Abstract
A novel copper-catalyzed electrophilic amination of cyclopropanols with O-benzoyl-N,-N-dialkylhydroxylamines to synthesize various β-aminoketones via a sequence of C-C bond cleavage and Csp3-N bond formation is reported. The reaction conditions are mild and tolerate a wide range of functional groups including benzoate, tosylate, expoxide, and α,β-unsaturated carbonyls, which are incompatible in the traditional amine nucleophilic conjugate addition and the Mannich reaction conditions. Preliminary mechanistic studies and a proposed catalytic cycle of this umpolung β-aminoketone synthesis process have been described as well.
Nitrogen-containing groups are indispensable moieties of pharmaceuticals, agrochemicals and materials. Transition-metal-catalyzed Csp2-N bond formations,1 particularly the Buchwald-Hartwig amination, 2 Chan-Lam coupling,3 and Csp2-H amination,4 have significantly advanced the installation of nitrogen-containing groups on aromatic and olefinic substrates. However, transition metal catalyzed Csp3-N bond formation at non-activated Csp3-centers for the syntheses of aliphatic amines are still very limited. Significant advances of Csp3-H animation reaction and amination of double bonds6 have been made recently to synthesize aliphatic nitrogen-containing compounds. Our interest in using cyclopropanols and the related systems as useful alkyl cross-coupling partners to form important Csp3-Csp3 and Csp3-heteroatom bonds (Figure 1A and 1B)7 as well as using polysubstituted amines as potential selective adenylyl cyclase 1 inhibitors for treating inflammatory and neuropathic pain8 prompted us to develop an efficient copper-catalyzed electrophilic amination9 of cyclopropanols to synthesize various β-aminoketones,10 which are commonly prepared by the conjugation addition of amine nucleophiles to α,β-unsaturated carbonyls11 or the Mannich-type reactions between azomethines and enolates.12
Figure 1.
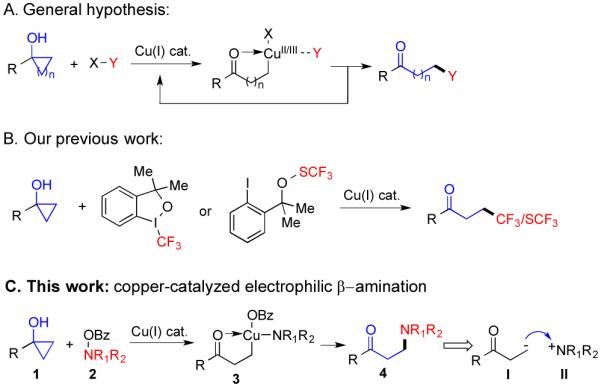
Our general hypothesis and work of copper-catalyzed ring-opening cross-coupling reactions.
Cyclopropanols are important and useful functional groups in organic synthesis and can be readily prepared via the Kulinkovich protocol or cyclopropanation (Simmons-Smith reaction) of enol ethers.13 Currently, most of the transition metal catalyzed cyclopropanol homoenolate chemistry focus on palladium-catalyzed C-C bond formation at the β-position (Scheme 1A).14 Copper-promoted or copper-catalyzed cyclopropanol ring-opening cross-coupling reactions have been very rare despite the low-cost and sustainable feature of copper catalyst.15 Ryu, Murai, and co-workers have discovered that Cu(II)-homoenolates derived from the treatment of 1-alkoxy-1-siloxycyclopropanes with a stoichiometric amount Cu(BF4)2 could undergo homodimerziation to form 1,6-diketones or be trapped by highly electron-deficient acetylenes to form cross-coupling products.16 Recently, Cha, and co-workers have developed elegant SN2’ alkylations of the metal-lo-homoenolate derived from treating cyclopropanols with more than stoichiometric amount CuCN and one equivalent of ZnEt2 as well as their applications in complex natural product synthesis.17
Electrophilic ring-opening amination of cyclopropanols could provide an umpolung and complementary strategy to synthesize β-amino carbonyls. We hypothesized that Cu(I) catalyst could be oxidized to Cu(III) by an O-benzoyl-N,-N-dialkylhydroxylamine18 (cf. 2, Figure 1C); the resulting Cu(III) species would promote a cyclopropanol ring opening reaction to generate copper-homoenolate 3, which would undergo reductive elimination to form a Csp3-N bond and regenerate the Cu(I) catalyst. We were also hoping that the use of catalytic amount of copper catalyst and the close interaction between the NR1R2 group and the copper-homoenolate could potentially suppress the homo-coupling, further oxidation, and other undesired reaction pathways. Herein, we report the first copper-catalyzed electrophilic amination of cyclopropanols with O-benzoyl-N,-N-dialkylhydroxylamines.
Our exploration started with model substrates 1a and O-benzoyl-morpholine 2a (Table 1). When a mixture of 1a and 2a were treated with a catalytic amount of CuI (0.1 equiv) in THF at 80 °C, the desired β-morpholinylketone 4a was produced in 85= yield. Further reaction condition optimization showed that (i) MeCN is superior to other solvents such as THF, toluene, DCE, and 1,4-dioxane presumably due to its coordinating capacity with the copper catalyst and (ii) CuBr works slightly better than CuCl and CuI. The reaction proceeds efficiently at 50 °C, but the yield dropped significantly when the reaction was conducted at room temperature. Increasing the amount of 2a to 1.5 equiv gave reduced reaction yield while using 1.5 equiv of 1a was beneficial for the production of 4a. Nitrogen-based bidentate ligand phenanthroline inhibited the product formation. Overall, we were able to obtain β-morpholinylketone 4a in 98= yield with CuBr (0.1 equiv) in MeCN at 50 °C (entry 11).
Table 1.
Reaction condition optimization.a
| ||||
|---|---|---|---|---|
|
| ||||
| entry | Cu cat. | solvent | temp (°C) | yield (%)a |
|
| ||||
| 1 | CuI | THF | 80 | 85b |
| 2 | CuI | THF | 80 | 88 |
| 3 | CuI | toluene | 80 | 75 |
| 4 | CuI | DCE | 80 | 65 |
| 5 | CuI | 1,4-dioxane | 80 | 82 |
| 6 | CuI | MeCN | 80 | 90 |
| 7 | - | MeCN | 80 | 0 |
| 8 | CuI | MeCN | rt | 39 |
| 9 | CuI | MeCN | 50 | 93 |
| 10 | CuCl | MeCN | 50 | 88 |
| 11 | CuBr | MeCN | 50 | 98 |
| 12 | CuBr | MeCN | 50 | 93b |
| 13 | CuBr | MeCN | 50 | 80c |
| 14 | CuBr | MeCN | 50 | traced |
General reaction conditions: A solution of 1a (0.15 mmol), 2a (0.1 mmol), and Cu(I) catalyst (0.1 equiv) were stirred until no more starting material left. The reaction process was monitored by thin-layer chromatography. Isolated yield from flash chromatography was given;
1/1 of 1a/2a (0.1 mmol);
1a (0.1 mmol) and 2a (0.15 mmol);
Phenanthroline (0.1 equiv).
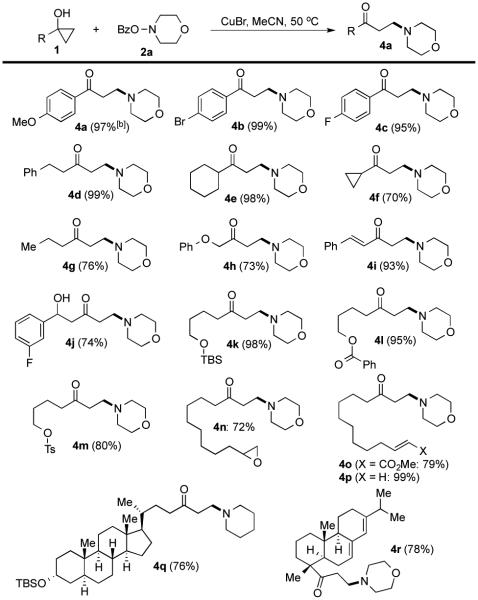
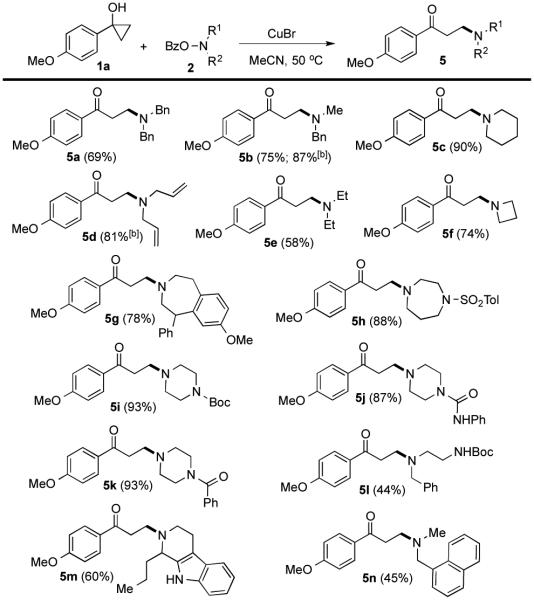
With the optimized reaction conditions, we then investigated the substrate scope of this electrophilic β-amination process in terms of both cyclopropanols (Figure 2) and O-benzoyl-N,N-dialkylhydroxylamines (Figure 3). To our satisfactory, both aryl and alkyl substituted cyclopropanols underwent the desired ring opening cross-coupling reaction to provide various aryl and alkyl β-aminoketones, respectively, in good to excellent yield. Ether (4a and 4h), bromide (4b), fluoride (4c), cyclopropyl (4f), TBS-ether (4k and 4q), terminal olefin (4p), free alcohol (4j), and diene (4r) functional groups are well tolerated under the reaction conditions. Notably, functional conjugate amine addition conditions and the Mannich reaction conditions to synthesize β-amino ketones survived the mild electrophilic amination conditions very well. In addition to O-benzoyl-morpholine (2a), many other O-benzoyl-N,-N-dialkylhydroxylamines work smoothly as the nitrogen-containing group donors (Figure 3). Benzyl (5a and 5b), allyl (5d), sulphonamide (5h), Boc-carbamate (5i and 5l), urea (5j) and amide (5k) groups are compatible with the reaction conditions. Notably, azitidine (5f), azepane (5g), 1,4-diazepane (5h), piperazine (5i-k), and indole or tryptoline (5m)-containing β-aminoketones could be obtained in good to excellent yields. The reaction could also be conducted in gram-scale as well (4a, Figure 2).
Figure 2.
Cyclopropanol substrate scope.a
[a] Yield of isolated products; [b] Gram-scale reaction.
Figure 3.
O-benzoyl-N,-N-dialkylhydroxylamine scope.a
[a] Yield of isolated products; [b] 80 °C.
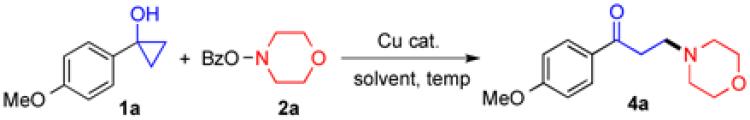
We then started to probe the reaction mechanism. When the reaction was conducted in the absence of O-benzoyl-N,-N-dialkylhydroxylamines, only a trace amount of ring opened product 6 was produced from 1a (Figure 4, Eq.1). Increasing the amount of CuBr to 1.0 equiv resulted in a 17/1 ratio of 1a/6. These results indicate that CuBr alone is not effective enough to induce cyclopropanol ring opening and a higher oxidation level of copper catalyst, Cu(II) or Cu(III), is required. When O-benzoyl-N,-N-dialkylhydroxylamine 2o was employed, desired product 5o was obtained in 69= yield and we didn’t observe the formation of pyrrolidine product 7 (Figure 4, Eq.2), which suggests that a nitrogen radical is not likely involved during the oxidation of Cu(I) catalyst. Similarly, cyclopropanol 1s gave desired product 4s in 76= yield and no cyclized product 8 was isolated indicating a non-radical cyclo-propanol ring-opening process (Figure 4, Eq.3).19 These two notions are further supported by the insensitivity of the reaction to the addition of 1.0 equiv of TEMPO (Figure 4, Eq.4).
Figure 4.
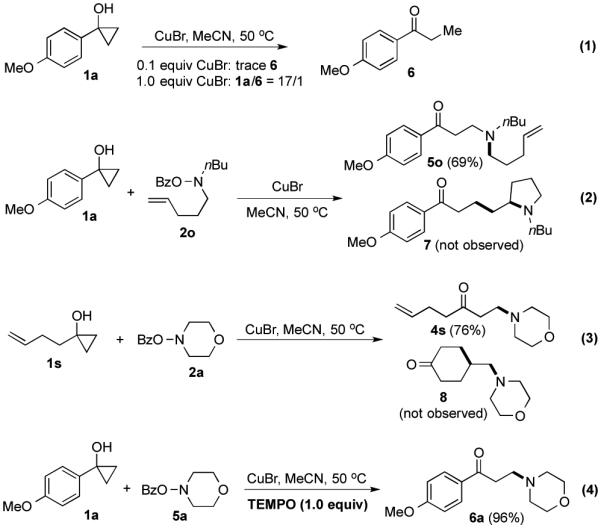
Preliminary probe of reaction mechanisms.
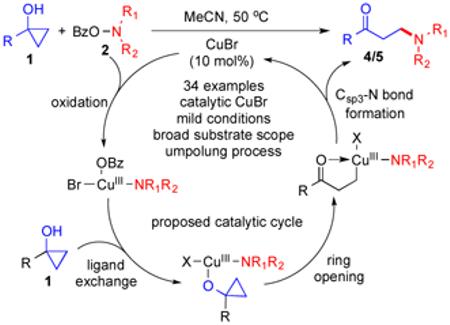
With these preliminary experimental results, we proposed the following reaction mechanism based on a Cu(I)/Cu(III) catalytic cycle (Figure 5). Cu(I) catalyst was first oxidized to Cu(III) complex A by O-benzoyl-N,-N-dialkylhydroxylamine 2.20 The latter would undergo ligand exchange and coordinate with cyclopropanol 1 to produce intermediate B, which then underwent β-carbon elimination to open the cyclopropane ring by breaking the Walsh bond and provide Cu(III) homoenolate C. Reductive elimination would form the desired Csp3-N bond, produce β-aminoketone 4/5, and regenerate Cu(I) catalyst.
Figure 5.
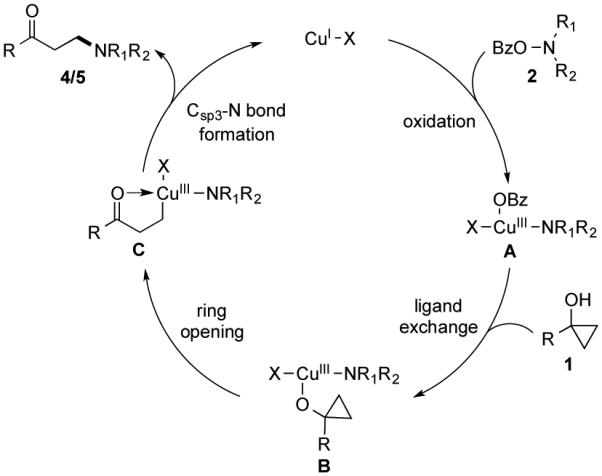
Proposed catalytic cycle.
In summary, we have developed a novel copper-catalyzed electrophilic amination of cyclopropanols with O-benzoyl -N,-N-dialkylhydroxylamines as oxidant. Various β-aminoketones can be synthesized in good to excellent yield. This novel synthetic transformation features mild reaction conditions, broad substrate scope, and excellent functional group compatibility, particularly those functional groups that are problematic in the traditional nucleophilic conjugate addition conditions and the Mannich reaction conditions. The reaction can also be conducted on gram-scale and in complex natural product and drug settings. Preliminary mechanism studies have been conducted and led us to propose a Cu(I)/Cu(III) catalytic cycle to account for the observed outcomes. While further mechanistic investigations are necessary to understand this reaction, it does open a new gate for the development of novel synthetic transformations involving the use of cyclopropanols and related systems as Csp3 cross-coupling partners.
Supplementary Material
ACKNOWLEDGMENT
We thank Prof. Y. Xia’s group at Purdue University for assistance with mass spectrometry and Purdue University for startup support. The NIH for supporting shared NMR resources to the Purdue Center for Cancer Research is acknowledged (P30CA023168). This work is also partially supported by the ACS Petroleum Research Foundation (PRF# 54896-DNI1).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures and characterization for new compounds are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
REFERENCES
- (1).Bariwal J, Van der Eycken E. Chem. Soc. Rev. 2013;42:9283. doi: 10.1039/c3cs60228a. For a review, see: [DOI] [PubMed] [Google Scholar]
- (2).(a) Wolfe JP, Wagaw S, Buchwald SL. J. Am. Chem. Soc. 1996;118:7215. [Google Scholar]; (b) Driver MS, Hartwig JF. J. Am. Chem. Soc. 1996;118:7217. [Google Scholar]
- (3).(a) Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; (b) Qiao JX, Lam PYS. Synthesis. 2011:829. [Google Scholar]
- (4).(a) Engle KM, Mei T-S, Wang X, Yu J-Q. Angew. Chem. Int. Ed. 2011;50:1478. doi: 10.1002/anie.201005142. For selected reviews, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thirunavukkarasu VS, Kozhushkov SI, Ackermann L. Chem. Commun. 2014;50:29. doi: 10.1039/c3cc47028h. [DOI] [PubMed] [Google Scholar]; (c) Whitfield SR, Sanford MS. J. Am. Chem. Soc. 2007;129:15142. doi: 10.1021/ja077866q. For leading references, see: [DOI] [PubMed] [Google Scholar]; (d) Tan Y, Hartwig JF. J. Am. Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. [DOI] [PubMed] [Google Scholar]; (e) Kawano T, Hirano K, Satoh T, Miura M. J. Am. Chem. Soc. 2010;132:6900. doi: 10.1021/ja101939r. [DOI] [PubMed] [Google Scholar]; (f) Kim JY, Park SH, Ryu J, Cho SH, Kim SH, Chang S. J. Am. Chem. Soc. 2012;134:9110. doi: 10.1021/ja303527m. [DOI] [PubMed] [Google Scholar]; (g) McDonald SL, Hendrick CE, Wang Q. Angew. Chem. Int. Ed. 2014;53:4667. doi: 10.1002/anie.201311029. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhu D, Yang G, He J, Chu L, Chen G, Gong W, Chen K, Eastgate MD, Yu J-Q. Angew. Chem. Int. Ed. 2015;54:2497. doi: 10.1002/anie.201408651. [DOI] [PubMed] [Google Scholar]
- (5).(a) Hili R, Yudin AK. Nat. Chem. Biol. 2006;2:284. doi: 10.1038/nchembio0606-284. For selected reviews, see. [DOI] [PubMed] [Google Scholar]; (b) Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Roizen JL, Harvey ME, DuBois J. Acc. Chem. Res. 2012;45:911. doi: 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jeffrey JL, Sarpong R. Chem. Sci. 2013;4:4092. [Google Scholar]; (e) Wu X, Zhao Y, Zhang G, Ge H. Angew. Chem. Int. Ed. 2014;53:3706. doi: 10.1002/anie.201311263. [DOI] [PubMed] [Google Scholar]
- (6).(a) Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795. doi: 10.1021/cr0306788. For a review, see: [DOI] [PubMed] [Google Scholar]; (b) Rucker RP, Whittaker AM, Dang H, Lalic G. J. Am. Chem. Soc. 2012;134:6571. doi: 10.1021/ja3023829. For selected examples, see: [DOI] [PubMed] [Google Scholar]; (c) Liu G-S, Zhang Y-Q, Yuan Y-A, Xu H. J. Am. Chem. Soc. 2013;135:3343. doi: 10.1021/ja311923z. [DOI] [PubMed] [Google Scholar]; (d) Lu D-F, Zhu C-L, Jia Z-X, Xu H. J. Am. Chem. Soc. 2014;136:13186. doi: 10.1021/ja508057u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Niljianskul N, Zhu S, Buchwald SL. Angew. Chem. Int. Ed. 2015;54:1638. doi: 10.1002/anie.201410326. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sakae R, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2015;54:613. doi: 10.1002/anie.201409104. [DOI] [PubMed] [Google Scholar]; (g) Matsuda N, Hirano K, Satoh T, Miura M. J. Am. Chem. Soc. 2013;135:4934. doi: 10.1021/ja4007645. [DOI] [PubMed] [Google Scholar]
- (7).Li Y, Ye Z, Bellman TM, Chi T, Dai M. Org. Lett. 2015;17 doi: 10.1021/acs.orglett.5b00782. DOI: 10.1021/acs.orglett.5b00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ye Z, Brust TF, Watts VJ, Dai M. Org. Lett. 2015;17:892. doi: 10.1021/ol503748t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Erdik E, Ay M. Chem. Rev. 1989;89:1947. For reviews, see: [Google Scholar]; (b) Dembeck P, Seconi G, Ricci A. Chem. Eur. J. 2000;6:1281. doi: 10.1002/(sici)1521-3765(20000417)6:8<1281::aid-chem1281>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]; (c) Barker TJ, Jarvo ER. Synthesis. 2011:3954. [Google Scholar]; (d) Zhang M, Zhang A. Synthesis. 2012:1. [Google Scholar]; (e) Corpet M, Gosmini C. Synthesis. 2014:2258. [Google Scholar]; (f) He C, Chen C, Cheng J, Liu C, Liu W, Li Q, Lei A. Angew. Chem. Int. Ed. 2008;47:6414. doi: 10.1002/anie.200801427. For selected examples: [DOI] [PubMed] [Google Scholar]; (g) Liu S, Liebeskind LS. J. Am. Chem. Soc. 2008;130:6918. doi: 10.1021/ja8013743. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Barker TJ, Jarvo ER. J. Am. Chem. Soc. 2009;131:15598. doi: 10.1021/ja907038b. [DOI] [PubMed] [Google Scholar]; (i) Barker TJ, Jarvo ER. Angew. Chem. Int. Ed. 2011;50:8325. doi: 10.1002/anie.201103700. [DOI] [PubMed] [Google Scholar]; (j) Ortiz GX, Jr., Kang B, Wang Q. J. Org. Chem. 2014;79:571. doi: 10.1021/jo4022666. [DOI] [PubMed] [Google Scholar]; (k) Hendrick CE, Wang Q. J. Org. Chem. 2015;80:1059. doi: 10.1021/jo502541t. [DOI] [PubMed] [Google Scholar]
- (10).(a) Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem. Rev. 2011;111:2626. doi: 10.1021/cr100204f. [DOI] [PubMed] [Google Scholar]; (b) Cardillo G, Tomasini C. Chem. Soc. Rev. 1996:117. [Google Scholar]
- (11).Liu M, Sibi MP. Tetrahedron. 2002;58:7991. For a review, see: [Google Scholar]
- (12).(a) Kobayashi S, Ishitani H. Chem. Rev. 1999;99:1069. doi: 10.1021/cr980414z. For reviews, see: [DOI] [PubMed] [Google Scholar]; (b) Arend M, Westermann B, Risch N. Angew. Chem. Int. Ed. 1998;37:1044. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; (c) Hart DJ, Ha D-C. Chem. Rev. 1989;89:1447. [Google Scholar]
- (13).(a) Gibson DH, DePuy CH. Chem. Rev. 1974;74:605. For reviews, see: [Google Scholar]; (b) Ryu I, Murai S. Houben-Weyl Methods of Organic Chemistry. E17c. Thieme; Stuttgart: 1997. p. 1985. [Google Scholar]; (c) Kulinkovich OG. Chem. Rev. 2003;103:2597. doi: 10.1021/cr010012i. [DOI] [PubMed] [Google Scholar]
- (14).(a) Rosa D, Nithiy N, Orellana A. Synthesis. 2013:3199. For a review, see: [Google Scholar]; (b) Aoki S, Fujimura T, Nakamura E, Kuwajima I. J. Am. Chem. Soc. 1988;110:3296. For examples, see: [Google Scholar]; (c) Fujimura T, Aoki S, Nakamura E. J. Org. Chem. 1991;56:2809. [Google Scholar]; (d) Aoki S, Nakamura E. Synlett. 1990:741. [Google Scholar]; (e) Kang S-K, Yamaguchi T, Ho P-S, Kim W-Y, Yoon S-K. Tetrahedron Lett. 1997;38:1947. [Google Scholar]; (f) Rosa D, Orellana A. Org. Lett. 2011;13:110. doi: 10.1021/ol1026409. [DOI] [PubMed] [Google Scholar]; (g) Rosa D, Orellana A. Chem. Commun. 2012;48:1922. doi: 10.1039/c2cc16758a. [DOI] [PubMed] [Google Scholar]; (h) Parida BB, Das PP, Niocel M, Cha JK. Org. Lett. 2013;15:1780. doi: 10.1021/ol400666x. [DOI] [PubMed] [Google Scholar]; (i) Cheng K, Walsh PJ. Org. Lett. 2013;15:2298. doi: 10.1021/ol4008876. [DOI] [PubMed] [Google Scholar]; (j) Rosa D, Orellana A. Chem. Commun. 2013;49:5420. doi: 10.1039/c3cc42080a. [DOI] [PubMed] [Google Scholar]; (k) Nithiy N, Orellana A. Org. Lett. 2014;16:5854. doi: 10.1021/ol5027188. [DOI] [PubMed] [Google Scholar]; (k) Nikolaev A, Nithiy N, Orellana A. Synlett. 2014:2301. [Google Scholar]
- (15).Krause N. Modern Organocopper Chemistry. Wiley; Hoboken: 2002. [Google Scholar]
- (16).(a) Ryu I, Ando M, Ogawa A, Murai S, Sonoda N. J. Am. Chem. Soc. 1983;105:7192. [Google Scholar]; (b) Ryu I, Matsumoto K, Kameyama Y, Ando M, Kusumoto N, Ogawa A, Kambe N, Murai S, Sonoda N. J. Am. Chem. Soc. 1993;115:12330. [Google Scholar]
- (17).(a) Das PP, Belmore K, Cha JK. Angew. Chem. Int. Ed. 2012;51:9517. doi: 10.1002/anie.201205190. [DOI] [PubMed] [Google Scholar]; (b) Rao NN, Parida BB, Cha JK. Org. Lett. 2014;16:6208. doi: 10.1021/ol503136s. [DOI] [PubMed] [Google Scholar]; (c) Rao NN, Cha JK. J. Am. Chem. Soc. 2015;137:2243. doi: 10.1021/jacs.5b00243. [DOI] [PubMed] [Google Scholar]
- (18).(a) Berman AM, Johnson JS. J. Am. Chem. Soc. 2004;126:5680. doi: 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]; (b) Berman AM, Johnson JS. J. Org. Chem. 2005;70:364. doi: 10.1021/jo048168g. [DOI] [PubMed] [Google Scholar]; (c) Berman AM, Johnson JS. J. Org. Chem. 2006;71:219. doi: 10.1021/jo051999h. [DOI] [PubMed] [Google Scholar]; (d) Campbell MJ, Johnson JS. Org. Lett. 2007;9:1521. doi: 10.1021/ol0702829. [DOI] [PubMed] [Google Scholar]; (e) Berman AM, Johnson JS. Synlett. 2005:1799. [Google Scholar]; (f) Matsuda N, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2012;51:3642. doi: 10.1002/anie.201108773. [DOI] [PubMed] [Google Scholar]; (g) Matsuda N, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2012;51:11827. doi: 10.1002/anie.201206755. [DOI] [PubMed] [Google Scholar]; (h) Rucker RP, Whittaker AM, Dang H, Lalic G. Angew. Chem. Int. Ed. 2012;51:3953. doi: 10.1002/anie.201200480. [DOI] [PubMed] [Google Scholar]; (i) Xiao Q, Tian LM, Tan R, Xia Y, Qiu D, Zhang Y, Wang J. Org. Lett. 2012;14:4230. doi: 10.1021/ol301912a. [DOI] [PubMed] [Google Scholar]; (j) Dumas AM, Molander GA, Bode JW. Angew. Chem. Int. Ed. 2012;51:5683. doi: 10.1002/anie.201201077. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Dong Z, Dong G. J. Am. Chem. Soc. 2013;135:18350. doi: 10.1021/ja410823e. [DOI] [PubMed] [Google Scholar]; (m) McDonald SL, Wang Q. Angew. Chem. Int. Ed. 2014;53:1867. doi: 10.1002/anie.201308890. [DOI] [PubMed] [Google Scholar]; (n) McDonald SL, Wang Q. Chem. Comm. 2014;50:2535. doi: 10.1039/c3cc49296f. [DOI] [PubMed] [Google Scholar]
- (19).(a) Hasegawa E, Tateyama M, Nagumo R, Tayama E, Iwamoto H. Beilstein J. Org. Chem. 2013;9:1397. doi: 10.3762/bjoc.9.156. For Cu(II) salt-promoted radical cyclopropanol ring opening, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hasegawa E, Yamaguchi N, Muraoka H, Tsuchida H. Org. Lett. 2007;9:2811. doi: 10.1021/ol0709937. [DOI] [PubMed] [Google Scholar]; (c) Li L-Z, Xiao B, Guo Q-X, Xue S. Tetrahedron. 2006;62:7762. [Google Scholar]; (d) Snider BB, Kwon T. J. Org. Chem. 1992;57:2399. [Google Scholar]; (e) Wang Y-F, Toh KK, Ng EPJ, Chiba S. J. Am. Chem. Soc. 2011;133:6411. doi: 10.1021/ja200879w. For a leading reference of other radical cyclopropanol ring-opening chemistry: [DOI] [PubMed] [Google Scholar]; (f) Zhao H, Fan X, Yu J, Zhu C. J. Am. Chem. Soc. 2015;137:3490. doi: 10.1021/jacs.5b00939. [DOI] [PubMed] [Google Scholar]
- (20).(a) Zhu S, Niljianskul N, Buchwald SL. J. Am. Chem. Soc. 2013;135:15746. doi: 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu S, Buchwald SL. J. Am. Chem. Soc. 2014;136:15913. doi: 10.1021/ja509786v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shi S-L, Buchwald SL. Nat. Chem. 2015;7:38. doi: 10.1038/nchem.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vikse KL, Chen P. Organometallics. 2015 DOI: 10.1021/acs.organomet.5b00038. [Google Scholar]; (d) Casitas A, Ribas X. Chem. Sci. 2013;4:2301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.