Abstract
The serine/threonine protein kinase Akt plays a critical role in regulating proliferation, growth and survival through phosphorylation of different downstream substrates. The mammalian target of rapamycin (mTOR) is a key target for Akt to promote tumorigenesis. It has been reported that Akt activates mTOR through phosphorylation and inhibition of the tuberous sclerosis complex (TSC) protein TSC2. Previously it was demonstrated that mTOR activates IKK/NF-κB signaling by promoting IKK activity downstream of Akt in conditions deficient of PTEN. In the current study, the mechanistic role of the tumor suppressor TSC2 was investigated in the regulation of IKK/NF-κB activity in PTEN-null prostate cancer and in TSC2 mutated tumor cells. The results demonstrate that TSC2 inhibits IKK/NF-κB activity downstream of Akt and upstream of mTORC1 in a PTEN deficient environment. However, TSC2 promotes IKK/NF-κB activity upstream of Akt and mTORC1 in TSC2 mutated tumor cells. These data indicate that TSC2 negatively or positively regulates IKK/NF-κB activity in a context-dependent manner depending on the genetic background.
Keywords: Prostate Cancer, Tuberous Sclerosis Complex, TSC2, IKK, NF-κB, PTEN, Akt, mTORC1, mTORC2
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder characterized by tumor growth in multiple organs including the brain, kidney, heart, lung and skin. TSC results from mutations in either the TSC1 gene which encodes for hamartin, or TSC2 gene which encodes for tuberin (1, 2). These two proteins form a complex that inhibits the mammalian target of rapamycin (mTOR) pathway through negative regulation of the small GTPase Ras homolog enriched in the brain, Rheb (3-11). The oncogenic kinase Akt is a serine/threonine kinase that promotes cell proliferation, growth, survival and energy metabolism through phosphorylation of specific substrates. In many types of cancers, Akt is constitutively activated due to activating mutations within the kinase itself, or due to upstream activation of receptor tyrosine kinases or Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), or due to loss of PTEN tumor suppressor function (12, 13). mTORC1 (mTOR complex 1), a key downstream effector of Akt, is a complex containing the serine/threonine kinase mTOR and a regulatory protein, Raptor, which phosphorylates S6K and 4E-BP1 to promote mRNA translation (14-16). The mTORC1 pathway plays an essential role in protein synthesis and translation necessary for cell proliferation. Akt activates mTORC1 by phosphorylating and inhibiting the tumor suppressor gene TSC2 to induce RheB which promotes mTORC1 activity (5, 7, 17-22). In addition, mTOR also associates with Rictor to form another mTOR complex, mTORC2 that phosphorylates Akt at serine 473 to promote fully the activation of Akt (16).
Importantly, in addition to up-regulation of growth signaling, mTORC1 and its downstream target, S6K, also mediate negative feedback loops that restrain PI3K/Akt signaling through insulin/IGF receptor and other tyrosine kinase receptors via phosphorylation as well as transcriptional repression of insulin receptor substrate 1 (IRS-1) (23-30). Therefore, blocking mTORC1 activity by mTOR inhibitors such as rapamycin reduces phosphorylation and degradation of IRS-1, which subsequently upregulates PI3K/Akt activity in several cancer cell lines (31). These studies suggest that the anti-cancer activity of mTOR inhibitors may be reversed by releasing feedback inhibition of PI3K/Akt (23, 24, 31). On the other hand, feedback inhibition of Akt signaling by loss of tumor suppressor gene TSC2 can limit the growth of TSC-related tumors, which is likely to explain the mostly benign nature of tumors arising in TSC patients (32, 33). In addition, these findings also suggest that TSC2 may play differing roles in downstream and upstream signaling pathways based on different genetic or physiological backgrounds (32)
The nuclear factor-κB (NF-κB) is critical in the regulation of immune function, cell survival, apoptosis, invasion, migration and cellular proliferation. NF-κB, a ubiquitously expressed nuclear factor, normally resides in the cytoplasm in its inactive form in association with IκBα, the inhibitor of κB (IκB) protein. Inflammatory cytokine induced NF-κB activation is mediated by the IκB kinase (IKK) complex, which is comprised of two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (NEMO). Once activated, the IKK complex phosphorylates IκBα leading to its rapid ubiquitination and proteasome-mediated degradation, which causes the release of NF-κB from IκBα inhibition. The activated NF-κB forms heterodimers with p50 and translocates to the nucleus to regulate NF-κB target gene transcription (34-37). The activated IKK complex also directly phosphorylates p65 NF-κB at serine 536 to promote its activity, which indicates that phosphorylation of NF-κB at serine 536 is a major marker of NF-κB activity (38-40). In many cancers including prostate cancer, IKK/NF-κB signaling is also constitutively active due to dysregulation/activation of oncogenic pathways such as Akt or loss of PTEN tumor suppressor function (41, 42).
We previously reported that IKK/NF-κB is activated downstream of Akt in PTEN deficient prostate cancer cells (43) and inhibition of mTORC1 significantly reduces NF-κB activity in these cells, which suggests that mTORC1 is involved in Akt-mediated activation of IKK/NF-κB in PTEN loss induced prostate cancer (43). In the current study, we investigated the role of TSC2, the upstream inhibitory protein of mTORC1, in regulating IKK/NF-κB activity in PTEN deficient prostate cancer cells and TSC2 mutant TSC tumor cells. We found that TSC2 inhibits IKK/NF-κB activity in PTEN deficient prostate cancer cells but promotes IKK/NF-κB activity in TSC tumor cells that have wild-type PTEN and mutated TSC2. These data demonstrate that TSC2 differentially modulates IKK/NF-κB pathway in cells with different genetic background and indicate that IKK/NF-κB play a pivotal role in both PTEN deficient prostate cancer as well as TSC2 mutated tumors including TSC.
Materials and Methods
Cell culture and reagents
The cell lines HeLa, TSC2 ang1 and PC3 were purchased from American Type Culture Collection (ATCC). The HeLa-myr-Akt2 Tet-On cell line was a kind gift from Dr. J Cheng of Moffitt Cancer Center. The EEF126-4 (Tsc2+/+) and EEF126-8 (Tsc2−/−) cells were also provided by Dr. J. Cheng and originally derived by R. Yeung. All cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 U/mL penicillin and streptomycin (Gibco). The respective reagents were obtained from the following sources: Protease and phosphatase inhibitor cocktails were from Roche; CHAPS from Pierce; LY294002 from Cell Signaling; and protein A and protein G agarose beads from Invitrogen Life Technologies. The radiochemicals used were obtained from New England Nuclear. Recombinant GST-p65/RelA C-terminal fragment and GST-IκBα were provided by Dr. Albert Baldwin (University of North Carolina at Chapel Hill).
Antibodies
Antibodies against IKKα, IKKβ, mTOR, Akt1, and Akt2 were obtained from Upstate Biotechnology. Anti-Raptor and anti-Rictor antibodies were obtained from Bethyl Laboratories. The anti-myc (9E-10), anti-TSC2 and control rabbit IgG, as well as HRP-labeled anti-mouse and anti-rabbit secondary antibodies were from Santa Cruz Biotechnology. All other antibodies were from Cell Signaling Technology.
Transfections
Transfections were performed using Lipofectamine and Plus (Invitrogen) following the manufacturer's instructions. 3–4 h after transfection, cells were recovered in full serum for 36 h before performing assays.
RNAi interference
siRNA SMARTpool human TSC2-(1) (catalog # M003029), RheB (catalog # M009692), Akt1 (catalog # M-003000), and Akt2 (catalog # M-003001) were from Dharmacon. Each of these represents four pooled SMART-selected siRNA duplexes that target the indicated mRNA. siRNA to human TSC2-(2) was from Cell Signaling Technology (CST-6476). siRNA to human Raptor was from Dharmacon. siRNA to rat Akt1, Akt2, Raptor, Rictor and ERK and siRNA to mouse Rictor, Raptor, Akt1, Akt2 and ERK were from Santa Cruz. PC3, TSC2−/− or TSC2 ang1 lines cells were transfected with indicated SMARTpool siRNA or nonspecific control pool siRNA using DharmaFECT 1 reagent (Dharmacon) according to the manufacturer's instructions. Briefly, 20 nM final concentration of siRNA was used to transfect cells at 60%–70% confluency. Cells were harvested 48–72 hours after siRNA transfection.
Cell lysis and western blot analysis
Cells were lysed and immuno-blotted as described previously (43, 44) with minor modifications. Briefly, cells grown in 100-mm dishes were rinsed twice with cold PBS and then lysed on ice for 20 min in 1 mL of lysis buffer (40 mM Hepes pH 7.5, 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 50 mM NaF, 0.5 mM orthovanadate, EDTA-free protease inhibitors [Roche]) containing 1% Triton X-100. After centrifugation at 13,000g for 10 min, samples containing 20–50 μg of protein were resolved by 4-12% SDS-PAGE gels (Invitrogen), and proteins were transferred to Pure Nitrocellulose Membrane (Bio-Rad), blocked in 5% nonfat milk, and probed with the indicated antibodies.
In vitro IKK Assay
PC3 cells were grown in 100-mm dishes for 48 h in DMEM containing 10% FBS and lysed in 1 mL of lysis buffer with 0.3% CHAPS. Half of the total cell lysate was incubated with IKKα or IKKβ antibodies for 6–12 h, followed by another hour of incubation with 25 μL of protein G agarose beads. Immuno-precipitates were washed three times with lysis buffer, and once with IKK kinase buffer without ATP (20 mM Hepes pH 7.7, 2 mM MgCl2, 2 mM MnCl2, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM p-Nitrophenyl Phosphate [PNPP], 300 μM orthovanadate, 1 mM Benzamidine, 2 mM PMSF, 1 mM DTT, 10 μg/mL aprotinin, 1 μg/mL Leupeptin, 1 μg/mL pepstatin, 1 mM DTT). IKK kinase assays were performed by incubating washed immuno-precipitates with recombinant GST-IκBα (amino acids 1–54) or GST-p65/RelA C-terminal fragment for 45 min at 30°C in 30 μL of IKK kinase buffer with 10 μM ATP and [γ-32P]ATP (0.5 μCi per reaction). To stop the reaction, 8 μL of 4× SDS sample buffer was added to each reaction, which was boiled for 10 min. The reaction was then separated by 4%–12% SDS-PAGE and transferred to a PVDF membrane. 32P incorporated into GST-IκBα or GST-p65/RelA C-terminal fragment was assessed by autoradiography.
Reporter assays
Cells were seeded in six-well plates. 200 ng of 3× κB luciferase reporter and 50 ng of pRL-SV40 (Renilla reporter control) DNA were cotransfected using Lipofectamine and Plus (Invitrogen) following the manufacturer's instructions. Cells were harvested after 24 hours of transfection, and luciferase assays were performed using the Dual Luciferase Assay System (Promega). All transfections were performed in triplicate.
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as described previously (43). In brief, nuclear extracts were obtained as described above, and 5 μg of nuclear proteins were incubated with 1 μg/μl polydIdC in binding buffer (50 mM Tris pH 7.6, 5 mM DTT, 2.5 mM EDTA, 50% glycerol) for 15 minutes. Subsequently, an oligonucleotide radiolabeled with [α32P]dCTP was added and allowed to incubate for an additional 15 minutes at room temperature. The probe contains a NF-κB consensus binding site for the H-2κB promoter (5′-GGGGATTCCC-3′). The samples were then separated on polyacrylamide gels and developed with autoradiography.
RNA extraction and real-time PCR
RNA extraction and real-time PCR were performed as described previously (43). Forty-eight hours after transfection of siRNA, total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA). Reverse transcription was conducted with 1 μg of total RNA using MMLV reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Real time RT-PCR was then performed using TaqMan gene expression assays for IκBα, cIAP1, cIAP2, XIAP, Survivin, Bcl-2, and Bcl-xL (Applied Biosystems Inc., Forest City, CA) on an ABI 7900HT real time PCR system (Applied Biosystems, Inc., Forest City, CA).
Statistics
Data from the in vitro experiments are expressed as mean ± SD from a minimum of 3 independent experiments. Comparison between groups were carried out by 2-way ANOVA or Student t test, and a P value of less than 0.05 was considered significant.
Results
TSC2 inhibits NF-κB/IKK activity in PTEN null PC3 cells
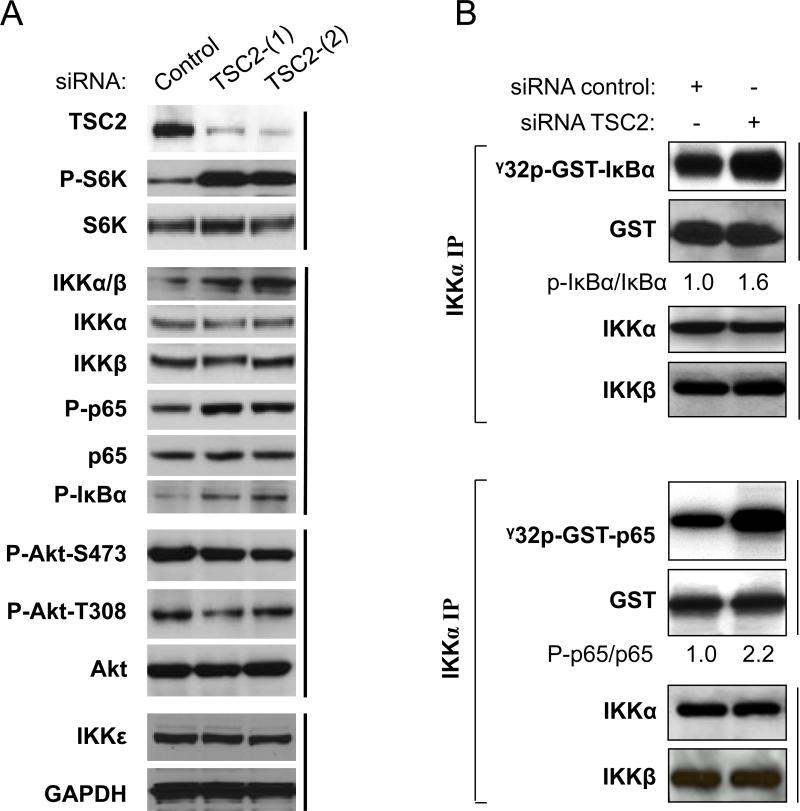
We previously reported that mTORC1 enhances the ability of the IKK complex to induce NF-κB p65 activity in PTEN null prostate cancer cells (43). While many studies have already shown that the tumor suppressor gene TSC2 inhibits mTORC1 activity and that this inhibition can be countered by Akt phosphorylation (4, 8-10), these observations raised the question of whether or not TSC2 suppresses NF-κB activity through mTORC1 inhibition. To address this question, we used siRNA against TSC2 in PC3 cells to knock down the expression of TSC2 and examine its effects on IKK/NF-κB activation. In order to minimize the possible off-target effect of the siRNA used, two independent siRNAs targeting TSC2 (siRNA TSC2-[1] and siRNA TSC2-[2]) or control siRNA were employed. As expected, knockdown of TSC2 markedly increases phosphorylation of S6K at threonine 389, which is the direct target site of mTORC1 while having no effect on S6K expression (Fig. 1A). Additionally, consistent with the increased S6K phosphorylation, augmented phosphorylation of NF-κB at serine 536, a critical marker of NF-κB activation, was observed. These data indicate that TSC2 negatively mediates NF-κB signaling in PC3 cells. Since it has been demonstrated that IKK is the pivotal kinase that phosphorylates NF-κB p65 at serine 536 (38-40, 43), we tested whether or not IKK activity is affected by TSC2 knockdown. Decreasing TSC2 expression increased endogenous phosphorylation of IKKα/β in their activation loops (serine 180/181) in a manner consistent with the increase in p65 phosphorylation while having no effect on expression of IKKα and IKKβ. In parallel with the enhanced p65 phosphorylation, basal phosphorylation of IκBα, another IKKα/β substrate, also increases (Fig. 1A). These data suggest that IKK activity and phosphorylation of its downstream targets are elevated upon TSC2 knockdown. It has been shown that IKKε (also named IKKe, IKBKE or IKKi) phosphorylates NF-κB/p65 at serine 536 to activate NF-κB (45). Therefore, we examined whether IKKε expression is elevated upon knockdown of TSC2. The results show that knockdown of TSC2 has no effect on the expression of IKKε, which suggests that IKKα/β, but not IKKε, is the major kinase that phosphorylates NF-κB at serine 536 in PC3 cells. Previous studies demonstrated that up-regulation of mTORC1 leads to inhibition of Akt activity (6, 12, 15). Thus, we determined if Akt activity is affected by TSC2 knockdown. Interestingly, there is no change observed in the basal phosphorylation status of Akt upon TSC2 knockdown (Fig. 1A). In order to confirm the results that IKK activity increases upon knockdown of TSC2, we also performed in vitro IKK kinase assays to examine the kinase activity of the IKK immunoprecipate for GST-IκBα and GST-p65, two typical IKK substrates that are upregulated by TSC2 knockdown as shown in Fig. 1A. As expected, we found that knockdown of TSC2, as expected, elevated IKK's kinase activity for both IκBα (Fig. 1B, upper panel) and p65 (Fig. 1B, lower panel) while having no effect on the expression of IKKα or IKKβ. These results indicate that TSC2 negatively modulates IKK/NF-κB activity through mTORC1 inhibition, but has no impact on Akt activity in PC3 cells in which Akt is constitutively activated due to loss of PTEN (3, 43, 46).
Figure 1.
TSC2 inhibits NF-κB/IKK signaling in PTEN null PC3 cells. A. Depletion of TSC2 induces activity of mTORC1, IKK and NF-κB without effects on Akt activity. PC3 cells were transfected with siRNA control or siRNA against TSC2 as indicated. The cells were lysed 48 h after transfection and western blots probed with the indicated antibodies. B. Endogenous IKK in the lysates (A) was immunoprecipitated with anti-IKKα and incubated with recombinant GST-p65 (upper panel) or GST-IκBα (lower panel) and with [γ-32P]-ATP for in vitro IKK kinase assay. Autoradiography was performed, followed by immunoblotting with the indicated antibodies. Densitometry analyses for phospho-IκBα and IκBα or p-p65 and p65 were performed employing ImageJ software (NIH) and presented as ratio of phospho-IκBα to IκBα or phosphop65 to p65 band signal intensity. All results are representative of three independent experiments.
TSC2 negatively regulates NF-κB luciferase activity and NF-κB targets gene expression in PTEN null PC3 cells through inhibition of mTORC1
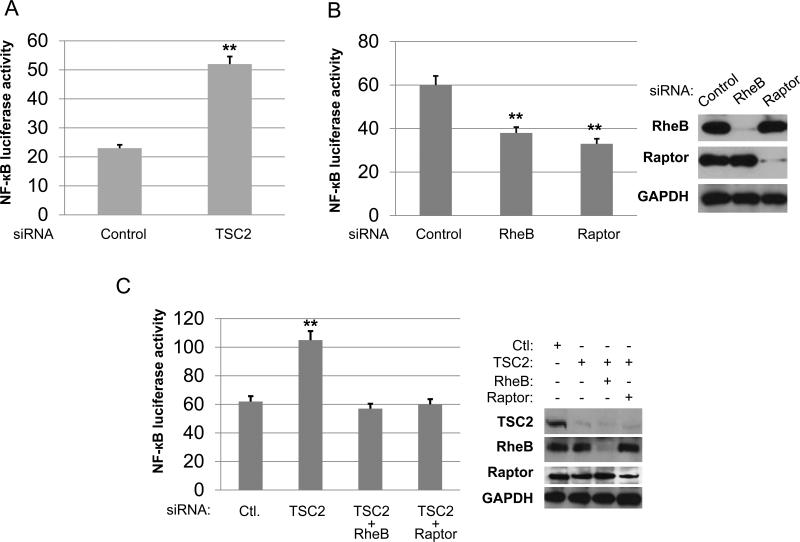
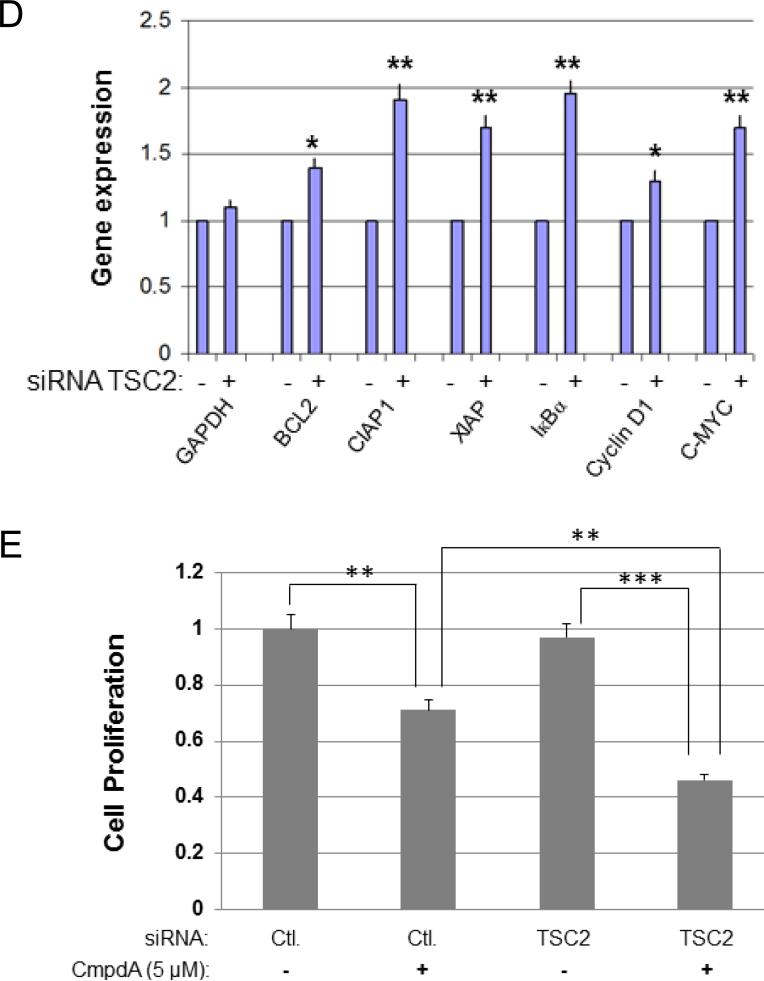
Next, we examined whether TSC2 modulates NF-κB transcriptional activity. We first determined whether TSC2 suppresses NF-κB activity in PC3 cells by using a luciferase reporter assay. After transfection of the TSC2 siRNA, NF-κB luciferase activity was markedly increased in TSC2 knockdown cells (Fig. 2A). We previously showed that mTORC1 promotes IKK/NF-κB activity in PC3 cells (43), and now our new data indicate that TSC2 inhibits mTORC1 and NF-κB in these cells. These data imply that TSC2 can abate NF-κB transcription through mTORC1. In order to test this hypothesis, we explored if Rheb, an upstream regulator of mTORC1 and downstream target of TSC2, is involved in NF-κB activation by mTORC1. RheB and Raptor, a known critical component of mTORC1 pathway, were knocked down by siRNA treatment, and their effects on NF-κB luciferase activity in PC3 cells were evaluated. Knockdown of either RheB or Raptor decreased NF-κB luciferase activity (Fig. 2B). These data indicate that RheB can induce NF-κB activation through mTORC1, similar to Raptor and mTOR. More importantly, we found that once the expression of RheB or Raptor is decreased, knockdown of TSC2 no longer induces NF-κB activity (Fig. 2C). These data suggest that TSC2 mediates NF-κB transcription through RheB-mTORC1 signaling. Next, we determined whether knockdown of TSC2 leads to upregulation of NF-κB target gene expression. We chose the typical NF-κB target genes including Bcl-2, Bcl-xl, CIAP1, IκBα, Cyclin D1 and C-Myc for analysis. As shown in fig. 2D, the expression of all NF-κB target genes listed were elevated upon knockdown of TSC2 compared to siRNA control. Taken together, TSC2 negatively modulates NF-κB transcription through mTORC1 in PTEN null PC3 cells. The observation that TSC2 suppression leads to upregulation of IKK/NF-κB signaling pathway prompted us to examine whether or not knockdown of TSC2 sensitizes cells to IKK inhibitor. In order to answer this question, the cells were treated with CmpdA, an IKKβ inhibitor (47), and cell proliferation measured. The result showed that knockdown of TSC2 has no effects on PC3 proliferation, but does make the cells more sensitive to CmpdA treatment (Fig. 2E).
Figure 2.
TSC2 inhibits NF-κB transcription and target gene expression in PC3 cells. A. PC3 cells were transfected with control siRNA or siRNA to TSC2 for 24 hours then followed by transfection with empty vector plus 200 ng of 3× κB luciferase reporter and 30 ng of pRL-SV40 (Renilla reporter control). Cells were harvested after 24 h and luciferase assays were performed. Three independent experiments were performed, each in triplicates. B. PC3 cells were transfected with siRNA control, RheB or Raptor for 24 hours and then transfected with the NF-κB luciferase reporter for another 24 hours and Luciferase assays were performed as in (A). Three independent experiments were performed, each in triplicates. C. PC3 cells were transfected with siRNA control, TSC2, TSC2 plus RheB, or TSC2 plus Raptor as indicated for 24 hours then followed by transfection of 200 ng of 3×κB luciferase reporter and 30 ng of Renilla reporter control. Cells were harvested and luciferase assays were performed. D. PC3 cells were transfected with siRNA control or TSC2 as indicated. RNA was extracted 48 h after transfection and RT–PCR was performed to assess changes in mRNA levels of NF-κB target gene expression. E. PC3 cells were transfected with siRNA control or TSC2 as indicated. Cells were treated with DMSO or IKKβ inhibitor (5μM) for another 48 hours and cell proliferation was measured by MTT assay. (* P<0.05, ** P<0.01, *** P<0.001).
TSC2 inhibits mTORC1/IKK/NF-κB signaling cascade in PTEN deficient prostate cancer cells downstream of Ak
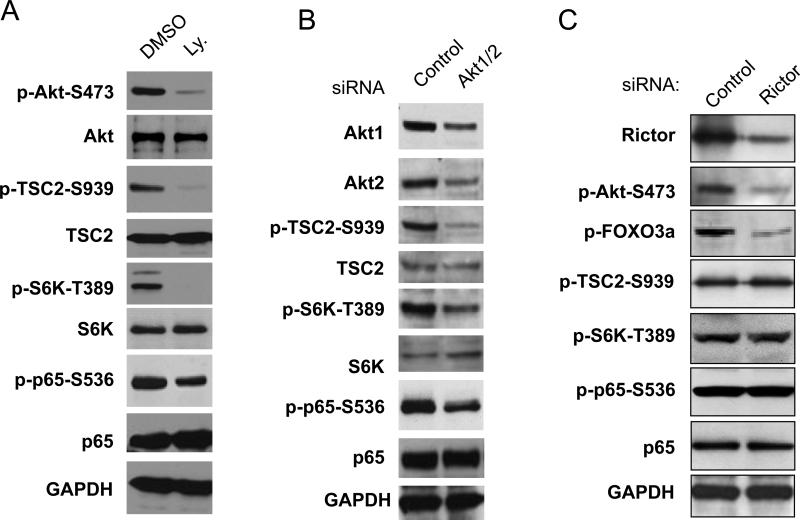
Given that PC3 cells have PTEN deletion and constitutively active Akt and mTORC1 (3, 45), we also investigated the potential relationship of Akt, TSC2, mTORC1 and NF-κB in these cells. First, we treated the PC3 cells with a PI3K inhibitor, LY294002, which blocks Akt activity and tested its effects on the activity of mTOR, Akt, TSC2 and NF-κB. As shown in Fig. 3 A, two hours of LY294002 treatment caused dramatic inhibition of phosphorylation of Akt, and subsequent decreases in the phosphorylation of TSC2, S6K, and p65 while having no effects on the expression of these proteins (Fig. 3A). Then, we treated the PC3 cells with siRNA to knock down both Akt1 and Akt2 and determined the effects on phosphorylation of TSC2, S6K and p65. Consistent with the results of PI3K inhibitor treatment, simultaneous knockdown of Akt1 and Akt2 markedly reduced phosphorylation of TSC2, S6K and p65 with no effect on the expression of S6K, TSC2 and p65 (Fig. 3B). These data suggest that TSC2 regulation of NF-κB through mTORC1 is downstream of Akt. Lastly, to further confirm that Akt controls TSC2-mTORC1-NF-κB signaling cascade through the TSC2-mTORC1 pathway in PC3 cells, the role of mTORC2 on TSC2, mTORC1 and NF-κB activities was examined. Several studies have already demonstrated that mTORC2 (mTOR/Rictor) phosphorylates Akt on serine 473 to activate Akt, but mTORC2 induced activation of Akt affects only Akt phosphorylation of FOXO3a, not TSC2 in cultured cells (48-50). We examined the effects of knockdown of Rictor, the critical component of mTORC2, on phosphorylation of NF-κB in PC3 cells. As expected, knockdown of Rictor decreased phosphorylation of Akt and FOXO3a while having no effects on phosphorylation of S6K (mTORC1 substrate) and the phosphorylation of TSC2 by Akt (Fig. 3C). More importantly, knockdown of Rictor did not affect p65 phosphorylation although Akt phosphorylation was reduced (Fig. 3C). Our data demonstrates that Akt controls NF-κB through TSC2-mTORC1-NF-κB signaling cascade independent of mTORC2 in PC3 cells.
Figure 3.
TSC2 regulates IKK/NF-κB signaling cascade downstream of Akt in PTEN deficient prostate cancer cells. A. Cells were treated with Ly 294002 (10 μM) or DMSO for 2 hours. Cell lysates were generated and blotted with the indicated antibodies. B. Cells were transfected with siRNA control or siRNA Akt1 and Akt2 as indicated and analyzed by western blots. C. Cells were transfected with siRNA control or siRNA Rictor as indicated. The cells were lysed 48 h after transfection and detected with the indicated antibodies. All results are representative of three experiments.
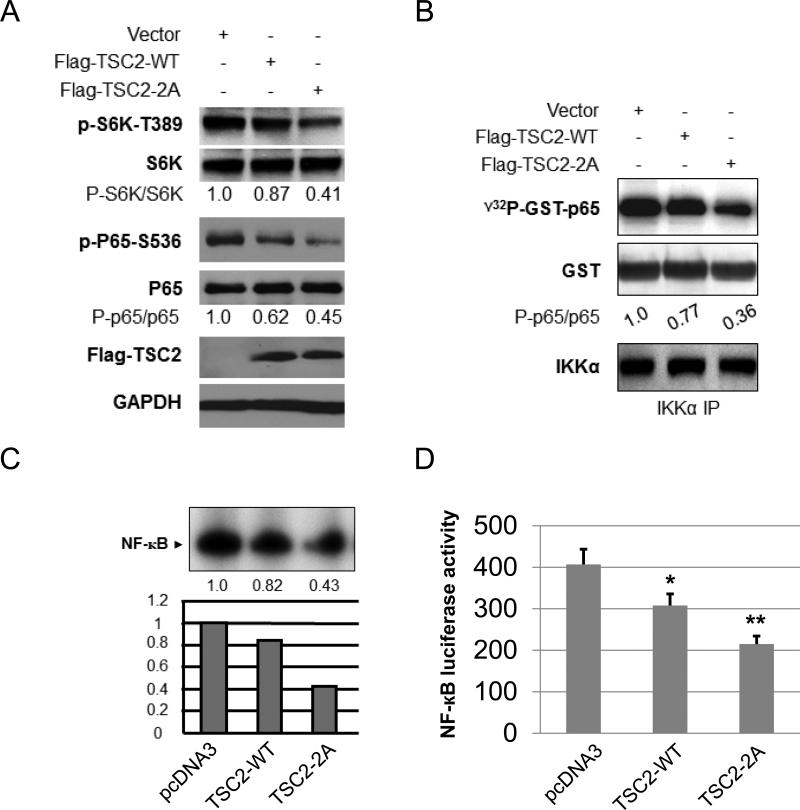
Akt controls TSC2 regulation of mTORC/IKK/NF-κB signaling through phosphorylation of TSC2
Next, we determined if Akt regulation of p65 NF-κB phosphorylation is mediated via phosphorylation of TSC2. Previous studies demonstrated that Akt phosphorylates TSC2 on serine 939 and threonine 1462 to promote mTORC1 activity (3, 8). Additionally, mutation of the two Akt phosphorylation sites (both serine 939 and threonine 1462 to alanine) in TSC2 (TSC2-2A: TSC2-S939A/T1462A) impaired Akt phosphorylation of TSC2 and activation of mTORC1 (3, 8). It would be important to determine if TSC2 wild type (TSC2-WT) and mutant (TSC2-2A) differentially affect mTORC1 and IKK/NF-κB pathways. Thus, we transiently transfected TSC2 wild type and TSC2-2A in PC3 cells and tested their effect on the activity of mTORC1 and IKK/NF-κB. As shown in figure 4A, over-expression of TSC2 wild type has a slight impact on the phosphorylation of S6K and p65, whereas over-expression of TSC2-2A significantly decreases the phosphorylation of S6K and p65. These results indicate that Akt controls mTORC1/IKK/NF-κB through TSC2 phosphorylation. We previously demonstrated that mTORC1 is involved in IKK/NF-κB activation through regulation of the kinase activity of IKK (43). Since Akt promotes mTORC1 activity through TSC2 phosphorylation, we thus examined if wild type TSC2 and mutant TSC2 (TSC2-2A) have differential effects on IKK kinase activity. The endogenous IKK complex was immuno-precipitated by IKKα antibody from TSC2 wild type or mutant transfected PC3 cell lysates as indicated in Figure 4A for in vitro kinase assays using GST-IκBα as substrate. The results show that transfection of wild type TSC2 led to minimal decreases in the phosphorylation of IκBα, whereas transfection of mutated TSC2 dramatically decreases IκBα phosphorylation (fig. 4B). Once activated, NF-κB translocates to the nucleus to bind to the promoter of its target gene to regulate gene transcription. We next examined the effects of transfecting TSC2 wild type and mutant on NF-κB DNA binding activity. Extracts (whole cell and nuclear) were prepared from PC3 cells transfected with control vector or with TSC2 wild type or TSC2 mutant. Electrophoretic mobility shift assays (EMSAs) were performed with nuclear extracts using a class I MHC NF-κB-binding site probe. As shown in figure 4C, while transfection of either TSC2 wild type or mutant reduced NF-κB DNA-binding activity, TSC2 mutant was more effective in blocking the EMSA complex. These results indicate that Akt regulates NF-κB DNA binding activity through TSC2 phosphorylation. Consistent with the results of EMSA, we found that TSC2 wild type reduces NF-κB luciferase activity only slightly but TSC2 mutant reduces NF-κB luciferase activity dramatically (fig. 4D). Lastly, to determine whether there is a difference between TSC2 wild type and TSC2 mutant in affecting NF-κB regulated gene induction, TSC2 wild type or the mutant were overexpressed in PC3 cells and RNA isolated. The expressions of cyclinD1, IκBα, XIAP, CIAP1 and Bcl-2 were determined by RT–PCR. GAPDH gene expression served as a control for these experiments. The results showed that while overexpression of TSC2 wild type blocked the expression of the experimental gene set ~10-20%, overexpression of TSC2 mutant reduced the expression of these genes ~40-50% (Fig. 4E). These results demonstrate that Akt phosphorylates TSC2 to promote mTOR1 signal and subsequently regulates endogenous NF-κB-dependent gene expression in PTEN-deficient prostate cancer cells.
Figure 4.
Akt regulation of mTORC1/IKK/NF-κB signaling involves TSC2 phosphorylation. A. Effect of Flag-wild-type and Flag-TSC2-2A on the phosphorylation of S6K and p65. Cells were transfected with Flag-wild-type or Flag-TSC2-2A and the phosphorylation p65 and S6K and total p65 and S6K, as well as Flag-TSC2 expression were tested by western blot. B. The endogenous IKK in the lysates (A) was immunoprecipitated with anti-IKKα and incubated with recombinant GST-p65 and [γ-32P]-ATP for in vitro IKK kinase assay. Autoradiography was performed, followed by immunoblotting with anti-GST and IKKα. C. Cells were transfected with Flag-wild-type and Flag-TSC2-2A as (A). EMSAs were performed using nuclear extracts from cells 48 h after transfection. D. Cells were transfected with vector, Flag-wild-type or Flag-TSC2-2A plus luciferase reporter. Cells were harvested after 24 h and luciferase assays were performed. Three independent experiments were performed, each in triplicates. E. PC3 cells were transfected as (A) and RNA was extracted 48 h after transfection and RT–PCR. A representative experiment from among three others is shown. The “*” indicates statistical significance compared with controls (t-test, P<0.05) and the “**” indicates P<0.01.
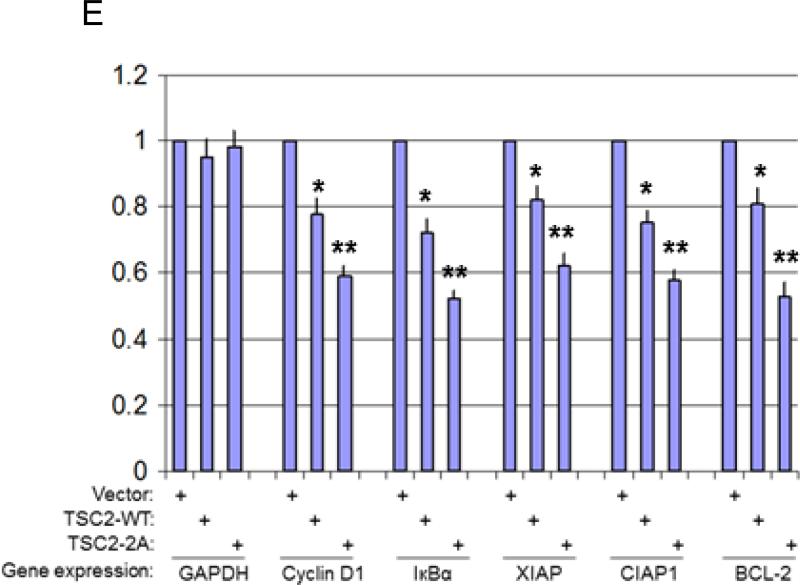
TSC2 differentially affects NF-κB activity through mTORC1 determined by Akt
Our data in PC3 cells indicate that TSC2 inhibits IKK/NF-κB, and that Akt plays a critical role in this regulation. PC3 cells harbor a mutant PTEN and hence have high endogenous basal Akt activity. We therefore also wanted to determine the effects of TSC2 on IKK/NF-κB upon introducing wild type PTEN (which would lower endogenous Akt activity). We transiently transfected vector control or the wild type PTEN gene in PC3 cells and then (36 hours post-transfection) knocked down TSC2 by siRNA. As shown in figure 5A and in line with the results from figure 1A, knockdown of TSC2 by siRNA increases phosphorylation of S6K and p65 without obvious impact on Akt phosphorylation in vector transfected PC3 cells (fig. 5A, lines 1 and 2). Moreover, as expected, wild type PTEN transfection led to dramatic reduction in Akt and S6K phosphorylation (fig. 5A, line 1 and 3). Interestingly, knockdown of TSC2 caused a strong induction of phosphorylation of S6K but obvious decrease in the phosphorylation of Akt and p65 in PTEN transfected cells (figure 5A, line 3 and 4). In addition, phosphorylation of IκBα-S32/36 also changed in a manner consistent with the modulation of Akt and p65 phosphorylation. These results indicate that wild type PTEN transfection reverses the effects of TSC2 from inhibition to promotion of IKK/NF-κB in PC3 cells. In addition, these results prompt us to hypothesize that TSC2 increases Akt and NF-κB activity in cells with lower Akt activity.
Figure 5.
Akt activity determines the effects of TSC2 on NF-κB activity through mTORC1. A. PC3 cells were transfected with vector control (lane 1 and 2) or PTEN (lane 3 and 4) for 24 hours and followed by transfection of siRNA control (lane 1 and 3) or siRNA against TSC2 (lane 2 and 4) as indicated. Cells were lysed 48 h after siRNA transfection and analyzed by western blots. B. PC3 and HeLa cells were lysed and analyzed by western blots. C. HeLa cells were transfected with siRNA control or against TSC2 as indicated. The cells were lysed and analyzed by western blots. D. HeLa-myr–HA-Akt2 tet-on cells were cultured with doxycycline (Dox) for 72 hours and the expression of HA-Akt2, phosphorylation of p65 and S6K, and total p65 and S6K were tested with the antibodies. E. HeLa cell with HA-myr-Akt2 expressed were transfected were siRNA control or siRNA against TSC2, lysed and analyzed by western blots. All the data are representative of three experimental repetitions.
In earlier work, we demonstrated that HeLa cells, which harbor wild type PTEN, have lower basal Akt activity than PC3 cells (46). In order to further define the role of TSC2, Akt and mTOR in the regulation of NF-κB, we compared the basal activity of Akt, mTOR and NF-κB between HeLa and PC3 cells. We confirm again that the phosphorylation of Akt, S6K and NF-κB in PC3 cells is much higher than in HeLa cells while the total protein levels in the two cell lines are similar (Fig. 5B). Next, we determined the effect of knocking down TSC2 on the activity of Akt, S6K and NF-κB in HeLa cells. As shown in figure 5C, siRNA against TSC2 led to decreased TSC2 protein expression which was accompanied by increased phosphorylation of S6K (Fig. 5C), and decreased phosphorylation of both Akt and p65. These results are consistent with those observed in the PTEN-transfected PC3 cells followed by siRNA TSC2 transfection, as shown in figure 5A. The data confirm that knock down of TSC2 increases NF-κB activity in PC3 cells (which have higher basal Akt activity) and decreases it in HeLa cells (which have lower basal Akt activity). These observations prompted us to then test whether Akt activity is involved in TSC2 regulation of NF-κB. Thus, we tested HeLa cells that were stably transfected with an inducible HA tagged myristoylated Akt2 construct (HeLa Akt2 Tet-On cell). The experiments showed that 72 hours after adding doxycycline (Dox) to the culture medium strong induction of HA-myr-Akt2 expression occurred, and it was also associated with increased phosphorylation of S6K, NF-κB and TSC2 in the Hela cells (Figure 5D). Next, we examined the effects of TSC2 knockdown on the activity of p65, Akt and S6K in the Akt2 active HeLa cells. siRNA against TSC2 dramatically lowered TSC2 protein expression in HeLa Akt2 tet-on cells and increased S6K and p65 phosphorylation (Figure 5E). These observations corroborate the findings in PC3 cells after TSC2 knockdown (Fig. 1A and 5A) and suggest that TSC2 can either down-regulate or up-regulate NF-κB in an Akt-dependent manner, depending upon cellular context.
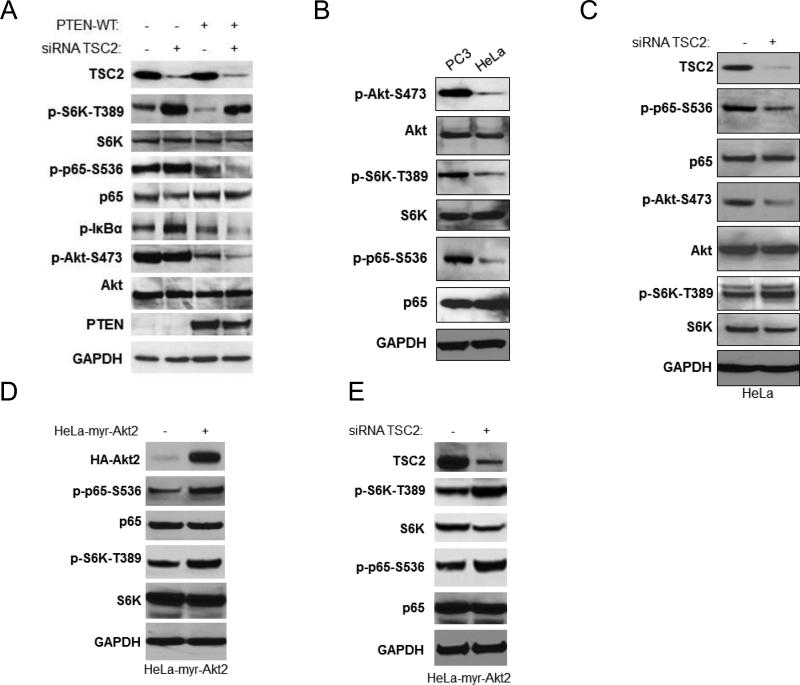
TSC2 promotes NF-κB activity in TSC2 null cells
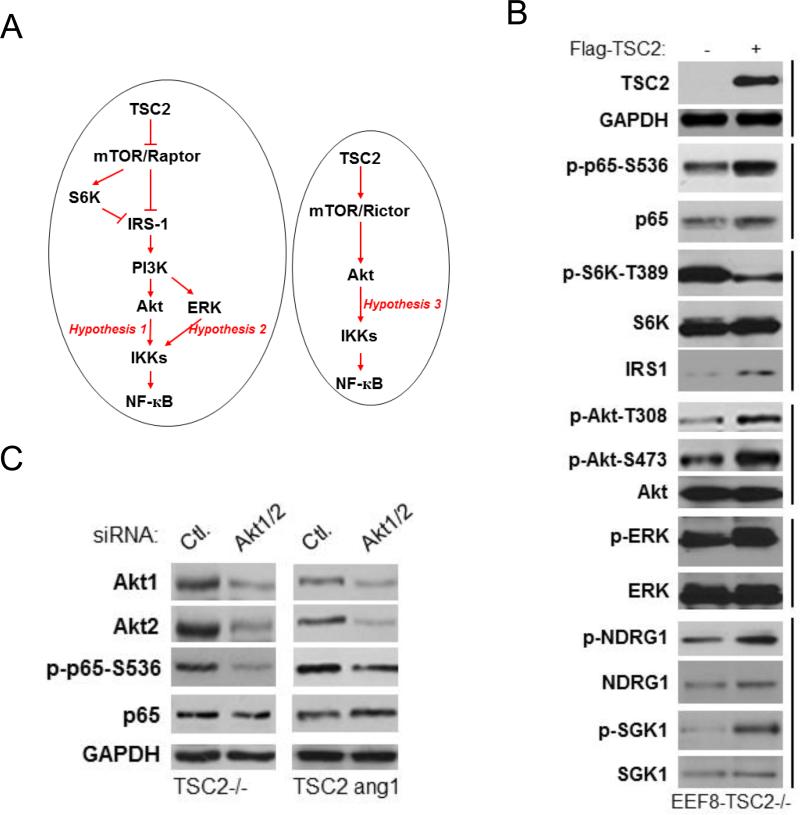
Both PTEN and TSC2 negatively affect mTOR activity. However, PTEN loss induces both Akt and mTORC1 activity whereas TSC2 loss induces mTORC1 but blocks Akt activity through a negative feedback regulatory mechanism (15, 23, 32). To further clarify the role of TSC2 in regulating NF-κB and Akt we carried out additional studies in cells without TSC2. As shown in figure 6A, EEF126-8 (Tsc2−/−) cells have higher phosphorylation of S6K, but lower phosphorylation of Akt and NF-κB, compared to EEF126-4 (Tsc2+/+) cells. These data imply that TSC2 can inhibit mTOR while simultaneously induce Akt and NF-κB in the EEF cells. We tested this hypothesis further using two approaches. First, the EEF TSC2 wild type cells were transfected with rat siRNA TSC2 and phosphorylation of S6K, Akt and NF-κB determined. As shown in figure 6B, the expression of TSC2 decreased and phosphorylation of S6K increased, while the phosphorylation of both Akt and NF-κB were reduced. Second, the EEF TSC2 null cells were transfected with wild type TSC2 which resulted in enhanced phosphorylation of Akt and p65 but decreased phosphorylation of S6K (Fig. 6C). Taken together, these data indicate that 1) TSC2 inhibits mTOR activity and induces Akt activity through mTOR inhibition, and 2) TSC2-mediated induction of Akt enhances NF-κB activity. We also examined the effects of expressing different doses of TSC2 on NF-κB luciferase activity in EEF TSC2−/− cells; NF-κB luciferase activity increased in a dose dependent manner (Fig. 6D), indicating that TSC2 induces NF-κB transcription in TSC2 null cells.
Figure 6.
TSC2 positively regulates NF-κB activity in TSC2 null cells. A. The EEF126-4 (Tsc2+/+) and EEF126-8 (Tsc2−/−) cells were lysed and western blots probed with the indicated antibodies. B. The EEF126-4 (Tsc2+/+) cells were transfected with rat siRNA TSC2 for 48 hours, lysed and analyzed by western blots. C. EEF126-8 (Tsc2−/−) cells were transfected with different doses of TSC2 (wild type). Cells were lysed 48 hours after the transfection and analyzed by western blots. D. EEF126-8 (Tsc2−/−) cells were transfected with different doses of TSC2 plus 3×κB luciferase reporter and Renilla reporter control. Cells were harvested and luciferase assays were performed. A representative experiment from among three others is shown. The “*” indicates statistical significance compared with controls (t-test, P<0.05) and the “**” indicates P<0.01. E. EEF8 cells were treated with DMSO, IKKβ inhibitor (5μM), Rapamycin (100nM) or combination of IKKβ inhibitor and Rapamycin for 48 hours and cell proliferation was measured by MTT assay (* P<0.05, ** P<0.01, *** P<0.001).
That inhibition of mTOR upon expressing TSC2 in TSC2 null cells leads to upregulation of IKK/NF-kB suggests that dual targeting of IKK and mTOR may be more effective than targeting each individually in suppressing the proliferation of these cells. Thus, to test this hypothesis TSC2 null cells were treated with rapamycin or the IKK inhibitor CmpdA or both, and the effects on cell proliferation assessed. Our results indicate that indeed the combination of rapamycin and CmpdA has significantly greater anti-proliferative activity than does either agent alone (Fig 6E).
TSC2 up-regulation of NF-κB in TSC2 null and TSC2 mutated tumor cells through inhibition of mTORC1-induced Akt activation
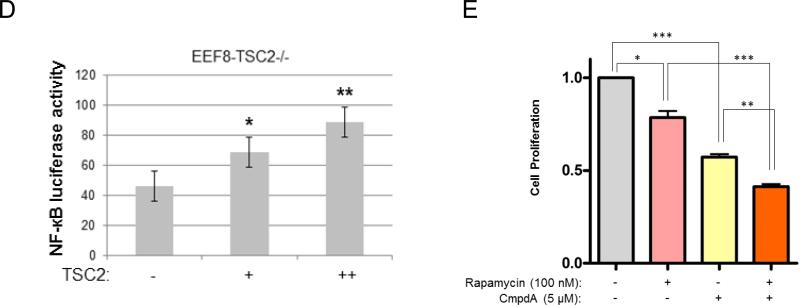
While many studies have demonstrated that Akt activates NF-κB and our data shows that TSC2 induces both Akt and NF-κB, we hypothesized that TSC2 induces NF-κB activity through Akt in TSC2 deficient cells. Previous studies have shown that TSC2 loss results in mTORC1 activation which in turn can suppress the PI3K-Akt pathway by phosphorylating IRS-1 and other receptors (23, 27-30). These results suggest that TSC2 transfection (overexpression) may induce a TSC2/mTORC1/IRS-1/PI3K-Akt cascade to upregulate IKK/NF-κB (Fig. 7A, left panel, hypothesis 1). Other studies have demonstrated that mTORC1 inhibition may lead to ERK activation via induction of IRS-1/PI3K/MEK/ERK signaling (31, 51), which could in turn potentially upregulate IKK/NF-κB (Fig. 7A, left panel, hypothesis 2). Finally, work by Huang and colleagues suggests that TSC2 is an upstream regulator of mTORC2 (52, 53); under this scenario TSC2 could induce IKK/NF-κB through a TSC2/mTORC2/Akt/IKK/NF-κB cascade (Fig. 7A, right panel, hypothesis 3).
Figure 7.
TSC2 promotes NF-κB signaling in TSC2 deficient cells through mTORC1 inhibition-induced activation of PI3K/Akt. A. The hypothesis of TSC2 regulation of IKK/NF-κB in TSC2 deficient cells. B. EEF126-8 (Tsc2−/−) cells were transfected with TSC2 (wild type), lysed, and analyzed by western blots. C. Cells were transfected with siRNA control or siRNA against Akt1 plus Akt2 as indicated, lysed and analyzed by western blots. D. Cells were transfected with siRNA control or siRNA against ERK as indicated. Cell lysates were analyzed by western blots. E. Cells were transfected with siRNA control or siRNA against Rictor as indicated, lysed and analyzed by western blots. F. Cells were transfected with siRNA control or siRNA against Raptor, lysed and analyzed by western blots. These experiments were repeated for three times.
To further clarify the underlying mechanisms involved in TSC2 induction of IKK/NF-κB in the TSC2 null cells, we carried out the following studies. As shown in figure 7B, transfection of TSC2 in TSC2 null cells led to inhibition of mTORC1 and increased NF-κB phosphorylation. In addition, the expression of IRS1 increased, indicating that TSC2 transfection releases mTORC1 inhibition of IRS1. Furthermore, phosphorylation of both Akt (at serine 473 and threonine 308) and ERK (which are downstream targets of IRS1), as well as phosphorylation of NDRG1and SGK1 (which are downstream targets of mTORC2), increased (fig. 7B). These findings indicate that TSC2 involves mTORC1 and mTORC2 signaling cascades in TSC2 null cells.
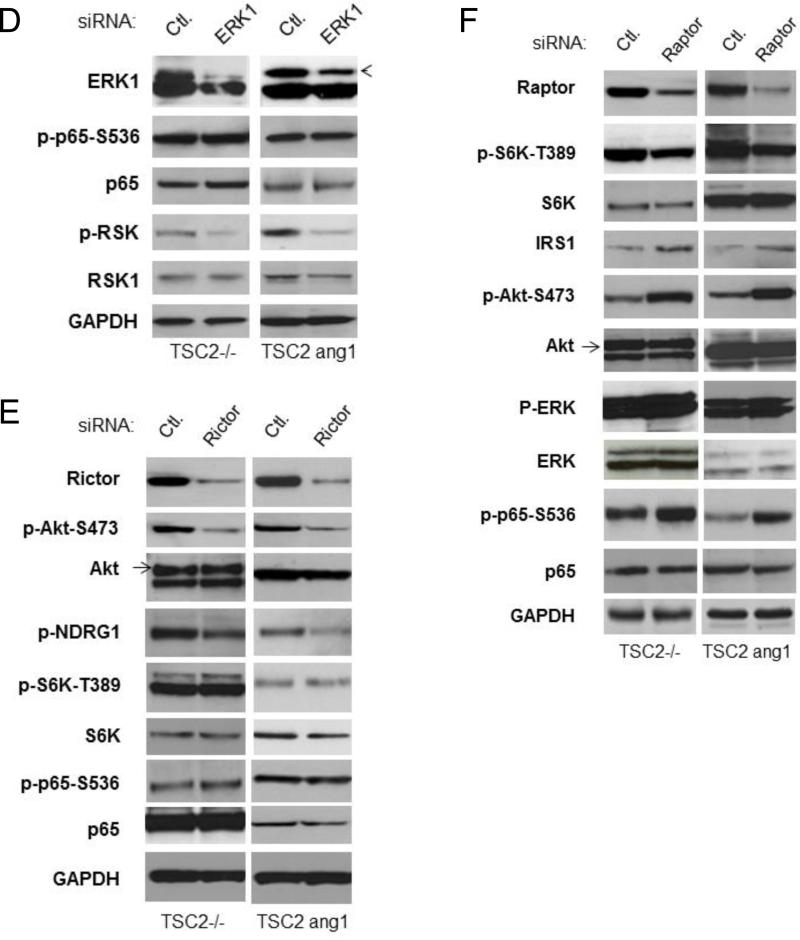
To clarify whether Akt is involved in TSC2 induction of IKK/NF-κB in TSC2 null and TSC2 deficient tumor cells we employed theTSC2 ang1 cell line which is derived from a cutaneous sarcoma taken from a Tsc2+/− mouse heterozygous for tuberin. This is the only genetically defined tuberous sclerosis cell line available that forms tumors in vivo (54, 55). Knockdown of Akt1 and Akt2 in both TSC2 null and TSC2 ang1 cells decreased NF-κB phosphorylation, suggesting that Akt is involved in the TSC2-mediated upregulation of NF-κB potentially through mTORC1 or/and mTORC2 (Fig 7C). On the other hand, knockdown of ERK affects RSK1 (downstream target of ERK (56)) but not NF-κB activity in both TSC2 null and TSC2 ang1 cells (Fig. 7D). Thus, Akt (hypothesis 1 or 3), but not ERK (hypothesis 2), appears to be involved in TSC2-mediated regulation of NF-κB activity.
To define which mTOR complex is involved in the above pathways, Rictor, a critical component of mTORC2, was knocked down and activity of mTORC2, Akt, S6K and NF-κB assessed. Although knock down of Rictor downregulated the activity of mTORC2 downstream targets, including phosphorylation of Akt-serine 473 and NDRG1, it did not impact the phosphorylation status of NF-κB (Fig. 7E). Thus, TSC2 regulation of NF-κB is mTORC2 independent in TSC2 null and TSC2 mutated cells.
Lastly, we determined whether TSC2 induction of NF-κB is mediated through inhibition of mTORC1 and the subsequent upregulation of PI3K/Akt in the TSC2 null and TSC2 ang1 cells (hypothesis 1, Fig 7A). If this loop is operative, then one would expect that knockdown of Raptor, a critical member of mTORC1, would cause inhibition of mTORC1 and activation of both Akt and NF-κB. Knock down of Raptor blocked mTORC1 activity and induced expression of IRS1 in both TSC2 null and TSC2 ang1 cells, which was also accompanied by increased activity of Akt (Figure 7F). In addition, enhanced phosphorylation of ERK was observed primarily in TSC2 null cells and to a lesser extent also in TSC2 ang1 cells (which already have high levels of basal ERK phosphorylation (54)). Importantly, the phosphorylation of NF-κB was markedly increased in both TSC2 null and TSC2 ang1 cells upon knockdown of Raptor (Fig. 7F). These results strongly imply that TSC2 induces PI3K/Akt/NF-κB through inhibition of phosphorylation of mTORC1 and induction of IRS1 in TSC2 null and TSC2 mutated backgrounds. Additionally, TSC2 induces Akt/NF-κB in TSC2 null cells via mTORC1-mediated feedback regulation of IRS-1/Akt.
Discussion
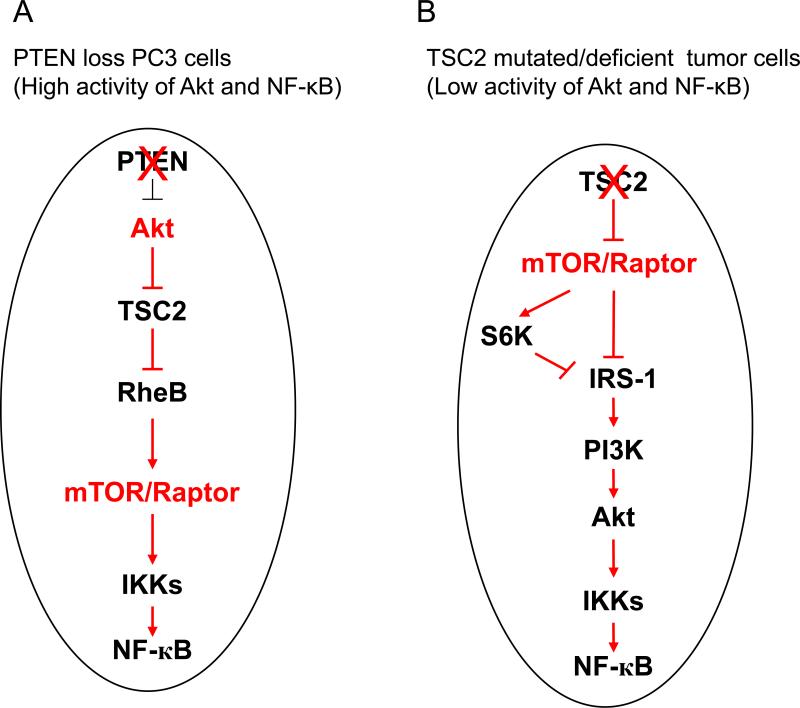
The role of TSC2, a critical gene that inhibits mTORC1 activity downstream of Akt, in the regulation of NF-κB is not clearly defined. We previously reported that Akt promotes NF-κB activity through mTORC1 in PTEN deficient prostate cancer (43). In the current study, we explored the functional interaction of Akt, TSC2 and mTORC1 in modulating NF-κB signaling in tumor cells with different genetic backgrounds. We found that in PTEN deficient cancer cells that have high basal Akt activity such as PC3 cells, TSC2 inhibits mTORC1 and subsequently blocks IKK/NF-κB function without significantly impacting Akt through mTORC1-mediated feedback (Fig. 8A). However, in a PTEN wild type background in which there is low basal Akt activity (such as TSC2 null and TSC2 mutated cells), although TSC2 still inhibits mTORC1, up-regulation of Akt and IKK/NF-κB occurs through a PI3K/Akt/IKK/NF-κB feedback regulatory loop (Fig. 8B). Of note is that TSC2 regulation of IKK/NF-κB (promotion or inhibition) appears to be independent of mTORC2 in both PTEN deficient prostate cancer cells as well as in TSC2 deficient and TSC2 mutated cancer cells.
Figure 8.
Propose model of TSC2 inhibition of IKK/NF-κB activity downstream of Akt and upstream of mTORC1 in PTEN null prostate cancer cells (A) and TSC2 promotion of IKK/NF-κB activity upstream of Akt and mTORC1 in TSC2 mutated tumor cells (B).
Previous studies have reported that mTOR inhibition induces upstream receptor tyrosine kinase signaling leading to activation of Akt in several cancer cell lines and cancer patients (31). We previously reported that rapamycin blocks mTOR and subsequently reduces IKK/NF-κB activity while increasing Akt phosphorylation in the PTEN deficient PC3 cells (43). These data suggest that mTORC1 lies downstream of Akt but upstream of IKK/NF-κB. The current study indicates that TSC2 is a key target for Akt activation of IKK/NF-κB in PTEN deficient prostate cancer in which PTEN loss causes constitutive activation of Akt and is insensitive to the feedback regulation by mTORC1 (Fig. 8A). A study by Ma et al showed that functional interactions exist between PTEN and TSC2 which can result in asymmetric haplo-insufficiency with respect to tumor suppression in mice (33). They found that both PTEN+/− and TSC2+/− mice display increased phosphorylation of S6, a marker of mTORC1 activity, compared to the wild type mice. However, PTEN+/−TSC2+/− mice show higher phosphorylation of S6 compared to Pten+/−/TSC2+/+ and PTEN+/+/TSC2+/− mice. More importantly, they found that Pten+/−Tsc2+/− mice displayed much higher staining of Ki-67, a proliferation marker, compared to Pten+/−TSC+/+ mice. Our data corroborates the transgenic mouse data by showing that knockdown of TSC2 increases mTOR and NF-κB activity in PTEN null cells, suggesting that loss of TSC2 function coupled with loss of PTEN function activates mTOR and NF-κB which may promote tumorigenesis.
Manning et al also reported that PTEN and TSC2 act synergistically to suppress the severity of a subset of tumors specific to loss of each of these genes (32). However, PTEN haploinsufficiency restores Akt signaling in these tumors and dramatically enhances tumorigenesis (32). Importantly, they found that Tsc2+/− mice exhibit defects in signaling downstream of Akt which could explain why TSC1/2 mutants induce mostly benign tumors. Our data in the EEF126-4 (Tsc2+/+) and EEF126-8 (Tsc2−/−) cells is consistent with the study by Ghosh et al which showed that NF-κB signaling is attenuated in TSC1- and TSC2-deficient MEFs (57). The rapamycin-mediated inhibition of mTORC1 restores NF-κB activation through ERK and PI-3K pathways (57). Our data, taken in context with those reported by others, confirms that TSC2 can either negatively or positively modulate NF-κB activity depending upon the genetic background, particularly as it relates to PTEN function and background Akt activity.
Our findings that TSC2 induces NF-κB activation in TSC2 deficient and mutated tumor cells are in accord with an earlier study by Weichhart and colleagues (58). They reported that the TSC-mTOR signaling pathway regulates inflammatory responses upon bacterial stimulation in monocytes, macrophages, and primary dendritic cells. They also found that TSC2 upregulates NF-κB signaling through mTORC1 inhibition, and they identified the mTORC1 pathway as a critical negative regulator of NF-κB and a positive regulator of STAT3 signaling in myeloid innate immune cells (58). Another study by Yoshida and colleagues reported that Rtp801, a suppressor of mTORC1 signaling, is essential in mediating cigarette smoke-induced pulmonary injury and emphysema (59). They reported that Rtp801 was necessary and sufficient for NF-κB activation in cultured cells and in mouse lungs. Consistent with this study, Weichhart and colleagues reported that mTOR inhibits NF-κB activity whereas rapamycin induces it in myeloid immune cells (60). In general, it is likely that TSC2 inhibits IKK/NF-κB activity in cancer cells, particularly in those cells with higher levels of Akt activity, but induces IKK/NF-κB in normal cells and benign tumor cells which have lower basal Akt activity (Fig. 8 A and 8B).
We propose that TSC2/mTORC1 differentially affects IKK/NF-κB in PTEN loss-induced prostate cancer and TSC2 mutated tumor cells. These observations implicate IKK/NF-κB as a therapeutic target in both PTEN deficient prostate cancer and TSC. In addition, our data suggest that mTOR inhibition when combined with IKK inhibition could be more effective in the treatment of TSC2 mutated tumors due to their dual blockade of activated mTOR and IKK/NF-κB. Lastly, it would be important to determine the therapeutic efficacy of mTOR inhibitors, in combination with IKK/NF-κB inhibitors, in prostate cancer and TSC in vivo.
Implications.
This study provides fundamental insight for understanding the molecular details by which TSC2/mTOR regulates NF-κB signaling in different tumors.
Acknowledgements
This project was initiated at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill. We thank Dr. Albert Baldwin for his invaluable support. We also thank Dr. Jin Cheng for constructs and cells.
Grant Support This work was supported, in whole or in part, by National Institutes of Health Grants K99CA149178 and R00CA149178 to H.C.D and startup funds from Marlene and Stewart Greenebaum Cancer Center, University of Maryland School of Medicine to H.C.D. and a Merit Review Award, Dept of Veterans Affairs A.H.
Footnotes
Authors' Contributions
Conception and design: H.C. Dan
Development of methodology: Y. Gao, Y. Zhang, H.C. Dan
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Y. Gao, Y. Zhang, H.C. Dan, X. Wang
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Y. Gao, H.C. Dan, R.B. Gartenhaus, R.G. Lapidus, X. Wang
Writing, review, and revision of the manuscript: H.C. Dan, R.G. Lapidus, A. Hussain, R.B. Gartenhaus,
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): H.C. Dan
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Short MP, Richardson EP, Jr, Haines JL, Kwiatkowski DJ. Clinical, neuropathological and genetic aspects of the tuberous sclerosis complex. Brain Pathol. 1995;5:173–179. doi: 10.1111/j.1750-3639.1995.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 2.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol. 2007;36:398–408. doi: 10.1165/rcmb.2006-0372TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14:251–258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 8.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signaling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 10.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke A, Hartkamp J, Saitoh M, Roworth W, Nobukuni T, Hodges A, et al. Tuberous sclerosis complex tumor suppressor-mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J Cell Biol. 2002;159:217–224. doi: 10.1083/jcb.jcb.200206108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw RJ, Cantley LC. Ras, PI3K, and mTOR signaling controls tumor growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 13.Rosen N, She QB. AKT and cancer--is it all mTOR? Cancer Cell. 2006;10:254–256. doi: 10.1016/j.ccr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes, and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 19.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 21.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 22.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 23.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah OJ, Hunter T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol. 2006;26:6425–6434. doi: 10.1128/MCB.01254-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Shah OJ, Hunter T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol. 2006;26:6425–6434. doi: 10.1128/MCB.01254-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiratani K, Haruta T, Tani A, Kawahara J, Usui I, Kobayashi M. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun. 2005;335:836–842. doi: 10.1016/j.bbrc.2005.07.152. [DOI] [PubMed] [Google Scholar]
- 30.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Teruya-Feldstein J, Behrendt N, Chen Z, Noda T, Hino O. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–1786. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basseres D, Baldwin A. NF-κB and IKK pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 35.Bonizzi G, Karin M. The two NF-κB activation pathways and their roles in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 37.Staudt LM. Oncogenic activation of NF-κB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274(43):30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 40.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen DP, Li J, Yadav SS, Tewari AK. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014;114:168–176. doi: 10.1111/bju.12488. [DOI] [PubMed] [Google Scholar]
- 42.Jain G, Cronauer MV, Schrader M, Möller P, Marienfeld RB. NF-κB signaling in prostate cancer: a promising therapeutic target? World J Urol. 2012;30:303–310. doi: 10.1007/s00345-011-0792-y. [DOI] [PubMed] [Google Scholar]
- 43.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 45.Adli M, Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell-associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281(37):26976–84. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- 46.Dan HC, Adli M, Baldwin AS. Regulation of mammalian target of rapamycin activity in PTEN-inactive prostate cancer cells by I kappa B kinase alpha. Cancer Res. 2007;67:6263–6269. doi: 10.1158/0008-5472.CAN-07-1232. [DOI] [PubMed] [Google Scholar]
- 47.Ziegelbauer K, Gantner F, Lukacs NW, et al. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol. 2005;145:178–92. doi: 10.1038/sj.bjp.0706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 51.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Govindarajan B, Mizesko MC, Miller MS, Onda H, Nunnelley M, Casper K, et al. Tuberous sclerosis-associated neoplasms express activated p42/44 mitogen-activated protein (MAP) kinase, and inhibition of MAP kinase signaling results in decreased in vivo tumor growth. Clin Cancer Res. 2003;9:3469–3475. [PubMed] [Google Scholar]
- 55.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999 May 25;151(1-2):65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh S, Tergaonkar V, Rothlin CV, Correa RG, Bottero V, Bist P, Verma IM, et al. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and cell survival. Cancer Cell. 2006;10:215–226. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, et al. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]