein solution. Both cells are maintained at a constant temperature by using a controller. The equipment has a syringe through which heparin (ligand) is injected into the measurement cell. The volume of the VP-ITC cell is 1.45 mL, whereas the injection volume is around 100 μL (25 × 4-μL injections). The solution in the measurement cell is constantly stirred for rapid mixing (see Notes 4–6).
The sample temperature should be just below the experimental temperature so that the equilibrium in the ITC instrument is attained quickly. We generally carry out our experiments at 25 °C. The protein and GAG solutions must be degassed to remove air bubbles as they can interfere with the feedback circuit and lead to poor baseline.
If necessary, run a blank titration of ligand into the buffer and buffer into protein to correct for any heat change due to contribution from dilution of the protein and/or GAG samples.
-
An important aspect of an ITC experiment is choosing the proper concentration for protein and ligand. The choice of protein concentration depends on the “c” value as given by Eq. 1:
where, Pt is the protein concentration in the measurement cell; “n” is the number of binding sites per protein molecule. Please note that multiple binding sites are present usually on the GAG and not on the protein. Thus the value of n could be <1. The shape of the binding isotherm depends on the c value. The c value should be between 20 and 100 to obtain a sigmoidal shape of the binding isotherm in order to get a good fit to estimate Kd, ΔH, ΔS, and n. If the c value is very high (∼1,000), one can get an estimate of ΔH and n, but not of the other parameters. If the c value is <5, then the shape of the binding isotherm does not permit accurate estimation of the thermodynamic parameters unless one of them is already known (such as the stoichiometry) and is not varied during the fitting process.(1) A simulation plot of heat released vs. mole ratio explains the importance of c value (Fig. 1). If the Kd and n are not known, assume that the Kd is between 1 mM and 1 nM. Start with a protein concentration of 10 μM. The shape of a step jump would indicate a high c value, and in which case reduce the protein concentration by tenfold. A hyperbolic shape would indicate a low c value, and in which case increase the protein concentration by tenfold. Optimize the protein concentration until a good shape for the isotherm is obtained (see Notes 7–9).
If an estimate of the dissociation constant (Kd) is known, then choose a protein concentration in the cell that is 30 times Kd. The ligand concentration should be ∼n × 30 times the protein concentration. For high MW GAGs, multiple binding sites are on the GAG and not on the protein, and therefore the n value could be <1. For more details, please refer to ref. [9].
The first injection is usually not accurate due to the diffusion of the ligand into the cell during the temperature equilibration. So, inject a small volume first (2 μL) and then inject 4 μL or more for the remaining titrations. One could also inject 2 μL for the first 4–5 injections and then increase the injection volume if one wants to switch between high data coverage at larger noise to lower data coverage at less noise.
We suggest that the protein is taken in the cell and the GAG in the syringe for two reasons. As discussed above, the concentration in the syringe must be higher (preferably n × 30) than the concentration in the cell. We observe that titrating protein into GAGs in the cell results in precipitation, and not if we titrate GAG into the protein. Secondly, more GAG will be required if taken in the cell compared to taken in the syringe. Though this may not be limiting for crude and cost-effective heparins, it could be limiting due to the high cost of the smaller and homogeneous GAGs. It is possible that precipitation may not be an issue for 1:1 stoichiometry, and in which case, the relative cost of the GAG and protein could determine what goes in the syringe and cell (see Note 10).
The affinity of protein–GAG interaction could increase with increasing GAG length. Therefore, if the c value is low for a particular chain length, longer GAGs could result in a better binding isotherm especially if the protein concentration cannot be increased (see Note 11).
Fig. 1.
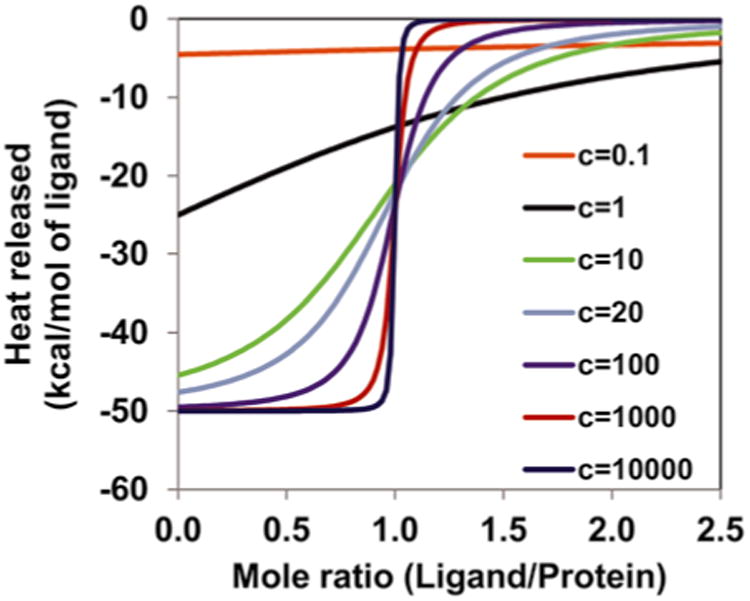
Simulated binding isotherm for a 1:1 system to show the importance of c value. The data were generated using the model proposed by Wiseman et al. [10] using ΔH = −50 kcal/mol. For low c values (<5), the binding isotherm loses the S-shape and thus determination of the thermodynamic parameters is not possible. At high c values (∼1,000), ΔH and stoichiometry (n) can be inferred from the shape of the binding isotherm but the data cannot be fitted to determine the other thermodynamic parameters
3.2 Data Analysis
Ensure that the concentrations of the protein and ligand are correct.
The peak integration baseline of each run should be inspected and adjusted manually if required.
If necessary, subtract the heats obtained from the protein and GAG dilution experiments.
Neglect the first data point, which may have a systematic error due to the diffusion effect.
- Choose an appropriate model using the fitting software provided by Microcal (Origin). The most common model is 1:1 binding. For a protein containing single set of n identical binding sites, the following equations are used:
(2)
where, Pt is the concentration of protein in the active volume, dV(i) is the displaced volume, V0 is the volume of the cell, Xt is the concentration of the ligand in the active volume, Q(i) and Q (i−1) are the total heat evolved or absorbed for ith and (i−1)th injections, respectively. Detailed derivation of the equations can be found in the VP ITC manual. Provide an initial estimate of n, Ka and ΔH, which calculates ΔQ(i) for each injection. Then the program calculates the values of n, Ka, and ΔH that provide the best fit to the calculated and experimental ΔQ(i). Note that good initial estimates are essential to prevent the algorithm from terminating at a sub-optimum.(3)
4 Notes
ITC can be used to describe the thermodynamics of how individual amino acids contribute to the binding process by characterizing a panel of mutants. ITC can be carried out at different conditions including as a function of pH, ionic strength, buffers, and temperature to gain insights into the role of electrostatic interactions and counter ions, ionization state, and binding mechanisms.
The protein and GAG concentrations must be accurate. If GAG is taken in the syringe, an error in the GAG concentration will result in systematic errors in all of the thermodynamic parameters as shown in Fig. 2b. For instance, if the measured GAG concentration is higher than the actual concentration, the experimental value of n and ΔH will be less than the actual n and ΔH, whereas the experimental Kd will be higher than the actual Kd. Therefore preparing stock solutions of high GAG concentration (>1 mM) could minimize the weighing error and hence the concentration error. If protein is taken in the cell, an error in the protein concentration will result in errors in stoichiometry alone and not in any other parameter as shown in Fig. 2c. The error in protein concentration will linearly change the estimated stoichiometry. Thus a 10 % inaccuracy in protein concentration will cause a 10 % error in stoichiometry. For instance, if the measured protein concentration is higher than the actual concentration (90 vs. 60 μM), then the measured stoichiometry will be higher (n = 16 vs. 11; the calculated numbers have been rounded to the nearest integer).
Errors in protein and GAG concentrations may not be limiting if the objective of the study is to compare different variants of a protein. For instance, any errors will propagate in the same manner when comparing thermodynamic parameters of a panel of protein mutants using the same GAG stock solution. However, the resulting dissociation constant values will have an unknown offset that will depend on the real concentration of the GAG stock solution. We recommend the following for those who use their own fitting procedures rather than the manufacturer-provided analysis package. In Eq. 2, the stoichiometry factor, n, is associated with the cell concentration of the protein, n × Pt. Alternatively, one could associate this factor with the syringe concentration of the ligand, n × Xt. The best practice is to have the factor “n” associated with the more uncertain of the two concentrations, Pt or Xt. In this way, the binding constant is least affected by concentration errors.
Meticulous cleaning of the cell and the syringe are very important. Please refer to the ITC manual for the instructions, and ensure that both the cell and syringe are as dry as possible to minimize dilution errors.
Take utmost care while loading samples in the cell and the syringe. There should be no bubbles in the cell or the syringe. The syringe should not bend. Even a small bending of the syringe will lead to a poor baseline.
Choose the time interval in such a way that heat released due to one injection does not overlap with that of another injection. The most common spacing is 300 s.
If protein is taken in the cell, 1.45 mL of sample is required for each experiment for the VP-ITC calorimeter. The protein concentration required depends on the affinity of protein–GAG complex; if unknown, we suggest a concentration between 10 and 100 μM.
A thorough knowledge of the protein of interest (or of related proteins) from literature including any prior studies on GAG binding, and knowledge of the structures and solution properties is essential. If the protein exists as monomers and dimers, knowledge of the dimerization constants and whether GAG binding influences dimerization would be useful. If GAG binding and dimerization are coupled, it is necessary to use appropriate equations which will be different from Eq. 2.
For a protein for which nothing is known regarding its GAG binding properties, initial experiments will determine whether ITC experiments are feasible and can provide meaningful results. Ideally, the protein must not be limiting, and so availability of milligram (mg) and preferably tens of mgs of protein would be useful. The apparent binding affinities could vary by orders of magnitude from mM to nM. In principle, ITC can measure binding affinities over this range, but multiple parameters must be optimized to obtain reliable thermodynamic data.
Stoichiometry of binding can vary among different proteins, and can also vary for a given protein depending on the size of the heparin used. The MW of GAG-binding proteins can also vary by orders of magnitude from a few kDa to 100 s of kDa. Further, GAG-binding residues could be highly clustered or span a large surface for any given protein. It has been proposed that chemokines and growth factors bind GAGs like beads on a string on the basis that the stoichiometry increases with increasing heparin length [6, 9]. As many as ten or more protein molecules have been reported to bind a single GAG. From an experimental perspective, this would mean that much less GAG is required for a titration on a mol:mol basis. Some studies also suggest that GAG-binding and chemokine dimerization are coupled. The measured binding affinities also vary for different heparin sizes and are also sensitive to solution pH and buffer conditions. These factors are also critical for deciding whether the protein or GAG should be in the syringe and the amount of protein or GAG that will be required.
The major challenges for characterizing protein–GAG complexes are large sample requirements, binding-induced protein dimerization/oligomerization, precipitation, and intrinsic heterogeneity of the GAGs. We suggest that the initial experiments use crude high MW GAGs, as these GAGs are less expensive and are also more likely bind with tighter affinity compared to homogeneous shorter oligosaccharides. Tighter binding enables experiments at lower protein concentrations thus minimizing binding-induced aggregation and precipitation issues. However, the thermodynamic parameters obtained using crude heterogeneous GAGs are average of all GAGs present in the sample. It is also possible that precipitation might occur with these GAGs due to high MW, in which case, we suggest using size-fractionated smaller GAGS. Size-fractionated GAGs are more homogeneous and also provide more defined thermodynamic parameters. Therefore, once feasibility is established and binding parameters more or less optimized, one could use size-fractionated GAGs whose results can be interpreted with greater confidence. However, the experimental design must take into consideration the fact that smaller GAGs could lead to reduced heat release and so would require larger quantities.
Fig. 2.
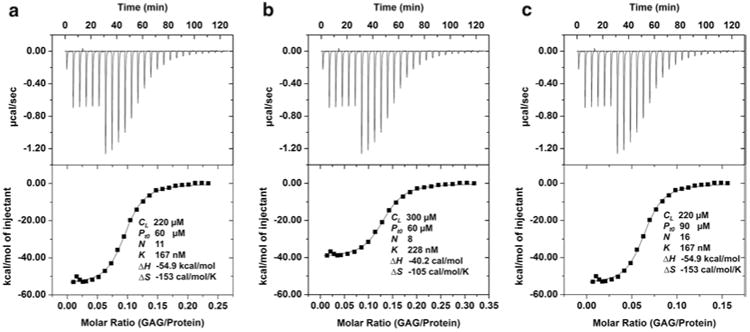
Relationship between concentration errors and thermodynamic parameters. (a) The protein (Pt0) and GAG (CL) concentrations were 60 and 220 μM, respectively. The first six injections were 2 μL and the rest were 4 μL. The stoichiometry (n), equilibrium dissociation constant (Kd), enthalpy and entropy data are shown. (b) An error of increased GAG concentration affected the values of all thermodynamic parameters. The stoichiometry and enthalpy decreased proportionally, and ΔS value also changed due to changes in ΔH and Kd. Note that the stoichiometry is protein/GAG. (c) An error of increased protein concentration affected the value of stoichiometry alone and all other values remained unchanged
Acknowledgments
This work was supported in part by grants P01HL1071521 and R21AI097975 to K.R. and R01GM049760 to J.R. from the National Institutes of Health. The authors would like to thank Dr. Luis Holthauzen for technical support.
References
- 1.Lindahl U, Kjellén L. Pathophysiology of heparan sulphate: many diseases, few drugs. J Intern Med. 2013;273:555–571. doi: 10.1111/joim.12061. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 3.Ladbury JE, Klebe G, Freire E. Adding calorimetric data to decision making in lead discovery: a hot tip. Nat Rev Drug Discov. 2010;9:23–27. doi: 10.1038/nrd3054. [DOI] [PubMed] [Google Scholar]
- 4.Salanga CL, Handel TM. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp Cell Res. 2011;317:590–601. doi: 10.1016/j.yexcr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangavarapu P, Rajagopalan L, Kolli D, Guerrero-Plata A, Garofalo RP, Rajarathnam K. The monomer-dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J Leukoc Biol. 2012;91:259–265. doi: 10.1189/jlb.0511239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuschert GSV, Coulin F, Power CA, Proudfoot AEI, Hubbard RE, Hoogewerf AJ, Wells TNC. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 7.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajarathnam K, Rösgen J. Biochim Biophys Acta. 2013. Isothermal titration calorimetry of membrane proteins – progress and challenges. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman G, Sasisekharan V, Herr AB, Ornitz DM, Waksman G, Cooney CL, Langer R, Sasisekharan R. Preferential self-association of basic fibroblast growth factor is stabilized by heparin during receptor dimerization and activation. Proc Natl Acad Sci. 1996;93:845–850. doi: 10.1073/pnas.93.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiseman T, Williston S, Brandts JF, Lin LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]


