Abstract
Background
Prescription sequence symmetry analysis (PSSA) is a signal detection method for adverse drug events. Its capacity to consistently detect adverse drug events across different settings has not been tested. We aimed to determine the consistency of PSSA results for detecting positive and negative control adverse drug events across different settings.
Methods
Using a distributed network model, we analyzed prescription dispensing data using PSSA in Australia, Hong Kong, Japan, Korea, and Taiwan. Positive control was amiodarone and thyroxine, as a marker of amiodarone-induced hypothyroidism, a known adverse event with a clear temporal relationship to amiodarone initiation. Negative controls were amiodarone and allopurinol, as a marker of amiodarone-induced gout and thyroxine and allopurinol, as a marker of thyroxine-induced gout. Gout is not recorded as an adverse event in product information for either medicine. Adjusted sequence ratios (ASR) were calculated for each country. Pooled estimates were obtained by using the generic inverse variance method.
Results
A positive association was identified between amiodarone and thyroxine in all settings with a pooled ASR 2.63 (95% confidence interval (CI) 1.47–4.72). Temporal analysis showed the effect occurred within the first few weeks of treatment. No significant associations were found for the negative controls in any setting; pooled ASR were 0.76 (95%CI 0.62–0.93) and 0.98 (95%CI 0.85–1.12) for amiodarone-allopurinol and thyroxine-allopurinol, respectively.
Conclusion
Despite different health settings, different populations, and different patterns of medicine utilization, PSSA gave consistent estimates across countries for a well-known positive association and two negative control adverse events. © 2015 The Authors Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.
Keywords: prescription symmetry analysis, pharmacovigilance, amiodarone, hyperthyroidism, pharmacoepidemiology
Background
Prescription sequence symmetry analysis (PSSA) is a signal detection method for adverse drug events utilizing administrative claims data.1 The method has the potential to become a tool to assist in global pharmacosurveillance of medicines, complementary to other methods, because of its ease of application. The method employs a simple algorithm, is computationally fast, and requires a minimal dataset of only three variables: drug name, date of supply, and a patient identifier. This minimal dataset is usually captured in electronically held prescription dispensing datasets, which are available in many developed countries, thus enabling multi-country analyses.
We have previously shown that PSSA has a sensitivity of 61%, a specificity of 93%, a positive predictive value of 77%, and a negative predictive value of 87% using adverse events identified in randomized controlled trials as the gold standard for positive events and using negative controls of adverse events identified in product information of unrelated products.2 We have also shown the feasibility of the method in a distributed network model to undertake multi-country analyses.3
The Bradford Hill criteria for causal association identify temporality and consistency as important criteria for assessing causation of events.4 One of the advantages of PSSA over other methods is that a visualization of temporal association is created in addition to the statistical output of an adjusted sequence ratio. By applying the method across multiple datasets, we also have the opportunity to test consistency across time. Thus, PSSA is a potentially valuable method for assessing both temporality and consistency. In our prior study, where we assessed the association between antipsychotics and acute hyperglycaemia,3 we found that the adjusted analyses for individual medicines were similar in the direction of the associations across countries; however, some countries had outlying results. Differences between countries in terms of stigma associated with mental health treatment were postulated as one of the reasons for the discrepancy. For this reason, the present validation study aimed to identify whether a well-known medicine adverse event that was unlikely to be confounded by health system factors could be consistently detected across countries.
In this study, we aimed to assess whether PSSA provides temporally associated and consistent results across multiple datasets in different countries.
This research was undertaken utilizing the network of databases established as part of the Asian Pharmacoepidemiology network (AsPEN).5 AsPEN enables studies to be undertaken in datasets within different countries, with different ethnic groupings and different health systems and regulations.
Method
In selecting the positive control, we identified a medicine that was available in every country and had a well-documented adverse event for which there was a specific prescription treatment and for which there was evidence of a temporal association. The positive control, or known adverse event, in this study was amiodarone-induced hypothyroidism. Amiodarone is an antiarrhythmic agent that contains iodine in its chemical structure and is known to exert effect on the thyroid gland6 with an estimated incidence of thyroid dysfunction of 15–20% in persons taking amiodarone.7 Amiodarone-induced hypothyroidism has been reported to occur early in the course of therapy and has an incidence of approximately 5–7%;8–10 thus, we would expect to see a temporal association of the adverse event with initiation. A nested case–control study found the risk of hypothyroidism was increased more than sixfold (Odds ratio 6.6, 95% confidence interval (CI) 3.9–11.1) in those taking amiodarone.11 A meta-analysis of adverse events from randomized controlled trials comparing amiodarone with placebo that grouped adverse events by organ class found a fourfold increased risk (OR 4.2, 95%CI 2.0–8.7) of adverse thyroid events with amiodarone.12 For the negative controls, or known negative events, we assessed amiodarone-induced allopurinol prescribing and thyroxine-induced allopurinol prescribing. Allopurinol, which is indicated for prevention of gout but not acute treatment of gout, was chosen as the negative control because neither amiodarone nor thyroxine is known to be associated with gout.13 Furthermore, given allopurinol is indicated for prevention, but not for treatment of an acute gout event, it is unlikely to be prescribed, even if a previously unrecognized adverse event occurred.
The AsPEN participants in this study were based in Australia, Hong Kong, Japan, Korea, and Taiwan. The datasets have been described previously.5 The Australian Government Department of Veterans’ Affairs healthcare claims database (Australia, 2001–2012) contains dispensing records for all veterans and their dependents in Australia (approximately 300 000) and represents a predominantly elderly cohort. The Korea Health Insurance Review and Assessment Service database (Korea, 2006–2008) includes all subsidized medicines for the entire population of approximately 50 million persons. The National Health Insurance Research Database (Taiwan, 2002–2008) includes all subsidized medicines covering the entire population; a random sample of one million persons was included in this study. The Hong Kong dataset was the Clinical Data Analysis and Reporting System from the Hong Kong Hospital Authority (2008–2012). The Hong Kong dataset includes data from publicly funded hospitals and primary care general physician services via the outpatient clinics in hospitals and general outpatient clinics in the community. The service is available to all Hong Kong residents. The dataset covers a population of approximately seven million. Data were also sourced from the Japan Medical Data Center insurance claims database (Japan, 2005–2010), which represents a privately insured population of approximately 300 000 adult working population and their dependents; however, there were insufficient records, and so data from this dataset were not included in the study. Data from a hospital-based database were also included, the Hamamatsu Medical University Database, Japan (1999–2010). The Japanese hospital dataset includes all patients attending the hospital (approximately 175 000) but does not include community records.
A distributive network model was employed.3 Participants created a dataset that included (i) a unique patient identifier; (ii) a variable to identify the medicine dispensed based on the World Health Organization standard anatomical therapeutic chemical (ATC) code; and (iii) a variable to identify the date of medicine supply. The coordinating center for this study, the University of South Australia, developed the statistical analysis code as a stand-alone Statistical Analysis System program for execution by each participant. The SAS program employed global macro variables that required participants to enter the variable names used in their datasets rather than forcing the creation of a data file with common data variable names. This approach eliminates a complex programming burden for participants and overcomes barriers because of language and disparate data structures. Participants executed the SAS code, and a standardized summary results file was returned to the coordinating center for collation. These standardized files included graphs of the number of people dispensed amiodarone, thyroxine, and allopurinol each month (prevalent population), the number of people starting amiodarone, thyroxine, and allopurinol each month (incident population), and the prescription symmetry analyses including the PSSA distribution graphs, which display the temporal relationship between dispensing. Medicines were selected by ATC codes in the Australian, Korean, and Taiwanese datasets: amiodarone C01BD01, allopurinol M04AA01, and thyroxine H03AA01. Codes for medicines in the Hong Kong and Japanese datasets were mapped to the ATC codes.
In the PSSA method, the date of incident dispensing of amiodarone, thyroxine, and allopurinol was determined for each individual patient. All incident dispensing that occurred within 1 year of each other for the same person were included in the analysis. We excluded patients who initiated any of the study medicines in the first year of data coverage in any dataset to ensure we limited the analysis to incident users. The crude sequence ratio (SR) was calculated by dividing the number of persons with thyroxine initiated after amiodarone initiation with the number of persons with thyroxine initiated prior to amiodarone initiation. The SR estimates the incidence rate ratio of the event in exposed compared with non-exposed person-time.14 The PSSA method uses a within-person design, making it robust towards confounders that are stable over time;1 however, it is sensitive to prescribing trends over time. Therefore, a null-effect SR was calculated to adjust for temporal trends. The null-effect SR is the expected SR in the absence of a causal association, given the incident medicine use and events in the background population. A description of the formula used for this value is provided elsewhere.14 An adjusted SR was obtained by dividing the crude SR by the null-effect SR, and 95%CI was calculated. The same analyses were undertaken for amiodarone and allopurinol, as well as thyroxine and allopurinol. The PSSA analyses were restricted to sequences of incident dispensing within 12 months of each other to limit the effect of age and other potential time-varying covariates on the probability of exposure and outcome. Moreover, a 12-month period has better specificity and positive predictive value compared with shorter periods.2 Pooled estimates were obtained by using the generic inverse variance method.15
Results
Positive control
Prescription sequence symmetry analysis identified statistically significant associations between incident amiodarone use and subsequent initiation of thyroxine in four of the five countries, with adjusted sequence ratios ranging from 1.52 to 5.30 (Table1). There were only limited numbers of persons dispensed amiodarone in the Japanese dataset, so while the ratio was positive, it did not reach a statistical significance. Temporal analysis showed that the observed effect of increased thyroxine dispensing was evident within a few weeks of initiation of therapy (Figure1). This temporal association was evident in all countries (data not shown). The pooled estimate was 2.63 (95%CI 1.47–4.72, Figure2).
Table 1.
Prescription sequence symmetry analysis results for the association between amiodarone and thyroxine: positive control
| Country | N pairs | Amiodarone first | Thyroxine first | Adjusted sequence ratio, 95% confidence intervals |
|---|---|---|---|---|
| Australia | 1979 | 1663 | 316 | 5.30 (4.69–5.96) |
| Hong Kong | 754 | 529 | 225 | 2.33 (1.99–2.72) |
| Japan | 16 | 11 | 5 | 1.77 (0.61–5.08) |
| Korea | 657 | 453 | 204 | 1.52 (1.29–1.80) |
| Taiwan | 153 | 115 | 38 | 3.26 (2.26–4.70) |
Figure 1.
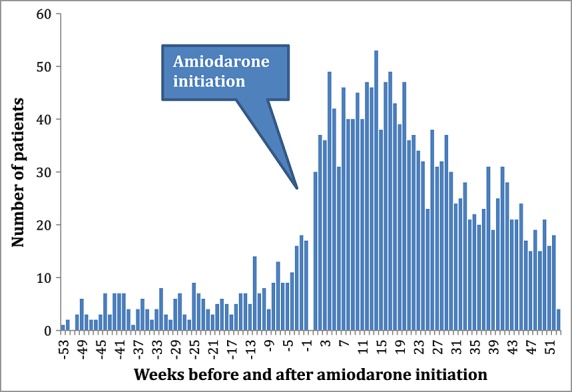
Temporal analysis positive control: amiodarone-thyroxine, Australia
Figure 2.
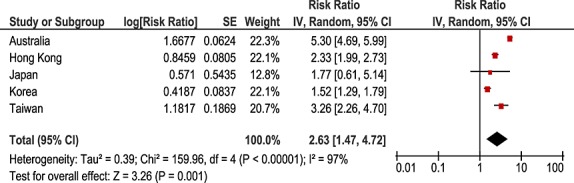
Pooled analysis positive control: amiodarone-thyroxine. CI, confidence interval; SE, standard error
Negative controls
The negative control of amiodarone and allopurinol showed no positive association in any of the countries, with adjusted sequence ratios ranging from 0.65 to 0.99 (Table2). No temporal association was observed (Figure3). The pooled estimate was 0.76 (95%CI 0.62–0.93, Figure4).
Table 2.
Prescription sequence symmetry analysis results for the association between amiodarone and allopurinol: negative control
| Country | N pairs | Amiodarone first | Allopurinol first | Adjusted sequence ratio, 95% confidence intervals |
|---|---|---|---|---|
| Australia | 1002 | 493 | 509 | 0.99 (0.88–1.12) |
| Hong Kong | 434 | 179 | 255 | 0.70 (0.58–0.85) |
| Japan | 23 | 9 | 14 | 0.65 (0.28–1.49) |
| Korea | 812 | 266 | 546 | 0.67 (0.57–0.77) |
| Taiwan | 384 | 148 | 236 | 0.72 (0.59–0.88) |
Figure 3.
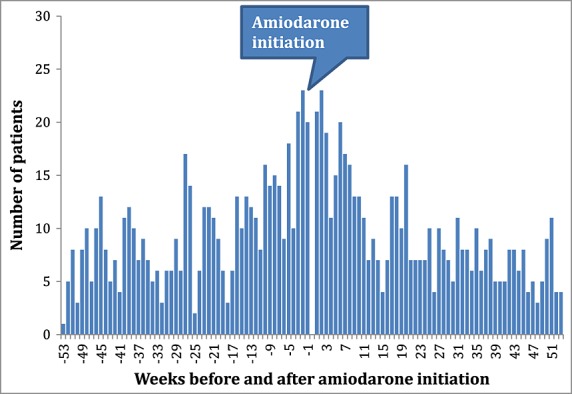
Temporal analysis negative control: amiodarone-allopurinol, Australia
Figure 4.
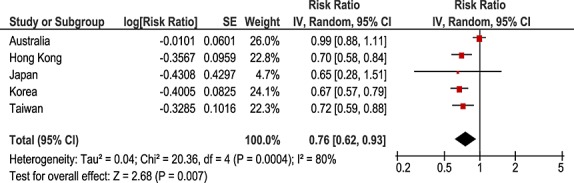
Pooled analysis negative control: amiodarone-allopurinol. CI, confidence interval; SE, standard error
For the negative control of thyroxine and allopurinol, no statistically significant positive association was observed; adjusted sequence ratios ranged from 0.74 to 1.73 (Table3). No temporal association was observed (Figure5). The pooled estimate was 0.98 (95%CI 0.85–1.12, Figure6).
Table 3.
Prescription sequence symmetry analysis results for the association between thyroxine and allopurinol: negative control
| Country | N pairs | Thyroxine first | Allopurinol first | Adjusted sequence ratio, 95% confidence intervals |
|---|---|---|---|---|
| Australia | 1092 | 519 | 573 | 0.92 (0.82–1.04) |
| Hong Kong | 215 | 105 | 110 | 0.97 (0.74–1.26) |
| Japan | 47 | 31 | 16 | 1.73 (0.95–3.16) |
| Korea | 933 | 471 | 462 | 1.05 (0.92–1.19) |
| Taiwan | 105 | 43 | 62 | 0.74 (0.50–1.09) |
Figure 5.
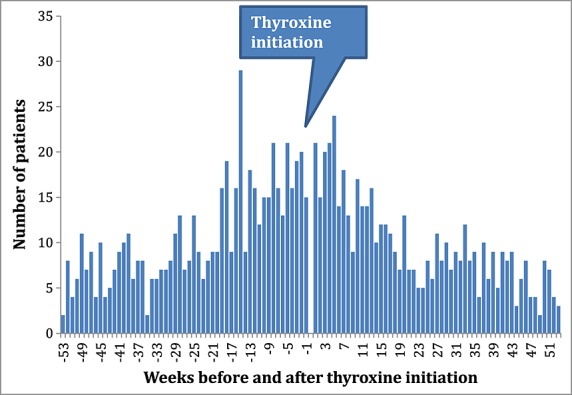
Temporal analysis negative control: thyroxine-allopurinol, Australia
Figure 6.
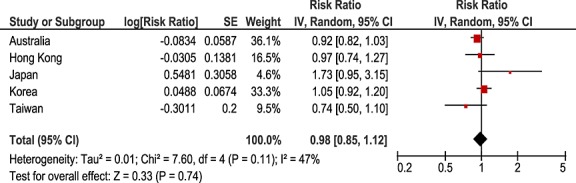
Pooled analysis negative control: thyroxine-allopurinol. CI, confidence interval; SE, standard error
Discussion
In this study, we used one positive and two negative controls to determine the risk, temporality, and consistency of prescription symmetry results, using datasets in different countries that included different age groups, different ethnic groups, different health systems, different insurance systems, and different patterns of utilization. In countries where there was sufficient sample size, the positive control analyses showed a statistically significant association. The negative controls were not statistically significantly positive in any dataset.
Consistent with the findings within each country, the pooled analyses showed a 2.6-fold increase in the association of initiation of thyroxine after initiation of amiodarone, while no effect was found in the pooled analyses for either negative control. The temporal results for amiodarone-induced thyroxine show use of thyroxine increasing within the first few weeks of amiodarone initiation. Given the long half-life of thyroxine, 6–7 days, and the fact that the adverse event is well known, it is likely that some degree of surveillance bias may be present, resulting in early initiation of thyroxine. These results reinforce the need to use multiple datasets to support signal generation for adverse event detection.
In one country, Japan, where a hospital only dataset was used, one of the negative controls, thyroxine-allopurinol, while not statistically significant did have an adjusted SR of 1.73. Hospital datasets, which may have some outpatient data but do not usually capture complete community medicine use, may contribute a biased estimate if, in this example, either thyroxine or allopurinol had significant community dispensing independent of hospital use. The extent of community use in this example is unknown. Hospital only datasets may be less suitable for routine surveillance using the PSSA method unless both medicines are initiated in the hospital setting.
In some countries, the negative control of amiodarone-induced allopurinol prescribing gave a result suggestive of reduced risk of initiating allopurinol after amiodarone. This pattern may represent detection bias as allopurinol is not known to cause arrhythmia.13 It may be that patients presenting with gout, an acute and painful condition that often precipitates a physician visit, are also assessed for other conditions at the time of their presentation, and arrhythmia subsequently are detected at some stage in the following 1 year time period.
In this study, we found that for a known positive and two negative controls, the method produced consistent, temporally associated results across countries, despite different settings, health systems, ethnic groups, and age groups. This provides further validation of the method as a tool for signal detection across the AsPEN. One of the advantages of PSSA is that the graphic enables temporal relationships to be observed, which demonstrates the strong temporal sequence for the positive control in all countries. The major limitation of the method is that it does not use diagnostic criteria, thus specific adverse events are not detected, rather medicines used to treat the adverse event are used. This method, therefore, is reliant on the existence of a medicine that could be used to treat the symptoms of the adverse event of interest. In countries where full electronic health records are available, the method could be trialed using specific adverse events instead of a medicine indicator, for example, hospitalization events with diagnostic codes. The advantage of the method in using medicine information is that it can be used to support pharmacovigilance in many countries that routinely collect dispensing data in electronic format in an efficient manner, alongside signal detection methods from spontaneous reporting systems.
Conflict of Interest
The authors declare no conflict of interest.
Key Points
Prescription sequence symmetry analysis provided consistent risk estimates and temporal analyses across different data sets in different health systems for both a positive control and two negative controls.
Ethic Statement
The research was approved by the University of South Australia Human Research Ethics Committee.
Acknowledgments
We acknowledge the contribution of Dr Tuan Nguyen in assisting with pooled estimates.
References
- Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology. 1996;7(5):478–484. [PubMed] [Google Scholar]
- Wahab IA, Pratt NL, Wiese MD, et al. The validity of sequence symmetry analysis (SSA) for adverse drug reaction signal detection. Pharmacoepidemiol Drug Saf. 2013;22(5):496–502. doi: 10.1002/pds.3417. [DOI] [PubMed] [Google Scholar]
- Pratt N, Andersen M, Bergman U, et al. Multi-country rapid adverse drug event assessment: the Asian Pharmacoepidemiology network (AsPEN) antipsychotic and acute hyperglycaemia study. Pharmacoepidemiol Drug Saf. 2013;22(9):915–924. doi: 10.1002/pds.3440. doi: 10.1002/pds.3440. [DOI] [PubMed] [Google Scholar]
- Lucas RM, McMichael AJ. Association or causation: evaluating links between “environment and disease”. Bull World Health Organ. 2005;83(10):792–795. [PMC free article] [PubMed] [Google Scholar]
- AsPEN collaborators. Andersen M, Bergman U, et al. The Asian Pharmacoepidemiology network (AsPEN): promoting multi-national collaboration for pharmacoepidemiologic research in Asia. Pharmacoepidemiol Drug Saf. 2013;22(7):700–704. doi: 10.1002/pds.3439. [DOI] [PubMed] [Google Scholar]
- Cohen-Lehman J, Dahl P, Danzi S, Klein I. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol. 2010;6(1):34–41. doi: 10.1038/nrendo.2009.225. [DOI] [PubMed] [Google Scholar]
- Bogazzi F, Tomisti L, Bartalena L, et al. Amiodarone and the thyroid: a 2012 update. J Endocrinol Invest. 2012;35(3):340–348. doi: 10.3275/8298. [DOI] [PubMed] [Google Scholar]
- Trip MD, Wiersinga W, Plomp TA. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med. 1991;91(5):507–511. doi: 10.1016/0002-9343(91)90187-3. [DOI] [PubMed] [Google Scholar]
- Batcher EL, Tang XC, Singh BN, et al. Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation. Am J Med. 2007;120(10):880–885. doi: 10.1016/j.amjmed.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Rouleau F, Baudusseau O, Dupuis JM, et al. Incidence and timing of thyroid dysfunction with long-term amiodarone therapy. Arch Mal Coeur Vaiss. 2001;94(1):39–43. [PubMed] [Google Scholar]
- Bouvy ML, Heerdink ER, Hoes AW, et al. Amiodarone-induced thyroid dysfunction associated with cumulative dose. Pharmacoepidemiol Drug Saf. 2002;11(7):601–606. doi: 10.1002/pds.735. [DOI] [PubMed] [Google Scholar]
- Vorperian VR, et al. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30(3):791–798. doi: 10.1016/s0735-1097(97)00220-9. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Campillos M, Letunic I, et al. A side effect resource to capture phenotypic effects of drugs. Mol Syst Biol. 2010;6:343. doi: 10.1038/msb.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):483–491. doi: 10.1002/pds.1736. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green SE. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. (editors). Version 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org. [Google Scholar]


