Links between human immunodeficiency virus (HIV) care quality indicators (QIs) and mortality rates are not well established. We assessed HIV-infected patients' baseline QIs; mortality rates during 24 805–person-years of follow-up were lower among patients receiving ≥80% of baseline HIV QIs.
Keywords: alcohol, quality of health care, HIV, health care, opioid-related disorders
Abstract
Background. The Patient Protection and Affordable Care Act encourages healthcare systems to track quality-of-care measures; little is known about their impact on mortality rates. The objective of this study was to assess associations between HIV quality of care and mortality rates.
Methods. A longitudinal survival analysis of the Veterans Aging Cohort Study included 3038 human immunodeficiency virus (HIV)–infected patients enrolled between June 2002 and July 2008. The independent variable was receipt of ≥80% of 9 HIV quality indicators (QIs) abstracted from medical records in the 12 months after enrollment. Overall mortality rates through 2014 were assessed from the Veterans Health Administration, Medicare, and Social Security National Death Index records. We assessed associations between receiving ≥80% of HIV QIs and mortality rates using Kaplan–Meier survival analysis and adjusted Cox proportional hazards models. Results were stratified by unhealthy alcohol and illicit drug use.
Results. The majority of participants were male (97.5%) and black (66.8%), with a mean (standard deviation) age of 49.0 (8.8) years. Overall, 25.9% reported past-year unhealthy alcohol use and 28.4% reported past-year illicit drug use. During 24 805 person-years of follow-up (mean [standard deviation], 8.2 [3.3] years), those who received ≥80% of QIs experienced lower age-adjusted mortality rates (adjusted hazard ratio, 0.75; 95% confidence interval, .65–.86). Adjustment for disease severity attenuated the association.
Conclusions. Receipt of ≥80% of select HIV QIs is associated with improved survival in a sample of predominantly male, black, HIV-infected patients but was insufficient to overcome adjustment for disease severity. Interventions to ensure high-quality care and address underlying chronic illness may improve survival in HIV-infected patients.
(See the Editorial Commentary by Horberg on pages 240–1.)
The Patient Protection and Affordable Care Act prioritizes the delivery of high-quality healthcare, encourages healthcare systems to track and report quality-of-care (QOC) measures, and allows healthcare funders to link provider payments to these measures [1]. QOC provided by a healthcare delivery system or individual provider is typically measured by disease-specific care process measures, called quality indicators (QIs). These QIs assess whether an individual patient receives a particular healthcare procedure during a specified time frame, if the patient is eligible to receive that care. QIs are used to inform healthcare providers, systems administrators, funders, and the general population of the overall QOC delivered by a healthcare system or individual providers. Summary measures of individual QIs are increasingly used to assess overall care quality [2, 3].
Human immunodeficiency virus (HIV) QIs are well-established, with growing consensus on a body of QIs endorsed by the US Health Resources Services Administration HIV/AIDS Bureau, the Veterans Health Administration (VHA), the Institute of Medicine, and the National Quality Forum [4, 5]. As in most chronic illnesses, individual process measures are often selected based on clinical trials that demonstrate their association with favorable outcomes [6, 7]. The link between a group of disease-specific process measure QIs and outcomes has been difficult to demonstrate in clinical practice. The few studies that have done so in patients with other chronic illnesses (eg, diabetes and acute myocardial infarction [8, 9]), did not focus on vulnerable populations, such as patients with HIV infection or substance use. Little is known regarding the association between aggregate receipt-of-care process measures, as a whole, and outcomes such as mortality rate.
Survival rates have historically varied among HIV-infected patients. Combined antiretroviral therapy (cART) use has increased survival for some HIV-infected patients from a few years to decades [10, 11]. Although historic sex and race/ethnicity gaps in life expectancy for HIV-infected patients have narrowed during the past decade, life expectancy has remained unchanged since 2000 for HIV-infected patients with a history of injection drug use and remains substantially lower than for other HIV risk groups (49 years for injection drug users vs 77 years for men who have sex with men) [12]. Similarly, unhealthy alcohol use is estimated to decrease survival by 3–6.4 years among HIV-infected patients [13].
Quality of HIV care is suboptimal for HIV-infected patients with substance use [14–17]. In a recent study of HIV-infected veterans, both recent unhealthy alcohol use and illicit drug use were inversely associated with the percentage of QIs received [16]. Whether decreased quality of HIV care is an independent predictor of decreased survival in HIV-infected patients with unhealthy alcohol or drug use is not known. Substance use may be an important confounder of an association between QOC and mortality rates.
We therefore chose to examine the association between QOC and mortality rates in a population of HIV-infected patients, where receipt of high-quality care might have the potential to close deficits in disease outcomes. Our objectives were to assess (1) the association between quality of HIV care and mortality rates and (2) the potential for high-quality care to close gaps in mortality for HIV-infected patients with past-year unhealthy alcohol and/or illicit drug use. We hypothesized that receiving more eligible HIV QIs would be associated with improved mortality rates and that mortality rates would be comparable for those who received the highest quality of HIV care, regardless of past-year unhealthy alcohol or illicit drug use.
METHODS
Setting and Participants
We conducted a longitudinal survival analysis among HIV-infected patients enrolled in the Veterans Aging Cohort Study (VACS), an ongoing study that has been described elsewhere [18, 19]. Briefly, VACS is a national cohort study of HIV-infected patients receiving care at 8 VHA infectious disease clinics and age-, race-, and site-matched HIV uninfected patients enrolled in general medicine clinics. Our analysis includes data from HIV-infected participants enrolled between June 2002 and July 2008. During this time period, VACS clinic sites were located at VHA facilities in Atlanta, Georgia; Baltimore, Maryland; Bronx, New York; Houston, Texas; Los Angeles, California; New York City, New York; Pittsburgh, Pennsylvania; and Washington, DC. VACS is approved by the institutional review boards at the coordinating center at the VA Connecticut Healthcare System and each of the participating VHA facilities and corresponding university affiliates. At enrollment, participants completed baseline surveys and provided permission to access all their information within the Department of Veterans Affairs (VA), including pharmacy, laboratory, diagnostic, healthcare utilization, and mortality data. Participants were excluded from the analysis if they died or were lost to follow-up in the 12 months after enrollment, because QOC was assessed during this time frame.
Measures
The independent variable for the current study was quality of HIV care in the 12 months after enrollment. We measured 9 process QIs from therapeutic, monitoring, screening, and prevention domains (Table 1). QIs were assessed only if a patient was eligible to receive the indicated care process (eg, the cART QI was considered “met” only if the participant's CD4 cell count nadir was <350/mL, consistent with treatment guidelines during the study period). All participants were eligible to receive ≥5 QIs and could receive as many as 9 depending on eligibility. The QIs were originally developed through modified Delphi methods [20], adapted for use in the VHA [14, 17, 21], reviewed in an Institute of Medicine Report [22], and belong to a set of national consensus panel QIs for HIV care [4].
Table 1.
Human Immunodeficiency Virus Quality-of-Care Indicators
| Quality Indicator | “Pass” Criteria | Eligibility Criteria | Proportion Meeting Criteria, if Eligible, % |
|---|---|---|---|
| Medications | |||
| ART | Receipt of ART in past 12 mo | CD4 cell count nadir ≤350/mL ever | 90.6 |
| Pneumocystis jiroveci pneumonia prophylaxis | Receipt of dapsone, trimethoprim-sulfamethoxazole, atovaquone, pentamidine in past 12 mo | CD4 cell count ≤200/mL in past 12 mo | 92.9 |
| Mycobacterium avium complex prophylaxis | Receipt of clarithromycin, azithromycin, or rifabutin in past 12 mo | CD4 cell count ≤50/mL | 87.6 |
| Screening | |||
| Hyperlipidemia | Lipid test in past 12 mo | Receiving ART | 80.0 |
| Hepatitis C | HCV antibody test ever | All | 95.3 |
| Prevention | |||
| Pneumovax | Pneumococcal vaccine ever | All | 89.2 |
| Influenza | Influenza vaccine in past 12 mo | All | 56.8 |
| Monitoring | |||
| CD4 cell count | ≥2 CD4 cell counts separated by ≥3 mo, within past 12 mo | All | 80.4 |
| HIV clinic visits | ≥2 HIV clinic visits separated by ≥3 mo, within 12 mo | All | 89.1 |
Abbreviations: ART, antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
We calculated the percentage of QIs each participant received in the 12 months after enrollment, if eligible, a strategy previously used to estimate the overall quality of US healthcare [23] and adapted for HIV-infected populations [24]. For example, if 6 QIs were met for a person who was eligible to receive 9, that person received 66.6% (6/9 × 100) of the QIs for which he or she was eligible. We dichotomized the percentage of quality of HIV care received as ≥80% of QIs versus <80%, because mortality rates were comparable for those with 100% and those with ≥80% of QIs and generally declined for those with <80% (Supplementary Table 1).
The primary outcome measure was overall mortality rate, assessed beginning 12 months after enrollment. Deaths were identified from 4 sources: (1) the Patient Treatment File, which records hospital deaths in the VA healthcare system; (2) the Beneficiary Identification Records Locating System, which tracks VA death benefits, (3) the Medicare Vital Status File; and (4) the Social Security National Death Index. Mortality rates were assessed through 31 July 2014, allowing for a follow-up period of up to 11 years, with a mean (standard deviation [SD]) follow-up of 8.2 (3.2) years.
Other independent variables and covariates included self-reported unhealthy alcohol use and illicit drug use from a survey at the time of study enrollment. Unhealthy alcohol use, which includes at-risk drinking, alcohol abuse, and dependence in the past 12 months, was defined by a 3-item Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) score of ≥4 [25, 26]. The AUDIT-C is a universal, annual screening measure for patients in primary care clinics in the VHA. Illicit drug use was defined by any self-reported use of nonprescribed stimulants, opioids, or injection drugs in the past 12 months [27, 28].
We also considered potential covariates that might influence mortality rate, including sex, race/ethnicity, age, education level, homelessness, baseline CD4 cell count, suppression of HIV viral replication, cART, hepatitis C virus (HCV) status, and the VACS Index as a measure of overall health. The VACS Index is a predictor of mortality rate in HIV-infected patients, comprised of measures of age, CD4 cell count, HIV-1 RNA level, hemoglobin, liver fibrosis, renal function (estimated glomerular filtration rate), and HCV status [29, 30].
Analysis
We report patient characteristics overall and by unhealthy alcohol use (yes/no) and illicit drug use (yes/no), using descriptive statistics. We compared continuous variables (age) using t tests and categorical variables using χ2 tests, and we calculated mortality rates in deaths per 100 person-years. We estimated the associations between the dichotomous percent of QIs received (≥80% vs <80%) and overall mortality rates using Kaplan–Meier survival analysis and univariate and multivariate Cox proportional hazards models. Analyses were stratified by past-year unhealthy alcohol and illicit drug use to test the hypothesis that receiving higher quality of HIV care might narrow gaps in mortality rates observed for these patients. We assessed a priori covariates of age, sex, race/ethnicity, CD4 cell count <200/µL, and site by adding each individually to the univariate models; if the model QI coefficient changed by >10% compared with the QI coefficient in the univariate model, the variable was included in the multivariate models. Only age met the criteria to be included in the multivariate models adjusted for demographic characteristics.
A second set of multivariate models was analyzed with adjustment for the VACS Index. We tested the proportional hazards assumption using Schoenfeld and scaled Schoenfeld residuals and found no violation of the global proportional hazards assumption. We conducted sensitivity analysis (1) limiting observation time to 2 years to explore the effect of healthy survivor bias; (2) stratifying by mutually exclusive categories of no unhealthy alcohol use or illicit drug use, unhealthy alcohol use only, illicit drug use only, and both unhealthy alcohol and illicit drug use; and (3) using alternative QOC thresholds of ≥50% and 100% of QIs received.
RESULTS
The majority of the 3038 HIV-infected participants were male (97.5%), black (66.8%), and had completed a high school education or General Educational Development certificate (59.5%); their mean (SD) age was 49.0 (8.8) years (Table 2). At study enrollment, 83.9% were prescribed cART, 79.7% had a CD4 cell count ≥200/mL, and 55.6% had an undetectable HIV viral load. Substance use was prevalent, with 25.9% reporting unhealthy alcohol use, 28.4% reporting illicit drug use, and 11.2% reporting both in the past year. Marijuana (27.6%) was the most frequently used drug, and 7.2% reported injection drug use. Compared with those without unhealthy alcohol use, participants with unhealthy alcohol use were younger, more likely to be HCV infected or drug users, and more likely to have ever been homeless; they were less likely to have a high school education, be receiving cART, or have an undetectable HIV viral load. Compared with those without illicit drug use, participants with illicit drug use were more likely to be HCV infected and have ever been homeless and less likely to have had a high school education, be receiving cART, or have an undetectable HIV viral load, or a CD4 cell count ≥200/mL A majority (69.6%) of participants received ≥80% of eligible QIs. Participants with current unhealthy alcohol and/or illicit drug use were less likely to receive ≥80% of eligible QIs than those without.
Table 2.
Participant Characteristics, Overall and by Substance Usea
| Characteristic | Overall | Current Unhealthy Alcohol Use |
Current Illicit Drug Use |
||||
|---|---|---|---|---|---|---|---|
| (n = 3038) | Yes (n = 788) | No (n = 2250) | P Valueb | Yes (n = 863) | No (n = 2175) | P Valueb | |
| ≥80% QIs received | 69.6 | 61.7 | 72.4 | <.001 | 61.0 | 73.2 | <.001 |
| ≥50% QIs received | 95.1 | 92.6 | 96.9 | <.001 | 92.5 | 96.2 | <.001 |
| ≥100% QIs received | 36.0 | 31.0 | 37.9 | <.001 | 28.3 | 39.2 | <.001 |
| Age, mean (SD), y | 49.0 (8.8) | 48.0 | 49.3 | <.001 | 48.6 | 49.1 | .17 |
| Male sex | 97.5 | 98.4 | 97.1 | .08 | 97.0 | 97.7 | .26 |
| Race/ethnicity | .84 | <.001 | |||||
| White | 19.9 | 19.2 | 20.1 | … | 13.0 | 22.6 | … |
| Black | 66.8 | 67.5 | 66.6 | … | 75.6 | 63.4 | … |
| Latino | 9.5 | 9.8 | 9.3 | … | 7.7 | 102 | … |
| Other | 3.8 | 3.4 | 4.0 | … | 3.7 | 3.9 | … |
| CD4 cell count >200/nadir | 79.7 | 79.6 | 79.7 | .92 | 75.8 | 81.2 | .001 |
| HIV RNA <500 copies/mL | 55.6 | 49.9 | 57.6 | <.001 | 48.7 | 58.4 | <.001 |
| cART prescribed | 83.9 | 80.2 | 85.2 | .001 | 80.8 | 85.1 | .003 |
| Education level at least high school/GED | 59.5 | 54.9 | 61.1 | .002 | 53.2 | 62.0 | <.001 |
| Ever homeless | 41.5 | 46.7 | 39.6 | .001 | 58.0 | 34.9 | <.001 |
| HCV positive | 53.5 | 57.7 | 52.0 | .006 | 66.9 | 48.2 | <.001 |
| Unhealthy alcohol use | 25.9 | … | … | … | 40.0 | 20.5 | <.001 |
| Drug use | |||||||
| Opiates | 9.4 | 11.8 | 8.5 | .006 | 32.9 | … | … |
| Cocaine | 21.9 | 38.0 | 16.2 | <.001 | 76.9 | … | … |
| Stimulants | 4.3 | 6.0 | 3.7 | .007 | 15.1 | … | … |
| Marijuana | 27.6 | 38.6 | 23.7 | <.001 | 48.9 | … | … |
| Injection drug use | 7.2 | 10.3 | 6.1 | <.001 | 25.3 | … | … |
| Mean VACS Index | 32.6 | 33.0 | 32.4 | .50 | 36.3 | 31.1 | <.001 |
| Mortality rate, deaths/100 person-years | |||||||
| <80% QIs received | 4.1 | 4.4 | 3.9 | .37 | 4.9 | 3.7 | .02 |
| ≥80% QIs received | 3.5 | 3.5 | 3.4 | .89 | 3.9 | 3.3 | .08 |
Abbreviations: cART, combination antiretroviral therapy; GED, General Educational Development certificate; HCV, hepatitis C virus; HIV, human immunodeficiency virus; QIs, quality indicators; SD, standard deviation; VACS, Veterans Aging Cohort Study.
a Data represent percentage of participants, unless otherwise specified.
b Differences were considered statistically significant at P < .05.
Participants were followed up for a mean (SD) of 8.2 (3.3) years. A total of 902 participants died (29.8%) during 24 805 person-years of follow-up. Mortality rates were higher for participants with past-year illicit drug use than for those without and comparable for those with and those without past-year unhealthy alcohol use. Mortality rates were lowest for those who received ≥80% of HIV QIs for which they were eligible, regardless of substance use (Table 2).
All participants who received ≥80% of eligible HIV QIs experienced lower hazards of age-adjusted mortality rates (Table 3). Overall, receiving ≥80% of eligible QIs was associated with a 25% decrease in mortality rate compared with receiving a lower percentage of eligible QIs (age-adjusted hazards ratio, 0.75; 95% confidence interval, .65–.86). Similar benefits were observed regardless of substance use. This association was attenuated in models adjusting for severity of illness using the VACS Index.
Table 3.
Unadjusted and Adjusted Hazard Ratios for All-Cause Mortality Rates, Overall and by Unhealthy Alcohol and Illicit Drug Use
| Participants Group | Hazard Ratio (95% CI) |
||
|---|---|---|---|
| Unadjusted | Adjusted for Age | Adjusted for VACS Indexa | |
| All participants (n = 3038) | |||
| ≥80% QIs received | 0.85 (.74–.98)b | 0.75 (.65–.86)b | 1.01 (.88–1.17) |
| Age (years) | … | 1.05 (1.04–1.05)b | … |
| Mean VACS Index | … | … | 1.19 (1.17–1.21)b |
| Unhealthy alcohol use (n = 788) | |||
| ≥80% QIs received | 0.80 (.62–1.03) | 0.74 (.57–.96)b | 1.00 (.78–1.30) |
| Age (years) | … | 1.05 (1.04–1.06)b | … |
| Mean VACS Index | … | … | 1.18 (1.15–1.21)b |
| No unhealthy alcohol use (n = 2250) | |||
| ≥80% QIs received | 0.89 (.750–1.05) | 0.76 (.64–.91)b | 1.02 (.86–1.21) |
| Age (years) | … | 1.05 (1.04–1.07)b | … |
| Mean VACS Index | … | … | 1.20 (1.18–1.21)b |
| Illicit drug use (n = 863) | |||
| ≥80% QIs received | 0.82 (.65–1.03) | 0.77 (.61–.97)b | 1.00 (.79–1.27) |
| Age (years) | … | 1.06 (1.05–1.08)b | … |
| Mean VACS Index | … | … | 1.19 (1.16–1.22)b |
| No illicit drug use (n = 2175) | |||
| ≥80% QIs received | 0.91 (.76–1.09) | 0.78 (.65–.94)b | 1.03 (.86–1.23) |
| Age (years) | … | 1.04 (1.04–1.05)b | … |
| Mean VACS Index | … | … | 1.19 (1.17–1.21)b |
Abbreviations: CI, confidence interval; QIs, quality indicators; VACS, Veterans Aging Cohort Study.
a VACS Index divided by 5; age is not adjusted for in this model because it is a component of the VACS Index.
b P < .05.
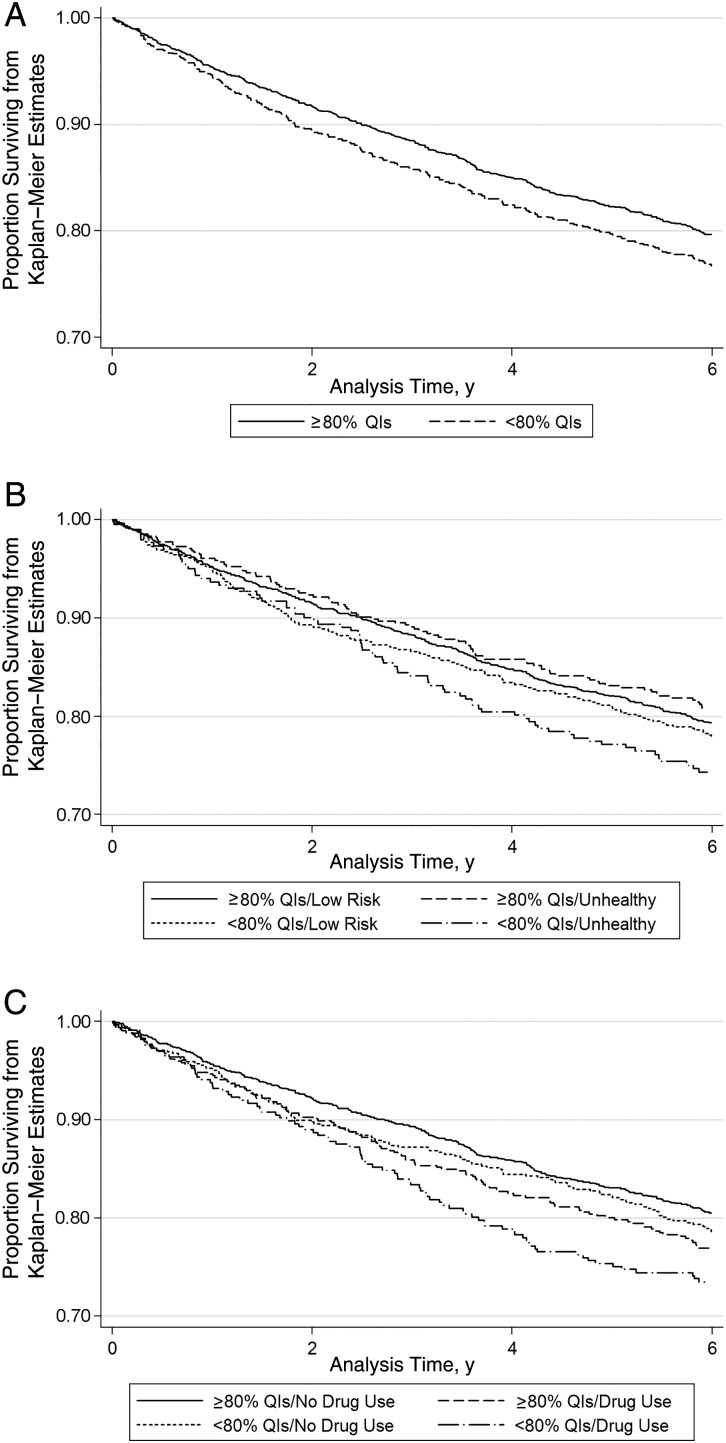
In survival analyses, overall survival was better for those who received ≥80% of HIV QIs (Figure 1A). This was true among those with or without unhealthy alcohol use and those with and without illicit drug use (Figure 1B and 1C).
Figure 1.
A, Survival for patients receiving ≥80% versus <80% of human immunodeficiency virus (HIV) quality indicators (QIs). B, Survival by past-year unhealthy alcohol use and receipt of ≥80% versus <80% of HIV QIs. C, Survival by past-year illicit drug use and receipt of ≥80% versus <80% of HIV QIs.
Sensitivity analyses limiting follow-up to 2 years, and using alternative QOC thresholds of ≥50% and 100% of QIs received yielded similar results. Stratifying by 4 mutually exclusive substance use categories (no unhealthy alcohol use or illicit drug use, unhealthy alcohol use only, illicit drug use only, and both unhealthy alcohol and illicit drug use) yielded point estimates similar in direction that did not achieve the threshold for statistical significance owing to small sample sizes (Supplementary Table 2).
DISCUSSION
The current study found improved mortality rates in HIV-infected patients who receive high-quality care. These findings support policies that promote monitoring and reporting of QIs, suggesting that high quality care provided by healthcare systems and providers may translate into decreased mortality rates for their patients. Improving QOC alone, however, may be insufficient for overcoming the contribution of underlying disease severity to mortality rate.
The current study provides an example of a chronic illness in which improvements in QIs are associated with improved mortality rates, and this finding requires replication in other chronic illnesses. Mortality rates have been associated with process measures for few other chronic illnesses [8, 9]. Of 14 diabetes QIs examined in a registry of 10 058 diabetic patients receiving care in the Netherlands, only 4 were associated with a decrease in a composite outcome of cardiovascular event or death [9]. In a survey of cardiac care units, optimal management scores were associated with decreased 30-day mortality rates for patients admitted for acute myocardial infarction [8]. Patient-level QOC measures were not considered. In a study of US Veterans admitted for acute myocardial infarction, the percentage of inpatient QIs received was not associated with 12-month all-cause mortality rate [31], but no primary care QIs were assessed. We were unable to identify studies that assessed associations between mortality rate and composite QIs for common conditions managed in outpatient settings.
When results were stratified by unhealthy alcohol and illicit drug use, all groups receiving ≥80% of QIs experienced lower all-cause unadjusted and age-adjusted mortality rates. This suggests that improvements in QOC may have beneficial effects on survival regardless of substance use status.
Associations between mortality rate and receipt of ≥80% of indicated QIs observed in a Cox-proportional hazards model were attenuated when adjusted for the VACS Index, a powerful predicator of mortality rate in HIV-infected patients that includes laboratory measures of HCV status, anemia, renal and liver function [29, 30]. The VACS Index was endogenous with other clinical predictors of mortality rate already included (eg, CD4 cell count) in a priori models. These findings suggest that improving adherence to QOC metrics may not be sufficient to overcome the role of severity of medical illness as the dominant driver of mortality rate, overall, or in patients with drug or alcohol problems. Treatment of patient comorbid conditions and substance use disorders remains crucial for improving survival for HIV-infected patients with unhealthy alcohol or illicit drug use, as demonstrated elsewhere [32–34].
Any potential for improved QOC to benefit survival in HIV-infected patients requires engagement in HIV care. Engagement and retention were high in a study of the continuum of HIV care at a large VHA HIV treatment center, with 90% of veterans linked to HIV care and 73% retained in care [35], but lower in nonveteran populations [36]. Unhealthy alcohol and illicit drug use are often associated with failure to be retained in care. Indeed, participants with unhealthy alcohol use and/or illicit drug use were less likely to receive ≥80% of indicated HIV QIs. Realizing potential survival benefits due to receipt of high-quality HIV care will require improved interventions to link and retain in medical care HIV-infected individuals with unhealthy alcohol or illicit drug use and engage them in treatment of substance use disorders.
The current study findings should be interpreted in the context of several potential limitations. First, our study population of HIV-infected patients was limited to predominantly male US military veterans who received care in the VHA, an organization whose systematic strategies to improve the QOC during the past 2 decades may have biased our results toward the null hypotheses [37–40]. Replications of the current study in other healthcare systems may find an even greater association between QOC and survival. Second, we were unable to measure some QIs owing to limitations of medical record data collection and validation (eg, high-risk sexual behavior screening and retention in care). Inclusion of these QIs in electronic medical record collection would facilitate assessment of such QIs for both clinical and research purposes.
As another limitation, we were unable to account for QIs delivered by non-VA providers. This is unlikely to bias results of most QIs (eg, most HIV-infected veterans fill prescription for cART at VA pharmacies) but may be important for QIs commonly delivered in non-VA settings (eg, influenza vaccinations). There is also little empirical evidence to support the use of an 80% of QIs threshold, though achieving ≥80% of QIs has strong face validity as a reflection of high-quality care and is often used as a performance target for non-HIV care in healthcare systems. Finally, we could not determine whether the association between QOC and survival was due to direct effects of care processes on survival or whether these measures were proxies for unmeasured patient characteristics or other aspects of care that influenced survival.
The 2010 US National HIV/AIDS Strategy identifies improving the QOC for persons living with HIV as a national priority [41]. The current study findings suggest that this policy may further improve survival among HIV-infected patients who engage in care but that increased adherence to QOC measures may not be sufficient for improving mortality rates without addressing underlying conditions. Future research should explore associations between QOC and other outcomes, such as patient satisfaction and cost of care, and the association between QOC and mortality rates for AIDS-related mortality and in other chronic illnesses.
Supplementary Data
Supplementary materials are available at (http://cid.oxfordjournals.org). Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The views expressed in this paper are those of the authors. No official endorsement by the National Institutes of Health or the Department of Veterans Affairs is intended or should be inferred.
Financial support. This work was supported by the National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA; grants U24-AA020794, NIAAA U01-AA020790, and NIAAA R01-AA022886) and the NIH, National Institute on Drug Abuse (grant K23 DA019809).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.The Patient Protection and Affordable CAre Act, 42 U.S.C. § 18001 2010.
- 2.Friedberg MW, Damberg CL. A five-point checklist to help performance reports incentivize improvement and effectively guide patients. Health Aff (Millwood) 2012; 31:612–8. [DOI] [PubMed] [Google Scholar]
- 3.Reeves D, Campbell SM, Adams J, Shekelle PG, Kontopantelis E, Roland MO. Combining multiple indicators of clinical quality: an evaluation of different analytic approaches. Med Care 2007; 45:489–96. [DOI] [PubMed] [Google Scholar]
- 4.Horberg MA, Aberg JA, Cheever LW, Renner P, O'Brien Kaleba E, Asch SM. Development of national and multiagency HIV care quality measures. Clin Infect Dis 2010; 51:732–8. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine Committee on Review Data Systems for Monitoring HIV Care. Monitoring HIV care in the United States: indicators and data systems. Washington, DC: National Academies Press, 2012. [PubMed] [Google Scholar]
- 6.Evans SM, Lowinger JS, Sprivulis PC, Copnell B, Cameron PA. Prioritizing quality indicator development across the healthcare system: identifying what to measure. Intern Med J 2009; 39:648–54. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press, 2001. [PubMed] [Google Scholar]
- 8.McConnell KJ, Lindrooth RC, Wholey DR, Maddox TM, Bloom N. Management practices and the quality of care in cardiac units. JAMA Intern Med 2013; 173:684–92. [DOI] [PubMed] [Google Scholar]
- 9.Sidorenkov G, Voorham J, de Zeeuw D, Haaijer-Ruskamp FM, Denig P. Do treatment quality indicators predict cardiovascular outcomes in patients with diabetes? PLoS One 2013; 8:e78821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore RD, Keruly JC, Gebo KA, Lucas GM. An improvement in virologic response to highly active antiretroviral therapy in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr 2005; 39:195–8. [PubMed] [Google Scholar]
- 11.Schackman BR, Gebo KA, Walensky RP et al. . The lifetime cost of current human immunodeficiency virus care in the United States. Med Care 2006; 44:990–7. [DOI] [PubMed] [Google Scholar]
- 12.Samji H, Cescon A, Hogg RS et al. . Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braithwaite RS, Conigliaro J, Roberts MS et al. . Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care 2007; 19:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Backus LI, Boothroyd DB, Phillips BR et al. . National quality forum performance measures for HIV/AIDS care: the Department of Veterans Affairs’ experience. Arch Intern Med 2010; 170:1239–46. [DOI] [PubMed] [Google Scholar]
- 15.Horberg M, Hurley L, Towner W et al. . HIV quality performance measures in a large integrated health care system. Aids Patient Care STDS 2011; 25:21–8. [DOI] [PubMed] [Google Scholar]
- 16.Korthuis PT, Fiellin DA, McGinnis KA et al. . Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J Acquir Immune Defic Syndr 2012; 61:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korthuis PT. Quality of HIV care within the Veterans Affairs health system: a comparison using outcomes from the HIV cost and services utilization study. J Clin Outcomes Manag 2004; 11:765–74. [Google Scholar]
- 18.Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med Care 2006; 44(8 suppl 2):S1–6. [DOI] [PubMed] [Google Scholar]
- 19.Justice AC, Dombrowski E, Conigliaro J et al. . Veterans Aging Cohort Study (VACS): Overview and description. Med Care 2006; 44(8 suppl 2):S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross PA, Asch SM, Kitahata MM et al. . Performance measures for guidelines on preventing opportunistic infections in patients infected with human immunodeficiency virus [published correction appears in Clin Infect Dis 2000 May;30(5):841] Clin Infect Dis 2000; 30:S85–93. [DOI] [PubMed] [Google Scholar]
- 21.Feussner JR, Kizer KW, Demakis JG. The Quality Enhancement Research Initiative (QUERI): from evidence to action. Med Care 2000; 38(6 suppl 1):I1–6. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Measuring what matters: allocation, planning, and quality assessment for the Ryan White CARE Act. 2003. Available at: http://iom.nationalacademies.org/Reports/2003/Measuring-What-Matters-Allocation-Planning-and-Quality-Assessment-for-the-Ryan-White-CARE-Act.aspx Accessed 3 September 2015. [PubMed]
- 23.McGlynn EA, Asch SM, Adams J et al. . The quality of health care delivered to adults in the United States. N Engl J Med 2003; 348:2635–45. [DOI] [PubMed] [Google Scholar]
- 24.Korthuis PT, Fiellin DA, Fu R et al. . Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr 2011; 56(suppl 1):S83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA; Ambulatory Care Quality Improvement Project (ACQUIP). The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med 1998; 158:1789–95. [DOI] [PubMed] [Google Scholar]
- 26.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med 2005; 352:596–607. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Core measures for HIV/STD risk behavior and prevention: sexual behavior and drug behavior questions, versions 4 & 5. Atlanta, GA: Centers for Disease Control and Prevention, 2002. [Google Scholar]
- 28.Green TC, Kershaw T, Lin H et al. . Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug Alcohol Depend 2010; 110:208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Justice AC, Modur SP, Tate JP et al. . Predictive accuracy of the veterans aging cohort study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013; 62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate JP, Justice AC, Hughes MD et al. . An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013; 27:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meterko M, Wright S, Lin H, Lowy E, Cleary PD. Mortality among patients with acute myocardial infarction: the influences of patient-centered care and evidence-based medicine. Health Serv Res 2010; 45(5 pt 1):1188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplehorn JR, Dalton MS, Haldar F, Petrenas AM, Nisbet JG. Methadone maintenance and addicts’ risk of fatal heroin overdose. Subst Use Misuse 1996; 31:177–96. [DOI] [PubMed] [Google Scholar]
- 33.Lepere B, Gourarier L, Sanchez M et al. . Reduction in the number of lethal heroin overdoses in France since 1994. Focus on substitution treatments [in French]. Ann Med Interne (Paris) 2001; 152(suppl 3):IS5–12. [PubMed] [Google Scholar]
- 34.Zanis DA, Woody GE. One-year mortality rates following methadone treatment discharge. Drug Alcohol Depend 1998; 52:257–60. [DOI] [PubMed] [Google Scholar]
- 35.Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a Veterans’ Affairs clinic. AIDS Res Hum Retroviruses 2014; 30:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr 2012; 60:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kizer KW, Dudley RA. Extreme makeover: transformation of the veterans health care system. Annu Rev Public Health 2009; 30:313–39. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi AN, Grebla RC, Wright SM, Washington DL. Despite improved quality of care in the Veterans Affairs health system, racial disparity persists for important clinical outcomes. Health Aff (Millwood) 2011; 30:707–15. [DOI] [PubMed] [Google Scholar]
- 39.Trivedi AN, Matula S, Miake-Lye I, Glassman PA, Shekelle P, Asch S. Systematic review: comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care 2011; 49:76–88. [DOI] [PubMed] [Google Scholar]
- 40.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med 2003; 348:2218–27. [DOI] [PubMed] [Google Scholar]
- 41.The White House Office of National AIDS Policy. National HIV AIDS strategy for the United States. Available at: http://aids.gov/federal-resources/policies/national-hiv-aids-strategy/nhas.pdf 2010. Accessed 25 March 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.