In human immunodeficiency virus–hepatitis C-coinfected, both protease inhibitor- and nonnucleoside reverse transcriptase inhibitor-based regimens were associated with progression of liver fibrosis over time when paired with a backbone of abacavir/lamivudine but not tenofovir/emtricitabine.
Keywords: HIV, hepatitis C, liver fibrosis, APRI, combination antiretroviral therapy
Abstract
Background. Liver diseases progress faster in human immunodeficiency virus (HIV)–hepatitis C virus (HCV)-coinfected persons than HIV-monoinfected persons. The aim of this study was to compare rates of liver fibrosis progression (measured by the aspartate-to-platelet ratio index [APRI]) among HIV-HCV–coinfected users of modern protease inhibitor (PI)- and nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens with a backbone of tenofovir/emtricitabine (TDF/FTC) or abacavir/lamivudine (ABC/3TC).
Methods. Data from a Canadian multicenter cohort study were analyzed, including 315 HCV polymerase chain reaction–positive persons who initiated antiretroviral therapy with a PI or NNRTI and a backbone containing either TDF/FTC or ABC/3TC. Multivariate linear regression analyses with generalized estimating equations were performed after propensity score matching to balance covariates across classes of anchor agent.
Results. A backbone of TDF/FTC was received by 67% of PI users and 69% of NNRTI users. Both PI and NNRTI use was associated with increases in APRI over time when paired with a backbone of ABC/3TC: 16% per 5 years (95% confidence interval [CI], 4%, 29%) and 11% per 5 years (95% CI, 2%, 20%), respectively. With TDF/FTC use, no clear association was found among PI users (8% per 5 years, 95% CI, −3%, 19%) or NNRTI users (3% per 5 years, 95% CI, −7%, 12%).
Conclusions. Liver fibrosis progression was more influenced by the backbone than by the class of anchor agent in HIV-HCV–coinfected persons. Only ABC/3TC-containing regimens were associated with an increase of APRI score over time, regardless of the class of anchor agent used.
With improvements in combination antiretroviral therapy (cART), the life expectancy of human immunodeficiency virus (HIV)–infected persons approaches that of the general population [1], resulting in long-term cART exposure and the potential for cART-related liver damage. HIV–hepatitis C virus (HCV)-coinfected persons experience more rapid progression of liver disease than HIV monoinfected persons [2]. However, to date, only a small proportion of coinfected persons have undergone HCV treatment, and liver damage may persist despite a cure. It is therefore essential to understand whether specific classes of cART agents are harmful in order to minimize the risk of additional liver disease in this population.
Both acute and long-term hepatotoxicities have been associated with protease inhibitors (PIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs). The metabolic effects of PIs, including increases in lipids, insulin resistance, and deposition of free fatty acids in the liver, could lead to steatosis and inflammation [3]. These metabolic changes are not commonly associated with NNRTI use, although efavirenz can cause lipid changes [4]. In several studies, PI-associated acute hepatotoxicity of HIV-infected persons has been reported, with or without HCV coinfection [5–11]. In coinfected persons, cumulative exposure to PIs was associated with liver steatosis [12]. However, lower risks of fibrosis [13, 14] and cirrhosis [15] and slower fibrosis progression rates [13–15] were reported when PI-based regimens were compared with either absence of treatment or mono/dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). Elevated risks of long-term hepatotoxicity have also been reported with NNRTI use [13, 16].
The inclusion of dideoxynucleoside-containing backbones complicates interpretation of the results of early studies as these NRTIs are highly disruptive of mitochondrial function and are associated with steatosis [17]. Currently, the recommended NRTI backbone combinations are tenofovir/emtricitabine (TDF/FTC) or abacavir/lamivudine (ABC/3TC) [18]. While these modern backbones have low levels of mitochondrial toxicity [17] and are not generally considered hepatotoxic, there is limited information on their long-term impact on liver fibrosis [18].
No long-term studies of modern cART regimens and liver disease in coinfected persons are available to indicate the long-term hepatic safety of PIs and NNRTIs. Our objective in this study was to assess the progression of liver damage among HIV-HCV–coinfected users of modern PI-based and NNRTI-based cART regimens, taking into account the backbone used. We sought to determine if either class of “anchor agent” (eg, PI or NNRTI) is associated with accelerating liver fibrosis.
METHODS
Study Population and Analytical Sample
The Canadian Co-infection Cohort (CCC) study is a multicenter cohort of HIV-HCV–coinfected persons followed every 6 months beginning in 2003. As of 1 July 2014, 1321 persons had been enrolled from 18 clinics in Canada. All participants are adults who have documented HIV infection, evidence of HCV infection, and have provided informed consent. The cohort has been described in greater detail elsewhere [19].
We included in the analyses all HCV polymerase chain reaction–positive persons who initiated cART with either a first-line PI or NNRTI as the anchor agent with a TDF/FTC or ABC/3TC backbone [18]. Although some individuals initiated cART prior to cohort entry, only those who had remained on the same class of anchor agent between cART initiation and cohort entry were eligible. We excluded those with chronic hepatitis B infection (because it increases the risk of liver-related outcomes and these individuals are preferentially prescribed TDF/FTC) and those with a history of dideoxynucleoside use. Person-time was censored after initiation of HCV treatment for those receiving treatment during follow-up, as HCV therapy can affect the platelet count and influence the liver fibrosis measure.
We created a propensity score–matched sample to minimize preexisting imbalances in selected covariates between PI and NNRTI users, thus reducing confounding. For example, the choice of anchor agent is closely related to the choice of backbone and to certain risk factors for liver disease. Propensity score matching can alleviate this concern by making the PI and NNRTI users more similar with respect to these characteristics. This score is obtained with logistic regression by calculating the predicted probability of initiating cART with an NNRTI vs a PI. The model included baseline values for age, sex, HCV duration, alcohol and injection drug use (IDU), income less than 1500 CAN$, CD4 cell count, HIV RNA <50 copies/mL, years since cART initiation, and the backbone used. Each individual was matched with replacement based on his or her propensity score using the nearest-neighbor approach [20].
Antiretroviral Use and Aspartate-to-Platelet Ratio Index Score Measurement
Information on current and past antiretroviral drugs was collected at the first study visit. At each follow-up visit, study coordinators recorded regimen changes. This information was validated with medical or pharmacy records. Chart reviews were conducted to collect additional information on the initiation and discontinuation date for each drug used before cohort entry.
Liver fibrosis was measured at each study visit with the aspartate-to-platelet ratio index (APRI) score, calculated using the aspartate aminotransferase (AST) levels and platelet count as APRI = 100[AST/upper limit of normal]/platelet count (109/L). The natural logarithm of this score was used as a continuous outcome to normalize its distribution [21, 22].
Statistical Analyses
We estimated the rate of change in ln(APRI) among those who initiated cART with a PI or NNRTI, using the anchor class at initiation as the exposure. This intention-to-treat analysis was selected to obtain the effect of initiating a new regimen.
We performed multivariate linear regression with generalized estimating equations to account for the correlated nature of the longitudinal measures. Frequency weights corresponding to the number of times each individual was matched to another were included in the model to account for certain individuals being selected more than once [20]. Years since cART initiation and the interaction term between time and NNRTI vs PI use served to estimate the average rates of change in ln(APRI) among PI and NNRTI users. The model was adjusted for the backbone used, age, sex, and years since HCV infection at cohort entry. We further adjusted for time-updated alcohol use in the previous 6 months, HIV RNA <50 copies/mL, and CD4 cell count at the previous visit.
NNRTI users are more likely to use TDF/FTC than PI users due to the availability of an efavirenz-TDF/FTC coformulation. We therefore explored the potential role of the backbone in fibrosis progression by adding an interaction term between time and TDF/FTC use to the model described above.
For both models, the correlation structure was selected based on the model fit, measured by the quasi-likelihood under the independence model criterion [23]. To handle missing data, multiple imputation implemented with chained equations was used to create 10 imputed datasets, using Rubin's rule to combine standard errors [24]. Because changes in ln(APRI) are difficult to interpret clinically, the coefficients obtained were exponentiated to represent the median change in APRI score on the multiplicative scale (percent change).
RESULTS
Study Population Characteristics
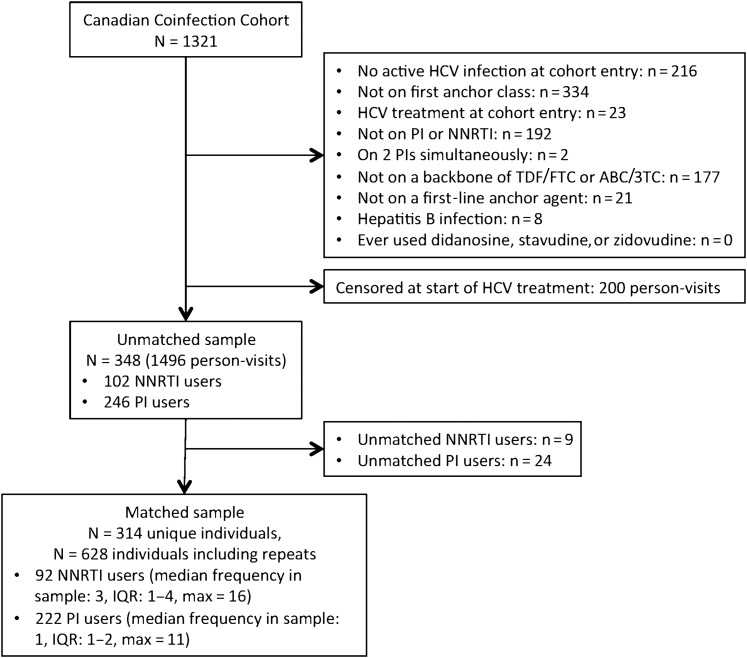
After matching, the sample consisted of the equivalent of 628 persons divided equally between NNRTI and PI users (Figure 1). Forty-one persons initiated HCV treatment during follow-up. Demographic and clinical characteristics at cohort entry are detailed in Table 1 for PI and NNRTI users before and after matching. Before matching, baseline imbalances were observed between PI and NNRTI users, notably in IDU, alcohol use, HIV RNA <50 copies/mL, and TDF/FTC use. These imbalances were reduced after matching on the propensity score. The majority (92%) of NNRTI users initiated cART with efavirenz, with the remaining receiving nevirapine (5%) or rilpivirine (3%). The most frequently used PIs were atazanavir/ritonavir (47%) and lopinavir/ritonavir (29%). Darunavir/ritonavir was used by 15% and atazanavir alone by 9%.
Figure 1.
Inclusion of participant in the study population. Abbreviations: ABC/3TC, abacavir/lamivudine; HCV, hepatitis C virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF/FTC, tenofovir/emtricitabine.
Table 1.
Demographic and Clinical Characteristics and Antiretroviral Use of the Participants at Cohort Entry, Stratified by Class of Anchor Agent Used in the Unmatched and Matched Samples
| Characteristic | Unmatched Sample |
Matched Sample |
||
|---|---|---|---|---|
| PI Users n (%) or Median (IQR) | NNRTI Users n (%) or Median (IQR) | PI Users n (%) or Median (IQR) | NNRTI Users n (%) or Median (IQR) | |
| Demographic characteristics at cohort entry | ||||
| Participants | 246 | 102 | 222 | 92 |
| Participants (including repeats) | NA | NA | 314 | 314 |
| Person-visits | 1019 | 477 | 927 | 443 |
| Person-visits (including repeats) | NA | NA | 1314 | 1409 |
| Frequency in the sample | NA | NA | 1 (1–2) | 3 (1–4) |
| Calendar year at cohort entry | 2009 (2008, 2012) | 2009 (2008, 2012) | 2009 (2008, 2012) | 2009 (2009, 2012) |
| Number of study visits | 3 (2, 6) | 4 (2, 7) | 3 (2, 6) | 4 (2, 7) |
| Male | 180 (73) | 78 (76) | 225 (72) | 210 (67) |
| Age at cohort entry, y | 45 (39–50) | 44 (38–49) | 45 (38–49) | 44 (39–50) |
| Monthly income of 1500 $CAN or lower | 192 (78) | 77 (75) | 237 (75) | 261 (83) |
| Homeless | 29 (12) | 7 (7) | 42 (13) | 21 (7) |
| Alcohol use in the 6 mo before cohort entry | 131 (53) | 63 (62) | 167 (53) | 172 (55) |
| Injection drug use in the 6 mo before cohort entry | 97 (39) | 34 (33) | 130 (41) | 121 (38) |
| Clinical characteristics at cohort entry | ||||
| Years of hepatitis C virus infection | 19 (11–27) | 17 (7–24) | 18 (11–25) | 20 (9–25) |
| Years of HIV infection | 10 (5–16) | 10 (5–17) | 9 (5–16) | 9 (5–16) |
| CD4 cell count | 379 (250–579) | 430 (280–580) | 380 (250–610) | 420 (270–540) |
| Undetectable HIV viral load (<50 copies/mL) | 156 (63) | 76 (74) | 213 (68) | 207 (66) |
| HIV viral load if detectable | 350 (89–4147) | 3048 (121–17 000) | 349 (82–4147) | 3116 (285–17 000) |
| APRI score | 0.56 (0.38–1.17) | 0.71 (0.40–1.28) | 0.54 (0.36–1.10) | 0.70 (0.40–1.31) |
| Significant liver fibrosis (APRI ≥ 1.5) at cohort entry | 46 (19) | 17 (17) | 55 (18) | 46 (15) |
| Liver cirrhosis (APRI ≥ 2) | 36 (15) | 11 (11) | 43 (14) | 32 (10) |
| End-stage liver disease | 25 (10) | 8 (8) | 33 (10) | 38 (12) |
| Antiretroviral use at cohort entry | ||||
| Years since initiation of combination antiretroviral therapy | 3.3 (0.4–8.0) | 2.3 (0.2–9.6) | 3.3 (0.4–7.2) | 2.3 (0.2–7.9) |
| Backbone : tenofovir/emtricitabine | 155 (63) | 73 (72) | 211 (67) | 218 (69) |
| Backbone : abacavir/lamivudine | 90 (37) | 29 (28) | 103 (33) | 96 (31) |
Abbreviations: APRI, aspartate-to-platelet ratio index; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Progression of Liver Fibrosis Over Time
In the first analysis, PI users experienced a significant median increase in APRI score of 11% per 5 years (95% confidence interval [CI], 1%, 21%). The median increase was slower in NNRTI users at 7% per 5 years (95% CI, −1%, 14%; Table 2). The overall median APRI score was 32% higher (95% CI, 14%, 50%) among TDF/FTC backbone users compared with ABC/3TC backbone users, although it remained below the cutoff for significant fibrosis (median APRI, 0.79 among TDF/FTC users and 0.61 among ABC/3TC users).
Table 2.
Median Changes in Aspartate-to-Platelet Ratio Index Score on the Multiplicative Scale Associated With Time on Combination Antiretroviral Therapy Among Protease Inhibitors or Nonnucleoside Reverse Transcriptase Users Estimated by Linear Regression With Generalized Estimating Equations
| Model 1a |
Model 2b |
|
|---|---|---|
| Exp(β) (95% CI) | Exp(β) (95% CI) | |
| Age (5 y) at cohort entry | 1.00 (.96, 1.04) | 1.00 (.96, 1.04) |
| Female | 0.90 (.79, 1.02) | 0.90 (.79, 1.02) |
| Time since hepatitis C virus infection at cohort (5 y)c | 1.04 (1.01, 1.07) | 1.04 (1.01, 1.07) |
| CD4 cell count at the previous study visit (100 cells/mL) | 0.99 (.98, 1.00) | 0.99 (.98, 1.00) |
| Undetectable viral load(<50 copies/mL) | 0.98 (.91, 1.06) | 0.98 (.91, 1.06) |
| Alcohol use in the past 6 mo | 1.03 (.95, 1.11) | 1.03 (.95, 1.11) |
| TDF/FTC backbone at cohort entry | 1.32 (1.14, 1.50) | 1.44 (1.20, 1.68) |
| cART initiated with NNRTI | 1.00 (.84, 1.16) | 1.00 (.84, 1.16) |
| Time on cART among PI users (5 y)d | 1.11 (1.01, 1.21) | NA |
| Time on cART among NNRTI users (5 y)d | 1.07 (.99, 1.14) | NA |
| Time on cART among PI-ABC/3TC users (5 y)e | NA | 1.16 (1.04, 1.29) |
| Time on cART among PI-TDF/FTC users (5 y)e | NA | 1.08 (.97, 1.19) |
| Time on cART among NNRTI-ABC/3TC users (5 y)e | NA | 1.11 (1.02, 1.20) |
| Time on cART among NNRTI-TDF/FTC users (5 y)e | NA | 1.03 (.93, 1.12) |
Abbreviations: ABC/3TC, abacavir/lamivudine; APRI, aspartate-to-platelet ratio index; cART, combination antiretroviral therapy; CI, confidence interval; NA, not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF/FTC, tenofovir/emtricitabine.
a Model 1: E[ln(APRI)] = β0 + βiNNRTI + β2Years + β3NNRTI × Years + ∑ βjcovariatesj.
b Model 2: E[ln(APRI)] = β0 + β1NNRTI + β2Years + β3NNRTI × Years + β4TDF/FTC + β5TDF/FTC × Years + ∑ βjcovariatesj.
c Based on the date of hepatitis C virus (HCV) seroconversion, if known, or year of first injection drug use or blood product exposure as a proxy of HCV infection.
d Represents the rate of change in APRI score over 5 years. Obtained with the interaction term between NNRTI use and time since cART initiation.
e Represents the rate of change in APRI score over 5 years. Obtained with the interaction terms between NNRTI use and time since cART initiation and between TDF/FTC use and time since cART initiation from the equation.
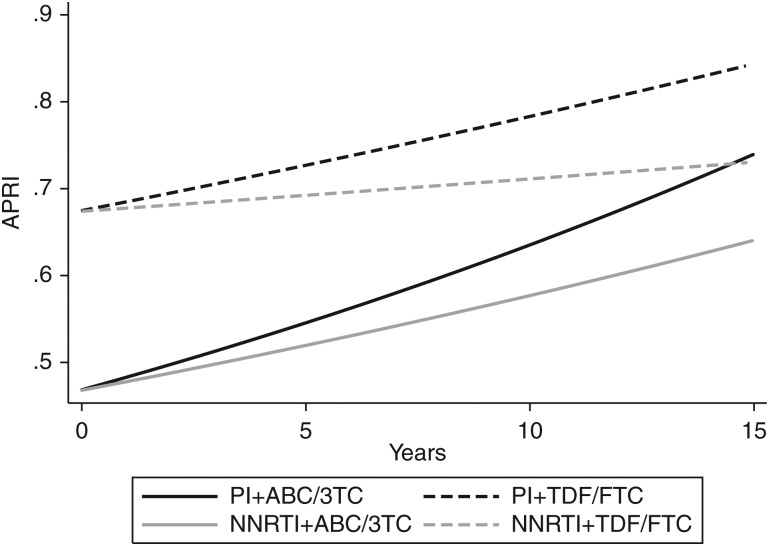
After including an interaction term between the backbone and time, rates of change in APRI score appeared driven by ABC/3TC use, with a 16% median increase in APRI score per 5 years (95% CI, 4%, 29%) when ABC/3TC was used in combination with a PI and an 11% increase per 5 years (95% CI, 2%, 20%) when used with an NNRTI. However, TDF/FTC users did not experience a statistically significant change in APRI score over time with PI-based (8% increase per 5 years; 95% CI, −3%, 19%) nor with NNRTI-based cART (3% increase per 5 years; 95% CI, −7%, 12%). Table 2 presents the back-transformed results for the full models. Although APRI scores are higher overall with TDF/FTC use than with ABC/3TC, the rate of increase in APRI score is greater over time with ABC/3TC use (Figure 2).
Figure 2.
Predicted aspartate-to-platelet ratio index score over time since combination antiretroviral therapy (cART) initiation, stratified cART regimen. Abbreviations: ABC/3TC, abacavir/lamivudine; APRI, aspartate-to-platelet ratio index; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF/FTC, tenofovir/emtricitabine.
DISCUSSION
To our knowledge, this is the first study to explore the rates of liver fibrosis progression according to the class of anchor agent and the backbone used in HIV-HCV–coinfected persons on modern cART regimens, with results applicable to current clinical practice. Virologic control of HIV through successful cART is known to be associated with slower fibrosis progression rates in coinfected persons overall. However, whether specific regimens affect fibrosis rates in treated patients remains unclear [25]. In this longitudinal study, we attempted to isolate the effect of modern cART regimens on long-term liver outcomes by emulating a randomized controlled trial using propensity score matching and an intention-to-treat analysis of new users of the anchor agent class. Initiation of cART with a PI appeared to be associated with increases in APRI score over time, whereas NNRTI use was not. This study was not designed to explain the role of the backbone on liver fibrosis progression. However, when the backbone was accounted for, ABC/3TC use with either a PI or NNRTI was associated with changes in APRI over time regardless of the anchor class. In contrast, use of TDF/FTC did not result in significant changes in the APRI score over time. It remains possible that PI use itself could contribute to fibrosis progression given that the estimate among PI-TDF/FTC users was 1.08 (95% CI, .97, 1.19) per 5 years, although we lacked the power to confirm this.
Several studies including HIV-HCV–coinfected persons have shown an association between hepatotoxicity, fibrosis, or clinical liver outcomes and nevirapine use, but not efavirenz [6, 26–28], which represented 92% of NNRTI use in this cohort. The results of a sensitivity analysis including only efavirenz users were not appreciably different from those reported here.
There have been several reports of acute PI-associated hepatotoxicity in cohorts of HIV-infected persons, in which some participants were coinfected with HCV [5–10] and in 1 coinfection cohort [11]. Only 1 study reported an increased risk of steatosis with PI use in coinfected persons [12], but others showed a decreased risk of liver fibrosis [13, 14] and cirrhosis [15] and slower rates of fibrosis progression [13–15]. These studies compared PI users with untreated persons or mono/dual-NRTI therapy users. These protective effects may have been driven by a better control of HIV infection among cART users on a PI. Ignoring the backbone used, including dideoxynucleoside-containing regimens and selecting improper comparison groups biased the conclusions of previous studies. Our group has investigated the relationship between PI and NNRTI use and changes in APRI score in 2 previous studies, with conflicting results [21, 22]. Unlike the present study, these studies were not restricted to coinfected persons, allowed for switches in the class of anchor agent, and failed to account for the backbone used.
Although TDF, FTC, and 3TC are associated with low levels of mitochondrial toxicity, ABC can reduce hepatocyte proliferation and increase intracellular lipids and lactate levels [17]. ABC use can also cause hypersensitivity reactions, which are associated with transient and mild liver enzyme elevations [29], but they usually occur within 6 weeks of initiation [30]. Because cART was initiated for a median of 3.5 years before cohort entry, hypersensitivity reactions are unlikely to have caused the elevated APRI scores observed. Finally, ABC is extensively metabolized by the liver. Bioactivation of ABC to a conjugated aldehyde has been recently identified as a potential trigger for ABC-induced toxic events [31]. Acetaldehyde is a principal mediator of fibrogenic and mutagenic effects of alcohol in the liver, raising the possibility of additive effects in the setting of alcohol use, which is frequent in the coinfected population [32].
Although there was no statistically significant increase in APRI score over time with TDF/FTC use, the use of a TDF/FTC backbone was associated with a higher median APRI score overall compared with ABC/3TC use. In the Data collection on Adverse events of Anti-HIV Drugs (D:A:D) study, chronic alanine aminotransferase (ALT) elevation was unexpectedly associated with current use of regimens containing TDF or FTC in HIV monoinfected persons, particularly in the first 2 years [33]. A randomized controlled trial comparing TDF/FTC and ABC/3TC also found stable elevations in AST, ALT, and alkaline phosphatase after 96 weeks of treatment in the TDF/FTC group only [34]. However, in treatment-experienced adults, there was no difference in AST and ALT between TDF/FTC and ABC/3TC in a combined analysis of 2 trials (BICOMBO trial in Spain and Simplification of antiretroviral therapy with Tenofovir-Emtricitabine or Abacavir-Lamivudine trial (STEAL) trial in Australia) [35]. HCV status was not reported in these trials. In our study, while TDF/FTC users had higher APRI scores overall, they did not experience statistically significant changes in APRI score over time. AST levels, but not the platelet counts (P = .48), were statistically higher among TDF/FTC users compared with ABC/3TC users (P = .01), resulting in higher overall APRI scores, which suggests that the elevation does not reflect development of fibrosis.
While this study presents limitations, these were mitigated by careful design and analysis. One shortcoming was the impossibility of applying a strict new-user design, in which no changes of specific anchor agent would be tolerated, because of the small number of eligible persons. We therefore implemented a design of new users of the anchor class, deemed adequate because we were interested in a class effect rather than the effect of a specific drug. Modifications of the anchor agent within the same class are usually triggered by an adverse reaction in the first 3 months. The most frequently reported drug intolerances resulting in a modification of the regimen are gastrointestinal tract intolerance, hypersensitivity reactions, and central nervous system adverse events [36]. These intolerances are generally acute and occur early after treatment initiation. The drugs that provoke these reactions are therefore likely to have been discontinued close to the time of their initiation. With the exception of hypersensitivity reactions, these adverse events do not impact the liver and are unlikely to affect the relationship studied.
Another limitation is the presence of left truncation because APRI measurements were not available prior to cohort entry and follow-up started after cART initiation for most participants. However, the new-user design that was implemented ensured that the class of anchor agent did not change between treatment initiation and cohort entry, limiting the impact of left truncation on our analyses. At cohort entry, the median time since cART initiation was 3.5 years in the matched sample; however, therapy had been initiated a year or less before cohort entry in 30% of the sample. It is possible that the changes in APRI score that occurred soon after treatment initiation would differ; however, our aim was to study long-term fibrosis development, not acute toxicity, and early effects are likely to be moderate.
The gold standard for liver fibrosis assessment is liver biopsy, which was performed only in a limited number of participants during clinical care and could not be performed ethically every 6 months for research purposes. Transient elastography (FibroScan) is replacing liver biopsy for the assessment of liver fibrosis; however, all study sites did not perform this test, and the number of repeated measures is limited. The APRI score is a widely used alternative that performs similarly to other markers [37]. It has been validated in HIV-HCV–coinfected populations [38] and predicts occurrence of liver complications [21] and all-cause mortality [39]. It is usually used as a dichotomous measure [38], but the continuous score can be useful for research purposes as it predicts overall 5-year survival in HCV-infected persons (hazard ratio, 2.8; 95% CI, 1.6, 4.7) [39]. Known predictors of liver disease also predict the continuous APRI score [40].
Finally, this study is limited by the close relationships between anchor agents and backbones, which are hard to dissociate and are subject to confounding. PIs are often favored for persons with poor adherence in order to lower risks of resistance [41]. Confounding by indication could bias the results if more PI users had unstable lives, resulting in poorer adherence to treatment and exposure to potential risk factors for liver injury such as alcohol use and uncontrolled HIV replication. NNRTI users are more likely to use a TDF/FTC backbone than PI users due to the availability of a fixed dose coformulation of efavirenz with TDF/FTC [18]. Preexisting differences in demographic and clinical characteristics between treatment groups were reduced by implementation of propensity score matching and adjustment for time-updated alcohol use and HIV RNA, thus reducing confounding.
Despite these limitations, this study has several strengths. The CCC is a large prospective cohort broadly representative of the coinfected population accessing care in Canada (eg, women, Aboriginal people, current and past injection drug users, men who have sex with men). The results obtained are also relevant to current clinical practice because all participants received modern cART regimens [18] and never received dideoxynucleosides, which are known to have high levels of mitochondrial toxicity [17].
Another strength is the emulation of a randomized controlled trial. Propensity score matching balanced baseline differences in potential confounders between treatment groups, removing part of the confounding bias. The intention-to-treat analysis takes a clinician's perspective, investigating the effect of initiating a certain treatment, regardless of future changes in the class of anchor agent that could be caused by factors associated with liver fibrosis and would result in a carryover effect of the previous regimen. Finally, several sensitivity analyses were conducted to test the robustness of our findings. No clear patterns were apparent when stratified by year of cART initiation. Censoring when changes to the class of anchor agent or backbone occurred or including only boosted PI and efavirenz recipients produced results comparable to those presented here. The conclusion remained unchanged with another sensitivity analysis in which we considered inverse probability weighting for end of follow-up (ie, censoring).
In conclusion, the rate of change in APRI score seemed more influenced by the backbone than by the class of anchor agent in coinfected persons. Both PI- and NNRTI-based regimens were associated with increases in APRI over time when combined with ABC/3TC. However, the APRI score did not increase significantly over time when PI- and NNRTI-based regimens were used with a backbone of TDF/FTC. This study was designed to investigate the role of the class of anchor agent, not the backbone, on progression of liver fibrosis. Therefore, further investigation is required to better understand how different backbone/anchor drug combinations can affect the liver of HIV-HCV–coinfected persons in the long term.
Supplementary Material
Notes
Acknowledgments. We thank all study coordinators and nurses for their assistance with study coordination, participant recruitment, and patient care. The Canadian Co-infection Cohort (CCC) cohort is comprised of 18 sites across Canada that recruit and follow human immunodeficiency virus (HIV)-hepatitis C virus–coinfected patients. The following co-investigators have taken part in data collection pertaining to this article and have reviewed its content. However, they did not partake in the analysis, writing, or editing of this article nor has anyone received compensation for any contributions.
The CCC investigators (CTN222) include the following: Drs Jeff Cohen, Windsor Regional Hospital Metropolitan Campus, Windsor, ON; Brian Conway, PENDER Downtown Infectious Diseases Clinic, Vancouver, BC; Curtis Cooper, the Ottawa Hospital Research Institute, Ottawa ON; Pierre Côté, Clinique du Quartier Latin, Montréal, QC; Joseph Cox, McGill University Health Centre Immune Deficiency Treatment Centre-Montréal General Hospital, Montréal, QC; John Gill, Southern Alberta HIV Clinic, Calgary, AB; Shariq Haider, McMaster University Medical Centre–SIS Clinic, Hamilton, ON; Aida Sadr, Native BC Health Center, St-Paul's Hospital, Vancouver, BC; Lynn Johnston, Queen Elizabeth II Health Science Center for Clinical Research, Halifax, NS; Mark Hull, BC Centre for Excellence in HIV/AIDS, Vancouver, BC; Julio Montaner, St. Paul's Hospital, Vancouver, BC; Erica Moodie, McGill University, Montreal, QC; Neora Pick, Oak Tree Clinic, Children's and Women's Health Centre of British Columbia, University of British Columbia, Vancouver, BC; Anita Rachlis, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON; Danielle Rouleau, Centre Hospitalier de l'Université de Montréal, Montréal, QC; Roger Sandre, Health Sciences North–the HAVEN/Hemophilia Program, Sudbury, ON; Joseph Mark Tyndall, Department of Medicine, Infectious Diseases Division, University of Ottawa, Ottawa, ON; Marie-Louise Vachon, Centre Hospitalier Universitaire de Québec, Québec, QC; Steve Sanche, SHARE University of Saskatchewan, Saskatoon, SK; Stewart Skinner, Royal University Hospital & Westside Community Clinic, University of Saskatchewan, Saskatoon, SK; and David Wong, University Health Network, Toronto, ON.
Financial support. M. B. K. is supported by a Chercheurs nationaux career award from the Fonds de recherche du Québec–Santé, Réseau SIDA/maladies infectieuses (FRQ-S). E. E. M. M. is supported by a Chercheurs boursier junior 2 career award from the FRQ-S. L. B. received travel support from the Canadian Network for Advanced Interdisciplinary Methods for Comparative Effectiveness Research, Drug Safety and Effectiveness Network. This work was supported by the FRQ-S, the Canadian Institutes of Health Research (CIHR) (MOP-79529), and the CIHR Canadian HIV Trials Network (CTN222).
Potential conflicts of interest. A. R. has received institutional grant support from CIHR. C.C. has received institutional grant support from Merck and Abbvie not related to the submitted work, consulting fees from Merck and Vertex; honoraria for lectures from Merck and Roche; and funding support from The Ottawa Hospital Department of Medicine. S. W. reports grants, personal fees, and nonfinancial support from Merck, ViiV Health Care (GSK-Pfizer), Gilead, Abbott, Bristol-Myers Squibb (BMS), Schering-Plough, Janssen, Boegringer Ingelheim, Tibotec not related to the submitted work. M. H. reports grant support from the National Institute on Drug Abuse and personal fees from Merck, Vertex, BMS, Ortho-Janssen, Pfizer, Gilead, and Viiv not related to the submitted work. M. K. has received institutional grant support from CIHR, Fonds de recherché en Sante du Quebec, Reseau SIDA/maladies infectieuses, and CIHR Canadian HIV Trails Network for this work, institutional grant support from Merck, Viiv, Janssen, BMS and Gilead not related to the submitted work and consulting fees from Viiv, Merck and BMS. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Canadian Co-infection Cohort Study, Jeff Cohen, Brian Conway, Curtis Cooper, Pierre Côté, Joseph Cox, John Gill, Shariq Haider, Aida Sadr, Lynn Johnston, Mark Hull, Julio Montaner, Erica Moodie, Neora Pick, Anita Rachlis, Danielle Rouleau, Roger Sandre, Joseph Mark Tyndall, Marie-Louise Vachon, Steve Sanche, Stewart Skinner, and David Wong
References
- 1.Samji H, Cescon A, Hogg RS et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol 2008; 48:353–67. [DOI] [PubMed] [Google Scholar]
- 3.Flint OP, Noor MA, Hruz PW et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol 2009; 37:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Leth F, Phanuphak P, Stroes E et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med 2004; 1:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA 2000; 283:74–80. [DOI] [PubMed] [Google Scholar]
- 6.Wit FWNM, Weverling GJ, Weel J, Jurriaans S, Lange JMA. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis 2002; 186:23–31. [DOI] [PubMed] [Google Scholar]
- 7.Becker S. Liver toxicity in epidemiological cohorts. Clin Infect Dis 2004; 38(suppl 2): S49–55. [DOI] [PubMed] [Google Scholar]
- 8.den Brinker M, Wit FW, Wertheim-van Dillen PM et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS 2000; 14:2895–902. [DOI] [PubMed] [Google Scholar]
- 9.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS 2004; 18:2277–84. [DOI] [PubMed] [Google Scholar]
- 10.Bonfanti P, Ricci E, Penco G et al. Low incidence of hepatotoxicity in a cohort of HIV patients treated with lopinavir/ritonavir. AIDS 2005; 19:1433–4. [DOI] [PubMed] [Google Scholar]
- 11.Neukam K, Mira JA, Ruiz-Morales J et al. Liver toxicity associated with antiretroviral therapy including efavirenz or ritonavir-boosted protease inhibitors in a cohort of HIV/hepatitis C virus co-infected patients. J Antimicrob Chemother 2011; 66:2605–14. [DOI] [PubMed] [Google Scholar]
- 12.Sulkowski MS, Mehta SH, Torbenson M et al. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS 2005; 19:585–92. [DOI] [PubMed] [Google Scholar]
- 13.Macías J, Castellano V, Merchante N et al. Effect of antiretroviral drugs on liver fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS 2004; 18:767–74. [DOI] [PubMed] [Google Scholar]
- 14.Macias J, Mira JA, Lopez-Cortes LF et al. Antiretroviral therapy based on protease inhibitors as a protective factor against liver fibrosis progression in patients with chronic hepatitis C. Antivir Ther 2006; 11:839–46. [PubMed] [Google Scholar]
- 15.Benhamou Y, Di Martino V, Bochet M et al. Factors affecting liver fibrosis in human immunodeficiency virus– and hepatitis C virus–coinfected patients: impact of protease inhibitor therapy. Hepatology 2001; 34:283–7. [DOI] [PubMed] [Google Scholar]
- 16.Dieterich DT, Robinson PA, Love J, Stern JO. Drug-induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis 2004; 38(suppl 2):S80–9. [DOI] [PubMed] [Google Scholar]
- 17.Walker UA, Setzer B, Venhoff N. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS 2002; 16:2165–73. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 8 December 2014.
- 19.Klein MB, Saeed S, Yang H et al. Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 20.Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat 2002; 84:151–61. [Google Scholar]
- 21.Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr 2007; 44:463–9. [DOI] [PubMed] [Google Scholar]
- 22.Moodie EE, Pant Pai N, Klein MB. Is antiretroviral therapy causing long-term liver damage? A comparative analysis of HIV-mono-infected and HIV/hepatitis C co-infected cohorts. PLoS One 2009; 4:e4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics 2001; 57:120–5. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB Multiple imputation for nonresponse in surveys. New York: Wiley, 1987. [Google Scholar]
- 25.Brau N, Salvatore M, Rios-Bedoya CF et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol 2006; 44:47–55. [DOI] [PubMed] [Google Scholar]
- 26.Martínez E, Blanco JL, Arnaiz JA et al. Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS 2001; 15:1261–8. [DOI] [PubMed] [Google Scholar]
- 27.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 2002; 35:182–9. [DOI] [PubMed] [Google Scholar]
- 28.Sanne I, Mommeja-Marin H, Hinkle J et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis 2005; 191:825–9. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health, US Department of Health & Human Services. Clinical and Research Information on Drug-Induced Liver Injury: Abacavir. Available at: http://livertox.nih.gov/Abacavir.htm Accessed 15 March 2015.
- 30.Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis 2002; 34:1137–42. [DOI] [PubMed] [Google Scholar]
- 31.Grilo NM, Charneira C, Pereira SA, Monteiro EC, Marques MM, Antunes AM. Bioactivation to an aldehyde metabolite—possible role in the onset of toxicity induced by the anti-HIV drug abacavir. Toxicol Lett 2014; 224:416–23. [DOI] [PubMed] [Google Scholar]
- 32.Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev 2010; 3:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovari H, Sabin C, Ledergerber B et al. Antiretroviral drugs associated with chronic ALT elevations in persons without HCV and HBV infection. In: Conference on Retroviruses and Opportunistic Infections Seattle, 2015. [Google Scholar]
- 34.Martin A, Bloch M, Amin J et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: a randomized, 96-week trial. Clin Infect Dis 2009; 49:1591–601. [DOI] [PubMed] [Google Scholar]
- 35.Amin J, De Lazzari E, Emery S et al. Simplification with fixed-dose tenofovir-emtricitabine or abacavir-lamivudine in treatment experienced, virologically suppressed adults with HIV infection: combined analysis of two randomised, non-inferiority trials Bicombo and Steal. J AIDS Clinic Res 2010; 1:1000103-1–1000103-5. [Google Scholar]
- 36.Elzi L, Marzolini C, Furrer H et al. Treatment modification in human immunodeficiency virus–infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med 2010; 170:57–65. [DOI] [PubMed] [Google Scholar]
- 37.Nunes D, Fleming C, Offner G et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J Acquir Immune Defic Syndr 2005; 40:538–44. [DOI] [PubMed] [Google Scholar]
- 38.Lin ZH, Xin YN, Dong QJ et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011; 53:726–36. [DOI] [PubMed] [Google Scholar]
- 39.Vergniol J, Foucher J, Terrebonne E et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011; 140:1970–9.e3. [DOI] [PubMed] [Google Scholar]
- 40.Price JC, Seaberg EC, Badri S, Witt MD, D'Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis 2012; 203:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangsberg DR, Acosta EP, Gupta R et al. Adherence–resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS 2006; 20:223–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.