Abstract
Coral reefs are in decline worldwide, and land-derived sources of pollution, including sewage, are a major force driving that deterioration. This review presents evidence that sewage discharge occurs in waters surrounding at least 104 of 112 reef geographies. Studies often refer to sewage as a single stressor. However, we show that it is more accurately characterized as a multiple stressor. Many of the individual agents found within sewage, specifically freshwater, inorganic nutrients, pathogens, endocrine disrupters, suspended solids, sediments, and heavy metals, can severely impair coral growth and/or reproduction. These components of sewage may interact with each other to create as-yet poorly understood synergisms (e.g., nutrients facilitate pathogen growth), and escalate impacts of other, non-sewage–based stressors. Surprisingly few published studies have examined impacts of sewage in the field, but those that have suggest negative effects on coral reefs. Because sewage discharge proximal to sensitive coral reefs is widespread across the tropics, it is imperative for coral reef–focused institutions to increase investment in threat-abatement strategies for mitigating sewage pollution.
Keywords: marine conservation, sanitation, coral disease, eutrophication, multiple stressors, reef management
Introduction
Coral reefs play a critical role in coastal ecosystem function in the tropics, providing food and habitat for 550,000 to 1,330,000 species.1 Along with the inherent biodiversity these habitats support, reefs built by corals also provide many valuable services for humans, including shoreline protection, livelihoods from ecotourism, fisheries production, and a living synthesis engine of biomedical and industrially valuable compounds.2–5 The value of these services varies globally, but is estimated at over $31 billion (US$, 2014) annually for all reefs combined.6 Unfortunately, reefs and the many benefits they provide are under severe threat, with evidence of a general pattern of habitat degradation.7,8
Spatial variation and forces behind coral reef decline
Coral reefs are exposed to a multitude of stressors emanating from human activities7–10 and, as a result, have experienced drastic declines in spatial coverage and diversity over the past 50 years.7,8 At a regional level in the Indo-Pacific, live coral cover has declined at an annual rate of 1% from the early 1980s to 2003, while in the Caribbean, the annual rate of coral cover loss was 1.5% between 1977 and 2001.11 Recent work cataloging the status of reefs has estimated that we have functionally lost at least 25% of coral reefs globally, and one-third of all coral species are threatened with extinction.12 Chief among threats identified in Reefs at Risk Revisited (RRR) are overfishing, pollution, coastal development, and climate change.8 For example, increasing temperature of surface waters from climate change has led to increased bleaching events and subsequent reef loss.13 Bleaching owing to elevated water temperatures is perhaps the most notable stress, with some reefs experiencing over 85% mortality in the 1998 mass bleaching event.14–17 While the 1998 bleaching event resulted in significant losses, coral reefs were already in a state of decline when this event occurred.10,18 The additive and synergistic effects of long-term overfishing, chronic coastal pollution, and poorly regulated coastal development had already compromised coral reefs, making it difficult for reefs to withstand more stressful conditions associated with increasing frequency and intensity of bleaching events.10,18,19
Over the past two decades, the conservation community has generally considered overfishing as the threat to coral reefs that warrants the most attention.8 For example, RRR emphasizes that more than 55% of the world’s reefs are under immediate threat from overfishing,8 which can lead to phase shifts from coral-dominated reefs to algal-dominated reefs as the number of algae-eating fish decreases significantly.20 Halpern et al.21 also suggest that overfishing is one of the most severe causes of coral reef decline. The extensive scientific literature on overfishing has prompted coral reef management responses that include limiting or banning fishing in some areas, regulations that prohibit the take of certain key fish species, and global efforts to influence consumer choice by limiting the demand for ecologically important species. Notably, the threat to coral reefs from pollution and eutrophication, although potentially just as important as overfishing, as suggested by the assessments of RRR8 and Halpern et al.,21 has received much less attention from conservation organizations (S. Wear, personal observation). Reasons for this disparity may include the practical challenges of dealing with a large-scale diffuse threat, the diversity of pollutants involved, the high cost of water-treatment facilities, and bureaucracy. The solutions to reducing and understanding the exact impacts of coastal pollution, where it is likely to be strong, have been lacking because of the inherent difficulties of monitoring and evaluating nonpoint sources of pollution, along with jurisdictional issues such as agency and private land conflicts.
The largest component of coastally derived pollution is sewage.22–25 Most coral reefs are located along the shorelines of developing countries, where tertiary sewage treatment is rare. Most sewage enters tropical waters as either poorly or completely untreated discharge or stormwater runoff.25,26 In fact, the United Nations Environmental Program estimated that 85% of the wastewater entering the sea in the Caribbean is untreated.27 As our global population likely expands by 2 billion over the next 35 years,28 the amount of sewage polluting reefs will also increase. It is thus critically important to understand the role of sewage discharge in coral reef declines and identify ways to minimize its impact on reef health. In this review, we synthesize what is known about the composition of sewage and how each component may affect coral reef health. We explore interactions between and among these components to evaluate synergisms. We also present a synthesis of previously conducted studies on the impacts of sewage discharge on coral reefs. Finally, we present a summation of the geographic extent of sewage pollution, in regions where coral reefs occur.
What is in sewage and how do those components affect corals?
Most reports addressing the impact of sewage on coral reefs cite high inorganic nutrient content as the primary reason for alarm—as those nutrients could lead to increased growth of algae and coral diseases.29,30 However, sewage in its raw form contains many more compounds than just inorganic nutrients (e.g., see Refs. 24, 25, and 31). In particular, sewage discharged into tropical coastal seas contains hundreds of different compounds, the most common of which are freshwater, inorganic nutrients, pathogens, endocrine disrupters, suspended solids, sediments, heavy metals, and other toxins.25,31 Below, we describe each of these constituents in detail and briefly summarize what is known about negative impacts on coral reefs and the mechanism(s) underlying the impact (Table1). Importantly, this understanding does not come from studies on sewage itself, but rather from work investigating how explicit sewage components (e.g., freshwater, ammonium) affect corals.
Table 1.
Examples of coral reef (corals and associated organisms) responses to common stressors found in sewage
| Stressor | Response | References |
|---|---|---|
| Freshwater | Increased coral mortality (with lowered salinity for >24 h). | 32, 33 |
| Dissolved inorganic nutrients (ammonium, nitrite + nitrate, and phosphate) | Increased coral bleaching, increased coral disease prevalence and severity, decreased coral fecundity, algal overgrowth, decreased coral skeletal integrity, decreased coral cover and biodiversity, and increased phytoplankton shading. | 30, 47, 50, 51, 54, 55, 57, 70, 107 |
| Endocrine disrupters (e.g., steroidal estrogens) | Reduction in coral egg–sperm bundles, slowed coral growth rates, coral tissue thickening. | 95, 103, 105 |
| Pathogens | Source of white pox disease pathogen for corals and associated mortality, and increased pathogenicity in corals. | 88–90 |
| Solids | Reduced photosynthesis of coral symbionts, coral species richness, coral growth rates, coral calcification, coral cover, and coral reef accretion rates, and increased coral mortality. | 107–112 |
| Heavy metals | Coral mortality, coral bleaching, reduction of basic functions such as respiration and fertilization success; Fe2+ may increase growth of coral disease. | 126–128 |
| Toxins | Lethal and sublethal effects on corals—highly variable and dependent on specific toxin. Reduced photosynthesis of coral symbionts, coral bleaching, coral mortality, reduced coral lipid storage, reduced coral fecundity, death of coral symbionts, and decreased coral growth. | 133 and references therein |
Freshwater
The primary component of sewage is freshwater, a known stressor to corals. Although there are surprisingly few studies examining impacts of freshwater on coral health, classic laboratory studies conducted over 80 years ago revealed that most corals die after prolonged exposure to fresh or brackish water sources and that the lower salinity tolerance of corals is ∼15–20 ppt.32 In the field, the effect of freshwater discharge onto coral reefs has been studied in a limited number of cases using correlational methods.32,33 In these studies, increased freshwater input into coastal waters associated with stormwater runoff was correlated with rapid drops in near-shore salinity and, in turn, significant loss of nearby corals. Reef mortality associated with these flood-related reductions in salinity has been documented around the world (e.g., see Ref. 32). Understanding the specific limits and tolerances of corals to freshwater exposure, however, is relatively underexplored.
Nutrients
Sewage discharging into coastal tropical waters contains very high concentrations of inorganic nutrients, such as ammonium, nitrite, nitrate, and phosphate. A number of studies have examined the effects of these compounds on specific components of coral health. Impacts can be categorized as either direct, having effects on the coral animal or its symbionts, or indirect, whereby nutrients influence other aspects of the reef that in turn negatively affect coral health. One of the most influential single mechanisms is indirect, whereby nutrient enrichment enhances macroalgal overgrowth, killing corals and thereby removing a foundation species. A growing body of new literature has also examined direct impacts, such as how inorganic nutrients modify microbial communities found on and in corals, coral symbionts, and calcification rates. Here, we briefly review key findings related to each of these topics.
Nutrients and algae
Since tropical reefs are generally nutrient poor or oligotrophic, any significant input of limiting macronutrients into coastal waters could cause shifts in reef community composition.34 Most research on nutrient impacts on reefs has focused on the direct effects of inorganic nutrients on primary producers, such as phytoplankton or macroalgae, both of which compete with corals for light and space. For example, increases in nutrient concentrations can facilitate large, often monospecific blooms of algae.35–37 It is also well documented that increasing inorganic nutrient levels increases macroalgal cover on reefs, to the detriment of coral cover.20,29,38–43
This reduction in coral cover is owing to the increased proliferation of macroalgal biomass in the presence of elevated dissolved inorganic nitrogen, which translates to increased competitive ability for macroalgae as they interact with corals and compete for space.44,45 This increase in macroalgal competition, when combined with nutrient pollution, may further reinforce a coral-depauperate state by reducing the growth and survival of adult corals46–48 and preventing the recruitment and establishment of juveniles.45,48 Increased macroalgal growth and competitive displacement of corals in response to increasing nutrients from human activities has been documented in enrichment studies in the Caribbean Sea and the Indian and Pacific Oceans.29,49
Nutrients, coral disease, and bleaching
Nutrient enrichment has also been hypothesized to be a driver of coral disease and bleaching. Recent studies on the Great Barrier Reef50 and in the Florida Keys51 found a positive correlation between bleaching prevalence and inorganic nitrogen (N) levels. Field surveys have also found that coral disease prevalence is often positively correlated with ambient seawater nutrient concentrations.52,53 For example, increasing nutrient availability is positively correlated with increased disease progression rates (i.e., the rate of movement of the disease over a coral’s surface) of some coral diseases, such as yellow blotch and black band disease.54,55 Recent experimental evidence has confirmed predictions from these observational studies and shown that nutrients can cause an increase in both the prevalence of coral disease and the extent of bleaching on natural reefs.30 Researchers have enriched replicate portions of a coral reef with inorganic N and phosphorus (P), to levels within the nutrient ranges experienced by contaminated reefs.56 After 3 years of this nutrient enrichment, disease incidence in corals increased more than twofold and bleaching prevalence in one coral species increased by more than 3.5-fold.30 Perhaps most importantly, after termination of nutrient additions, there was a return to preenrichment water quality, followed by rapid recovery (within 6 months) of the enriched reef sites, such that disease and bleaching levels returned to those in control reef sites lacking the enrichment treatment. These findings demonstrate that measures to reduce inorganic nutrient pollution through water quality mitigation efforts may successfully reduce coral disease and bleaching levels, perhaps even very rapidly.
Nutrients and coral growth
Nutrients have long been hypothesized to reduce coral growth rates. A recent meta-analysis showed that exposure to nitrate and ammonium over a wide range of concentrations (0.5–26 μM) generally had negative effects on corals, but increased P (0.11–26 μM) actually enhanced calcification.57 Nevertheless, although elevated P concentrations increased calcification rates, this response also involved losses of skeletal integrity. The effects were also context dependent such that different morphologies (mounding versus branching) and different species of corals exhibited varying calcification responses and varying impacts of N, depending on type (nitrate or ammonium) and source (natural or anthropogenically derived).57 The variable effects of nutrient pollution across coral morphology and species carries implications for how different habitat types will uniquely respond to nutrient enrichment. In particular, mounding and poritid corals were shown to be more susceptible to the negative effects of increased nutrients, and habitats or ecosystems dominated by these taxa are more likely to suffer impacts from increased inorganic nutrient concentrations that often accompany reduced water quality.
Nutrients can also decrease coral growth by acting on the autotrophic algal partner Symbiodinium, which is a symbiont in corals. Nutrients have long been hypothesized to decrease coral growth rates via bleaching, through elevating the abundance of algal symbionts.58,59 Increased symbiont density leads to corresponding increases in reactive oxygen species, which may result in damage to host cells and/or death and expulsion of the symbiont.60 It is this loss of the pigmented Symbiodinium that causes coral bleaching, decreased growth rates, and even whole-colony mortality. It should be noted, however, that recent research has revealed that increased nutrient levels do not always have a negative impact on coral growth but instead can have a unimodal relationship, where increasing nutrient levels first increase coral growth but then decrease coral growth as levels of nutrients rise.61
Nutrients and microbial communities
Coral-associated microbes (i.e., eubacteria and archaea) have a multitude of context-dependent roles in health and physiological homeostasis of scleractinian corals.62,63 For example, mucus-associated bacteria are believed to regulate the settlement and/or growth of opportunist microbes by occupying space or producing effective antibiotics.64–66 Alterations in ambient conditions, such as water temperature and nutrient concentrations, have been shown to induce shifts in the associated microbes or microbiome of a coral.67,68 These shifts can be the result of both direct and indirect effects of inorganic nutrients. For example, tank experiments suggest that addition of inorganic N can induce growth of potential bacterial pathogens.68,69 An increase in nutrients also can stimulate growth of macroalgae and turf algae,70 which have been shown to have multiple negative effects on the coral microbiome, such as depletion of local oxygen concentrations,37,71,72 transferal of allelotoxins,73–76 and transmission or vectoring of pathogens.77,78 Shifts in the microbiome can ultimately lead to coral health declines and sometimes death.37,62,79
Pathogens
Coral disease has increased in prevalence in the Caribbean, with as much as 20% of reefs affected in some places.80 While the Pacific has not yet experienced the devastating consequences of coral diseases, it is clear that many diseases are present, and the problem is expected to grow with environmental change (e.g., see Refs. 81 and 82). For example, at least seven diseases have been documented in Australia’s Great Barrier Reef, including cyanobacterial, protozoan, and Vibrio spp. infections.80 The impacts of disease on corals can be profound, ranging from minor tissue loss to entire-colony mortality. For example, in the 1980s, the two dominant Acroporid species, Acroporid palmata and Acroporid cervicornis, experienced Caribbean-wide die-offs owing to white band disease, with estimates reaching as high as 95% of colonies lost.83,84 Such losses are unprecedented and have led to dramatic management responses, including the listing of both taxa under the Endangered Species Act.
Recent work has started to link certain environmental conditions,30,54,85 as well as a changing climate, to the emergence of disease.86,87 However, we understand very little about reservoirs for coral disease. One such likely reservoir for pathogens is sewage. In fact, sewage effluent has been identified as the source of the pathogen complex that causes white pox disease in Caribbean corals.88–90 Using Koch’s postulates, Patterson et al.88 first identified Serratia marcescens as the disease-causing agent for white pox disease. At the time of this study, the elkhorn coral, A. palmata, was experiencing a major die-off in the Florida Keys, with more than 70% of coral cover lost owing to white pox disease.88 During a subsequent outbreak of white pox disease in 2003, a unique strain of S. marcescens was identified (PDR60) from samples taken from live A. palmata, as well as two other species of non-Acroporid corals, reef water, and nearby sewage sources.89
In their most recent publication, Sutherland et al.90 used experimental laboratory manipulations to demonstrate that sewage was indeed the source of the disease, and that a human strain of the pathogen was the causal agent. These findings marked the first time that a human pathogen has been demonstrably transmitted to a marine invertebrate, providing strong evidence for the linkage between sewage exposure and disease in the marine environment. While evidence showing that sewage is an important disease reservoir is limited to one type of disease and its associated causal agent, the potential for discovery of more examples is considerable, given the sheer numbers of microbes and viruses present in the average human gut and consequently in the average sewage effluent (e.g., see Refs. 91–93).
Endocrine disrupters
Endocrine disrupters are common pollutants in coastal waters. They include both natural and synthetic estrogens, polychlorinated biphenyls (PCBs), plasticizers, pharmaceuticals, parabens, phthalates, dioxins, petrochemicals, organochlorinated pesticides, microplastics, and detergents.94–98 Endocrine disrupters are chemicals with the ability to disrupt the endocrine or hormone system in living organisms. They can act on multiple processes in animals, including reproduction, immune response, and growth.99 Endocrine disrupters are commonly identified in sewage effluent delivered by human excretion,96 as well as through general household wastewater. They have also been detected in sediments adjacent to coral reefs.95,100,101
Both distance from the source of sewage and the physical characteristics of an area affect the concentrations of endocrine disrupters.96,100,102 As is the case for some other pollutants, well-flushed areas have lower concentrations of endocrine disrupters, whereas areas that are enclosed, or semi-enclosed, tend to have higher concentrations.96 Studies on the effects of endocrine disrupters on corals have shown that impacts are similar to those they have on other organisms (i.e., suppressing growth and reproduction).95,103 Early work on understanding the role of endocrine disrupters, specifically steroidal estrogens, established the presence of estrogens in the water column and in the tissues and skeletons of corals.96,104–106 Subsequent studies demonstrated that corals take up estrogens, incorporate them into their tissues and skeletons, and metabolize them.103 The metabolic mechanisms are poorly understood, but what has been shown is that certain estrogens affect coral reproductive abilities, growth rates, and morphological features. For example, Tarrant et al.95 showed that additions of estradiol to Montipora spp. over 3 weeks resulted in a 29% reduction of egg–sperm bundles, whereas additions of estrone to Porites spp. over 2–8 weeks slowed growth rates by 13–24%. Tarrant et al.95 also added estrone to Montipora spp. nubbins over several weeks, and found an increase in tissue thickness. Much more study is needed to better understand these dynamics, so that informed strategies for minimizing exposure to these and other endocrine disrupters can be developed.
Suspended solids and sedimentation
Both suspended solids and sediments accompany sewage discharge and are threats to coral health.25,107–110 Sewage typically contains high concentrations of suspended solids, primarily organic. Suspended solids increase turbidity and block sunlight, which can reduce growth of coral symbionts.108,111,112 Corals may survive for many days under severely reduced sunlight, but after a few weeks, excessive shading can result in reduced photosynthetic activity, growth, and, ultimately, coral cover.113 When chronic shading owing to increased suspended solids occurs, this can result in coral depth distribution shifts.114 Thus, the impact of suspended solids on corals will depend on how long solids remain in the water column and how much sunlight they block.
High rates of sedimentation may also co-occur with sewage discharge, especially coinciding with storm events.115 The range of impacts from prolonged sediment cover includes shading and thus suppression of food production by coral symbionts, smothering of corals,108,116,117 energetic losses owing to effort spent to reject sediments,118 and disease.110,119 Corals differ in their susceptibility to sedimentation based on differences in morphology,117,120,121 size,122 and ability to reject sediments.120 Regardless of any coping mechanisms that corals may have, sedimentation impacts are pervasive. Fabricius conducted an extensive review on field studies that provided evidence that sedimentation has negatively affected reefs across all major coral reef geographies (see Table1 in Ref. 107). This work also highlighted specific stress responses of individual corals (e.g., reduced growth rates, reduced calcification, and increased mortality), communities (e.g., reduction in species richness and coral cover), and ecosystems (e.g., net productivity and accretion rates) to different levels of sedimentation.
Besides the physical stress that sedimentation and suspended solids can generate, there may also be chemical stress generated, especially from sewage-derived sediments, because they contain a wide range of compounds. For instance, suspended solids associated with sewage that eventually settle on corals often have a different profile, both in chemical composition and toxicology, from those originating from other sources, such as agricultural runoff and natural erosion flows.24 Suspended solids may contain toxic compounds and high levels of nutrients, each of which can result in negative responses in corals, such as disease and mortality.25,123 The highly organic particles derived from sewage can chemically stress corals by greatly increasing biological oxygen demand in surrounding waters, as bacterial consumption of oxygen rises with increasing availability of organic material.25,123
Heavy metals
Heavy metals are commonly present in sewage worldwide.124 Metals routinely found in sewage include mercury, lead, cadmium, chromium, copper, nickel, zinc, cobalt, and iron.124,125 In general, increasing levels of heavy metals in the tissues of organisms interfere with metabolism and influence the activity of a wide range of enzymes, suppressing important physiological processes, such as respiration and nerve communication. Numerous studies have shown that exposure to elevated levels of metals can result in coral mortality, bleaching, and decreased fertilization success.126,127 Heavy metals also have the potential to damage corals by increasing success of certain microbes. For example, Fe2+, which is common in raw sewage, plays an important role in increasing both the virulence of pathogenic microbes (e.g., Vibrio spp.), and the growth rates of microalgae. This occurs because Fe2+ is a limiting nutrient for microbe reproduction, and thus its addition leads to increased microbial growth.128 When iron is in excess and freely available, it is taken up by pathogenic microbes, allowing them to further multiply and increase their success in attacking and infecting live corals.128 Finally, increases in this essential bacterial micronutrient have been implicated in altering reef community structure and function in extremely oligotrophic environments, such as isolated coral atolls.129
Other toxins
The range of other toxins potentially present in sewage is wide, but which toxins actually are present is dependent on local conditions, such as type and abundance of local industries and agriculture. Chemicals commonly found in sewage beyond the metals and endocrine disrupters discussed above include PCBs, chlorine, pesticides, herbicides, petroleum hydrocarbons, and pharmaceuticals.24,25,130–132 Numerous laboratory studies and field studies have examined the impacts of these toxins on corals. This work was summarized by van Dam et al.,133 who reported that the response of corals depended both on the type of toxin and its concentration, with responses varying from mortality, to bleaching, to reduced lipid concentrations (see Table1 for examples of responses).
Field evidence linking sewage exposure and coral reef health
The section above reviews the impacts that individual components of sewage have on coral reef health and suggests that sewage as a whole has the potential to have strong negative impacts. However, this prediction is based on studies that did not experimentally expose corals in the field to sewage. To evaluate the findings of field experiments and observational studies assessing the effects of sewage and its constituents on coral reefs, we conducted a search of the literature (Web of Science with following search terms: TOPIC: “coral reef*” and TOPIC: “sewage” and TOPIC: “pollution”). Remarkably, we did not find one experimental field study that investigated impacts of sewage on coral reef health. Most studies looking at linkages between sewage and coral reefs focused on identifying indicators of sewage presence and intensity, rather than on the actual impacts of sewage on coral reef constituents, the general untested assumption being that sewage had a negative impact, and so should be monitored and abated.134–139 We did, however, identify eight observational studies that surveyed coral reef areas with substantial sewage input and compared them to nearby, environmentally similar areas with little or no known suspected sewage input.115,140–147
In each of these correlational studies, scientists investigated how the condition of coral reef communities varied with decreased water quality (e.g., fecal coliform counts, turbidity, and inorganic nutrients) associated with sewage outflows. In seven of the eight studies, a negative impact of sewage on reefs was implicated, and, in one study, no effect was suggested. Below, I briefly review the findings of these studies. Caution should be taken in interpreting the results of these studies, as none used the most robust design (i.e., before–after–control–impact)148 for correlational testing of contaminant effects. Nonetheless, taken together, their quantitative results allow us to make informed hypotheses about the probable impacts of sewage on coral health.
Two of these observational studies focused on the incidence of coral disease in response to sewage exposure. Kaczmarsky et al.141 examined two different sites in St. Croix, U.S. Virgin Islands—a sewage-impacted site, and an ecologically and geologically similar site nearby with no known sewage exposure. Water quality sampling by the Virgin Islands Department of Planning and Natural Resources showed high counts of fecal coliforms (1460/100 mL) after a sewage overflow event at the sewage-impacted site, but no indication of fecal coliforms (0/100 mL) at the nonimpacted site (approximately 1.5 km from the sewage pipe). The authors conducted surveys to determine the prevalence of black band disease and white plague type II at both sites, and found significantly (P < 0.0001) more disease cases at the sewage-impacted sites, with 7 of the 10 species surveyed showing an increased incidence of disease. Redding et al.147 reported similar trends of increasing coral disease with exposure to sewage. In this study on reefs in Guam, the authors found that increasing sewage (estimated from measurements of sewage-derived N) correlated significantly with increases in white syndrome disease on Porites spp. and that the level of δ15N was a strong predictor of severity of this disease.147
Five other field studies implicated increased sewage exposure as the factor generating inferred changes in community structure on reefs, with the most common responses being an increase in macroalgae and a decrease in coral cover.115,144,146 For example, a study examining two bays in Thailand, one sewage impacted and the other not, found that the sewage-impacted bay had significant increases in turbidity and inorganic nutrients.115,143 The authors then correlated these differences to changes at multiple ecological levels of organization in the nearby coral reef community, including increased macroalgal density and diversity, reduced cover of reef-building corals, and reductions in fish abundance on the reef.115,143 Similarly, a study of reefs in Taiwan that examined the impacts of sewage found that higher levels of sewage (as estimated by measurements of nutrient and suspended sediment levels) were linked to algal blooms and sediment smothering of corals in shallow areas.145 Finally, during a bleaching event in 1995, scientists examined the interactions between bleaching and sewage pollution in Curaçao and found that the highest levels of coral tissue mortality occurred on reefs chronically exposed to sewage.142
Our search yielded only one published field study purporting to find no detectable effect of sewage outflow on coral communities. Grigg used a control–impact design to investigate effects of sewage outflow coming from pipes deployed in the coastal waters of Hawaii.140 Grigg stated that there were no statistically significant impacts of sewage outflow on coral species richness and cover,140 a negative result that has been cited over 180 times in the literature. Close examination of the methods and results of Grigg,140 however, call into question this inference and thus challenge the wisdom and rigor of the widespread use of the conclusions of this paper in the scientific literature. Specifically, for the case of coral cover, no statistical results were reported in the figures, tables, or text. In addition, visual inspection of the differences in coral cover at shallow depths (Fig. 1 in Ref. 140) next to outflow pipes versus coral cover in control sites suggests the opposite effect—significantly less coral cover around outflow pipes. These concerns, along with the fact that there were no before–after data, suggest that Grigg’s strongly worded conclusions140 that sewage does not impact coral reef ecosystems should be reevaluated.
Figure 1.
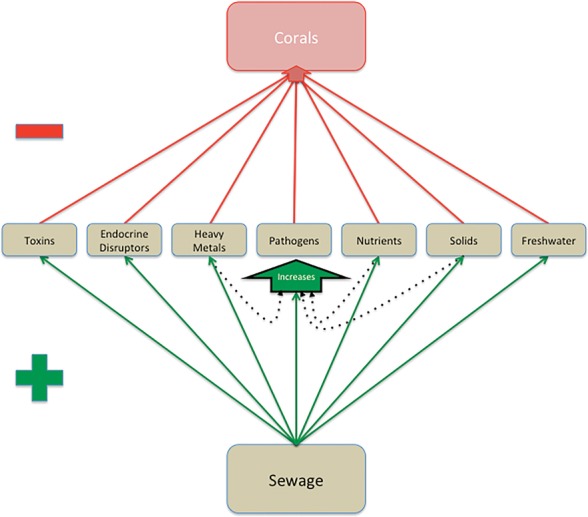
Interaction diamond illustrating impacts of sewage on concentrations of known stressors to corals and the positive feedbacks those stressors can have.
In summary, seven of eight of these observational field studies show positive correlations between increasing sewage concentration on reefs and increasing coral disease and degradation of coral reef communities. The eighth study reports no effect; however, we have concerns about the analysis and interpretation of data provided. Future investigations should use both experimental manipulations of sewage presence in the field and more rigorously designed before–after–control–impact studies148 to test for this putative causal relationship. Furthermore, new studies should (1) employ varying degrees of sewage exposure, in order to produce a functional relationship between increasing sewage concentration and metrics of coral health and reef community condition; and (2) measure concentrations of as many sewage-associated toxins as possible to help begin to decipher which toxin(s) within sewage is most correlated with declines in coral health.
Synergistic impacts of sewage
When organisms experience multiple stressors, synergistic impacts can occur.149 In particular, exposure to multiple stressors has been cited as a key factor in habitat loss in marine ecosystems150,151 and to decreasing growth rates in many marine species (e.g., see Refs. 149 and 152).
This is an important point, because sewage discharge is often mischaracterized as a single stressor in coral reef management. This review challenges that view and documents that sewage is a conglomerate of many potentially toxic and distinct coral and coral reef stressors, including freshwater, inorganic nutrients, pathogens, endocrine disrupters, suspended solids, sediments, heavy metals, and other toxins. Given the high number of individual stressors found in sewage and that the negative impacts of many of these pollutants are likely to combine at least additively because of positive feedbacks (see Fig. 1 and discussion below), we argue that sewage should be viewed primarily as a multiple, rather than a single stressor.
We propose a conceptual model to highlight common direct and indirect negative impacts that stressors found in sewage can have on corals (Fig. 1). This model also highlights common directional interactions that those stressors may have with each other, and therefore additionally points out opportunities for positive feedbacks, additive effects, and subsequent multiple-stressor effects. For example, sedimentation generated by sewage can stress corals and deplete their energy resources, resulting in increased susceptibility to pathogens that are found in high concentrations in sewage.107,153 Sediment-facilitated coral disease has the potential to be fueled to an even greater degree by increased nutrients54 derived from sewage. The most important conclusion that can be taken away from this model is that the pathways for multiple-stressor effects generated by the multitude of component pollutants within sewage are high both in diversity and abundance, making sewage a potentially lethal cocktail for coral reefs.
In addition to the synergistic effects that can occur among the component stressors found within sewage, there is also the strong possibility for synergistic interactions between sewage discharge and the many non-sewage stressors that affect coral reefs worldwide. For example, warming seas are hypothesized to play a role in facilitating disease outbreaks by increasing the susceptibility of coral to disease through temperature stress and increasing the virulence of pathogens.80,154 Evidence to support this hypothesis is present in recent work examining temperature anomalies and disease outbreaks.86,155 Furthermore, overfishing can lead to release of small corallivores from predatory control, such that they increase surface wounds on corals.156 Increased wounding of corals is subsequently followed by greater disease susceptibility in these foundation species.136,157–159 Sewage discharge, through introduction of heavy metals and inorganic nutrients, could also interact with ocean warming and acidification to decrease coral growth and reproduction in an additive or synergistic way.87,160 These interactions with sewage are likely to lead to greater declines in coral cover and ultimately more disease, as stressed corals are more susceptible to disease.87,160 We would expect sewage impacts to be strongest in areas in close proximity to human populations, especially in areas with low flushing.96
A common mechanism leading to synergies between stressor impacts in both of these examples is that non-sewage stressors increase susceptibility to infection, while the addition of sewage renders disease delivery more likely and disease progression more rapid. The various effects that combined anthropogenic stressors have on the complex microbial community in the surface mucous layer of corals have not been well explored. As we learn more about the role this mucous layer plays in coral health, we may learn that even small disturbances have the potential to tip the balance in favor of more harmful bacteria and viruses, ultimately leading to serious outbreaks of coral disease. Given the high potential for these synergistic interactions to occur when stress levels are high, future scientific studies and conservation efforts focused on sewage discharge should take their potential occurrence into careful consideration.
How extensive is the sewage discharge problem?
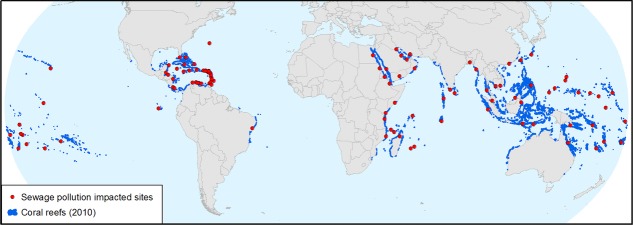
We conducted a literature review to determine how many coral reef geographies had a documented sewage pollution problem. Using the World Atlas of Coral Reefs7 list of coral reef geographies, we conducted a Web of Science search with the following terms: TOPIC: “coral reef*” and TOPIC: “sewage” and TOPIC: “pollution” and TOPIC: “Location Name” (e.g., “Bahamas”). We identified the majority of our cases of sewage-impacted coral reef geographies in this way, with the remainder identified through a Google search using the same key words. In these cases, we typically found a local government report, but a few were noted only in newspaper articles. Our review revealed that, for almost every coral reef geography, raw or partially treated sewage is polluting the local environment. Figure 2 illustrates the spatial extent of the sewage contamination problem in the tropics, and clearly shows that no region is immune to this problem. Of the 112 coral reef geographies, including territories, states, and countries, 104 have documented sewage contamination problems, with the majority having documentation of direct ocean discharge. Only three of those geographies are uninhabited, and therefore have no potential for sewage contamination. Although the amount of sewage discharged into the environment is difficult to quantify with accuracy, this survey reveals that the spatial extent of the problem is global in that it occurs in almost all coral reef geographies. However, the magnitude of the problem in a particular place is not represented in this assessment.
Figure 2.
Global map showing 104 of 112 distinct coral reef geographies listed in the World Atlas of Coral Reefs7 (including 80 countries, 6 states, and 26 territories) with documented coastal sewage pollution problems.
The ways by which sewage reaches waters bathing coral reefs are diverse, including intentional sewage contamination through direct-discharge outfall pipes (e.g., Hollywood, Florida sewage outfall),161 and treatment systems that allow sewage overflows or bypasses during rain events or system failures (e.g., U.S. Virgin Islands Frederiksted sewage bypass outfall).141 Unintended sewage contamination also often occurs through faulty systems, attributable to engineering design flaws, especially inadequate capacity for flooding waters, a leaking infrastructure, shifts in soils and rock that surround the sewerage system, or lack of maintenance.162 Even when state-of-the-art sewage treatment plants are installed, the governments of developing countries often do not have the staff or long-term funding to properly maintain the facility; thus, these facilities often fall into disrepair, leaving the communities to once again deal with a sewage problem.162,163
Along with the faulty sewer and sewage treatment systems comes the issue of a widespread lack of proper sanitation. There are 2.4 billion people without access to sanitation, many in tropical, developing countries.163 This lack of proper sanitation is linked to public health problems, including significant illness and death rates associated with diarrheal disease in developing countries.164,165 There are many geographies where the ocean is used as a toilet in common practice (open defecation), with this disposal method widely socially accepted.163 While there is much progress being made on the Millennium Development Goals,166 which are specifically working to address the lack of access to sanitation, there is still much work to be done to reduce overall sewage contamination in the environment. The World Health Organization expects to fall short of its sanitation goal in 2015 by half a billion people.163 As human populations continue to grow and sea level continues to rise, the problem of sewage contamination in the environment will persist in the absence of truly significant interventions and likely grow as a function of human population growth.
Research and conservation recommendations
This review documents sewage discharge as a global and intense threat to coral reefs. Remarkably, despite the extent of this threat, both scientists and conservationists have paid relatively less attention (e.g., in comparison to overfishing) to understanding and abating sewage impacts on coral reefs. This is surprising because it is well documented that sewage contains a range of contaminants that individually are known stressors of coral reef ecosystems. Furthermore, the additive or synergistic impacts of these multiple contaminants have the potential to combine with one another and with other stressors beyond sewage, such as warming waters, to accelerate coral reef ecosystem declines. Mitigating this growing global threat will require future research that focuses on (1) understanding tolerance thresholds that corals have to sewage exposure, evaluating individual contaminants as well as additive and synergistic combinations of contaminants; (2) quantifying the spatial extent and magnitude of the sewage discharge problems; and, most importantly, (3) testing both proactive and reactive strategies that can be employed to reduce the adverse impacts of the massive amounts of human sewage that enter tropical coastal waters. Pursuing only advanced treatment options for sewage systems is not an appropriate, viable solution to this problem. In many cases, this approach is not even feasible because of high costs. We must think creatively to solve this problem, by forging partnerships among human health organizations, sewage infrastructure and treatment experts, entrepreneurial groups, and development and environmental conservation organizations. Sewage pollution is a global threat that humans and coral reefs share. Combining forces across organizations in traditionally non-interacting sectors (e.g., conservation and economic development) is essential if we are to address the strain of human sewage in our reef systems and their associated human communities.
Acknowledgments
This work was funded in part from the following sources: the Nature Conservancy’s NatureNet Fellowship to S. Wear and a National Science Foundation Grant (OCE-1130786) to R. Vega Thurber. Special thanks go to P. Kareiva, R. Noble, C. Peterson, B. Silliman, and two anonymous reviewers for critical reviews and improving the manuscript, and C. Adams and T. Boucher for assistance in developing the sewage pollution map.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Fisher R, O’Leary RA, Low-Choy S, et al. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 2015;25:500–505. doi: 10.1016/j.cub.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Carté BK. Biomedical potential of marine natural products. Bioscience. 1996;46:271–286. [Google Scholar]
- Peterson CH. Lubchenco J. Marine ecosystem services. In: Daily GC, editor; Nature’s Services: Societal Dependence on Natural Ecosystems. Washington, DC: Island Press; 1997. pp. 1–9. [Google Scholar]
- Moberg F. Folke C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999;29:215–233. [Google Scholar]
- Barbier EB, Hacker SD, Kennedy C, et al. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011;81:169–193. [Google Scholar]
- Cesar H, Burke L. Pet-Soede L. The Economics of Worldwide Coral Reef Degradation. Zeist, the Netherlands. Cesar Environmental Economics Consulting. 2003 [Google Scholar]
- Spalding M, Ravilious C. Green EP. World Atlas of Coral Reefs. Los Angeles: University of California Press; 2001. p. 424. [Google Scholar]
- Burke L, Reytar K, Spalding M. Perry A. Reefs at Risk Revisited. Washington, DC: World Resources Institute; 2011. [Google Scholar]
- De’ath G, Fabricius KE, Sweatman H. Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. U.S.A. 2012;109:17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JBC, Donovan MK, Cramer KL, editors; Lam VV, editor. Status and Trends of Caribbean Coral Reefs. Gland, Switzerland: Global Coral Reef Monitoring Network, IUCN; 2014. pp. 1970–2012. [Google Scholar]
- Bruno JF. Selig ER. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One. 2007;2:e711. doi: 10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KE, Abrar M, Aeby G, et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321:560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Berkelmans R. Oliver JK. Large-scale bleaching of corals on the Great Barrier Reef. Coral Reefs. 1999;18:55–60. [Google Scholar]
- Spencer T, Teleki KA, Bradshaw C. Spalding MD. Coral bleaching in the southern Seychelles during the 1997–1998 Indian Ocean warm event. Mar. Poll. Bull. 2000;40:569–586. [Google Scholar]
- Bruno JF, Siddon CE, Witman JD, Colin PL, et al. El Niño related coral bleaching in Palau, Western Caroline Islands. Coral Reefs. 2001;20:127–136. [Google Scholar]
- McClanahan T, Muthiga N. Mangi S. Coral and algal changes after the 1998 coral bleaching: interaction with reef management and herbivores on Kenyan reefs. Coral Reefs. 2001;19:380–391. [Google Scholar]
- Bryant D, Burke L, McManus J. Spalding M. Reefs at Risk: Map-Based Indicator of Threats to the World’s Coral Reefs. Washington, DC: World Resources Institute; 1998. [Google Scholar]
- Knowlton N. Jackson JB. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008;6:e54. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- Halpern BS, Walbridge S, Selkoe KA, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- Doty MS. The ecology of Honaunau Bay, Hawaii. Univ. Hawaii Bot. Sci. Paper. 1969;14:1–221. [Google Scholar]
- Banner AH. Proceedings of the 2nd International Symposium Coral Reefs. Vol. 2. Brisbane: 1974. Kaneohe Bay, Hawaii: urban pollution and a coral reef ecosystem; pp. 685–702. [Google Scholar]
- Pastorok RA. Bilyard GR. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 1985;21:175–189. [Google Scholar]
- Islam S. Tanaka M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar. Poll. Bull. 2004;48:624–649. doi: 10.1016/j.marpolbul.2003.12.004. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme. 1994. . Regional overview of land-based sources of pollution in the wider Caribbean region. UNEP Caribbean Environment Programme. CEP Technical Report No. 33. Kingston.
- United Nations Environment Programme. 2006. . The state of the marine environment: regional assessments. Global Programme of Action for the Protection of the Marine Environment from Land-based Activities. Hague, the Netherlands.
- Gerland P, Raftery A, Ševčíková H, et al. Wilmoth. World population stabilization unlikely this century. Science. 2014;346:234–237. doi: 10.1126/science.1257469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, De’ath G, McCook L, et al. Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Poll. Bull. 2005;51:384–398. doi: 10.1016/j.marpolbul.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Vega Thurber R, Burkepile DE, Fuchs C, et al. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global Change Biol. 2014;20:544–554. doi: 10.1111/gcb.12450. [DOI] [PubMed] [Google Scholar]
- Pantsar-Kallio M, Mujunen SP, Hatzimihalis G, et al. Multivariate data analysis of key pollutants in sewage samples: a case study. Anal. Chim. Acta. 1999;393:181–191. [Google Scholar]
- Coles SL. Jokiel PL. Pollution in Tropical Aquatic Systems. London: CRC Press; 1992. “Effects of salinity on coral reefs; pp. 147–166. & ” In D.W. Connell & D.W. Hawker, Eds.: [Google Scholar]
- Jokiel PL, Hunter CL, Taguchi S. Watarai L. Coral Reefs. Vol. 12. 1993. Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu, Hawaii; pp. 177–184. [Google Scholar]
- Thacker R, Ginsburg D. Paul V. Effects of herbivore exclusion and nutrient enrichment on coral reef macroalgae and cyanobacteria. Coral Reefs. 2001;19:318–329. [Google Scholar]
- Hunter CL. Evans CW. Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bull. Mar. Sci. 1995;57:501–515. [Google Scholar]
- Lapointe BE. Nutrient thresholds for bottom-up control of macroalgal blooms and coral reefs. Limnol. Oceanogr. 1997;44:1586–1592. [Google Scholar]
- Smith JEM, Shaw RA, Edwards D, et al. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol. Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- Hughes T, Szmant AM, Steneck R, et al. Algal blooms on coral reefs: what are the causes. Limnol. Oceanogr. 1999;44:1583–1586. [Google Scholar]
- Smith J, Smith C. Hunter C. An experimental analysis of the effects of herbivory and nutrient enrichment on benthic community dynamics on a Hawaiian reef. Coral Reefs. 2001;19:332–342. [Google Scholar]
- Burkepile DE. Hay ME. Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology. 2006;87:3128–3139. doi: 10.1890/0012-9658(2006)87[3128:hvncom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ostrander GK, Armstrong KM, Knobbe ET, et al. Rapid transition in the structure of a coral reef community: the effects of coral bleaching and physical disturbance. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5297–5302. doi: 10.1073/pnas.090104897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MJ, Paredes GA, Sala E. Jackson JB. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol. Lett. 2006;9:1216–1227. doi: 10.1111/j.1461-0248.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Sweatman H, Precht WF, et al. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology. 2009;90:1478–1484. doi: 10.1890/08-1781.1. [DOI] [PubMed] [Google Scholar]
- Lirman D. Competition between macroalgae and corals: effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral Reefs. 2001;19:392–399. [Google Scholar]
- McCook L. Competition between corals and algal turfs along a gradient of terrestrial influence in the nearshore central Great Barrier Reef. Coral Reefs. 2001;19:419–425. [Google Scholar]
- Kinsey DW. Davies PJ. Effects of elevated nitrogen and phosphorus on coral reef growth. Limnol. Oceanogr. 1979;24:935–940. [Google Scholar]
- Koop K, Booth D, Broadbent A, et al. ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar. Pollut. Bull. 2001;42:91–120. doi: 10.1016/s0025-326x(00)00181-8. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Rodrigues MJ, Bellwood DR, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Williams I. Polunin N. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs. 2001;19:358–366. [Google Scholar]
- Wooldridge SA. Done TJ. Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 2009;19:1492–1499. doi: 10.1890/08-0963.1. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Kramer P. Van Woesik R. Species composition, habitat, and water quality influence coral bleaching in southern Florida. Mar. Ecol. Prog. Ser. 2010;408:65–78. [Google Scholar]
- Haapkylä J, Unsworth RK, Flavell M, et al. Seasonal rainfall and runoff promote coral disease on an inshore reef. PLoS One. 2011;6:e16893. doi: 10.1371/journal.pone.0016893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarsky L. Richardson LL. Do elevated nutrients and organic carbon on Philippine reefs increase the prevalence of coral disease? Coral Reefs. 2011;30:253–257. [Google Scholar]
- Bruno JF, Petes LE, Harvell CD. Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 2003;6:1056–1061. [Google Scholar]
- Voss JD. Richardson LL. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs. 2006;25:569–576. [Google Scholar]
- Dinsdale EA, Pantos O, Smriga S, et al. Microbial ecology of four coral atolls in the Northern Line Islands. PLoS One. 2008;3:e1584. doi: 10.1371/journal.pone.0001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantz AA. Burkepile DE. Context-dependent effects of nutrient loading on the coral-algal mutualism. Ecology. 2014;95:1995–2005. doi: 10.1890/13-1407.1. [DOI] [PubMed] [Google Scholar]
- Marubini F. Davies PS. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar. Biol. 1996;127:319–328. [Google Scholar]
- Wooldridge SA. A new conceptual model for the warm-water breakdown of the coral–algae endosymbiosis. Mar. Freshwater Res. 2009;60:483–496. [Google Scholar]
- Lesser MP. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceangr. 1996;41:271–283. [Google Scholar]
- Gil MA. Unity through nonlinearity: a unimodal coral–nutrient interaction. Ecology. 2013;94:1871–1877. doi: 10.1890/12-1697.1. [DOI] [PubMed] [Google Scholar]
- Rosenberg E, Koren O, Reshef L, et al. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- Ainsworth TD, Vega Thurber R. Gates RD. The future of coral reefs: a microbial perspective. Trends Eco. Evol. 2010;25:233–240. doi: 10.1016/j.tree.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Ritchie KB. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 2006;322:1–14. [Google Scholar]
- Mao-Jones J, Ritchie KB, Jones LE. Ellner SP. How microbial community composition regulates coral disease development. PLoS Biol. 2010;8:e1000345. doi: 10.1371/journal.pbio.1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypien KL, Ward JR. Azam F. Antagonistic interactions among coral-associated bacteria. Environ. Microbiol. 2010;12:28–39. doi: 10.1111/j.1462-2920.2009.02027.x. [DOI] [PubMed] [Google Scholar]
- Sunagawa S, DeSantis TZ, Piceno YM, et al. Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 2009;3:512–521. doi: 10.1038/ismej.2008.131. [DOI] [PubMed] [Google Scholar]
- Vega Thurber R, Willner-Hall D, Rodriguez-Mueller B, et al. Metagenomic analysis of stressed coral holobionts. Environ. Microbiol. 2009;11:2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- Kuntz NM, Kline DI, Sandin SA. Rohwer F. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol. Prog. Ser. 2005;294:173–180. [Google Scholar]
- River GF. Edmunds PJ. Mechanisms of interaction between macroalgae and scleractinians on a coral reef in Jamaica. J. Exp. Mar. Biol. Ecol. 2001;261:159–172. doi: 10.1016/s0022-0981(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Haas AF, Jantzen C, Naumann MS, et al. Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ O2 availability. Mar. Ecol. Prog. Ser. 2010;409:27–39. [Google Scholar]
- Haas AF, Nelson CE, Kelly LW, et al. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS One. 2011;6:e27973. doi: 10.1371/journal.pone.0027973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasher DB. Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9683–9688. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barott KL, Rodriguez-Brito B, Janouškovec J, et al. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ. Microbiol. 2011;13:1192–1204. doi: 10.1111/j.1462-2920.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- Barott KL, Rodriguez-Mueller B, Youle M, et al. Microbial to reef scale interactions between the reef-building coral Montastraea annularis and benthic algae. Proc. R. Biol. Soc. B. 2011;279:1655–1664. doi: 10.1098/rspb.2011.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow KM, Paul VJ, Liles MR. Chadwick NE. Allelochemicals produced by Caribbean macroalgae and cyanobacteria have species-specific effects on reef coral microorganisms. Coral Reefs. 2011;30:309–320. [Google Scholar]
- Nugues MM, Smith GW, Hooidonk RJ, et al. Algal contact as a trigger for coral disease. Ecol. Lett. 2004;7:919–923. [Google Scholar]
- Vega Thurber R, Burkepile DE, Correa AM, et al. Macroalgae decrease growth and alter microbial community structure of the reef-building coral, Porites astreoides. PLoS One. 2012;7:e44246. doi: 10.1371/journal.pone.0044246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C, Mao-Jones J, Ritchie KB, et al. How Microbial community composition regulates coral disease development. PLoS Biol. 2010;8:e1000345. doi: 10.1371/journal.pbio.1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell CD, Jordán-Dahlgren E, Merkel S, et al. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20:172–195. [Google Scholar]
- Willis BL, Page CA. Dinsdale EA. Coral disease on the Great Barrier Reef. In: Loya Y, editor; Rosenberg E, editor. Coral Health and Disease. Berlin: Springer; 2004. pp. 69–104. [Google Scholar]
- Haapkylä J, Melbourne-Thomas J, Flavell M. Willis BL. Disease outbreaks, bleaching and a cyclone drive changes in coral assemblages on an inshore reef of the Great Barrier Reef. Coral Reefs. 2013;32:815–824. [Google Scholar]
- Aronson RB. Precht WF. White-band disease and the changing face of Caribbean coral reefs. In: Porter JW, editor; The Ecology and Etiology of Newly Emerging Marine Diseases. Dordrecht, the Netherlands: Springer; 2001. pp. 25–38. [Google Scholar]
- Miller M, Bourque A. Bohnsack J. An analysis of the loss of acroporid corals at Looe Key, Florida, USA: 1983–2000. Coral Reefs. 2002;21:179–182. [Google Scholar]
- Garren M, Raymundo L, Guest J, et al. Resilience of coral-associated bacterial communities exposed to fish farm effluent. PLoS One. 2009;4:e7319. doi: 10.1371/journal.pone.0007319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Selig ER, Casey KS, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007;5:e124. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt ME. McManus JW. Disease incidence is related to bleaching extent in reef-building corals. Ecology. 2009;90:2859–2867. doi: 10.1890/08-0445.1. [DOI] [PubMed] [Google Scholar]
- Patterson KL, Porter JW, Ritchie KB, et al. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8725–8730. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland KP, Porter JW, Turner JW, et al. Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral. Acropora palmata. Environ. Microbiol. 2010;12:1122–1131. doi: 10.1111/j.1462-2920.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- Sutherland KP, Shaban S, Joyner JL, et al. Human pathogen shown to cause disease in the threatened eklhorn coral Acropora palmata. PLoS One. 2011;6:e23468. doi: 10.1371/journal.pone.0023468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DW, Gibson CJ, Lipp EK, et al. Detection of viral pathogens by reverse transcriptase PCR and of microbial indicators by standard methods in the canals of the Florida Keys. Appl. Environ. Microbiol. 1999;65:4118–4125. doi: 10.1128/aem.65.9.4118-4125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetz JJ, Lipp EK, Griffin DW, et al. Presence, infectivity, and stability of enteric viruses in seawater: relationship to marine water quality in the Florida Keys. Mar. Poll. Bull. 2004;48:698–704. doi: 10.1016/j.marpolbul.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Blinkova O, Rosario K, Li L, et al. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J. Clin. Microbiol. 2009;47:3507–3513. doi: 10.1128/JCM.01062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek AO, Vonier PM, Oberdörster E, et al. Environmental signaling: a biological context for endocrine disruption. Environ. Health Persp. 1998;106:5. doi: 10.1289/ehp.106-1533276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant AM, Atkinson MJ. Atkinson S. Effects of steroidal estrogens on coral growth and reproduction. Mar. Ecol. Prog. Ser. 2004;269:121–129. [Google Scholar]
- Singh SP, Azua A, Chaudhary A, et al. Occurrence and distribution of steroids, hormones and selected pharmaceuticals in South Florida coastal environments. Ecotoxicology. 2010;19:338–350. doi: 10.1007/s10646-009-0416-0. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Halsband C. Galloway TS. Microplastics as contaminants in the marine environment: a review. Mar. Poll. Bull. 2011;62:2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Hall NM, Berry KLE, Rintoul L. Hoogenboom MO. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015;162:725–732. [Google Scholar]
- Snyder SA, Westerhoff P, Yoon Y. Sedlak DL. Pharmaceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environ. Eng. Sci. 2003;20:449–469. [Google Scholar]
- Kawahata H, Ohta H, Inoue M. Suzuki A. Endocrine disrupter nonylphenol and bisphenol A contamination in Okinawa and Ishigaki Islands, Japan—within coral reefs and adjacent river mouths. Chemosphere. 2004;55:1519–1527. doi: 10.1016/j.chemosphere.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Kitada Y, Kawahata H, Suzuki A. Oomori T. Distribution of pesticides and bisphenol A in sediments collected from rivers adjacent to coral reefs. Chemosphere. 2008;71:2082–2090. doi: 10.1016/j.chemosphere.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Armoza-Zvuloni R, Kramarsky-Winter E, Rosenfeld H, et al. Reproductive characteristics and steroid levels in the scleractinian coral Oculina patagonica inhabiting contaminated sites along the Israeli Mediterranean coast. Mar. Poll. Bull. 2012;64:1556–1563. doi: 10.1016/j.marpolbul.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Tarrant AM, Blomquist CH, Lima PH, et al. Metabolism of estrogens and androgens by scleractinian corals. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2003;136:473–485. doi: 10.1016/s1096-4959(03)00253-7. [DOI] [PubMed] [Google Scholar]
- Atkinson S. Atkinson MJ. Detection of estradiol-17β during a mass coral spawn. Coral Reefs. 1992;11:33–35. [Google Scholar]
- Tarrant A, Atkinson M. Atkinson S. Uptake of estrone from the water column by a coral community. Mar. Biol. 2001;139:321–325. [Google Scholar]
- Atkinson S, Atkinson MJ. Tarrant AM. Estrogens from sewage in coastal marine environments. Environ. Health Persp. 2003;111:531. doi: 10.1289/ehp.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Poll. Bull. 2005;50:125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Rogers CS. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 1990;62:185–202. [Google Scholar]
- Fabricius KE. Wolanski E. Rapid smothering of coral reef organisms by muddy marine snow. Estuar. Coast. Shelf Sci. 2000;50:115–120. [Google Scholar]
- Pollock FJ, Lamb JB, Field SN, et al. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS One. 2014;9:e102498. doi: 10.1371/journal.pone.0102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomascik T. Sander F. Effects of eutrophication on reef-building corals. Mar. Biol. 1985;87:143–155. [Google Scholar]
- Lewis JB. Abundance, distribution and partial mortality of the massive coral Siderastrea siderea on degrading coral reefs at Barbados, West Indies. Mar. Poll. Bull. 1997;34:622–627. [Google Scholar]
- Anthony KRN. A tank system for studying benthic aquatic organisms at predictable levels of turbidity and sedimentation: case study examining coral growth. Limnol. Oceanogr. 1999;44:1415–1422. [Google Scholar]
- Sheppard C. Coral populations on reef slopes and their major controls. Mar. Ecol. Prog. Ser. 1982;7:83–115. [Google Scholar]
- Reopanichkul P, Schlacher TA, Carter RW. Worachananant S. Sewage impacts coral reefs at multiple levels of ecological organization. Mar. Poll. Bull. 2009;58:1356–1362. doi: 10.1016/j.marpolbul.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Roy KJ. Smith SV. Sedimentation and coral reef development in turbid water: Fanning Lagoon. Pac. Sci. 1971;25:234–248. [Google Scholar]
- Rogers CS. Sublethal and lethal effects of sediments applied to common Caribbean reef corals in the field. Mar. Poll. Bull. 1983;14:378–382. [Google Scholar]
- Telesnicki GJ. Goldberg WM. Effects of turbidity on the photosynthesis and respiration of two south Florida reef coral species. Bull. Mar. Sci. 1995;57:527–539. [Google Scholar]
- Brandt ME, Smith TB, Correa AM. Vega Thurber R. Disturbance driven colony fragmentation as a driver of a coral disease outbreak. PLoS One. 2013;8:e57164. doi: 10.1371/journal.pone.0057164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak RPM. Elgershuizen JHBW. Patterns of oil-sediment rejection in corals. Mar. Biol. 1976;37:105–113. [Google Scholar]
- Dryer S. Logan A. Holocene reefs and sediments of Castle Harbor, Bermuda. J. Mar. Res. 1978;36:399–425. [Google Scholar]
- Dodge RE. Vaisnys JR. Coral populations and growth patterns: responses to sedimentation and turbidity associated with dredging. J. Mar. Res. 1977;35:715. [Google Scholar]
- Johannes RE. Pollution and degradation of coral reef communities. Elsevier Oceanogr. Ser. 1975;12:13–51. [Google Scholar]
- Grillo V, Parsons ECM. Shrimpton JH. 2001. pp. 3–16. & A review of sewage pollution in Scotland and its potential impacts on harbor porpoise populations. Paper presented to the Scientific Committee at the 53rd Meeting of the International Whaling Commission in London.
- Ščančar J, Milačič R, Stražar M. Burica O. Total metal concentrations and partitioning of Cd, Cr, Cu, Fe, Ni and Zn in sewage sludge. Sci. Total Environ. 2000;250:9–19. doi: 10.1016/s0048-9697(99)00478-7. [DOI] [PubMed] [Google Scholar]
- Howard LS. Brown BE. Heavy metals and reef corals. Oceanogr. Mar. Biol. Annu. Rev. 1984;22:195–210. [Google Scholar]
- Reichelt-Brushett AJ. Harrison PL. The effect of copper, zinc and cadmium on fertilization success of gametes from scleractinian reef corals. Mar. Poll. Bull. 1999;38:182–187. [Google Scholar]
- Griffiths E. Iron and bacterial virulence—a brief overview. Biol. Met. 1991;4:7–13. doi: 10.1007/BF01135551. [DOI] [PubMed] [Google Scholar]
- Kelly LW, Barott KL, Dinsdale E, et al. Black reefs: iron-induced phase shifts on coral reefs. ISME J. 2012;6:638–649. doi: 10.1038/ismej.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton CG. Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Persp. 1999;107:907. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel S, Berger U, Jensen E, et al. Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø, Norway with emphasis on ibuprofen and its metabolites. Chemosphere. 2004;56:583–592. doi: 10.1016/j.chemosphere.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Fang TH, Nan FH, Chin TS. Feng HM. The occurrence and distribution of pharmaceutical compounds in the effluents of a major sewage treatment plant in Northern Taiwan and the receiving coastal waters. Mar. Poll. Bull. 2012;64:1435–1444. doi: 10.1016/j.marpolbul.2012.04.008. [DOI] [PubMed] [Google Scholar]
- van Dam JW, Negri AP, Uthicke S. Mueller JF. Chemical pollution on coral reefs: exposure and ecological effects. In: Mann RM, editor; Sanchez-Bayo F, van den Brink PJ, editors. Ecological Impact of Toxic Chemicals. Bentham Science Publishers Ltd; 2011. pp. 187–211. [Google Scholar]
- Costanzo SD, O’Donohue MJ, Dennison WC, et al. A new approach for detecting and mapping sewage impacts. Mar. Poll. Bull. 2001;42:149–156. doi: 10.1016/s0025-326x(00)00125-9. [DOI] [PubMed] [Google Scholar]
- McKenna SA, Richmond RH. Roos G. Assessing the effects of sewage on coral reefs: developing techniques to detect stress before coral mortality. Bull. Mar. Sci. 2001;69:517–523. [Google Scholar]
- Baker DM, MacAvoy SE. Kim K. Relationship between water quality, δ 15N, and aspergillosis of Caribbean sea fan corals. Mar. Ecol. Prog. Ser. 2007;343:123–130. [Google Scholar]
- Bonkosky M, Hernandez-Delgado EA, Sandoz B, et al. Detection of spatial fluctuations of non-point source fecal pollution in coral reef surrounding waters in southwestern Puerto Rico using PCR-based assays. Mar. Poll. Bull. 2009;58:45–54. doi: 10.1016/j.marpolbul.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Risk MJ, Lapointe BE, Sherwood OA. Bedford BJ. The use of δ15N in assessing sewage stress on coral reefs. Mar. Poll. Bull. 2009;58:793–802. doi: 10.1016/j.marpolbul.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Baker DM, Jordán-Dahlgren E, Maldonado MA. Harvell CD. Sea fan corals provide a stable isotope baseline for assessing sewage pollution in the Mexican Caribbean. Limnol. Oceanogr. 2010;55:2139–2149. [Google Scholar]
- Grigg RW. Effects of sewage discharge, fishing pressure and habitat complexity on coral ecosystems and reef fishes in Hawaii. Mar. Ecol. Prog. Ser. 1994;103:25–34. [Google Scholar]
- Kaczmarsky LT, Draud M. Williams EH. Is there a relationship between proximity to sewage effluent and the prevalence of coral disease. Caribb J. Sci. 2005;41:124–137. [Google Scholar]
- Nagelkerken I. Relationship between anthropogenic impacts and bleaching-associated tissue mortality of corals in Curaçao (Netherlands Antilles) Rev. Biol. Trop. 2006;54:31–43. [Google Scholar]
- Reopanichkul P, Carter RW, Worachananant S. Crossland CJ. Wastewater discharge degrades coastal waters and reef communities in southern Thailand. Mar. Environ. Res. 2010;69:287–296. doi: 10.1016/j.marenvres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Jones R, Parsons R, Watkinson E. Kendell D. Sewage contamination of a densely populated coral ‘atoll’ (Bermuda) Environ. Monit. Assess. 2011;179:309–324. doi: 10.1007/s10661-010-1738-3. [DOI] [PubMed] [Google Scholar]
- Liu PJ, Meng PJ, Liu LL, et al. Impacts of human activities on coral reef ecosystems of southern Taiwan: a long-term study. Mar. Poll. Bull. 2012;64:1129–1135. doi: 10.1016/j.marpolbul.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Huang H, Li XB, Titlyanov EA, et al. Linking macroalgal δ15N-values to nitrogen sources and effects of nutrient stress on coral condition in an upwelling region. Bot. Mar. 2013;56:471–480. [Google Scholar]
- Redding JE, Myers-Miller RL, Baker DM, et al. Link between sewage-derived nitrogen pollution and coral disease severity in Guam. Mar. Poll. Bull. 2013;73:57–63. doi: 10.1016/j.marpolbul.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Stewart-Oaten A. Problems in the analysis of environmental monitoring data. In: Osenberg CW, editor; Schmitt RJ, editor. Detecting Ecological Impacts in Coastal Habitats. San Diego: Academic Press; 1996. pp. 109–131. [Google Scholar]
- Crain CM, Kroeker K. Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008;11:1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- Lotze HK, Lenihan HS, Bourque BJ, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- Silliman BR, McCoy MW, Angelini C, et al. Consumer fronts, global change, and runaway collapse in ecosystems. Annu. Rev. Ecol. Evol. Syst. 2013;44:503–538. [Google Scholar]
- Sanford E, Gaylord B, Hettinger A, et al. Ocean acidification increases the vulnerability of native oysters to predation by invasive snails. Proc. R. Biol. Soc. B. 2014;281:20132681. doi: 10.1098/rspb.2013.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. Sediment and the settlement of larvae of the reef coral, Pocillopora damicornis. Coral Reefs. 1990;9:41–43. [Google Scholar]
- Burge CA, Eakin CM, Friedman CS, et al. Climate change influences on marine infectious diseases: implications for management and society. Annu. Rev. Mar. Sci. 2014;6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- Selig ER, Harvell CD, Bruno JF. Analyzing the relationship between ocean temperature anomalies and coral disease outbreaks at broad spatial scales. In: Strong A, et al., editors; Phinney JT, Hoegh-Guldberg O, Kleypas J, Skirving W, editors. Coral Reefs and Climate Change: Science and Management. Washington, DC: American Geophysical Union; 2006. pp. 111–128. [Google Scholar]
- Burkepile DE. Hay ME. Predator release of the gastropod Cyphoma gibbosum increases predation on gorgonian corals. Oecologia. 2007;154:167–173. doi: 10.1007/s00442-007-0801-4. [DOI] [PubMed] [Google Scholar]
- Nicolet KJ, Hoogenboom MO, Gardiner NM, et al. The corallivorous invertebrate Drupella aids in transmission of brown band disease on the Great Barrier Reef. Coral Reefs. 2013;32:585–595. [Google Scholar]
- Katz SM, Pollock FJ, Bourne DG. Willis BL. Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals. Coral Reefs. 2014;33:705–716. [Google Scholar]
- Lamb JB, True JD, Piromvaragorn S. Willis BL. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Cons. 2014;178:88–96. [Google Scholar]
- Brandt ME, Ruttenberg BI, Waara R, et al. Bull. Mar. Sci. Vol. 88. USA: 2012. Dynamics of an acute coral disease outbreak associated with the macroalgae Dictyota spp; pp. 1035–1050. . in Dry Tortugas National Park, Florida, [Google Scholar]
- Project Baseline. 2014. . Hollywood Sewage Outfall. Cited December 11, 2014. http://www.projectbaseline.org/gulfstream/project-baseline-gulfstream-projects/hollywood-sewage-outfall/
- Caribbean Environment Programme of the United Nations Environment Programme. 2014. . Wastewater, Sewage, and Sanitation. Cited November 16, 2014. http://www.cep.unep.org/publications-and-resources/marine-and-coastal-issues-links/wastewater-sewage-and-sanitation.
- World Health Organization and UNICEF. Progress on Sanitation and Drinking-Water—2013 Update. Geneva, Switzerland: WHO Press; 2013. [Google Scholar]
- World Health Organization. 2004. . Facts and figures: water, sanitation and hygiene links to health. Cited December 8, 2014. http://www.who.int/water_sanitation_health/publications/factsfigures04/en/print.html.
- Prüss-Üstün A, Bos R, Gore F. Bartram J. Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health. Geneva: World Health Organization; 2008. [Google Scholar]
- United Nations. 2014. . The Millennium Development Goals Report 2014. New York. Cited December 11, 2014. http://www.un.org/millenniumgoals/2014%20MDG%20report/MDG%202014%20English%20web.pdf.