Abstract
Coelomic cavity–derived B-1 and splenic marginal zone (MZ) B lymphocytes play principal roles in frontline host protection at homeostasis and during primary humoral immune responses. Although they share many features that enable rapid and broad-based defense against pathogens, these innate-like subsets have disparate B cell receptor (BCR) signaling features. Members of the Fc receptor–like (FCRL) family are preferentially expressed by B cells and possess tyrosine-based immunoregulatory function. An unusual characteristic of many of these cell surface proteins is the presence of both inhibitory (ITIM) and activating (ITAM-like) motifs in their cytoplasmic tails. In mice, FCRL5 is a discrete marker of splenic MZ and peritoneal B-1 B cells and has both ITIM and ITAM-like sequences. Recent work explored its signaling properties and identified that FCRL5 differentially influences innate-like BCR function. Closer scrutiny of these differences disclosed the ability of FCRL5 to counter-regulate BCR activation by recruiting SHP-1 and Lyn to its cytoplasmic motifs. Furthermore, the disparity in FCRL5 regulation between MZ and B-1 B cells correlated with relative intracellular concentrations of SHP-1. These findings validate and extend our understanding of the unique signaling features in innate-like B cells and provide new insight into the complexity of FCRL modulation.
Keywords: B cells, BCR signaling, regulation, kinase, phosphatase
Roles of innate-like B cells in humoral immunity
B-1 B cells are chiefly responsible for maintaining preimmune humoral defense at homeostasis.1 Their biased immunoglobulin (Ig) repertoire and capacity for generating broadly reactive antibodies are not only tailored for basal protection and primary immune reactivity, but are also fundamental for housekeeping functions, such as removing potentially damaging apoptotic cells and oxidized lipids from the circulation.2 The B-1 lineage was originally defined based on expression of the Ly1/CD5 surface antigen, but these cells can be further discriminated by their differential expression of CD5 and CD11b/Mac1 into B-1a (CD19hiCD5hi) and B-1b (CD19hiCD5loCD11bhi) subsets.3 B-1 cells also phenotypically differ from B-2 cells (CD19+CD5loCD11blo).
The distinct enrichment of B-1 lymphocytes in the coelomic body cavities, such as the peritoneum and lungs, underscores their ability to serve as sentinels at the front lines of pathogen entry. B-1a and B-1b cells are especially suited for prompt reactivity to innate agonists that initiate thymus-independent (TI) humoral responses. However, their capacity to produce and secrete antibodies requires their emigration from these anatomic sites to the bone marrow and spleen.4,5 Aside from eliciting early protection from pathogenic challenge, B-1b cells also have adaptive recall capability to certain types of bacteria.6,7
A second distinctive B cell subset that is apparently B-2 lineage derived occupies a special location in the spleen and has similar innate-like characteristics to B-1 cells. Marginal zone (MZ) B cells are situated in a unique microanatomical environment that facilitates surveillance of blood-borne and particulate antigens including encapsulated bacteria such as Pneumococcus.8,9 Like B-1 cells, MZ B cells possess a distinct Ig repertoire and differentiate into plasma cells within hours upon exposure to innate agonists, such as lipopolysaccharide and TI antigens.10 Their CD19hiCD21hiCD23loCD1dhi phenotype distinguishes them from the more abundant B-2 follicular (FO) population that bears a CD19hiCD21loCD23hi surface signature, can recirculate, and participates in germinal center (GC)–based affinity maturation.11 Antigen-induced differentiation of B-2 FO B cells via this more complex diversification pathway requires additional time and regulatory oversight, but equips the host with memory B cells that enable brisk secondary responses to future foreign antigenic encounters.
Despite their many shared features, B-1 and MZ B cells have different B cell receptor (BCR) signaling characteristics. Although engagement of the BCR in MZ B cells drives robust calcium signaling, whole-cell tyrosine phosphorylation (pTyr), and activation of the MAP kinase and NF-κB cascades,12,13 these signal transduction outcomes are significantly blunted in B-1 cells.14,15 For example, B-1a or B-1b cell BCR engagement does not provoke the same degree or quality of calcium flux or whole-cell pTyr observed in MZ B cells. Furthermore, ERK is constitutively activated in B-1 cells, but the NF-κB pathway is impaired.16,17 Given the crucial roles of these innate-like B cell types in preimmune and primary humoral responses, it is surprising that the basis for their robust responsivity to TI antigens and the differences in their antigen receptor triggering capacity remain poorly understood. The importance of improved understanding of how these cells integrate and regulate signals is highlighted by their roles in disease. It has been long appreciated that their biased Ig repertoires, unique phenotypes, and other characteristic features resemble pathogenic B cells implicated in autoimmune diseases and certain B cell lymphoproliferative disorders like B cell chronic lymphocytic leukemia.18
The FCRL family and B cell regulation
A gene family related to the classical Fc receptors (FCR) for IgG and IgE, termed FCR-like (FCRL), encodes type I transmembrane proteins with tyrosine-based signaling properties that are preferentially expressed by B cells. In humans, six FCRLs (FCRL1–6) are located on human chromosome 1q21–23, whereas in mice there are only three family members, located on chromosomes 1 and 3 (Fcrl1, Fcrl5, and Fcrl6).19 The interspecies diversity of these receptor genes has provided a challenge as well as an opportunity to unravel their physiology. Their evolutionary conservation portends a fundamental role for these receptors in immunity and involvement in multiple immune disorders including infections, autoimmunity, malignancies, and immunodeficiencies.20–22
FCRLs have variable numbers of extracellular Ig-like domains (2–9) and at least one immunoreceptor tyrosine-based inhibitory motif (ITIM), immunoreceptor tyrosine-based activation motif (ITAM), or both motif types in their cytoplasmic tails (Fig.1). Their autonomous signaling potential and regulatory complexity distinguishes this subfamily from the classical FCR proteins that have either a single ITIM or ITAM or associate with an ITAM-bearing adaptor subunit (e.g., FCRγ, CD3ζ). Ligands have recently been identified for several FCRLs. In humans, FCRL4 and FCRL5 bind IgA and IgG, respectively,23,24 whereas FCRL6 interacts with major histocompatibility complex class II (MHCII) protein HLA-DR.25 In mice, FCRL5 was discovered as a target for the immunoevasin orthopox MHCI-like protein, which was initially found to bind NKG2D and alter NK cell cytotoxicity.26,27 However, more study is required to clarify the functional consequences of these interactions and the other FCRL ligands.
Figure 1.
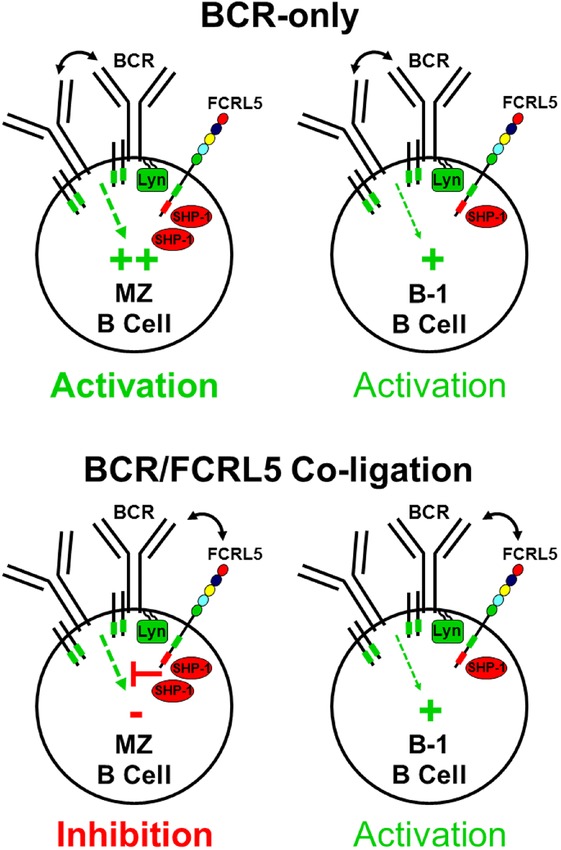
Overview of BCR and FCRL5 tyrosine-based signaling in mouse innate-like B cells. FCRL5 is shown in relation to the BCR and its Ig-α and Ig-β ITAM-bearing (green boxes) signaling adaptor subunits. The FCRL5 cytoplasmic tail has both an ITAM-like sequence and a consensus ITIM (red box). Differences in FCRL5 function between the MZ and B-1 B cell subsets directly correlate with their relative intracellular pools of the protein tyrosine phosphatase SHP-1. BCR signaling is robust in splenic MZ B cells, but the comparatively higher SHP-1 levels in these cells enables FCRL5 to inhibit BCR stimulation when the two receptors are cross-linked. In contrast, peritoneal cavity–derived B-1 B cells have more blunted BCR activation and a lower abundance of SHP-1. With the relatively balanced activity of SHP-1 and Lyn in B-1 B cells, FCRL5 has no apparent influence on BCR activation.
Significant progress has been made in investigating FCRL regulatory properties.28 Among these receptors in humans, FCRL1 has two potential ITAM-like motifs. Initial experiments explored its impact on BCR stimulation and disclosed that co-ligation of these two receptors induced pTyr of FCRL1 and enhanced calcium signaling in addition to B cell proliferation.29 However, the molecular basis for these downstream events, and which signaling proteins arbitrate these effects through FCRL1, remains under investigation. More extensive biochemical work has been carried out with the other four B cell–expressed human FCRLs. In general, studies of FCRL2–5, which all have intracellular ITIMs, have found a negative influence on BCR activation.30–33 Several groups have employed B cell line transfectants expressing Y→F mutant FCRLs to help define their cytoplasmic effector–recruitment associations in the context of BCR ligation. Indeed, treatment with the phosphatase inhibitor pervanadate, or cross-linking with the BCR, stimulates pTyr of each of these receptors. This primary modification event, presumably induced by Src family kinases (SFK) such as Lyn, coincides with the recruitment of the SH2 domain–containing phosphatases SHP-1 and/or SHP-2 to respective ITIM tyrosines. As a result of these interactions, FCRL2–5 are each capable of suppressing calcium mobilization, whole-cell pTyr, and MAPK activation to various degrees. These findings have aided in the determination that FCRL2–5 can dominantly repress antigen-receptor activation in B cells. However, the added presence of cytoplasmic ITAM-like sequences in most of these molecules suggests a complex, multifaceted mode of regulation. In fact, indications that they also positively influence B cell signaling were subtly apparent in several of the aforementioned studies, for example, several mutants with disrupted ITIM tyrosines acquired the potential to enhance BCR-mediated calcium activation. These outcomes provided indirect evidence that tandemly positioned intracellular ITAM-like sequences in the tails of ITIM-bearing FCRLs are also potentially active.
FCRL5 is a unique immunoregulatory marker of innate-like B cells
To examine the possibility that many FCRLs have both inhibitory and activating function, we recently focused on the FCRL5 member in mice. FCRL5 has five extracellular Ig-like domains, an uncharged transmembrane region, and cytoplasmic ITIM and ITAM-like sequences (Fig.1). This receptor resembles human FCRL3 in its primary structure, but mouse FCRL5 differs in the lack of a sixth membrane-proximal extracellular Ig-like domain and the absence of a third tyrosine-based hemi-ITAM sequence at the carboxy-terminus of its cytoplasmic tail.34 Notably, within the B lymphocyte lineage, FCRL3 expression peaks on a circulating population of effector memory B cells that are also found in the spleen and have been considered a human MZ B cell equivalent.35,36
Shortly after it was cloned, a survey of mouse tissues failed to detect Fcrl5 transcripts in total RNA samples by northern blotting, indicating that the gene might be expressed by only a minority of cells.34 Accordingly, analyses of cell lines and sorted lymphocyte subsets disclosed the expression of Fcrl5 transcripts by WEHI231 and primary MZ B cells. The generation of receptor-specific monoclonal antibodies confirmed the distribution of the FCRL5 protein by splenic MZ B cells as well as B-1a and B-1b cells isolated from the peritoneal cavity, but not by conventional B-2 cells that also occupy these sites.37 Immunohistology of the spleen confirmed the topographical concentration of FCRL5 in the splenic MZ, but general absence from the follicle. These results showed that, aside from CD1d, CD9, and CD36, FCRL5 was one of only a few surface antigens that can discretely mark innate-like splenic MZ and/or peritoneal cavity B-1 cells.37–40 Its specific pattern of expression by these unique subsets, along with its tyrosine-based signaling potential, implies that FCRL5 has a distinct role in modulating the B cells principally responsible for orchestrating primary humoral defense.
This early descriptive work also validated its capacity for tyrosine-based signaling. Similar to human FCRL2–5, mouse FCRL5 could undergo pTyr and inhibit BCR-induced calcium flux in co-ligation assays performed with WEHI231 and primary MZ B cells. To determine the mechanistic basis for these effects, and assess whether its activity differs in MZ and B-1 cells given their disparate BCR signaling profiles, an expanded biochemical analysis of FCRL5 regulation was undertaken. These studies focused on the receptor’s cytoplasmic ITIM and ITAM-like sequences and took advantage of a panel of Y→F chimeric receptor mutants and mouse FCRL5-specific monoclonal antibodies. These tools were employed to dissect its impact in cell line transductants as well as in primary B cells isolated from wild-type (WT) and genetic mutant mouse models.
FCRL5 counter-regulates innate-like B cells via Lyn and SHP-1
In MZ B cells, FCRL5 could inhibit BCR-triggered calcium signaling as well as whole-cell pTyr assessed by phosphoflow analysis, but a parallel investigation revealed that it exerted remarkably little influence on these events in B-1a or B-1b cells.41 Expectedly, the amplitude of calcium influx and the magnitude of pTyr induced by BCR engagement in both B-1 cell subsets was markedly lower compared to MZ B cells. These findings revealed that FCRL5 has differential regulatory properties in innate-like splenic MZ and peritoneal cavity B-1 cells that occupy different anatomical compartments.
To dissect the cause of these subset-specific differences, define the individual contributions of its cytoplasmic tyrosines, and determine the nature of intracellular proteins recruited to them, a panel of FcγRIIb/FCRL5 chimeric receptor constructs was generated. By fusing the extracellular and transmembrane portions of mouse FcγRIIb in-frame with different FCRL5 cytoplasmic Y→F variants, the effects of the ITAM-like sequence (Y543/Y556), ITIM (Y566), and all three tail tyrosines (FFF) could be analyzed in parallel. These molecules were expressed in the A20IIA1.6 (IgG2aκ) mouse B cell line that lacks endogenous FcγRIIb expression. This approach, initially used by the Honjo group,42 enabled functional comparisons of BCR engagement alone with F(ab′)2 fragments, versus co-ligation of the BCR and chimeric FcγRIIb/FCRL5 tail mutants by means of intact (Fc-containing) rabbit anti-mouse-IgG.
With this system, we first performed a global comparison of FCRL5 tyrosine-based regulation upon antigen receptor stimulation. Chimeric receptors harboring an intact unmodified tail (WT) versus a cytoplasmic FFF mutation were examined after BCR ligation or co-ligation by immunoblotting whole-cell lysates. Consistent with its effects on BCR calcium signaling and pTyr in MZ B cells, FCRL5 co-ligation in A20 cells attenuated whole-cell pTyr as well as MAPK ERK activation.41 These downstream effects correlated with pTyr of the WT FCRL5 tail itself, but were absent in the FFF transfectant. Moreover, these data also verified that a fourth tyrosine located at amino acid position 544, which does not fit a typical signaling motif, failed to be pTyr. These assays confirmed that FCRL5’s ability to be pTyr and modulate BCR signaling is confined to its intracellular ITIM and ITAM-like sequences.
The impact of FCRL5’s two cytoplasmic motifs, and individual tyrosines therein, was further examined in calcium flux assays. The WT chimera strongly suppressed calcium mobilization, but this effect was lost when the FFF variant was cross-linked with the BCR.41 Curiously, studies with the ITAM-like mutant (FF), which leaves the ITIM intact, revealed a near complete shutdown of calcium signaling that was even greater than that observed for the WT. A similar inhibitory phenotype was evident for the Y543F mutant, but BCR co-ligation with the Y556F chimera resulted in effects analogous to the WT. These data suggested differential functional properties for these two ITAM-like tyrosines. Surprisingly, the ITIM Y566F chimera, which has an unaltered ITAM-like sequence, augmented calcium mobilization over that of BCR triggering alone and thus disclosed FCRL5’s potential for activation. In line with its impact on calcium flux, immunoblotting analyses of lysates isolated from these stimulated transfectants showed equivalent results for these FCRL5 tail constructs on MAPK ERK activation. These experiments41 demonstrated that FCRL5’s cytoplasmic ITIM and ITAM-like sequences are both functional and that the receptor has counter-regulatory properties. Furthermore, within the ITAM-like module, the amino-terminal Y543 residue is critical for offsetting repressive effects of the Y566 ITIM, whereas the Y556 site appears to be dispensable.
The molecular elements that mediate these outcomes were then defined in immunoprecipitation and blotting analyses. After engagement with the BCR, pTyr was evident for all of the mutant constructs except for the FFF.41 Candidate phosphatases that might bind to the ITIM—SHP-1, SHP-2, and SHIP—were probed for, but only SHP-1 was found to associate, and this was dependent on the Y566 ITIM residue. To determine what effector component(s) was responsible for the receptor’s ITAM-like capability, blotting studies were performed to define suspect kinases and adaptor proteins. No direct interaction was apparent for the Syk, Btk, PI3K, or PLCγ2 kinases or BLNK or Grb2 adaptor proteins. However, the Lyn SFK was clearly identified in immunoprecipitates and its interaction was dependent on the ITAM-like amino-terminal Y543 residue. SFK can exist in active and inactive conformations, and by use of a discriminating antibody we could show that the SFK bound at Y543 was in the active state. These experiments41 provided the first molecular evidence that FCRLs may have dual regulatory function in B cells and can balance activating and inhibitory signaling by virtue of recruiting a kinase and phosphatase to independent tyrosines.
The ability of FCRL5 to integrate Lyn and SHP-1 via tandem motifs in its cytoplasmic tail is intriguing given the role of these signaling proteins in B cell selection and immunoreceptor function.43 Mice strains that possess genetic alterations in Lyn and SHP-1 have a progressive loss in B-2 lineage B cells, including MZ B cells, but develop a relative expansion of B-1 B cells and lupus-like autoimmunity.44–46 The importance of these two constituents in driving FCRL5’s integrated regulation was further explored in primary B-1 B cells from conventional Lyn-deficient mice as well as the motheaten variable (mev) mutant strain that harbors a point mutation compromising SHP-1 activity to approximately 10–20% of normal.47,48 Calcium flux and pTyr phosphoflow analyses of B-1 B cells from these mice confirmed the bifunctionality of FCRL5 and the requirement of Lyn and SHP-1 in exerting these effects. In contrast to WT B-1 B cells, where FCRL5 failed to influence BCR activation, the absence of Lyn or SHP-1 produced inverse outcomes and tipped the balance of its activating or inhibitory function in the opposite direction. In mev SHP-1 mutant B-1 B cells, FCRL5 enhanced BCR-mediated calcium activation and pTyr. However, through this analysis, a non-redundant role for Lyn also became apparent. Although other SFKs are present in B-1 B cells, in the absence of Lyn, FCRL5 acquired inhibitory features. In this context, the receptor developed SHP-1 dominant activity, and its ITAM-like capability failed to offset this suppression. This suggests a differential hierarchy and site-specific role for SFK binding to the FCRL5 substrate. The duality of FCRL5 regulation was also unique compared to other well-characterized B-1 B cell inhibitory immunoreceptors like CD5, CD22, CD32, and CD72. These molecules either rely on SHP-1 (CD5 or CD72) or SHIP (CD22 and CD32) to wield their repressive effects.49–52 However, in contrast to FCRL5, these receptors are all inhibitory in WT B-1 B cells, were not affected by Lyn deficiency, and failed to enhance BCR signaling in SHP-1 mutant B-1 cells. These findings illuminate FCRL5’s distinct binary function and sensitivity to the relative activity of Lyn and SHP-1.
With the identification of Lyn and SHP-1 as the primary mediators recruited to the FCRL5 cytoplasmic tail, the receptor’s compartment-specific disparity in splenic MZ and peritoneal cavity B-1 B cells was revisited. After verifying the molecular associations of Lyn and SHP-1 with FCRL5 in primary MZ and B-1 B cells, a flow cytometry–based intracellular and phosphoflow-staining analysis was used to compare the relative pTyr status as well as the quantity and activity of Lyn and SHP-1 among spleen and peritoneal cavity B cell subsets.41 Consistent with prior studies,12 constitutive pTyr was greatest for splenic MZ B cells; whereas B-1a and B-1b cells also exhibited high basal pTyr, peritoneal cavity B-2 B cells as well as splenic FO and newly formed B cells had similarly low pTyr levels. A comparable pattern of pTyr among these subsets was produced by BCR engagement; however, newly formed cells demonstrated a potential for higher activation than splenic FO or peritoneal B-2 cells. The quantity and activation status of Lyn was also examined at homeostasis in these subpopulations. Although slightly higher levels of active and inactive forms of Lyn were detected in B-1 cells compared to B-2 B cells, the total quantities and activation status among B cell subsets from these tissues did not markedly differ. Of significant interest was the striking abundance of basal SHP-1 levels found in MZ B cells. Quantities of this phosphatase were nearly threefold lower in FO B cells. Although slightly higher amounts of SHP-1 were present in peritoneal cavity B-1 compared to B-2 cells, they were twofold lower than in MZ B cells. These results41 were thus consistent with the differential inhibitory properties of FCRL5 among innate-like B cells. The elevated SHP-1 concentration in MZ B cells causes FCRL5 to adopt predominantly inhibitory function in this subset. However, in B-1 B cells, which have a relatively lower pool of SHP-1, FCRL5 assumes more balanced activity that appears to be equally compensated for by Lyn (Fig.1).
Conclusions and future studies
These observations not only provide new perspectives on MZ and B-1 B cell biology beyond their shared responsibilities in basal and primary host defense, but also reinforce previously recognized differences in their regulation. A detailed investigation of FCRL5 has illustrated that members of this immunoreceptor family can positively and negatively modulate B cells and serve as dynamic signaling platforms. Moreover, data demonstrating that FCRL5 function differs according to the relative expression of signaling elements in a given subset, as well as its tissue of origin, has important implications for disease. Alterations in the quantity or activity of these effector proteins could have a profound impact on recruitment to these receptors, perturb their downstream regulation, and have a detrimental effect on humoral immunity. Consequently, the growing number of disease associations for the FCRLs is not unexpected. The studies reviewed here have primarily focused on BCR regulation; however, the chief responsibility of MZ and B-1 B cells is their primed ability to sense, engage, and combat innate agonists. A role for the FCRLs in this aspect of B cell function has recently been examined by several groups. For example, human FCRL3 and FCRL4 can both promote TLR9-mediated activation.35,53 Collectively, these findings indicate that the FCRLs differentially modulate innate and adaptive immune responses. With this new insight into FCRL5 regulation, the development of genetically deficient mice is expected to enable a more systematic understanding of the in vivo physiology of the FCRLs and their fascinating properties in innate-like B cells.
Acknowledgments
The author appreciates Peter Burrows and members of the Davis laboratory for critical reading of the manuscript. This work was supported in part by funding from the Lupus Research Institute and NIH Grants AI110553, AI097729, CA175912, and CA161731.
Conflicts of interest
The author declares no conflict of interest.
References
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- Shaw PX, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR. Hayakawa K. B cell development pathways. Annu. Rev. Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- Choi YS, et al. B-1 cells in the bone marrow are a significant source of natural IgM. Eur. J. Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T, et al. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- Haas KM, et al. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Martin F. Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A. Moran ST. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- Martin F. Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory. Immunol. Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- Pillai S. Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Antigen receptor proximal signaling in splenic B-2 cell subsets. J. Immunol. 2001;166:3122–3129. doi: 10.4049/jimmunol.166.5.3122. [DOI] [PubMed] [Google Scholar]
- Oliver AM, et al. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- Chumley MJ, Dal Porto JM. Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J. Immunol. 2002;169:1735–1743. doi: 10.4049/jimmunol.169.4.1735. [DOI] [PubMed] [Google Scholar]
- Holodick NE. Rothstein TL. Atypical response of B-1 cells to BCR ligation: a speculative model. Front. Immunol. 2013;4:457. doi: 10.3389/fimmu.2013.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL. Rothstein TL. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J. Exp. Med. 1993;177:857–861. doi: 10.1084/jem.177.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodick NE, Tumang JR. Rothstein TL. Continual signaling is responsible for constitutive ERK phosphorylation in B-1a cells. Mol. Immuno. 2009;46:3029–3036. doi: 10.1016/j.molimm.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr. Opin. Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Davis RS. Fc receptor-like molecules. Annu. Rev. Immunol. 2007;25:525–560. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- Akula S, Mohammadamin S. Hellman L. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS One. 2014;9:e96903. doi: 10.1371/journal.pone.0096903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayngerts SA, Najakshin AM. Taranin AV. Species-specific evolution of the FcR family in endothermic vertebrates. Immunogenetics. 2007;59:493–506. doi: 10.1007/s00251-007-0208-8. [DOI] [PubMed] [Google Scholar]
- Li FJ, et al. Emerging roles for the FCRL family members in lymphocyte biology and disease. Curr. Top. Microbiol. Immunol. 2014;382:29–50. doi: 10.1007/978-3-319-07911-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Fuchs A. Colonna M. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J. Immunol. 2012;188:4741–4745. doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, et al. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J. Immunol. 2013;190:5739–5746. doi: 10.4049/jimmunol.1202860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreeder DM, et al. Cutting edge: FcR-like 6 is an MHC class II receptor. J. Immunol. 2010;185:23–27. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, et al. Cutting edge: FcR-like 5 on innate B cells is targeted by a poxvirus MHC class I-like immunoevasin. J. Immunol. 2010;185:28–32. doi: 10.4049/jimmunol.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, et al. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J. Exp. Med. 2007;204:1311–1317. doi: 10.1084/jem.20062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt GR. Cooper MD. Immunoregulatory roles for fc receptor-like molecules. Curr. Top. Microbiol. Immunol. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]
- Leu CM, et al. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–1126. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- Haga CL, et al. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9770–9775. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TA, et al. FcR-like 2 inhibition of B cell receptor-mediated activation of B cells. J. Immunol. 2010;185:7405–7412. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt GR, et al. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13489–13494. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi Y, et al. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J. Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- Davis RS, et al. Differential B cell expression of mouse Fc receptor homologs. Int. Immunol. 2004;16:1343–1353. doi: 10.1093/intimm/dxh137. [DOI] [PubMed] [Google Scholar]
- Li FJ, et al. FCRL3 promotes TLR9-induced B-cell activation and suppresses plasma cell differentiation. Eur. J. Immunol. 2013;43:2980–2992. doi: 10.1002/eji.201243068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won WJ, et al. Fc receptor homolog 3 is a novel immunoregulatory marker of marginal zone and B1 B cells. J. Immunol. 2006;177:6815–6823. doi: 10.4049/jimmunol.177.10.6815. [DOI] [PubMed] [Google Scholar]
- Won WJ. Kearney JF. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J. Immunol. 2002;168:5605–5611. doi: 10.4049/jimmunol.168.11.5605. [DOI] [PubMed] [Google Scholar]
- Roark JH, et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- Won WJ, Bachmann MF. Kearney JF. CD36 is differentially expressed on B cell subsets during development and in responses to antigen. J. Immunol. 2008;180:230–237. doi: 10.4049/jimmunol.180.1.230. [DOI] [PubMed] [Google Scholar]
- Zhu Z, et al. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E1282–E1290. doi: 10.1073/pnas.1215156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornall RJ, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Nishizumi H, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Chan VW, et al. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- Shultz LD, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Ono M, et al. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- Poe JC, et al. CD22 forms a quaternary complex with SHIP, Grb2, and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J. Biol. Chem. 2000;275:17420–17427. doi: 10.1074/jbc.M001892200. [DOI] [PubMed] [Google Scholar]
- Adachi T, et al. The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J. Immunol. 1998;160:4662–4665. [PubMed] [Google Scholar]
- Sen G, et al. Negative regulation of antigen receptor-mediated signaling by constitutive association of CD5 with the SHP-1 protein tyrosine phosphatase in B-1 B cells. Eur. J. Immunol. 1999;29:3319–3328. doi: 10.1002/(SICI)1521-4141(199910)29:10<3319::AID-IMMU3319>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sohn HW, et al. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood. 2011;118:6332–6341. doi: 10.1182/blood-2011-05-353102. [DOI] [PMC free article] [PubMed] [Google Scholar]


