Antiretroviral therapy (ART) timing is more critical than its duration for normalizing CD8 T-cell counts in HIV infection. In addition to CD4 T-cell recovery, early ART may help reduce future risks of non–AIDS-related events by alleviating CD8 T-cell elevation.
Keywords: HIV, primary infection, antiretroviral therapy, CD8 T-cell count, CD4/CD8 ratio
Abstract
Background. CD8 T-cell counts remain elevated in human immunodeficiency virus (HIV) infection even after long-term antiretroviral therapy (ART), which is associated with an increased risk of non–AIDS-related events. We assessed the impact of ART initiation in early versus chronic HIV infection on trajectories of CD8 cell counts over time.
Methods. Of 280 individuals enrolled during primary HIV infection (PHI), 251 were followed up for 24 months; 84 started ART before 6 months of infection (eART), 49 started between 6 and 24 months, and 118 remained untreated. Plasma HIV viral load (VL), CD4 and CD8 cell counts were assessed at each study visit. CD8 counts were also examined in 182 age-matched HIV-infected individuals who started ART during chronic infection and maintained undetectable plasma VL for ≥5 years.
Results. At PHI baseline, higher CD8 cell counts were associated with more recent infection (P = .02), higher CD4 cell counts (P < .001), and higher VL (P < .001). The CD8 count in the eART group decreased from 797 to 588 cells/µL over 24 months (P < .001), to a level lower than that in untreated PHI (834 cells/µL; P = .004) or in long-term–treated patients with chronic HIV infection (743 cells/µL; P = .047). More prominent CD4 T-cell recovery was observed in the eART group than in the delayed ART group.
Conclusions. ART initiated in early HIV infection is associated with improved resolution of CD8 T-cell elevation compared with long-term ART initiated in chronic infection. Early ART may help reduce the risk of non–AIDS-related events by alleviating this elevation.
Human immunodeficiency virus (HIV) infection is featured by profound immune dysfunction and a skewed T-cell homeostasis [1]. In addition to the gradual loss of CD4 T cells in most untreated HIV-infected individuals, elevation of CD8 T-cell counts (hereinafter CD8 counts) persists until the very late phase when T cells are depleted [2, 3]. Robust expansion of both HIV-specific and nonspecific CD8 T cells occurs soon after HIV acquisition. Broader bystander activation has been observed in CD8 than in CD4 T cells, probably through antigen-independent mechanisms, and it persists throughout the course of infection [4, 5]. It is also reported that circulating CD8 T cells exhibit more features of exhaustion and immunosenecence than CD4 T cells over the disease progression, including increased expression of PD-1, CD160, Tim-3, and CD57 [6–9].
Although antiretroviral therapy (ART) achieves a progressive recovery of CD4 T cells in the majority of treated HIV-infected individuals, quantitative and functional defects in CD8 T cells continue to exist even after a decade of effective treatment [10, 11]. The CD8 counts exhibit a modest change with ART initiated in chronic HIV infection but remain consistently elevated with prolonged duration of treatment [12, 13]. Mechanisms underlying CD8 persistence remain unclear and may include immune activation due to residual viral replication, alteration of lymph node architecture, gut mucosal dysfunction, microbial translocation, and T-cell trafficking or redistribution [14–16]. Nevertheless, the unremitting elevation of CD8 counts and the resulting low CD4/CD8 ratio after long-term ART have been associated with an increased non–AIDS-related morbidity and mortality risk independent of CD4 T-cell recovery [12, 13, 17].
Considered a window of opportunity, early ART initiated in primary HIV infection (PHI) especially before 6 months of infection has been associated with decreased seeding in latent reservoirs and an almost back-to-normal level of immune activation [18–20]. However, damage of gut mucosal integrity and elevation of microbial product due to microbial translocation are expected to persist despite early or extremely early ART initiation [15, 21]. It remains unknown whether this early approach could alleviate the ongoing elevation of CD8 counts. In the present study, we examined factors associated with trajectories of CD8 counts in early HIV infection. By comparing individuals receiving early versus delayed ART, we also assessed the impact of timing and duration of ART on elevated CD8 T-cell counts over time.
METHODS
Study Population
The Montreal Primary HIV Infection Study is a prospective cohort study established in 1996 and implemented in 6 university medical centers and 5 private medical centers in Montreal, Quebec, Canada. HIV-1–infected individuals aged ≥18 years with an estimated date of HIV acquisition <180 days were recruited and followed for an intended 24 months. Diagnosis of HIV infection was established based on positivity for p24 antigen and/or detectable HIV RNA, subsequently confirmed by Western blot. In the present study, all participants enrolled in the Montreal PHI study from 1 May 1996 to 31 December 2012 were retrospectively assessed. Participants were evaluated at a maximum interval of 3 months, when clinical and laboratory assessments were conducted including testing for plasma viral load (VL) and CD4 and CD8 counts. The initiation of ART during follow-up was based on CD4 counts and the decision of physicians and participants.
To better understand the effects of ART on T-cell dynamics, we also assessed HIV-1–infected adults receiving care during a similar period (1996–2010) at McGill University Health Centre (MUHC), Montreal, including 182 age-matched individuals who started ART during chronic infection and maintained undetectable VLs for ≥5 years. We used 5 years as an inclusive criterion to allow enough time for CD8 T-cell homeostasis after viral control, because most studies suggest an average of 2–3 years after ART initiation for stabilization of CD8 counts [11, 12]. We examined the CD4 and CD8 counts measured each calendar year after VLs became undetectable. Another 40 age-matched uninfected healthy volunteers enrolled at MUHC were included as uninfected controls. This study was approved by the MUHC Ethics Review Board, and all study subjects provided written informed consent for participation in the study.
Laboratory Methods
Testing for HIV-1 p24 antigen and testing for HIV-1 antibodies with enzyme immunoassay were performed in laboratories of the involved university medical centers. Confirmatory Western blot testing was performed at the Laboratoire de Santé Publique du Québec. The Architect HIV Ag/Ab Combo assay was used for concurrent testing of p24 antigen and HIV-1 antibodies after 2011 (Abbott Laboratories). Plasma VL was measured using the Roche Amplicor HIV Monitor assay (Roche Diagnostics) or the Abbott RealTime HIV-1 assay (Abbott Laboratories), depending on the time of testing. Virological suppression was defined as undetectable if plasma VL was <50 copies/mL with the Roche or <40 copies/mL with the Abbott assay. Absolute CD4 and CD8 counts were determined using 4-color flow cytometry performed with a FACSCalibur cytometer (Becton-Dickinson Immunocytometry Systems) as reported elsewhere [22].
Statistical Analyses
Statistical analyses were performed using SPSS 22.0 (SPSS) and GraphPad Prism 6.0 (GraphPad Software). Summary statistics were used to describe the study samples. Univariate linear regression analysis was conducted to examine factors potentially associated with CD8 counts at baseline. All variables from univariate analyses were included in a multivariate linear regression model.
For follow-up analyses, time was calculated from the date of ART initiation or study entry until the date of measurement (±15 days). Correlations between CD8 counts and VLs were assessed with Spearman rank correlation. All tests were 2 sided, and differences were considered statistically significant at P < .05. For further comparisons between N groups, differences were considered significant at P < .05/N(N − 1).
RESULTS
Study Population
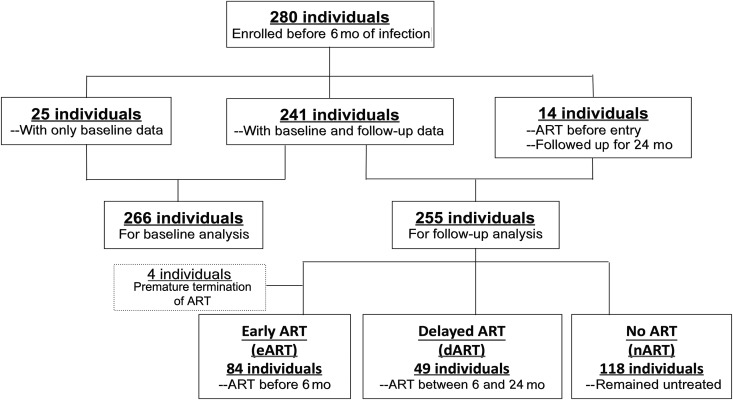
A total of 280 individuals were enrolled in the Montreal PHI study from May 1996 to December 2012 (Figure 1); 266 were included in the baseline analysis, because the other 14 had started ART before study entry. Of those in the baseline analysis, 246 (92.5%) were white, 254 (95.5%) were men, and 207 (77.8%) were men who have sex with men; the mean (standard deviation [SD]) age was 36.1 (9.4) years. Participants were recruited at a median (interquartile range [IQR]) of 82 (56–121) days after the estimated date of infection (EDI), including 40 patients (15%) in Fiebig stage II–III, 81 (30.5%) in stage IV, and 145 (54.5%) in stage V–VI [23]. The mean (SD) VL at baseline was 4.59 (1.07) log copies/mL, and the median (IQR) CD4 count was 500 (380–658) cells/µL.
Figure 1.
Flow diagram illustrating enrollment of participants with primary human immunodeficiency virus infection. ART, antiretroviral therapy.
For follow-up analysis, 251 participants with PHI were included; 84 (33.5%) had started ART within 6 months after EDI (eART) and 49 (19.5%) between 6 and 24 months (dART), and the other 118 (47.0%) remained untreated over the follow-up (no ART [nART]) (Figure 1 and Table 1). Twenty-nine individuals were excluded from this analysis owing to lack of CD8 data (n = 25) or premature termination of treatment followed by a viral rebound (n = 4). The median time of ART initiation was day 81 after EDI in the eART group, and day 436 in the dART group. A total of 2648 CD8 counts were measured for these participants over a median (IQR) follow-up of 24 (15.6–24) months. Age, sex, VLs, and CD8 counts at entry were comparable between the 3 groups. However, the eART group had lower CD4 counts (P < .001) and lower CD4/CD8 ratios (P < .01) than the other 2 groups, partly accounting for early ART initiation in this group.
Table 1.
Characteristics of Participants with Primary HIV Infection or Chronic Human Immunodeficiency Virus Infection and Uninfected Controls
| Characteristic | Participants With PHI |
Chronic Infection, Long-term ART (n = 182) |
Uninfected Controls (n = 40) |
||
|---|---|---|---|---|---|
| eART (n = 84) | dART (n = 49) | nART (n = 118) | |||
| Age, mean (SD), y | 37.0 (9.7) | 36.0 (9.8) | 36.0 (9.0) | 42.2 (5.3) | 45.4 (7.4) |
| Sex, male (%) | 78 (92.9) | 47 (95.9) | 114 (96.6) | 157 (86.3) | 27 (67.5) |
| Interval between EDI and enrollment, median (IQR), d | 71 (49–108) | 96 (76–145) | 91 (66–135) | … | … |
| Interval between EDI and ART initiation, median (IQR), d | 81 (57–133) | 436 (302–642) | … | … | … |
| Interval between enrollment and ART initiation, median (IQR), d | 9 (0–28) | 357 (187–526) | … | … | … |
| VL and T-cell measurementsa | |||||
| HIV VL, mean (SD), log copies/mL | 4.67 (1.30) | 4.53 (0.91) | 4.24 (0.94) | Undetectable | … |
| T-cell count, median (IQR), cells/µL | |||||
| CD8 | 770 (532–1343) | 780 (660–1450) | 825 (605–1152) | 743 (600–926) | 376 (256–458)b |
| CD4 | 403 (305–610)c | 510 (407–600) | 565 (460–735) | 539 (431–677) | 858 (570–1000)b |
| CD4/CD8 ratio, median (IQR) | 0.54 (0.26–0.86)c | 0.61 (0.36–0.79) | 0.68 (0.47–1.00) | 0.75 (0.56–1.03) | 2.1 (1.81–3.02)b |
Abbreviations: ART, antiretroviral therapy; dART, delayed ART (initiated 6–24 months after EDI); eART, early ART (initiated within 6 months after EDI); EDI, estimated date of infection; HIV, human immunodeficiency virus; IQR, interquartile range; nART, no ART during follow-up; PHI, primary HIV infection; SD, standard deviation; VL, viral load.
a In the PHI groups, these values were measured at enrollment; in the individuals with chronic infection, in the fifth year with undetectable VLs.
b The control group had higher CD4 and CD8 cell counts and higher CD4/CD8 ratio (all P < .001) than all the infected groups.
c The eART group had lower CD4 cell counts (P < .001) and CD4/CD8 ratios (P < .01) than the dART and nART groups.
We also assessed 182 age-matched individuals receiving long-term ART initiated during chronic HIV infection. These individuals have been treated for a median (IQR) of 8 (6–11) years, and maintained undetectable VLs for ≥5 years (median, 6 years; range, 5–8 years). Their demographic characteristics and T-cell measurements at the fifth year with undetectable VLs are shown in Table 1.
The uninfected control group included 27 men and 13 women with a mean (standard deviation) age of 45.4 (7.4) years. Compared with the HIV-infected individuals, they had higher CD4 counts, lower CD8 counts, and higher CD4/CD8 ratios, as expected (Table 1).
Baseline CD8 Counts in PHI Group
The distribution of CD8 counts in 266 participants with PHI at baseline is summarized in Table 2. Overall, the median CD8 count at baseline was 800 cells/µL, remarkably higher than that in the uninfected controls (376 cells/µL; P < .001). In the univariate analysis, white ethnicity, higher plasma VLs, and higher CD4 counts were associated with higher CD8 counts before the start of ART. However, in multivariate analysis with all factors in Table 1 included, CD8 counts were much higher with more acute and recent infection (Fiebig stage II/III; P = .02). Higher CD8 counts were also associated with higher VLs (P < .001) and higher CD4 counts (P < .001) in multivariate analysis. Otherwise, CD8 counts were comparable in the adjusted analysis despite differences in ethnicity, age, sex, route of infection, coinfection with hepatitis B or C virus, or cytomegalovirus seropositivity.
Table 2.
Univariate and Multivariate Analyses of Factors Associated With CD8 T-Cell Counts for Individuals With Primary Human Immunodeficiency Virus Infection at Baseline
| Factor | Participants, No. (%) | CD8 T-Cell Count, Median (IQR), cells/µLa |
P Value |
|
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Overall | 266 | 800 (598–1265) | … | … |
| Race/ethnicity | ||||
| White | 246 (92.5) | 817 (600–1284) | .02 | .17 |
| Nonwhite | 20 (7.5) | 720 (549–992) | ||
| Age, y | ||||
| <50 | 247 (92.9) | 826 (600–1265) | .77 | .99 |
| ≥50 | 19 (7.1) | 710 (566–1211) | ||
| Sex | ||||
| Male | 254 (95.5) | 824 (600–1281) | .64 | .31 |
| Female | 12 (4.5) | 715 (627–1076) | ||
| Presumed route of infection | ||||
| MSM | 207 (77.8) | 853 (602–1417) | .15 | .82 |
| Heterosexual | 21 (7.9) | 660 (560–830) | ||
| IDU | 38 (14.3) | 790 (594–1156) | ||
| Fiebig staging | ||||
| II/III | 40 (15.0) | 935 (709–1790) | .12 | .02 |
| IV | 81 (30.5) | 810 (557–1470) | ||
| V/VI | 145 (54.5) | 787 (607–1148) | ||
| HIV RNA load, log copies/mL | ||||
| <3 | 19 (7.1) | 650 (525–794) | <.001 | <.001 |
| 3–5 | 152 (57.1) | 782 (592–1088) | ||
| >5 | 95 (35.7) | 1140 (702–1783) | ||
| CD4 T-cell count, cells/µL | ||||
| <500 | 124 (46.6) | 740 (549–1140) | .005 | <.001 |
| ≥500 | 142 (53.4) | 880 (665–1450) | ||
Abbreviations: HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men.
a CD8 T-cell counts did not differ significantly by coinfection status—including cytomegalovirus seropositivity (P = .35), hepatitis C virus coinfection (P = .43), and hepatitis B virus coinfection (P = .13)—or by time of enrollment (P = .33).
Trajectories of CD4 and CD8 Counts and CD4/CD8 Ratios in Early-Treated Individuals With PHI
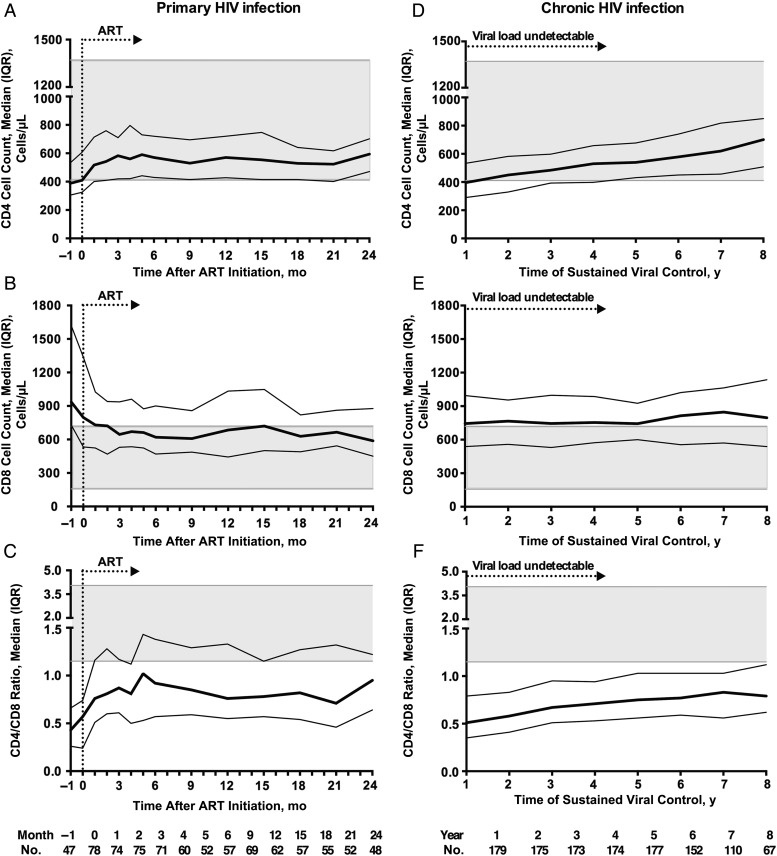
After 24 months of ART, mean (SD) VLs in the eART group decreased from 4.67 (1.30) to 1.89 (0.61) log copies/mL. Sixty-eight of the 84 individuals had their VLs tested at 24 months, with undetectable VLs achieved in 54. Changes in T-cell counts and ratio in the eART group over time are depicted in Figure 2A–C.
Figure 2.
Trajectories of T-cell counts over time in early-treated primary human immunodeficiency virus (HIV) infection and chronic infection with long-term treatment. Values represent medians with interquartile ranges (IQRs); the 5th–95th percentiles of values for the control population are shaded gray. In primary HIV infection, when antiretroviral therapy (ART) was initiated within 6 months of infection, CD8 cell counts were prominently decreased at 24 months compared with the level at ART initiation (B; P = .01) and fell within the upper limit of CD8 counts for normal controls. CD4 cell counts (A; P = .002) and CD4/CD8 ratios (C; P < .001) were significantly increased compared with when treatment started. In patients with chronic infection receiving long-term treatment, CD4 cell counts (D) and CD4/CD8 ratio (F) continued to increase over the 8 years with sustained viral suppression. However, CD8 counts (E) remained stable over time and were higher than those in the early ART group at 24 months of treatment.
CD8 counts underwent a median (IQR) change of 1 (−156 to 121) cells/µL during the month before treatment. The median (IQR) CD8 count before ART was 797 (533–1338) cells/µL, which decreased to 588 (450–877) cells/µL (P = .01) by 24 months of treatment, with a median change of −170 (−560 to −29) cells/µL). The decline in CD8 counts was most prominent during the first month after ART initiation (median [IQR] change, −73 [−347 to 22] cells/µL). After the first 3 months, CD8 counts became relatively stable, with the median fluctuating within 600 and 700 cells/µL. At 24 months, CD8 counts in the eART group were lower than those of the long-term–treated individuals with chronic infection (743 cells/µL at the fifth year with undetectable VLs; P = .01), although they still remained elevated compared with the uninfected controls (median, 376 cells/µL; P < .001).
Meanwhile, the median CD4 count in the eART group increased from a median (IQR) of 410 (325–607) cells/µL to 594 (471–702) cells/µL (P = .002) over 24 months, with a median change of 87 (I−21 to 257) cells/µL. At 24 months, CD4 counts in this group were similar to those in the chronic infection, long-term ART group at the fifth year with undetectable VLs (median, 534 cells/µL; P = .63), but were still lower than in the uninfected group (median, 858 cells/µL; P = .003). The median [IQR] CD4/CD8 ratio increased from 0.47 (0.22–0.75) before ART to 0.95 (0.62–1.22; P < .001) at 24 months, which was slightly higher than that of the chronic infection, long-term ART group at the fifth year with undetectable VLs (median, 0.75; P = .08), but remained lower than in the uninfected group (median, 2.1; P < .001).
Changes in CD4 and CD8 Counts and CD4/CD8 Ratios in Long-term–Treated Individuals With Chronic Infection
Dynamics of CD4 and CD8 counts and CD4/CD8 ratios in the context of undetectable VLs after long-term ART are also depicted in Figure 2D–F. The median CD4 count in these individuals increased from 397 to 578 cells/µL over the 8 years with undetectable VLs (P < .001). Specifically, since the fourth year, CD4 counts in the chronic infection, long-term ART group had become comparable to those of early-treated individuals. Meanwhile, due to the recovery in CD4 T cells, the CD4/CD8 ratio increased from 0.67 to 0.77 over 8 years (P < .001), to a level slightly lower than that in the eART group at 24 months (P = .08). In contrast, CD8 counts in these individuals remained rather stable over as long as 8 years of successful viral control and were constantly higher than those of the uninfected controls (P < .001) or the eART group at 24 months (P = .047).
Trajectories of CD4 and CD8 Counts and CD4/CD8 Ratio in Individuals With PHI
Trajectories of CD4 and CD8 counts in the eART, dART, and nART groups over time are shown in Figure 3. CD8 counts remained relatively stable in untreated individuals, in contrast to the decline in both eART and dART groups. Interestingly, although ART was started at different time points in the eART and dART groups, only a modest difference in CD8 counts was observed between the 2 at 24 months (median, 627 and 668 cells/µL, respectively). In both treated PHI groups, CD8 counts were lower than those in the chronic infection, long-term ART group and fell within the limits of normal for the uninfected controls.
Figure 3.
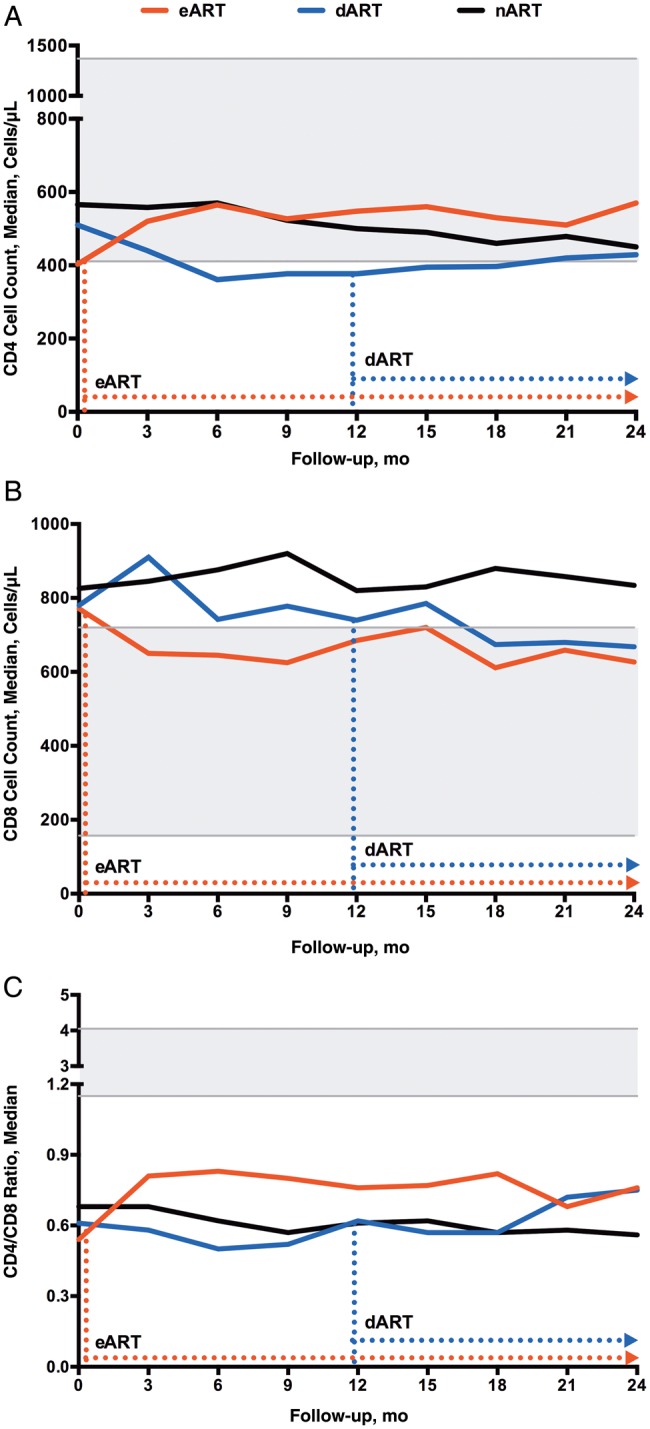
Trajectories of CD4 (A) and CD8 (B) T-cell counts and CD4/CD8 ratios (C) over time in primary human immunodeficiency virus (HIV) infection. Dashed arrows show the median time of antiretroviral therapy (ART) initiation in the early ART (eART) (red) or delayed ART (dART) (blue) group. The 5th–95th percentiles of values in the control population are shaded gray. A, CD4 cell counts in the eART group were higher than in the dART (P = .048) or no-ART (nART) (P = .01) group at 24 months. B, CD8 cell counts in the nART group remained stable; at 24 months, CD8 counts in the eART group were lower than in the nART group (P = .004) though still elevated compared with the control group (P < .001). C, CD4/CD8 ratios in the nART group declined over time; those in the eART group were higher than in the nART group (P < .001) at 24 months.
Compared with the other 2 PHI groups, the eART group achieved the most prominent CD4 increase at 24 months (median, from 403 to 570 cells/µL; P < .001). In contrast, the dART and nART groups underwent a gradual loss in CD4 T cells over time. Of note, the decrease was so prominent in the dART group (lowest point at 6 months, 361 cells/µL) that even the eventual initiation of ART for approximately 1 year did not reconstitute its baseline level.
Regarding the CD4/CD8 ratio, increases were observed in both eART and dART groups; the medians (IQRs) at 24 months were 0.76 (0.58–1.22) and 0.75 (0.53–0.91), respectively. The eART group exhibited a more significant increase as a result of concurrent CD4 T-cell recovery and CD8 T-cell reduction (P < .001). However, all the infected individuals, even the early-treated ones, continued to display a decreased ratio compared with the uninfected controls.
We also assessed CD8 counts by VLs at baseline and at 12 and 24 months in individuals with PHI. At baseline, CD8 counts were strongly correlated to VL in all PHI groups. However, at the end of 24 months, no significant correlation was observed between CD8 counts and VLs for all 3 groups (Supplementary Table 1).
DISCUSSION
The dynamic of CD8 counts during PHI remains poorly studied, especially in the context of early ART initiation. In this study, we examined trajectories of CD8 counts in individuals with acute and early HIV infection, with or without early ART initiation. We found that CD8 counts were markedly increased in untreated PHI. Early ART initiated in PHI was associated with a significant decrease in CD8 counts coupled with VL reduction and CD4 T-cell recovery, which was even more prominent when ART was initiated within 6 months of infection. In contrast, although prolonged ART initiated in chronic HIV infection was associated with progressive recovery in CD4 counts and CD4/CD8 ratio, CD8 counts in these individuals remained persistently elevated and were higher than those observed with ART initiation in PHI.
Elevation and expansion of CD8 T cells occurs from the very early days of HIV infection, as a general situation in viral infections such as Epstein-Barr virus and cytomegalovirus infections. During the acute phase, the robust expansion of CD8 T cells, particularly in the viral-specific subsets, almost always indicates a strong immune reaction followed by a rapid control of viremia, as suggested by our cohort, in which higher CD8 counts were also associated with higher CD4 counts in acute infection. However, the HIV-specific CD8 T cells rapidly undergo exhaustion and senescence during the course of HIV infection, while the circulating bystander CD8 T cells remain elevated, representing the major part of CD8 persistence. Although long-term ART can induce a contraction in the CD8 compartment, normalization of CD8 counts is achieved in only a small proportion of individuals, as observed in our long-term–treated patients with chronic HIV infection and in other studies [10, 17].
Unlike the situation in chronic HIV infection, when ART was initiated early in PHI, we observed a rapid decrease in CD8 counts to a level within the upper limit of normal for uninfected persons. In addition to this quantitative improvement, previous studies indicated that some of the CD8 functional defects could also be partially reversed by early treatment [24, 25]. Therefore, early initiation of ART is probably more promising than prolonged duration for the normalization of CD8 counts, which may further contribute to reducing future risks of non–AIDS-related events. Our result is consistent with previous reported association between CD4/CD8 ratio and ART timing [13]. However, we further demonstrated that CD8 persistence might be more responsible for the long-term dynamics of the CD4/CD8 ratio, given that optimal CD4 count recovery has been achieved in the majority of treated HIV-infected individuals.
Although the decline of CD8 counts occurred soon after ART initiation in individuals with PHI, it gradually slowed down, leading to relatively stable CD8 counts for the rest of follow-up. Similar findings have been reported when ART is started during chronic HIV infection [12, 13]. Although our study design does not allow further assessment of CD8 T-cell trends with prolonged early ART, some early-treated participants with PHI continue to be followed up in our center. Available data based on a median (IQR) follow-up of 5 (3–6) years showed no further significant changes in CD8 counts compared with levels documented at 2 years. Based on these observations, we propose that before the very late stage of infection, elevation of circulating CD8 T cells in HIV infection may contain 2 major compartments: a fast-responsive compartment, which responds and decays rapidly on initiation of effective ART, and a persistent compartment, which may endure even with prolonged ART. Progression of HIV infection may lead to accumulation in the persistent compartment over time, and ART initiated at different time points may result in different levels of CD8 persistence. Furthermore, it is possible that expansion of the persistent compartment is limited in early infection, because similar levels of post-ART CD8 counts were observed in our study even when treatment was initiated 1 year apart in PHI.
Most probably, the fast-responsive compartment mainly includes activated CD8 T cells such as the CD38+/HLA-DR+ subsets, which are associated with viral antigenic stimulation. In one of our recent studies, CD8 T-cell activation (CD38+/HLA-DR+) was examined longitudinally in selected participants from the eART and nART groups [15]. Compared with the elevated level of CD8 activation in the untreated individuals, the frequencies of activated CD8 T cells rapidly normalized after ART initiation, which corresponds to the partial recovery of CD8 counts on treatment. In contrast, the persistent compartment may have a more profound origin, including gut mucosal damage, T-cell exhaustion, or acquired “immunoaging,” which persist even after early initiated ART [15, 26]. The impaired distribution and homing capacity of T cells may also contribute to the CD8 persistence [27]. However, the situation would be quite different in late stage of infection with overwhelming destruction of T cells, in which ART induced viral suppression will first lead to reconstitution of both CD4 and CD8 T cells, as reported by us and others [12, 28].
Although a definite improvement in CD8 counts was observed in early-treated individuals with PHI, our study has several limitations as a nonrandomized retrospective study. With growing evidence showing the benefit of early ART, it will be increasingly difficult to observe and follow up persons with early infection as per randomization. Nevertheless, the similar shapes of T-cell curves after ART initiation indicated the impact of ART on CD8 counts. Our PHI cohort was composed mainly of young men who have sex with men, and outcomes described here may be different for groups with older or female subjects. However, as also indicated by other studies, the effects of age and sex may be minimal in terms of longitudinal assessment.
In summary, timing rather than duration of ART seems be more critical for normalization of CD8 counts in HIV infection. In addition to the recovery of CD4 T cells and delay in disease progression, ART initiation during PHI may further contribute to reducing future non–AIDS-related morbidity and mortality risk by alleviating CD8 elevation. Our findings bring more insights into the underlying mechanisms for CD8 T-cell dynamics in HIV infection. More research should be conducted on CD8 T-cell persistence in the context of treated infection, assessing in-time contributions of viral persistence, T-cell homing, trafficking, and turnover in circulating blood and, more importantly in tissues such as lymph nodes and gut mucosa.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the following physicians for collaboration in screening, recruiting, and following up participants with primary or chronic HIV infection: Drs S. Vézina, L. Charest, M. Milne, E. Huchet, S. Lavoie, J. Friedman, M. Duchastel, and F. Villielm, at l'Actuel Medical Clinic; P. Côté, M. Potter, B. Lessard, M. A. Charron, S. Dufresne, and M. E. Turgeon, at Quartier Latin Medical Clinic; Drs D. Rouleau, L. Labrecque, C. Fortin, A de Pokomandy, V. Hal-Gagné, M. Munoz, B. Deligne, and V. Martel-Laferrière, at UHRESS CHUM Hôtel-Dieu and Notre-Dame; and N. Gilmore, M. Fletcher, and J. Szabo, at MUHC Chest Institute. We thank Mario Legault and Costas Pexos for database management; Angie Massicotte for coordination and assistance in manuscript writing; Jacquie Sas and Jim Pankovich, from the Canadian Institutes of Health Research (CIHR) Canadian HIV Trials Network (CTN), for coordinating the international research collaboration between MUHC and Peking Union Medical College Hospital with the support of CIHR/CTN.
Financial support. This work was supported by the Fonds de la Recherche Québec-Santé (FRQ-S): Thérapie Cellulaire and Réseau SIDA/Maladies Infectieuses, the Canadian Institutes of Health Research (grants MOP 103230 and CTN 257), the Canadian Foundation for AIDS Research (CANFAR; grant 023–512), and the Canadian HIV Cure Enterprise Team (grant HIG-133050 from the CIHR in partnership with CANFAR). W. C. is supported by a CTN postdoctoral fellowship award, V. M. is supported by an FRQ-S postdoctoral fellowship award, and J. P. R. holds the Louis Lowenstein Chair in Hematology & Oncology at McGill University.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: the Montreal Primary HIV Infection Study Group, S. Vézina, L. Charest, M. Milne, E. Huchet, S. Lavoie, J. Friedman, M. Duchastel, F. Villielm, P. Côté, M. Potter, B. Lessard, M. A. Charron, S. Dufresne, M. E. Turgeon, D. Rouleau, L. Labrecque, C. Fortin, A. de Pokomandy, V. Hal-Gagné, M. Munoz, B. Deligne, V. Martel-Laferrière, N. Gilmore, M. Fletcher, and J. Szabo
References
- 1.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lempicki RA, Kovacs JA, Baseler MW et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc Natl Acad Sci U S A 2000; 97:13778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellerstein M, Hanley MB, Cesar D et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med 1999; 5:83–9. [DOI] [PubMed] [Google Scholar]
- 4.Bastidas S, Graw F, Smith MZ, Kuster H, Gunthard HF, Oxenius A. CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. J Immunol 2014; 192:1732–44. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasula S, Lempicki RA, Adelsberger JW et al. Differential effects of HIV viral load and CD4 count on proliferation of naive and memory CD4 and CD8 T lymphocytes. Blood 2011; 118:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peretz Y, He Z, Shi Y et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS Pathog 2012; 8:e1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, Karandikar NJ, Betts MR et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003; 101:2711–20. [DOI] [PubMed] [Google Scholar]
- 8.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep 2010; 7:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercier F, Boulassel MR, Yassine-Diab B et al. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7R∝ on central and effector memory CD+ and CD8+ T cell subsets. Clin Exp Immunol 2008; 152:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emu B, Moretto WJ, Hoh R et al. Composition and function of T cell subpopulations are slow to change despite effective antiretroviral treatment of HIV disease. PLoS One 2014; 9:e85613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. T-cell subset distribution in HIV-1-infected patients after 12 years of treatment-induced viremic suppression. J Acquir Immune Defic Syndr 2012; 61:270–8. [DOI] [PubMed] [Google Scholar]
- 12.Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. J Infect Dis 2015; 211:1726–34. [DOI] [PubMed] [Google Scholar]
- 13.Serrano-Villar S, Sainz T, Lee SA et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano-Villar S, Gutierrez C, Vallejo A et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect 2013; 66:57–66. [DOI] [PubMed] [Google Scholar]
- 15.Jenabian MA, El-Far M, Vyboh K et al. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015; 212:355–66. [DOI] [PubMed] [Google Scholar]
- 16.Catalfamo M, Wilhelm C, Tcheung L et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol 2011; 186:2106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mussini C, Lorenzini P, Cozzi-Lepri A et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 18.Strain MC, Little SJ, Daar ES et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005; 191:1410–8. [DOI] [PubMed] [Google Scholar]
- 19.Jain V, Hartogensis W, Bacchetti P et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013; 208:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W, Mehraj V, Vyboh K, Li T, Routy JP. Antiretroviral therapy in primary HIV-1 infection: Influences on immune activation and gut mucosal barrier dysfunction. AIDS Rev 2015; Accepted. [PubMed] [Google Scholar]
- 21.Utay J, Ananworanich J, Slike B et al. Inflammation persists despite early initiation of ART in acute HIV infection [abstract 47]. Presented at: 22nd Conference on Retroviruses and Opportunistic Infections; 23–24 February 2015; Seattle, WA. [Google Scholar]
- 22.Boulassel MR, Mercier F, Gilmore N, Routy JP. Immunophenotypic patterns of CD8+ T cell subsets expressing CD8∝ and IL-7R∝ in viremic, aviremic and slow progressor HIV-1-infected subjects. Clin Immunol 2007; 124:149–57. [DOI] [PubMed] [Google Scholar]
- 23.Fiebig EW, Wright DJ, Rawal BD et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. [DOI] [PubMed] [Google Scholar]
- 24.Plana M, Garcia F, Gallart T et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS 2000; 14:1921–33. [DOI] [PubMed] [Google Scholar]
- 25.Lee SA, Sinclair E, Jain V et al. Low proportions of CD28− CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis 2014; 210:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolz JC, Harty JT. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest 2014; 124:1013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015; 18:20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.