Our epidemic-economic model of human immunodeficiency virus (HIV) in the United States quantifies excess HIV transmissions and deaths due to incomplete engagement in HIV care. Improving retention has greater epidemiologic impact at better cost-effectiveness ratios than expanded HIV screening.
Keywords: HIV, cost-effectiveness, mathematical model, economics
Abstract
Background. Recent guidelines advocate early antiretroviral therapy (ART) to decrease human immunodeficiency virus (HIV) morbidity and prevent transmission, but suboptimal engagement in care may compromise impact. We sought to determine the economic and epidemiologic impact of incomplete engagement in HIV care in the United States.
Methods. We constructed a dynamic transmission model of HIV among US adults (aged 15–65 years) and conducted a cost-effectiveness analysis of improvements along the HIV care continuum. We evaluated enhanced HIV testing (annual for high-risk groups), increased 3-month linkage to care (to 90%), and improved retention (50% relative reduction in yearly disengagement and 50% increase in reengagement). Our primary outcomes were HIV incidence, mortality, costs and quality-adjusted life-years (QALYs).
Results. Despite early ART initiation, a projected 1.39 million (95% uncertainty range [UR], 0.91–2.2 million) new HIV infections will occur at a (discounted) cost of $256 billion ($199–298 billion) over 2 decades at existing levels of HIV care engagement. Enhanced testing with increased linkage has modest epidemiologic benefits and could reduce incident HIV infections by 21% (95% UR, 13%–26%) at a cost of $65 700 per QALY gained ($44 500–111 000). By contrast, comprehensive improvements that couples enhanced testing and linkage with improved retention would reduce HIV incidence by 54% (95% UR, 37%–68%) and mortality rate by 64% (46%–78%), at a cost-effectiveness ratio of $45 300 per QALY gained ($27 800–72 300).
Conclusions. Failure to improve engagement in HIV care in the United States leads to excess infections, treatment costs, and deaths. Interventions that improve not just HIV screening but also retention in care are needed to optimize epidemiologic impact and cost-effectiveness.
(See the Editorial Commentary by Gardner on pages 230–2.)
In recent years, antiretroviral therapy (ART) for human immunodeficiency virus (HIV) has become more potent with less side effects and simpler dosing schedules. Viral suppression is associated with improved immunologic function and reductions in both infectious and noninfectious morbidity and mortality rates [1]. ART is also increasingly recognized as an effective tool to prevent HIV transmission [2, 3]. Earlier models have suggested that a “test and treat” policy, if widely implemented, could reduce HIV prevalence significantly over the coming decades [4]. In light of these findings, current US treatment guidelines recommend ART initiation without regard to CD4 cell count [1, 5].
Despite widespread focus on testing and initiation of treatment, arguably the greatest barrier to prevention of both HIV-related morbid conditions and HIV transmission is suboptimal engagement in care [6]. In the United States, nearly 20% of all persons living with HIV remain unaware of their infection [7], and the median CD4 cell count at the time of first presentation remains unacceptably low at <350 cells/ μL [8]. As a result, the US Centers for Disease Control and Prevention and others have advocated for routine testing for HIV among patients aged 13 to 64 years in most healthcare settings [9, 10]. However, once HIV infection is diagnosed, engagement in care remains suboptimal. A sizeable proportion of individuals do not link (eg, keep a first appointment) to HIV care in a timely manner [7, 11–16]; among those who do link to care, lack of long-term retention remains an important challenge [17, 18]. Ultimately, current estimates suggest that fewer than half of persons living with HIV in the United States are virologically suppressed, even though nearly 80% are probably aware of their serostatus [7, 11, 12, 19]. Each step in this “continuum of care” results in potentially preventable morbid effects and HIV transmission.
The impact and cost-effectiveness of expanded HIV screening and ART initiation have been studied [20, 21]. By contrast, the effects of suboptimal engagement in care on both the epidemiology and the economics of HIV care in the United States have not been adequately quantified. As guidelines continue to favor early testing and prolonged therapy, interruptions in care may increase. We therefore constructed a model of the US continuum of HIV care to estimate the economic and epidemiologic consequences of incomplete or intermittent engagement in care and explore the potential impact of interventions that strengthen such engagement relative to early treatment initiation alone.
METHODS
HIV Epidemic-Economic Model
Model Structure
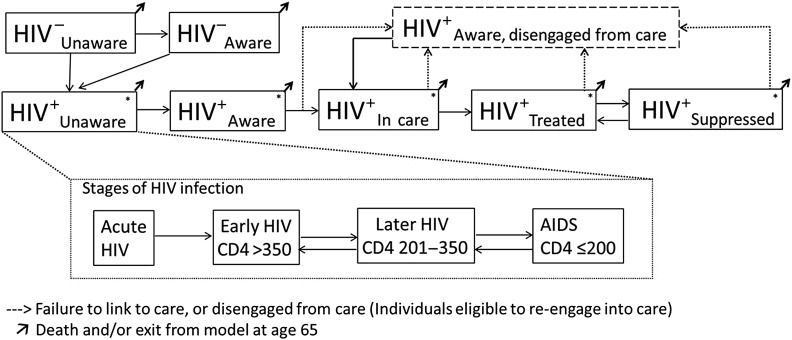
Our model incorporates HIV transmission, disease progression, and health system engagement in the United States. The model partitions the adult population (aged 15–65 years) based on sex, age, HIV infection status, and risk profile (heterosexuals, men who have sex with men [MSM], and persons who inject drugs [PWID]) (Figure 1). Among persons living with HIV, the population is further characterized by CD4 cell count, engagement in care, and treatment status (eg, unaware of HIV status, aware but out of care, in care but not receiving ART, receiving ART but not virologically suppressed, and virologically suppressed). The size of each subpopulation changes over time based on a system of ordinary differential equations (Supplementary Materials).
Figure 1.
Model schematic of human immunodeficiency virus (HIV) transmission, disease progression, and engagement in HIV care. The population is divided into compartments based on HIV status (and stage of HIV for HIV-infected), and engagement with HIV care. HIV+ represents HIV-infected individuals; HIV−, HIV-uninfected individuals. Each compartment is stratified further by sex and risk group (heterosexual, men who have sex with men, persons who inject drugs). The model incorporates transmission through sex and injection drug use. *Persons living with HIV (at any point in the HIV continuum of care) progress through a series of HIV stages from acute HIV to AIDS if not receiving antiretroviral therapy (ART), shown in subset. Individuals experience immunologic recovery if receiving ART and virally suppressed. CD4 represents CD4 cell count (in cells per microliter).
The model incorporates HIV transmission through sex (heterosexual or male homosexual) and through needle sharing. The risk of transmission is based on the frequency of sexual partnerships (and needle-sharing partnerships) and associated HIV transmission probabilities and was calibrated to match the observed HIV epidemiology in the United States. The probability of HIV transmission was modified by sex, stage of HIV, awareness of serostatus, and ART usage.
Among subpopulations with untreated HIV, the CD4 cell count declined at rates based on existing literature (Table 1). We assumed that awareness of HIV serostatus occurs through both routine screening and symptomatic presentation, the rates of which vary according to risk group. We incorporated benefits of virologic suppression through immunologic recovery and reduced transmission potential. We explicitly modeled rates of discontinuation in care, calibrating those rates to reflect current estimates of engagement in the continuum of care [59].
Table 1.
Key Model Parameters
| Variable | Value (Range for Sensitivity Analysis) | Source (References) |
|---|---|---|
| Demographics | ||
| Total adult population (aged 15–65 y) | 207 million | 22 |
| PWID (men) | 1.2 million | 21, 23, 24 |
| PWID (women) | 600 000 | 21, 23, 24 |
| MSM | 3.5 million | 25 |
| HIV disease dynamics without ART | ||
| Duration of acute infection, mo | 2.9 (1–4) | 5, 26, 27 |
| Duration of chronic infection, y | ||
| CD4 >350 | 6.5 (3–10) | 28–30 |
| CD4 200–350 | 2.5 (1–5) | 28, 29 |
| Duration of AIDS (CD4 ≤200), y | 2 (1–5) | 26, 27, 30–33 |
| Excess HIV mortality rate in persons not receiving ART (CD4 >200), %/y | 0.14 (0.1–1) | 34–36 |
| HIV disease dynamics with ARTa | ||
| Reduction in transmission rate, % | 93 (80–99.5) | 3, 21, 37, 38 |
| Time to viral suppression with ART, mo | 6 (2–12) | 32 |
| Reduction in rate of AIDS death with ART (CD4 ≤200), % | 90 (50–95) | 33, 39 |
| Transmission dynamicsb | ||
| Annual partnerships, No./y | 1.5–5 (0.2–6) | 21, 40–42, Calculated |
| Transmission per partnership, % | ||
| Male to female | 4.75 (2.4–7.1) | 21, Calculated |
| Female to male | 3.75 (1.8–5.6) | 21, Calculated |
| MSM | 5 (2.5–7.5) | 21, Calculated |
| Transmission probability per needle-sharing partnership (PWID) | 0.0025 (0.0025–0.0075) | 21, 43, 44, Calculated |
| Increase in transmission probability during acute HIV infection, relative risk | 12 (2–24) | 26, 27 |
| Engagement in care dynamicsc | ||
| HIV testing in past 12 mo, % | 5–20 (2.5–30) | 40, 45 |
| Patients with newly diagnosed HIV infection linked to care, % | 55–75 (20–100) | 7, 11–16 |
| Annual rate of disengagement from care | 0.15–0.32 (0.05–0.63) | 13, 46, 47 |
| Annual rate of reengagement in care | 0.20 (0.1–0.4) | 47, 48 |
| Costs, $d | ||
| HIV test | 32 (10–50) | 49–51 |
| HIV viral load | 106 (50–150) | 52 |
| Genotyping | 351 (25–500) | 52 |
| Outpatient visit | 118 (50–250) | 52 |
| CD4 test | 45 (20–90) | 52 |
| Annual ART costs | 16 263 (5000–20 000) | 21, 52 |
| “Rapid linkage to care” intervention (per individual linked to care) | 500 (10–3000) | 53, 54, Assumption |
| “Increased retention in care intervention” (per person per year) | 1000 (100–7500) | 53, 55, 56, Assumption |
| Utility weights | ||
| Uninfected | 1 (…) | 57, 58 |
| Acute HIV | 0.84 (0.8–0.9) | 57, 58 |
| HIV unsuppressed (CD4 >350) | 0.94 (0.9–0.99) | 57, 58 |
| HIV unsuppressed (CD4 200–350) | 0.84 (0.8–0.99) | 57, 58 |
| HIV/AIDS unsuppressed (CD4 ≤200) | 0.70 (0.5–0.9) | 57, 58 |
| Reduction in disability with viral suppression, % | 50 (0–90) | Assumption |
| Usage of ART | 0.96 (0.94–1) | 57, 58 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MSM, men who have sex with men; PWID, persons who inject drugs.
a Rapid ART initiation occurred for all populations in care regardless of CD4 cell count (see Supplementary Materials). Reduction in AIDS mortality rate with ART assumes usage of prophylaxis for opportunistic infections when indicated.
b The number of partnerships per year and probability of transmission per partnership were calibrated (see Supplementary Table 1, Supplementary Figure 2, and Supplementary Figure 3) to fit observed HIV incidence and prevalence in the United States and varied by sex and risk group. The probability of transmission per partnership was further modified by condom usage, male circumcision, stage of HIV infection, and awareness of HIV serostatus.
c Annual HIV screening rates, percentage linkage, and disengagement from care were varied by sex and risk group. Linkage was defined as an initial HIV clinic visit within 3 months of diagnosis. We also incorporated symptomatic testing and engagement in care (stratified by HIV stage). Reengagement refers to a return to care among persons living with HIV aware of serostatus but not in care (see Supplementary Table 2).
d We also included annual healthcare costs for individuals not in care or receiving ART (eg, hospitalizations, emergency department visits) (see Supplementary Table 3).
Economic and Epidemiologic Impact of HIV Continuum of Care
We estimated health-care costs, HIV incidence, AIDS mortality rate, and quality-adjusted life-years (QALYs), over a 20-year time horizon. In the base-case analysis, we assumed implementation of current guidelines on timing of ART initiation (ie, initiation at any CD4 cell count), with continuation of current trends in the HIV care continuum [1, 59, 60]. We then sequentially projected the epidemiologic impact and incremental cost-effectiveness of improvements in the HIV care continuum. We specifically examined the following interventions (under assumptions of immediate implementation), either independently or jointly:
Enhanced targeted screening: annual testing for high-risk individuals (MSM, persons who inject drugs, and heterosexuals aged 15–24 years) [61], concordant with US Preventive Services Task Force guidelines [62];
Enhanced targeted and general screening: annual testing for high-risk individuals, plus testing every 3 years for the general population aged 25–65 years;
Increased linkage to care: increasing the proportion of newly diagnosed persons completing an HIV care visit within 3 months from current level of approximately 70% [13, 63] to 90%; and
Improved retention in care: 50% reduction in yearly rate of disengagement from care, plus 50% increase yearly rate of return to care for those not in care (relative to current rates of disengagement and reengagement; Table 1).
Costs and QALYs were calculated from a societal perspective with a unit-costing (Table 1) approach that considers the person-time spent in each model compartment (eg, person-time receiving ART) and the number of transitions between compartments (eg, transition from unaware to aware as a result of HIV testing). All costs are reported in 2014 US dollars; costs and QALYs were discounted at an annual rate of 3% [64]. Estimates of disease burden (eg, infections or deaths averted) are reported without discounting.
There are limited data on the cost of interventions seeking to improve the HIV care continuum [53–55, 65]. For intervention scenarios involving increased screening, we assumed a 20% increase in per-test costs to account for added resources dedicated to an expanded testing program; for linkage to care interventions, we assumed an intervention cost of $500 (range, $10–$3000) per individual linking to care to represent intensified case management after diagnosis [53, 54]. We estimated the costs of retention in care based on staffing for social work, nurse managers, and case managers at local HIV clinics (base case, $300 per patient in care per year [range, $50–$1500]) (Baltimore City Health Department HIV/EII program, personal communication). We assumed additional costs of $1000 (range, $100–$7500) per engaged patient per year for an intervention (assumed to consist of intensified case management) capable of reducing the yearly rate of care disengagement by 50% (compared with current rates) and increasing reengagement among those lost from care by 50% [53, 55, 56].
Model Calibration and Sensitivity Analysis
We calibrated the annual number of sexual partnerships, probability of transmission per partnership, and rates of care engagement to reported epidemiologic data on the incidence, prevalence, and care continuum from 2006 to 2010 (Supplementary Materials) [7, 12, 13, 46, 66–69]. We conducted sensitivity analyses on all parameter values over the ranges specified in Table 1 and report on the parameters that most influenced model results. We also conducted a probabilistic uncertainty analysis by simultaneously varying all parameter values over beta distributions bounded by their ranges. We report 95% uncertainty ranges (URs) as the 2.5th and 97.5th percentiles of those simulations and report the proportion of simulations falling under different willingness-to-pay thresholds [70–73]. We performed all analyses using R software, version 3.0.1 (R Foundation for Statistical Computing).
RESULTS
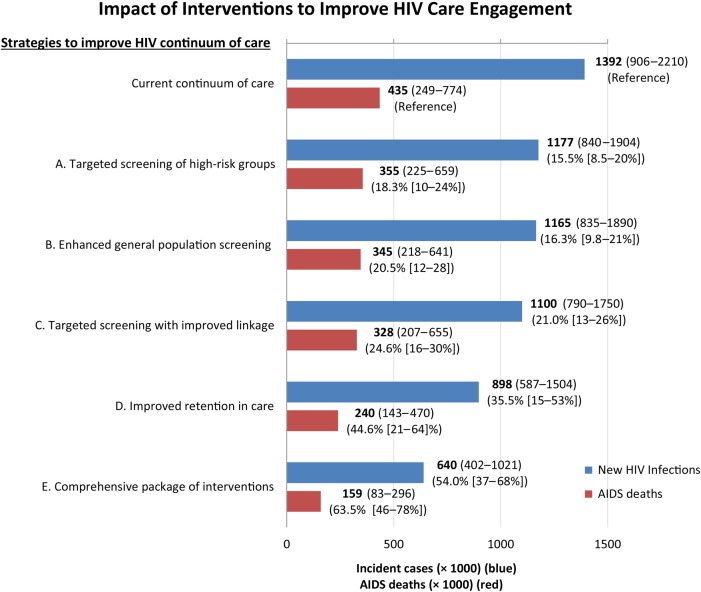
In the base-case, incorporating early ART initiation at any CD4 cell count but at current levels of retention in care, we projected that 1.39 million (95% UR, 0.91–2.2 million) new HIV infections would occur from 2015 to 2035 (Figure 2), with 435 000 AIDS deaths (95% UR, 249 000–774 000). Among new HIV cases, we estimated that 784 000 (56%) would occur as transmission from individuals aware of their HIV diagnosis. The majority (54%) of incident HIV occurred among MSM. HIV diagnosis and care was projected to cost the US health system $256 billion (with 3% discounting; $344 billion without discounting) over the next 2 decades (95% UR, $199–$298 billion) (Table 2).
Figure 2.
Impact of interventions to improve human immunodeficiency virus (HIV) screening and engagement in care. Shown are the model projections of total numbers (boldface) and percentage reductions (in parentheses) of new HIV infections (blue) and AIDS deaths (red) during the next 20 years, after implementation of 5 different interventions (95% UR). Intervention A includes yearly screening of young heterosexuals, all men who have sex with men, and all persons who inject drugs; intervention B, general population screening every 3 years, coupled with intervention A; intervention C, an intervention that results in 90% of newly diagnosed individuals achieving linkage to care within 3 months, coupled with intervention A (targeted screening); intervention D, an intervention that reduces the annual rate of disengagement by 50%, and increases the rate of reengagement in care by 50%; and intervention E, a comprehensive package of interventions that includes interventions C (targeted screening plus improved linkage to care) and D (improved retention in care). All scenarios (including current standard of care) assume antiretroviral eligibility at all CD4 cell counts. Abbreviation: UR, uncertainty range.
Table 2.
Cost-effectiveness of Alternative Strategies for Enhanced Engagement in Human Immunodeficiency Virus Care
| Intervention | Total Health System Costs (95% UR), $ Billiona | Incremental Costs (95% UR), $ Billion | Incremental QALYs (95% UR), ×1000a | Incremental Cost-effectiveness (95% UR), $/QALY Gainedb | Incremental Cost-effectiveness (95% UR), $/QALY Gainedc |
|---|---|---|---|---|---|
| Early ART initiation: current levels of engagement in care | 256 (199–298) | Reference | Reference | Reference | … |
| A. Enhanced targeted screeningd | 305 (241–343) | 49.2 (34–65) | 582 (313–828) | 84 700 (57 200–160 000) | Dominated |
| B. Enhanced general screeninge | 327 (267–370) | 71.1 (52–95) | 650 (352–913) | 109 000 (74 600–208 000) | Dominated |
| C. Enhanced targeted screening with improved linkage to caref | 309 (247–350) | 52.9 (39–70) | 805 (485–1139) | 65 700 (44 500–111 000) | Dominated |
| D. Improved retention in careg | 303 (241–348) | 47.7 (23–83) | 1413 (536–2811) | 33 700 (20 000–60 600) | Reference |
| E. Comprehensive package of interventionsh | 352 (291–407) | 96.0 (67–138) | 2120 (1155–3695) | 45 300 (27 800–72 300) | 68 300 (24 000–95 000) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; QALY, quality-adjusted life-year; UR, uncertainty range.
a Costs in 2014 US dollars with 3% discounting of future costs and QALYs.
b Incremental cost-effectiveness in which all interventions are compared with a common reference (current HIV continuum of care).
c Incremental cost-effectiveness ranked according to ascending order of cost-effectiveness.
d Intervention A: yearly screening of young heterosexuals, all men who have sex with men, and all persons who inject drugs.
e Intervention B: intervention A (yearly targeted screening) with general population screening every 3 years.
f Intervention C: targeted yearly screening (intervention A), coupled with an intervention that results in 90% of newly diagnosed individuals achieving initial linkage to care.
g Intervention D: an intervention that reduces the annual rate of disengagement by 50% and also increases the rate of reengagement in care by 50%.
h Intervention E: a comprehensive package of interventions that strengthens the full spectrum of the HIV continuum of care (ie, intervention C [Enhanced targeted screening and linkage to care] plus intervention D [improved retention in care]).
Strategies focused on increasing testing alone had only modest benefits. Annual targeted screening of high-risk individuals would avert 215 000 new HIV infections (16% reduction; 95% UR, 9%–20%) over the next 20 years, at an incremental (discounted) cost of $49.2 billion (95% UR, $34–$65), or $84 700 per QALY gained (95% UR, $57 200–160 000; Figure 2 and Table 2). Screening the entire general population every 3 years (in addition to high-risk individuals yearly) would require an additional $21.9 billion over 20 years to avert only 11 600 additional infections. Overall, enhanced population screening (whether high-risk only or general population) averted 18%–21% of AIDS-related deaths (95% UR, 10%–28%) during the analysis period. Increasing the proportion of persons linked to care (within 3 months) after a new HIV diagnosis to 90%, coupled with targeted yearly screening of high-risk individuals, would avert an estimated 292 000 HIV infections (21% reduction; 95% UR, 13%–26%) and 107 000 AIDS-related deaths (25% reduction; 95% UR, 16%–30%) at an incremental (discounted) cost of $52.9 billion dollars (95% UR, $39–$70 billion) compared with current levels of testing and linkage. This intervention was projected to cost $65 700 per QALY gained (95% UR, $44 500–$111 000).
In contrast to interventions limited to screening and linkage, interventions targeting retention and reengagement in care were projected to have larger population-level impact (Figure 2). Even at current levels of awareness and linkage, an intervention that would reduce the current rate of disengagement from care and increase the rate of reengagement in care (for individuals lost to follow-up) by 50% was projected to avert 494 000 HIV infections (95% UR, 186 000–984 000) over 20 years, a 36% reduction (95% UR, 15%–53%). The cost-effectiveness ratio of this intervention was also more favorable, at $33 700 per QALY gained (95% UR, $20 000–60 600) (Table 2).
Alternatively, a comprehensive package of interventions that coupled targeted screening of high-risk groups, improved linkage, and enhanced retention and reengagement in care was projected to have the greatest population benefit, averting a projected 752 000 new HIV infections (54% reduction; 95% UR, 37%–68%) and 276 000 AIDS deaths (64% reduction; 95% UR, 46%–78%) (Figure 2). The incremental cost of such an approach was projected to be $96 billion (95% UR, $67–$138 billion) over 20 years, or $45 300 per QALY gained (95% UR, $27 800–$72 300).
Sensitivity Analysis
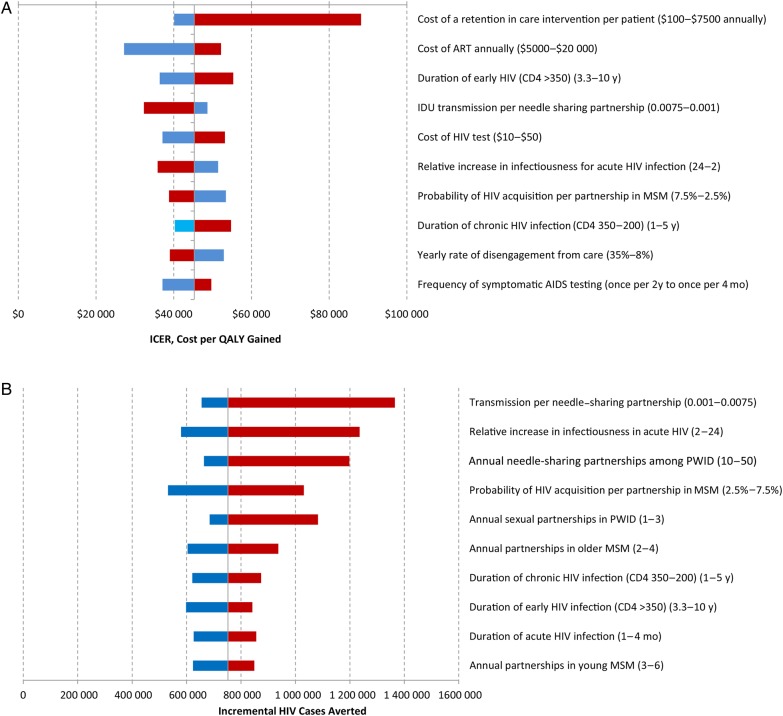
In sensitivity analysis, there was no single dominant driver of the cost-effectiveness of the comprehensive intervention, with incremental cost-effectiveness mostly varying between $28 000 and $55 000 per QALY gained (Figure 3A). We initially modeled improved retention at a cost of $1000 per person per year; a 7.5-fold increase in intervention costs (to $7500 per patient-year) resulted in a 1.9-fold increase in cost-effectiveness (from $45 300 to $89 800 per QALY gained). Similarly, varying the effectiveness of retention interventions (from 25% to 75% reductions in the disengagement rate) caused minimal changes in cost-effectiveness (from $51 400 to $42 000 per QALY gained), though the effect on epidemiologic impact was greater (from 593 000 [43% reduction] to 939 000 infections averted [67% reduction]). In a “worst-case scenario” when all component intervention costs (ie, linkage, testing, and retention) and ART costs were simultaneously set to their highest estimates, the incremental cost-effectiveness of the combined intervention rose to $118 000 per QALY gained. In probabilistic sensitivity analysis evaluating this combined intervention compared with the base case, 70% of simulations fell below a threshold of $50 000 per QALY gained, rising to 100% of simulations at a threshold of $100 000 per QALY gained [70–73].
Figure 3.
Sensitivity analysis of key parameters comparing current levels of engagement in care with comprehensive enhancements in human immunodeficiency virus (HIV) continuum of care (targeted yearly screening of high-risk groups, improved linkage to care, and improved yearly retention). A, Incremental cost-effectiveness ratio (ICER) comparing a comprehensive intervention to improve HIV continuum of care with current HIV care. B, Incident HIV cases averted comparing a comprehensive intervention to improve HIV continuum of care with current HIV care. Solid vertical line represents base-case values (ie, base-case ICER [$45 300 per QALY gained] in A, and base-case incremental HIV cases averted [n = 752 000] in B); blue bars, low values of parameter range; red bars, high values of parameter range. Abbreviations: ART, antiretroviral therapy; CD4, CD4 cell count (in cells per microliter); IDU, injection drug use; MSM, men who have sex with men; PWID, persons who inject drugs; QALY, quality-adjusted life-year.
Model projections of averted HIV infections were sensitive to estimates of risk behaviors and the infectiousness of acute period (Figure 3B). Under scenarios with increased risk behavior (eg, higher number of sexual partnerships), comprehensive improvements to the HIV care continuum avert a greater number of new HIV cases. Relative reductions in incidence were less sensitive to model estimates than were absolute projections; for example, assuming a 2-fold increase in relative infectiousness during the acute HIV period compared with chronic HIV led the model to project that the comprehensive intervention would reduce incident cases from 1.1 million to 520 000, an absolute decline of 580 000 and a relative decline of 53%. Increasing the infectiousness of the acute period by a factor of 24 compared with infectiousness during chronic HIV led the model to project a reduction from 2.1 million incident cases with current levels of care to 920 000 with the comprehensive intervention, a much greater absolute decline of 1.2 million but a similar relative decline of 57% (Figure 3B, second bar).
DISCUSSION
Although guidelines often focus on testing and early treatment, suboptimal linkage and retention in care are also important drivers of ongoing HIV transmission and cost of care in the United States today. This epidemic-economic model suggests that, even if ART is initiated irrespective of CD4 cell count, nearly 1.4 million HIV infections and >400 000 AIDS deaths may occur in the United States over the next 20 years, at a (discounted) cost of >$250 billion. Increased screening and ensuring rapid linkage to care for 90% of all those testing positive was projected to reduce the burden of HIV incidence and mortality rate by 20%–25%. By contrast, adding interventions to improve retention and reengagement in HIV care could more than double this epidemiologic impact while also improving cost-effectiveness.
In an era of constrained resources for HIV prevention in the United States, these findings are relevant for resource allocation decisions. Recent emphasis has been placed on increasing screening, particularly among persons at high risk [9, 74]. However, our model suggests that focusing some of these resources toward retention and reengagement of persons with known HIV—even at an additional cost of several thousand dollars per person-year—might be a more cost-effective use of resources. This finding reflects the fact that more than half of HIV infections were projected to occur after serostatus awareness. Thus, ensuring that persons with known HIV infection remain in care addresses the largest avertable burden of HIV.
This study adds an important perspective to a growing body of literature estimating the impact and cost-effectiveness of HIV care interventions in the United States. Previous studies have demonstrated that expanded HIV screening and earlier treatment initiation are cost-effective [4, 20, 21, 75]. The current model supports these findings but adds the comparative effectiveness and cost-effectiveness of linkage and of retention in care. Earlier ART initiation has the benefit of improved survival but also leads to increased opportunities for interruptions in care which can lead to additional transmission. In accounting for such “real-world” gaps in the continuum of care, our estimate of 1.4 million new HIV infections over 20 years despite rapid ART initiation is greater than some prior estimates (eg, 1.2 million with more limited ART use) [21]. However, our findings suggest that a substantial proportion of these infections can be averted through improving retention in HIV care.
Improving retention will require innovative strategies. Relatively few studies have evaluated interventions to enhance retention, especially in the context of treatment as prevention [76]. Patient navigators decreased disengagement from 36% to 21% over a 12-month period in 1 study [77]. An HIV Prevention Trials Network study (HPTN 065) is ongoing to assess, among other end points, whether patient financial incentives can improve clinic and medication adherence [78]. Peer counseling [77, 79], directed youth case management [80], and buprenorphine or methadone treatment for opioid-dependent patients [81] are examples of targeted interventions that may also improve retention in care.
Although our findings speak to the urgency of identifying locally relevant interventions for improving care engagement, existing mechanisms that support retention should be recognized. Ryan White Act funding currently provides outpatient visit coverage for the uninsured, emergency medication coverage, treatment education [82], transportation [83], housing assistance [84], and support groups [85]. These ancillary services help maintain current levels of care engagement. It may be extrapolated from our results that removing support for such existing programs may have a large negative epidemiologic impact.
As with any modeling analysis, our study has certain limitations. We adopted a population-level approach; as such, we did not perform a detailed costing of specific interventions, the cost of which will differ according to local conditions. Rather, we projected the epidemiologic impact of interventions capable of achieving a certain effect immediately and varied unit costs of those interventions widely, with little resultant changes in our projected cost-effectiveness ratios. Thus, as with prior analyses of national HIV guidelines [65], our model cannot speak to the cost-effectiveness of specific interventions implemented on the local level, but we provide broad estimates of the likely impact and cost-effectiveness of such interventions on a population level. Sexual partnerships and preferences, needle sharing, and risk behaviors all occur within complex and heterogeneous networks. We used a compartmental modeling approach that simplifies these dynamics. For example, key parameters (such as partnerships per year) may be very heterogeneous at the individual level, and we model these parameters as population averages (within each risk group). To the extent that heterogeneous behavior within subpopulations of age and risk behavior is not well represented by average values, our results may be biased. However, such simplifying assumptions increase the transparency of results, and we present URs based on a probabilistic sensitivity analysis that consider a wide range for each of these population-average parameter values. Our model also uses a fixed time horizon of 20 years to estimate costs and effects (ie, QALYs), which is likely to give conservative estimates of cost-effectiveness of interventions, relative to analyses using lifetime closed-cohort time horizons. Given this potential limitation, we also provide data on HIV incident cases averted, as well as averted AIDS deaths for each intervention scenario to allow examination of the relative effectiveness of varying interventions.
Our model has several important strengths. In contrast to decision-analytic or strict Markov models, we are able to capture transmission dynamics and their impact on HIV-associated costs and epidemiology over time. We also incorporate key subpopulations and risk groups with differential behaviors, and explicitly model steps in the HIV continuum of care. Finally, our combined economic-epidemiologic framework generates estimates of cost and cost-effectiveness as well as epidemiologic impact (incidence and mortality rate) at the level of the US population.
In conclusion, to alter the course of the HIV epidemic in the United States, strategies of “test and treat” alone may be insufficient; attention to the full continuum of care will be essential. Although targeted HIV screening, rapid linkage to care, and early ART initiation are all effective interventions, improved retention may ultimately have a more transformative impact on the HIV epidemic in the United States over the next 20 years.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. Funders had no role in the design or conduct of the study, analysis or interpretation of the results, manuscript writing, or decision to publish results.
Financial support. This work was supported the National Institute of Allergy and Infectious Diseases, National Institutes of Health, (grants K23AI089259, K23AI084854, and T32AI102623), the B. Frank and Kathleen Polk Assistant Professorship in Epidemiology, and the Canadian Institutes of Health Research (grant 131558).
Potential conflicts of interest. S. A. B. has served as a consultant to Bristol-Myers Squibb (BMS) for work unrelated to this project; B MS had no role in the design or conduct of the study, analysis or interpretation of the results, manuscript writing, or decision to publish results. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2013. Available at: http://aidsinfo.nih.gov/guidelines Accessed 15 January 2014.
- 2.Quinn TC, Wawer MJ, Sewankambo N et al. Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs fro treating and preventing HIV infection. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 Accessed 5 June 2014.
- 6.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis 2007; 44:1500–2. [DOI] [PubMed] [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Althoff KN, Gange SJ, Klein MB et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis 2010; 50:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branson BM, Handsfield HH, Lampe MA et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17. [PubMed] [Google Scholar]
- 10.Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med 2009; 150:125–31. [DOI] [PubMed] [Google Scholar]
- 11.Muthulingam D, Chin J, Hsu L, Scheer S, Schwarcz S. Disparities in engagement in care and viral suppression among persons with HIV. J Acquir Immune Defic Syndr 2013; 63:112–9. [DOI] [PubMed] [Google Scholar]
- 12.Hall HI, Frazier EL, Rhodes P et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173:1337–44. [DOI] [PubMed] [Google Scholar]
- 13.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS 2010; 24:2665–78. [DOI] [PubMed] [Google Scholar]
- 14.Thompson MA, Mugavero MJ, Amico KR et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012; 156:817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paz-Bailey G, Pham H, Oster AM et al. Engagement in HIV care among HIV-positive men who have sex with men from 21 cities in the United States. AIDS Behav 2014; 18(suppl 3):348–58. [DOI] [PubMed] [Google Scholar]
- 16.Hall HI, Tang T, Westfall AO, Mugavero MJ. HIV care visits and time to viral suppression, 19 U.S. jurisdictions, and implications for treatment, prevention and the national HIV/AIDS strategy. PLoS One 2013; 8:e84318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y, L'Italien G, Mukherjee J, Iloeje UH. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort. HIV Med 2006; 7:156–62. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Margolick JB, Conover CS et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2005; 38:320–8. [PubMed] [Google Scholar]
- 19.Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a Veterans’ Affairs clinic. AIDS Res Hum Retroviruses 2014; 30:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paltiel AD, Weinstein MC, Kimmel AD et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–95. [DOI] [PubMed] [Google Scholar]
- 21.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med 2010; 153:778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Census Bureau. Current population survey (CPS). Available at: https://www.census.gov/cps/ Accessed 6 March 2014.
- 23.US Department of Health and Human Services. National Survey on Drug Use and Health, 2010. Available at: https://nsduhweb.rti.org/respweb/homepage.cfm. Accessed 14 September 2014.
- 24.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The NSDUH report: injection drug use and related risk behaviors. Available at: http://www.samhsa.gov/data/2k9/139/139IDU.htm Accessed 14 September 2014.
- 25.Centers for Disease C, Prevention. Estimated percentages and characteristics of men who have sex with men and use injection drugs—United States, 1999–2011. MMWR Morb Mortal Wkly Rep 2013; 62:757–62. [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis 2008; 198:687–93. [DOI] [PubMed] [Google Scholar]
- 27.Wawer MJ, Gray RH, Sewankambo NK et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005; 191:1403–9. [DOI] [PubMed] [Google Scholar]
- 28.Wolbers M, Babiker A, Sabin C et al. Pretreatment CD4 cell slope and progression to AIDS or death in HIV-infected patients initiating antiretroviral therapy—the CASCADE collaboration: a collaboration of 23 cohort studies. PLoS Med 2010; 7:e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyles RH, Munoz A, Yamashita TE et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men: Multicenter AIDS Cohort Study. J Infect Dis 2000; 181:872–80. [DOI] [PubMed] [Google Scholar]
- 30.Vergis EN, Mellors JW. Natural history of HIV-1 infection. Infect Dis Clin North Am 2000; 14:809–25. [DOI] [PubMed] [Google Scholar]
- 31.Katz DA, Cassels SL, Stekler JD. Replacing clinic-based tests with home-use tests may increase HIV prevalence among Seattle men who have sex with men: evidence from a mathematical model. Sex Transm Dis 2014; 41:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Currie S, Rogstad KE, Piyadigamage A, Herman S. Time taken to undetectable viral load, following the initiation of HAART. Int J STD AIDS 2009; 20:265–6. [DOI] [PubMed] [Google Scholar]
- 33.CASCADE Collaboration. Survival after introduction of HAART in people with known duration of HIV-1 infection: the CASCADE Collaboration—Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet 2000; 355:1158–9. [PubMed] [Google Scholar]
- 34.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emery S, Neuhaus JA, Phillips AN et al. Strategies for Management of Antiretroviral Therapy Study Group. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis 2008; 197:1133–44. [DOI] [PubMed] [Google Scholar]
- 36.Lodwick RK, Sabin CA, Porter K et al. Study Group on Death Rates at High CD4 Count in Antiretroviral Naive Patients. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per μL in Europe and North America: a pooled cohort observational study. Lancet 2010; 376:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnell D, Baeten JM, Kiarie J et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lingappa JR, Hughes JP, Wang RS et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One 2010; 5:e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy EL, Collier AC, Kalish LA et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med 2001; 135:17–26. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Trends in prevalence of sexual behaviors and HIV testing, 2012. Available at: http://www.cdc.gov/healthyyouth/yrbs/pdf/trends/us_sexual_trend_yrbs.pdf Accessed 5 June 2014.
- 41.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data 2005; 362:1–55. [PubMed] [Google Scholar]
- 42.Rosenberg ES, Sullivan PS, Dinenno EA, Salazar LF, Sanchez TH. Number of casual male sexual partners and associated factors among men who have sex with men: results from the National HIV Behavioral Surveillance system. BMC Public Health 2011; 11:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health 2000; 90:1100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr 1992; 5:1116–8. [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Persons tested for HIV—United States, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:845–9. [PubMed] [Google Scholar]
- 46.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA; HIV Research Network. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr 2012; 60:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. AIDS 2013; 27:2271–9. [DOI] [PubMed] [Google Scholar]
- 48.Cunningham CO, Buck J, Shaw FM, Spiegel LS, Heo M, Agins BD. Factors associated with returning to HIV care after a gap in care in New York State. J Acquir Immune Defic Syndr 2014; 66:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Medicaid and Medicare Services. Medicaid fee schedule, 2014. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed 22 July 2014.
- 50.Cragin L, Pan F, Peng S et al. Cost-effectiveness of a fourth-generation combination immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen for the detection of HIV infections in the United States. HIV Clin Trials 2012; 13:11–22. [DOI] [PubMed] [Google Scholar]
- 51.Hutchinson AB, Ethridge SF, Wesolowski LG et al. Costs and outcomes of laboratory diagnostic algorithms for the detection of HIV. J Clin Virol 2013; 58(suppl 1):e2–7. [DOI] [PubMed] [Google Scholar]
- 52.Gebo KA, Fleishman JA, Conviser R et al. Contemporary costs of HIV healthcare in the HAART era. AIDS 2010; 24:2705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardner LI, Metsch LR, Anderson-Mahoney P et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 2005; 19:423–31. [DOI] [PubMed] [Google Scholar]
- 54.Spaulding AC, Pinkerton SD, Superak H et al. Cost analysis of enhancing linkages to HIV care following jail: a cost-effective intervention. AIDS Behav 2013; 17(suppl 2):S220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sansom SL, Anthony MN, Garland WH et al. The costs of HIV antiretroviral therapy adherence programs and impact on health care utilization. AIDS Patient Care STDS 2008; 22:131–8. [DOI] [PubMed] [Google Scholar]
- 56.Shrestha R. Estimating the cost of increasing retention in care for HIV-infected patients: results of the CDC/HRSA Retention in Care Trial. In: 5th Biennial Conference of the American Society of Health Economists; 2014; Los Angeles, CA. [Google Scholar]
- 57.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making 2002; 22:27–38. [DOI] [PubMed] [Google Scholar]
- 58.Honiden S, Sundaram V, Nease RF et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res 2006; 15:69–82. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep 2011; 60:1618–23. [PubMed] [Google Scholar]
- 60.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2009. Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001419.pdf Accessed 27 May 2014.
- 61.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2012. Atlanta, GA: US Department of Health and Human Services, 2013. [Google Scholar]
- 62.United States Preventive Services Task Force. Screening for HIV. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm Accessed 22 July 2014.
- 63.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Available at: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf Accessed 6 August 2014.
- 64.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996; 276:1253–8. [PubMed] [Google Scholar]
- 65.Gopalappa C, Farnham PG, Hutchinson AB, Sansom SL. Cost effectiveness of the National HIV/AIDS Strategy goal of increasing linkage to care for HIV-infected persons. J Acquir Immune Defic Syndr 2012; 61:99–105. [DOI] [PubMed] [Google Scholar]
- 66.Finlayson TJ, Le B, Smith A et al. HIV risk, prevention, and testing behaviors among men who have sex with men—National HIV Behavioral Surveillance System, 21 U.S. cities, United States, 2008. MMWR Surveill Summ 2011; 60:1–34. [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention. HIV surveillance—United States, 1981–2008. MMWR Morb Mortal Wkly Rep 2011; 60:689–93. [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention. National HIV Prevention Progress Report, 2013. Available at: http://www.cdc.gov/hiv/pdf/policies_NationalProgressReport.pdf Accessed 22 July 2014.
- 69.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data-United States and 6 dependent areas—2011. HIV surveillance supplemental report 2013, 2013. Available at: http://www.cdc.gov/hiv/pdf/2011_monitoring_hiv_indicators_hssr_final.pdf Accessed 1 September 2014.
- 70.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making 2000; 20:332–42. [DOI] [PubMed] [Google Scholar]
- 71.Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med 1998; 13:716–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braithwaite RS, Meltzer DO, King JT Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008; 46:349–56. [DOI] [PubMed] [Google Scholar]
- 73.Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 2010; 19:422–37. [DOI] [PubMed] [Google Scholar]
- 74.US Preventive Services Task Force. Human immunodeficiency virus (HIV) infection: screening. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm Accessed 22 July 2014.
- 75.Paltiel AD, Walensky RP, Schackman BR et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med 2006; 145:797–806. [DOI] [PubMed] [Google Scholar]
- 76.Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis 2014; 59(suppl 1):S21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS 2007; 21(suppl 1):S49–58. [DOI] [PubMed] [Google Scholar]
- 78.HIV Prevention Trials Network. HPTN 065: TLC-Plus: A study to evaluate the feasibility of an enhanced Test, Link to Care, Plus Treat approach for HIV prevention in the United States. Available at: http://www.hptn.org/research_studies/hptn065.asp Accessed 6 August 2014. [DOI] [PMC free article] [PubMed]
- 79.Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care 2009; 21:868–73. [DOI] [PubMed] [Google Scholar]
- 80.Wohl AR, Garland WH, Wu J et al. A youth-focused case management intervention to engage and retain young gay men of color in HIV care. AIDS Care 2011; 23:988–97. [DOI] [PubMed] [Google Scholar]
- 81.Uhlmann S, Milloy MJ, Kerr T et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction 2010; 105:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aggleton P, Clarke D, Crewe M, Kippax S, Parker R, Yankah E. Educating about HIV: prevention, impact mitigation and care. AIDS 2012; 26:1215–22. [DOI] [PubMed] [Google Scholar]
- 83.Andersen M, Hockman E, Smereck G et al. Retaining women in HIV medical care. J Assoc Nurses AIDS Care 2007; 18:33–41. [DOI] [PubMed] [Google Scholar]
- 84.Wolitski RJ, Kidder DP, Pals SL et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav 2010; 14:493–503. [DOI] [PubMed] [Google Scholar]
- 85.Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, Giordano TP. Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care 2013; 25:202–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.