Abstract
Objective
The second messenger cAMP is involved in both β3 adrenoceptor (β3-AR) mediated detrusor relaxation and the kinetics of Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Here we characterized the effect HCN channel activation and possible interaction with β3-AR in bladder.
Materials and Methods
Bladder tissues from Sprague-Dawley rats and Human organ donors were obtained for studying species-specific expression of HCN channels by real-time qPCR and Western Blot. Effect of β3-agonist on rat bladder strips (0.5 × 0.5 × 7 mm in size) was studied during activation and blockade of HCN channels by Lamotrigine and ZD7288, respectively.
Results
Expression of all four genes encoding for HCN channels (HCN1-4) was detected separately in bladder mucosa and detrusor from human and rat bladders. Species based differences were evident from relatively higher expression of HCN4 isoform in human bladder and that of HCN1 in rat bladder. Western blot confirmed the findings at mRNA level. Cumulative application β3-AR agonist CL316,243 produced a concentration dependent decrease in resting tension of rat bladder strips expressed as integral of mechanical activity. Pre-incubation of HCN channel blocker ZD 7288 opposed the relaxant effect of CL316,243, whereas co-administration of lamotrigine with CL316,243 at equal molar concentrations caused an additive decrease in resting tension. Cumulative addition of ZD7288 and lamotrigine in absence of CL316,243 showed opposing effects on detrusor contractility.
Conclusions
Species-specific differences were noted in expression of HCN channels in bladder. Opposing effects ZD7288 and Lamotrigine in the action of β3-AR agonist demonstrate possible functional interaction of HCN channels and β3-AR in detrusor contractility.
Keywords: HCN, ZD7288, detrusor relaxation, β3-AR
Introduction
Urinary bladder smooth muscle is characterized by spontaneous action potentials that trigger phasic contractions [1], whose relaxation is mediated by the second messenger cyclic adenosine monophosphate (cAMP) [2]. Elevation of intracellular cAMP by activated β3-adrenoceptors (β3-ARs) [3] ultimately causes detrusor smooth muscle relaxation following changes in intracellular [Ca2+] bought by changes in membrane potential through hyperpolarization.
Coincidentally, hyperpolarization in neurons and smooth muscles also activates a family of non-selective cationic channels that conduct Na+ and K+ current known as hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [4,5]. HCN channels are comprised of four subtypes encoded by four genes (HCN1-4), which form the structural component of a voltage-gated inwardly rectifying Ih current, which restores the resting membrane potential [4,5]. Direct binding of intracellular cAMP to HCN channels can activate them [4,5], raising the possibility of interaction with drugs acting upstream of cAMP second messenger pathway such as β3-AR agonist. The proposed research hypothesizes communication between β3-ARs and HCN channels in β-adrenoceptor-mediated detrusor muscle relaxation.
We have previously reported expression and function of HCN in neurons that govern voiding, but function of HCN channels expressed within the bladder is not completely known. Despite the observations that HCN channels are expressed in bladder [6], their physiological role is currently unknown. In the present study, we performed a series of functional and molecular experiments to investigate expression of HCN channels and their potential role in detrusor contractility.
Materials and Methods
Human bladder tissue
Using an honest broker system of the Health Sciences Tissue Bank at University of Pittsburgh, human bladder tissues were obtained after informed consent from the next of kin of 3 organ donors. The study was approved by Committee for Oversight of Research and Clinical Training Involving Decedents at University of Pittsburgh. The organ donors belonged to both sexes and aged between 18–69 years. The health and disease status of organ donors was unavailable to the study investigators. Removed bladder tissues were kept in ice-cold Krebs salt solution until the detrusor was separated from the bladder mucosa by microdissection under sterile conditions. The term bladder mucosa refers to both urothelial and suburothelial structures including the lamina propria. For quantitative polymerase chain reaction QPCR and Western blot studies, frozen detrusor and bladder mucosa samples were divided into 2 sections each. Total protein concentrations were determined by bicinchoninic acid protein assay (Pierce, Rockford, Illinois) with bovine serum albumin as the standard.
Rat bladder
In this study, we used 20 males Sprague–Dawley rats (Harlan Laboratories Inc., Frederick, MD, USA) of average weight 350 g. Rats were killed with CO2 inhalation following the procedures of the animal use protocol reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Isolated urinary bladders were cut open longitudinally from the lumen to isolate urothelium intact 7 mm long bladder strips for isometric tension recordings.
Chemicals
Lamotrigine and ZD7288, CL316243 (Disodium (R,R)-5- [2- [[2-(3-chlorophenyl)-2-hydroxyethyl]-amino] propyl]-1,3-benzodioxole-2,2-dicarboxylate), were obtained from Tocris bioscience Catalog (Ellisville, Missouri, USA). All solutions were prepared fresh daily. Stock solutions were all made in dimethyl sulfoxide. Further dilutions were carried out in Krebs solution. The concentrations are expressed as the final molar concentration in the tissue chamber.
Isometric tension recording
Contractile responses of urothelium-intact rat bladder strips (0.5 × 0.5 × 7 mm in size) were measured using isometric transducers (World Precision Instruments, Sarasota, Florida, USA) and recorded using a data acquisition system PowerLab Software. Tissues were allowed to equilibrate under a resting tension of 10 mN for 60 min in oxygenated Krebs solution as previously described [7] and washed with fresh solution every 15 min during equilibration period. Each tissue sample was suspended in a 5ml organ bath containing oxygenated Krebs solution (NaCl, 118 mM/L; KCl, 5.6 mM/L; NaHCO3, 25 mM/L; KH2PO4, 1.2 mM/L; CaCl2, 2.5 mM/L; MgSO4, 1.2 mM/L; glucose 6.1 mM/L; pH 7.4) and maintained at 37°C and constantly aerated with 95% oxygen and 5% carbon dioxide. Electrical field stimulation (EFS) was performed to verify the tissue viability [7]. Strips were exposed to either Lamotrigine [8-10] or ZD7288 in the concentration range of 10−10 to 10−4 M using a cumulative protocol with each addition separated by approximately 5–10 min intervals. Effect of ZD7288 on EFS evoked contractions was tested by delivering square wave pulses of 5ms width at 20 V for 2 s duration with 20 s intervals at 20 hz frequency in the presence and absence of tetrodotoxin (TTX) 10−6 M. Spontaneous activity evoked by ZD7288 was also tested in the presence of TTX to determine contribution of neural input. Mechanical responses of the bladder strips were recorded and the mechanical activity over an equal time period was integrated for comparative analysis. Cumulative effect of CL316,243 was studied with or without pre-incubation of ZD7288 in the tissue bath and equilibrated for ≥10 minutes. Effect of CL316,243 was also checked in the presence of equal molar concentration of Lamotrigine. The results were expressed as % of integral spontaneous activity relative to pretreatment value.
RNA isolation and RT-PCR
Total RNA was extracted from freshly separated bladder mucosa and detrusor as described previously [11]. Tissue specimens were kept in RNAlater and total RNA was isolated using RNeasy™ kits (Qiagen, Hilden, Germany) according to the manufacturer protocol and RNA was quantified by a spectrophotometer (Eppendorf, Hamburg, Germany). All gene-specific primers were designed using software based on the completed species specific mRNA sequences in Genebank. Total RNA from human heart was obtained for the control experiments from Clontech and total RNA from rat heart was obtained from rat heart tissue harvested at the time of bladder removal for isometric studies. QPCR reactions were performed according to the manufacturer protocol. Briefly, cDNA was prepared from 1 μg total RNA using an RT2 PCR array first strand kit (SABiosciences™). QPCR reactions were done in a 25 μl mixture, which included 12.5 μl RT2 Real-Time™ SYBR® Green/ROX PCR master mix (SABiosciences), 10.5 μl nuclease-free H2O, 1 μl specific primers, including 10 μM each of HCN1-4 gene-specific primers for human or rat and Actin-β (SABiosciences), and 1 μl template cDNA.
QPCR amplification was done with an initial 10-min step at 95°C, followed by 40 cycles at 95°C for 15 s and at 60°C for 1 min. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a unique product free of primer dimers. Quantitation was performed using the Mx3000P® QPCR detection system for fluorescence threshold Ct values and to determine relative expression. Reliability of specific products was checked by omission of the reverse transcriptase (RT) in RT-PCR reaction mixture for the negative control. HCN channel expression in human and rat bladder was normalized to β-Actin and expressed relative to expression in human heart and rat heart, respectively using the 2-ΔΔCt method.
Western blot analysis
Western blot analysis was carried out for different HCN proteins (1-4) in the bladder tissue. Briefly, bladder tissue was homogenized using CelLytic™ MT Mammalian Tissue Lysis/Extraction Reagent (Sigma, USA) in the presence of phenylmethylsulfonyl fluoride (1 mM), sodium orthovanadate (2 mM) and protein inhibitor cocktail (Sigma, USA). Protein estimation was done by BCA Protein Assay Kit (Pierce, Rockford, Illinois). An equal amount (50 μg/well) of denatured proteins was loaded in 10% tricine-SDS gel and blotted on polyvinylidene fluoride (PVDF) membranes using wet transfer system. After blocking (2 h at 37°C), membranes were incubated overnight at 4°C with primary antibodies specific for HCN1, HCN4 and Actin-β (Santa Cruz Biotechnology), HCN2 and HCN3 (Abcam, USA), in blocking buffer (pH 7.5). The membranes were then re-incubated for 2 h at room temperature with secondary immunoglobulin G (IgG)-conjugated with horseradish peroxidase (Santa Cruz Biotechnologies, USA). The blots were developed using luminol (Thermo Scientific, USA) and measured on Versa doc imaging system (Model 4000; BioRed, USA). Densitometry for protein specific band was done using AlphaEase FC StandAlone V. 4.0.0 software. β-Actin was used as an internal control to normalize the band density.
Data analysis and statistics
Contractile activity was quantified by measuring the muscle integral force over 5 min intervals (the area under the curve of the phasic contractions) post-application of each concentration of drugs [12]. To estimate the relative changes at each dose level, the data were normalized and expressed as percentages with respect to values measured prior to drug addition. Statistical analysis was performed with GraphPad Prism 4.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The data are expressed as mean ± SE. Statistical analysis was performed using two-tailed unpaired Student's t-test or Two way ANOVA followed by Bonferroni post test. A P-value < 0.05 was considered to be statistically significant.
Results
Expression of HCN genes in human bladder
We detected expression of all four genes HCN1-4 at mRNA level in both bladder mucosa and detrusor specimens of human bladder (Fig. 1A). The expression of HCN4 was predominant over other HCN isoforms in both bladder mucosa and detrusor. HCN4 expression in detrusor was 8 fold higher than in the human heart and 2 fold higher than the levels detected in bladder mucosa. Dominant mRNA expression of HCN4 in human detrusor was supported by the protein expression measured by Western Blot (Fig. 2A). The band density for HCN4 was normalized to the corresponding band density for the loading control β-Actin. The normalized band density for HCN4 was 3 fold higher than for other subtypes in detrusor. Normalized band density in bladder mucosa was 6 fold higher for HCN1 and HCN2 and 2 fold higher for HCN3 relative to the densities of respective subtypes measured in corresponding detrusor tissues. It is likely that expression of HCN channels in detrusor is predominantly contributed by neurons and interstitial cells.
Figure 1. Expression of HCN channels in the bladder mucosa and detrusor of human and rat bladder measured by real-time QPCR.
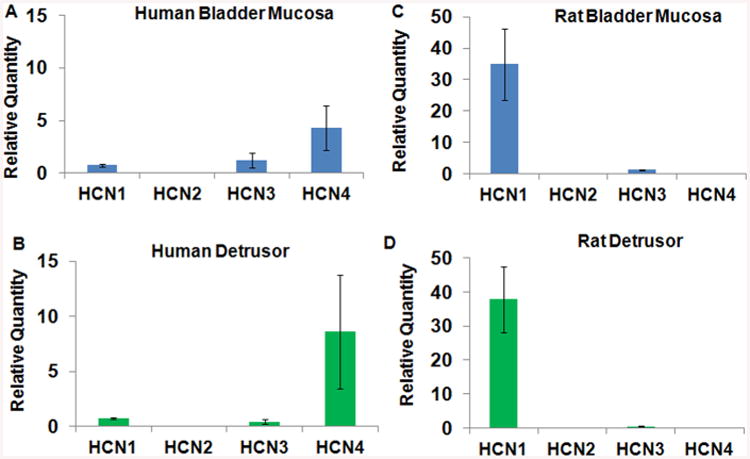
A and B. In human bladder, expression of HCN subtypes in bladder mucosa (A) was lower compared to expression in detrusor (B). Among the subtypes, expression of HCN4 was predominant in human detrusor with comparable expression of HCN1 and HCN3 subtype. Bars depict the relative quantity of expression level with respect to expression in human heart (mean ± SEM; n = 3 specimens). C and D. The expression of HCN1 subtype was predominant in rat bladder mucosa (C) and detrusor (D) with expression comparable for other subtypes in bladder mucosa and detrusor. HCN channel subtype expression in human and rat bladder was normalized to β-Actin and calculated relative to expression in human heart and rat heart, respectively using the 2-ΔΔCt method. Relative expression is plotted as relative quantity RQ.
Figure 2. Western blot images of HCN 1-4 subtype expression in human (A) and rat bladder (B).
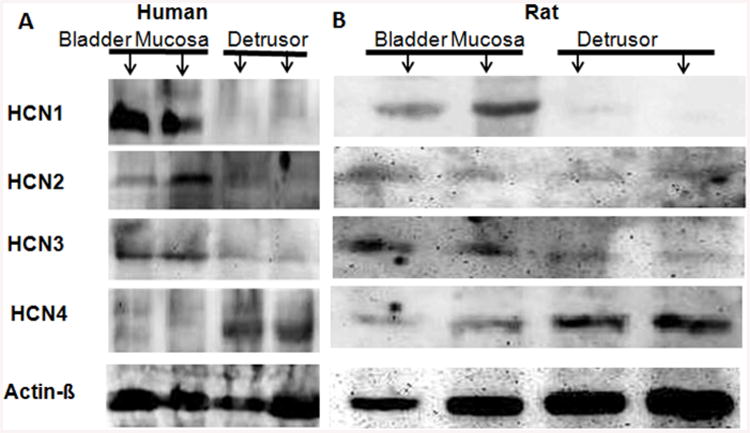
β-Actin was used as a loading control for each lane, where the mucosa containing urothelium and detrusor specimens were added to separate lanes indicated by arrows. A. As predicted by PCR findings, dense band corresponding to HCN4, 110 kDa protein was predominantly detected in human detrusor smooth muscle, with a faint band for HCN4 in urothelium. The bands for HCN1, HCN2 and HCN3 were only detectable in human bladder mucosa with only faint corresponding bands in detrusor. B. In rat bladder, the band corresponding HCN1 subtype was strongest in bladder mucosa with comparable expression of HCN3 and HCN4 in urothelium.
Expression of HCN in rat bladder
Expression of HCN1 was predominant in bladder mucosa (Fig. 1C) and detrusor (Fig. 1D), as also published elsewhere [13]. Expression of HCN1 in bladder mucosa was more than 30 times the levels in rat heart. The predominant HCN1 mRNA expression in rat bladder mucosa (Fig. 1C and D) is supported by Western blot results (Fig. 2B). Normalized band density for HCN1 was 10 fold higher in bladder mucosa relative to detrusor. In contrast, the normalized band densities for HCN2, HCN3 and HCN4 were comparable.
Effect of lamotrigine and ZD7288 on phasic contractions of rat bladder in the absence of TTX
When stretched under 10 mN tension, bladder strips displayed phasic contractions as shown in the representative tracings in Figure. 3. Cumulative application of Lamotrigine (Fig. 3A; blue tracing) caused a concentration dependent decrease in contractility as measured by total (integrated) mechanical activity of rat detrusor strips in separate 5 min intervals. At the 10−5 M concentration in the tissue bath, Lamotrigine decreased the contractile activity to 69.65± 2.21% (n = 5, P < 0.05; Fig. 3B) of the pretreatment values measured at baseline. To determine, if Lamotrigine elicited responses by activating HCN channels, bladder strips were pretreated with of ZD7288(Fig. 3A; purple tracing) at 10−5 M in the bath for 20min prior to cumulative addition of Lamotrigine, where 10−5 M concentration of ZD7288 was constantly present in the bath. The Lamotrigine dose responses with and without ZD7288 were performed on separate bladder strips. The concentration dependent relaxant effect of Lamotrigine was blocked in the presence of ZD7288 indicating that HCN channels activated by Lamotrigine facilitate relaxation. ZD7288 caused moderate decrease in the relaxant effect of Lamotrigine [10-9 M] 4.72 ± 0.79% and that of Lamotrigine [10-4 M] by 15.36 ± 4.24% (*P < 0.05; two-way ANOVA followed by a Bonferroni multiple comparison post hoc test).
Figure 3. Effect of ZD7288 on phasic contractions in absence of Tetrodotoxin (TTX).
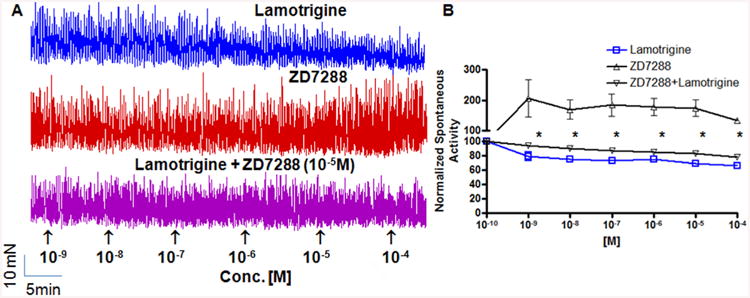
A. Typical traces of isometric tension recording from urothelium intact rat bladder strips. Cumulative addition of a non-selective blocker of HCN channels, ZD 7288 produced a concentration dependent increase in phasic detrusor contractions (red tracing), whereas Lamotrigine, which is known to activate HCN channels, caused a modest decrease in phasic contractions (blue tracing). In each trace, the timing of cumulative drug application is shown by arrowheads, and vehicle (DMSO) had no effect. The effect of Lamotrigine was blocked when HCN blocker ZD7288 was constantly present in the bath at the fixed conc. of 10-5 M (purple tracing), demonstrating that relaxant effect of Lamotrigine is dependent on opening of HCN channels. B. Comparison of ZD7288 (Δ), Lamotrigine (□) and combination of two drugs (∇) for effects on normalized integrated mechanical activity. The relaxant effect of Lamotrigine was reversed in the presence of ZD 7288(∇). Ordinate Scale is total integrated mechanical activity (above baseline) normalized to the activity recorded prior to drug application, which is defined as 100% (corresponds to arbitrary point of 10-10 M on x-axis). Each point in (B) represents mean ± SEM of 4–5 strips. ZD7288 caused moderate decrease in the relaxant effect of Lamotrigine [10-9 M] by 4.72 ± 0.79% and that of Lamotrigine [10-4 M] by 15.36 ± 4.24% (*P < 0.05; two-way ANOVA followed by a Bonferroni multiple comparison post hoc test). The y-axis is in two segments 0–100 and 100–300 to indicate the divergent effect of Lamotrigine and ZD7288.
Furthermore, cumulative application of ZD7288 per se (Fig. 3A; red tracing) to spontaneously contracting bladder strips also caused a concentration-dependent increase in muscle integral force. ZD7288 (10−5 M) increased the muscle integral force by 174.09 ± 28.2% (n = 4, P < 0.05; Fig. 3B) relative to baseline. Comparison of integral muscle force at 10−5 M between ZD7288 and Lamotrigine found significant difference (unpaired t test, P < 0.05). Increased tension of the phasic contractions on cumulative addition of ZD7288 supports our hypothesis that, ZD7288-sensitive HCN channels are constitutively active during bladder relaxation. The spontaneous contractions evoked by cumulative application of ZD7288 are not sensitive to the continued presence of TTX 10−6 M in the myobath.
Effect of ZD7288 on phasic and neurogenic contractions in the presence of TTX
Repeated control neurogenic contractions were evoked by 20 hz stimulation frequency, which were considerably blocked in the presence of TTX 1 μM in the myobath (Fig. 4A-C). In absence of TTX, addition of ZD7288 in the myobath at both low (10-8 M) and higher concentration of (10-4 M) caused a moderate inhibition of the contraction amplitude from control levels (Fig. 4A and B; blue tracing). There was 8.16 ±1.55% reduction from control levels at lower concentration of 10-8 M and 9.4 ± 6.95% reduction at higher concentration of 10−4 M for ZD7288. In the presence of TTX 1 μM, ZD7288 had no discernible effect on the TTX insensitive contractions evoked by EFS at 20 hz stimulation frequency (Fig. 4C; purple tracing). In the presence of TTX 1 μM, ZD7288 produced a dose dependent increase in the phasic contractions (Fig. 4D). ZD7288 at the concentration of 10-4 M in the myobath increased the muscle integral force by 217.3 ± 19.2% (n = 3) (Fig. 4E). Effect of ZD7288 on phasic contractions in the presence of TTX 1μM suggests that in absence of neural input, HCN inhibition in bladder is likely to cause increased pacemaker activity of interstitial cells.
Figure 4. Effect of ZD7288 on phasic and neurogenic contractions in the presence of Tetrodotoxin (TTX) (A-C) produced moderate inhibition of EFS evoked contractions at 20 hz frequency.
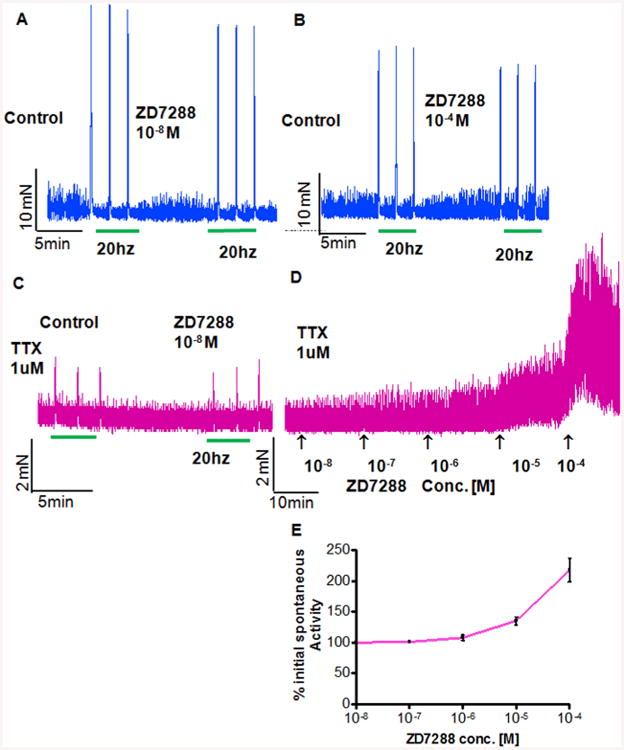
Three repeated pre-treatment control contractions were evoked with 20hz frequency before incubating strips with ZD7288 10-8 M (A) and 10-4 M (B) repeating the stimulation. TTX 1 μM dramatically reduced the amplitude of EFS contractions 20hz frequency and in presence of TTX 1 μM, ZD7288 at 10-8 M had no discernible effect on the TTX insensitive contractions evoked by EFS (C), notice the different scale compared to (A) and (B). In the presence of TTX 1 μM, ZD7288 produced a dose dependent increase in phasic contraction (D and E), the muscle integral force increased by 217.3 ± 19.2% (n = 3) at the concentration of 10-4 M in myobath relative to pretreatment values. Notice that amplitude of contractions in (C) and (D) is much lower than amplitude of neurogenic contractions measured in absence of TTX shown in (A) and (B).
Effect of CL316,243 on phasic contractions of rat bladder in the absence of TTX
Cumulative application of β3-agonist (CL316,243) concentration dependently reduced the phasic contractions of rat bladder strips (Fig. 5A; red tracing). At the highest concentration of 10−4M, CL316,243 was able to reduce the integral muscle activity to 28.88 ± 2.29%(n = 4, P < 0.05; Fig. 5B) of the pretreatment values. Pre-incubation of bladder strips with ZD7288 (Fig. 5A; green tracing) (10−5 M) significantly inhibited the relaxant effect of CL316,243 (10−4 M) as integral muscle activity doubled from 28.88 ± 2.29% to 51.23 ± 6.83% (n = 3, P < 0.05; Fig. 5B). ZD7288 increased the phasic contractions and slightly shifted the concentration-response curve of CL316,243 to the right.
Figure 5. Concentration dependent effect of β3-AR agonist and agents acting on HCN channels on spontaneous activity of rat bladder strips in absence of TTX.
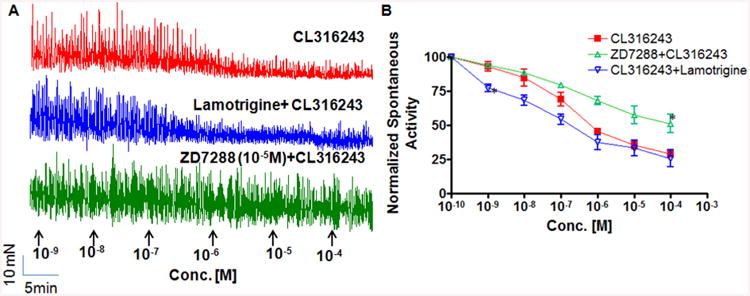
A. Typical traces showing CL316,243 (□) produced concentration dependent decrease in the integrated mechanical activity of rat bladder strips. Relaxant effect of CL316,243 [10-4 M] was decreased in the presence of ZD7288 (Δ) as measured by a doubled mechanical activity from 28.88 ± 2.29% to 51.23 ± 6.83% (*P < 0.05). In contrast, a decrease in mechanical activity caused by CL316,243 was concentration dependently enhanced in the presence of Lamotrigine(∇). Compared to constant concentration of ZD7288 used in combined experiment with CL316,243, in similar experiments involving Lamotrigine, it was added in equimolar concentrations at each concentration addition indicated by arrowhead. B. Lamotrigine induced significant enhancement of detrusor relaxation was most evident at 10-9 M (*P < 0.01). Ordinate Scale is total integrated mechanical activity (above baseline) expressed as a ratio of the pretreatment value (corresponds to 10-10 M on x-axis), which was defined as 100%. Each point in panel B represents mean ± SEM of 3–4 strips.
Considering the respective antagonist and agonist nature of ZD7288 and Lamotrigine, respectively on HCN channels, we followed different addition protocol to the tissue bath in combination experiments with CL316,243. Bladder strips were pre incubated with ZD7288 (10-5 M) and then it was constantly present in the bath during cumulative addition of CL316,243. In contrast, the Lamotrigine was added in equimolar concentrations to CL316,243 at each arrow point. Cumulative co-administration of lamotrigine (Fig. 5A; blue tracing) at equimolar concentrations with CL316,243 enhanced the relaxant effect of CL316,243. The integral muscle activity recorded when both drugs were at present at 10−9 M concentration in the bath was 77.32 ± 2.52% of the pretreatment values compared to 93.03 ± 3.38%, when CL316,243 at10−9 M was present alone in the tissue bath (n = 3, P < 0.01; Fig. 5B).
Discussion
We were able to confirm the expression of HCN channels in the human and rat bladder as has been reported in recent studies [6,13]. Compared to other studies, which reported the expression of HCN1-4 subtypes in the whole rat bladder [13], we were able to study region specific expression of HCN1-4 separately in bladder mucosa and detrusor portion of bladder. The observations of predominant expression of HCN4 mRNA in human bladder corroborate the recent findings of increased HCN4 protein expression [6]. In contrast, expression of HCN1 mRNA was predominant in rat bladder, which is also in agreement with published reports [13]. It is likely that expression of HCN channels in detrusor is predominantly contributed by neurons and interstitial cells, although as studies on enteric neurons indicates, HCN immunoreactivity does not correlate with functional activity of HCN channels [14]. Therefore, functional studies on HCN channels in bladder are important.
Our prior work in this field [15] demonstrated that HCN channels expressed in mechanosensitive {Aδ} bladder neurons [16] play an important role in the control of normal voiding [17]. Based on previous research in this field [12], we hypothesized a functional role of HCN channels expressed in the bladder in phasic contractions [18], which are believed to be triggered by bursts of spike potentials [1]. We hypothesized that basal levels of intracellular cAMP in bladder can constitutively activate HCN channels, and therefore the blockade of HCN channels should increase the phasic contractions.
Urothelium intact bladder strips were used for the contractility experiments, because molecular studies indicated prominent expression of HCN channels in bladder mucosa [13]. Moreover, interstitial cells of cajal in suburothelium expressing HCN channels are known to participate in spontaneous bladder contractility and voiding through different signaling pathways [19]. We found that application of HCN channel antagonist ZD7288 [15] increased the phasic contractions of bladder strips in a reversible manner. The effect of ZD7288 on the concentrations tested here agree with previously reported results [12] as well with the reported IC50 of ZD7288 for HCN channels, which is in the range of 0.2 μM for heart [20] and1.8 μM for brain neurons [21]. There is a risk that higher concentration of ZD7288 (50 μM) used in recent bladder strip studies [13,22] may receive confounding contribution from inhibition of other channels as ZD7288 can block T-type Ca2+ channels with an IC50 of 100 μM [23]. ZD7288 at lower dose levels, which are closer to the reported IC50 of ZD7288 for HCN channels, increases the amplitude and frequency of spontaneous contractions.
Considering the effect of ZD7288 on spontaneous activity, we expected a decrease in the activity of phasic contractions with agents that can cause pharmacological activation of HCN channels. Search for such agents was helped by a recent report, which claimed that anticonvulsants such as Lamotrigine and Gabapentin can also activate HCN channels [9,24]. Cumulative addition of Lamotrigine to the tissue bath caused a significant inhibition of phasic contractions in a concentration-dependent manner. The inhibitory effect of Lamotrigine was competitively opposed by ZD7288. Opposing effects of ZD7288 and Lamotrigine on bladder contractility illustrates that constitutive role of HCN channels is to attenuate the spontaneous contractions of rat bladder.
The origin of spontaneous activity in rat bladder has been a subject of intense interest and several tools have been used for its investigations. Earlier studies on optical imaging of bladder cross-sections [25] demonstrated that spontaneous activity (measured by propagating Ca2+ waves) often arises in the suburothelial layer of interstitial cells and then spreads to the detrusor layer. The spontaneous activity of urothelium intact bladder strips [25] is also usually higher perhaps due to better coordination of the activity of interstitial cells. Interstitial cells are considered pacemaker cells that activate the periodic spontaneous inward currents (pacemaker currents) responsible for the origin of Ca2+ waves.
It is postulated that interstitial cells [26] innervated by afferents and efferent nerves can modulate and coordinate activity of detrusor bundles [27] and our findings suggest that HCN channels are involved in the spread of evoked network activity from neurons to interstitial cells of cajal in bladder. A similar role for HCN channels is previously reported in neocortical slices [24]. Theoretically speaking, HCN inhibition by ZD7288 impairs the coordination of activity between urothelium and interstitial cells, which leads asynchronous generation of increased pacemaker activity by interstitial cells, ultimately leading to increased rhythmicity of phasic contractions. Furthermore, phasic contractions induced by ZD7288 were increased in the presence of TTX, which supports our postulate that blockade of neural input to interstitial cells increases the asynchronous generation of pacemaker activity by interstitial cells. The insensitivity of interstitial cells of bladder for tetrodotoxin is corroborated by a recent report [28].
Conversely, activation of HCN channels by lamotrigine can facilitate the coordination of spontaneous activity in urothelium and interstitial cells which leads to reduced asynchronous generation of pacemaker activity by interstitial cells and ultimately reduced rhythmicity of phasic contractions. At the concentrations tested, ZD7288 is not known to inhibit any potassium currents [12], which can explain the increase in phasic contractions. It is known that Lamotrigine can block Na+ channels, but inhibition of Na+ channels requires much higher concentrations [29] than those used in experiments described here. Furthermore, Lamotrigine is devoid of any effect on bladder contractility at higher concentrations required to block Na+ channels [30]. The effect of HCN channels on Ca2+ influx in detrusor smooth muscles needs to be studied in detail in order to understand the underlying mechanisms for these observations.
Since HCN channels are activated by nucleotides (cAMP), there is a potential for neurotransmitters or their mimetics to influence the activity of HCN channels by increasing or decreasing the cAMP levels inside the cell [31,32]. We demonstrated a role for HCN channels in β3-AR-mediated inhibition of phasic contractions. Previous studies have shown that CL316,243 exhibits a more potent β3-AR agonistic activity and detrusor relaxing effects in rats, but has less potency for human β3-AR activity and, as a consequence, for human bladder relaxation [33]. Pre-incubation of rat bladder strips with ZD7288 opposed the characteristic relaxant effect of CL316,243 and produced a slight rightward shift in the relaxing effect of CL316,243, which also accompanied a change in the maximal relaxing response. It can be inferred from these observations, that rat bladder relaxation is facilitated by activation of HCN channels. It is known that interstitial cells are also innervated by afferent nerves and they can potentially serve as the control point for regulation of spontaneous activity [27] and studies report that activation of β3-AR expressed on primary bladder afferents by CL316,243 contributed to detrusor relaxation [34,35]. Increased cAMP following β3-AR-agonism in afferents or interstitial cells can theoretically synchronize the generation of pacemaker activity in interstitial cells and this postulate is consistent with reduced pacemaker currents in interstitial cells of intestine reported following β3-AR activation [32].
We found that enhancement of CL316,243 mediated detrusor relaxation by Lamotrigine was most evident at lower concentration(10−9 M-10−6 M), which further underscores the selectivity of Lamotrigine for HCN channels. Enhancement of CL316,243 mediated bladder relaxation was hardly noticeable at higher concentration of Lamotrigine,10−4 M. The effect of Lamotrigine was altogether abolished by preincubation of strips with ZD7288. The concentration dependent enhancement of CL316,243 by Lamotrigine suggests that contribution of HCN channels to detrusor contractility can be unmasked during phasic contractions, but it need to be studied further. These results support our hypothesis of a communication between β3-ARs and HCN channels in detrusor relaxation.
One proposed role for phasic contractions observed here ex vivo, is when integrated over the entire bladder wall, they maintain a tone that can be relaxed during filling or synchronized via neuronal input to achieve voiding [1]. Furthermore, the characteristic frequent bladder contractions that are not associated with bladder emptying (non-voiding contractions) seen during pathological conditions such as neurogenic or idiopathic overactive bladder (OAB) may result from an increased level of spontaneous phasic contractions of the urinary bladder smooth muscle [1]. Therefore, it can be surmised that the relaxant effect of Lamontrigine involving HCN channels demonstrated here on bladder strips, is corroborated by its in vivo effect in spinal cord injury induced detrusor overactivity model. Chronic administration of Lamotrigine (20 mg/kg) [36] caused a urodynamic improvement in spinal cord injured rat model. It is interesting to note that HCN subtype (HCN4) expressed in human bladder is different from predominant expression of HCN2 in human heart [37]. HCN4 channels constitutively activated by basal intracellular cAMP production in bladder may serve as an effective therapeutic target for disorders affecting detrusor contractility such as OAB without any cardiac effects.
Conclusion
Based on the results of this study, we can conclude the functional presence of HCN channels in bladder. HCN channels expressed in bladder communicate with β3-ARs in mediating detrusor relaxation.
Acknowledgments
The work was partly supported by grants from NIH NIDDK DK088836 (PT, NY and MC).
Abbreviations
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- β3-AR
β3 adrenoceptor
- cAMP
cyclic adenosine monophospate
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol. 2006;570:13–22. doi: 10.1113/jphysiol.2005.097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyagi P, Tyagi V, Yoshimura N, Chancellor M, Yamaguchi O. Beta3-adrenoceptor agonists for the treatment of overactive bladder. Drugs Fut. 2009;34 doi: 10.1358/dof.2009.034.08.1401947. [DOI] [Google Scholar]
- 3.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 4.Cuttle MF, Rusznák Z, Wong AY, Owens S, Forsythe ID. Modulation of a presynaptic hyperpolarization-activated cationic current (I(h)) at an excitatory synaptic terminal in the rat auditory brainstem. J Physiol. 2001;534:733–744. doi: 10.1111/j.1469-7793.2001.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenwood IA, Prestwich SA. Characteristics of hyperpolarization-activated cation currents in portal vein smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:744–753. doi: 10.1152/ajpcell.00393.2001. [DOI] [PubMed] [Google Scholar]
- 6.Xue L, Li Y, Han X, Yao L, Yuan J, et al. Investigation of hyperpolarization-activated cyclic nucleotide-gated channels in interstitial cells of Cajal of human bladder. Urology. 2012;80:224. doi: 10.1016/j.urology.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Tyagi P, Thomas CA, Yoshimura N, Chancellor MB. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol. 35:76–83. doi: 10.1590/s1677-55382009000100012. [DOI] [PubMed] [Google Scholar]
- 8.Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- 9.Postea O, Biel M. Exploring HCN channels as novel drug targets. Nat Rev Drug Discov. 2011;10:903–914. doi: 10.1038/nrd3576. [DOI] [PubMed] [Google Scholar]
- 10.Surges R, Freiman TM, Feuerstein TJ. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia. 2003;44:150–156. doi: 10.1046/j.1528-1157.2003.36802.x. [DOI] [PubMed] [Google Scholar]
- 11.Tyagi V, Philips BJ, Su R, Smaldone MC, Erickson VL, et al. Differential expression of functional cannabinoid receptors in human bladder detrusor and urothelium. J Urol. 2009;181:1932–1938. doi: 10.1016/j.juro.2008.11.078. [DOI] [PubMed] [Google Scholar]
- 12.Green ME, Edwards G, Kirkup AJ, Miller M, Weston AH. Pharmacological characterization of the inwardly-rectifying current in the smooth muscle cells of the rat bladder. Br J Pharmacol. 1996;119:1509–1518. doi: 10.1111/j.1476-5381.1996.tb16066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He P, Deng J, Zhong X, Zhou Z, Song B, et al. Identification of a hyperpolarization-activated cyclic nucleotide-gated channel and its subtypes in the urinary bladder of the rat. Urology. 2012;79:1411. doi: 10.1016/j.urology.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Xiao J, Nguyen TV, Ngui K, Strijbos PJLM, Selmer I, et al. Molecular and functional analysis of hyperpolarisation-activated nucleotide-gated (HCN) channels in the enteric nervous system. Neuroscience. 2004;129:603–614. doi: 10.1016/j.neuroscience.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Masuda N, Hayashi Y, Matsuyoshi H, Chancellor MB, de Groat WC, et al. Characterization of hyperpolarization-activated current (Ih) in dorsal root ganglion neurons innervating rat urinary bladder. Brain Res. 2006;1096:40–52. doi: 10.1016/j.brainres.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 18.Sui G, Fry CH, Malone-Lee J, Wu C. Aberrant Ca2+ oscillations in smooth muscle cells from overactive human bladders. Cell Calcium. 2009;45:456–464. doi: 10.1016/j.ceca.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Xue L, Miao Q, Mao F, Yao L, et al. Expression and electrophysiological characteristics of P2X3 receptors in interstitial cells of Cajal in rats with partial bladder outlet obstruction. BJU Int. 2012;111:843–851. doi: 10.1111/j.1464-410X.2012.11408.x. [DOI] [PubMed] [Google Scholar]
- 20.BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghamari-Langroudi M, Bourque CW. Excitatory role of the hyperpolarization-activated inward current in phasic and tonic firing of rat supraoptic neurons. J Neurosci. 2000;20:4855–4863. doi: 10.1523/JNEUROSCI.20-13-04855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng T, Zhang Q, Wang Q, Zhong X, Li L. Changes in hyperpolarization-activated cyclic nucleotide-gated channel expression and activity in bladder interstitial cells of Cajal from rats with detrusor overactivity. Int Urogynecol J. 2015 doi: 10.1007/s00192-015-2632-x. [DOI] [PubMed] [Google Scholar]
- 23.Felix R, Sandoval A, Sanchez D, Gomora JC, De la Vega-Beltran JL, et al. ZD7288 inhibits low-threshold Ca(2+) channel activity and regulates sperm function. Biochem Biophys Res Commun. 2003;311:187–192. doi: 10.1016/j.bbrc.2003.09.197. [DOI] [PubMed] [Google Scholar]
- 24.Albertson AJ, Yang J, Hablitz JJ. Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia. J Neurophysiol. 2011;106:2189–2200. doi: 10.1152/jn.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2006;292:1065–1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- 27.Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 28.Lentle RG, Reynolds GW, Janssen PWM, Hulls CM, King QM, et al. Characterisation of the contractile dynamics of the resting ex vivo urinary bladder of the pig. BJU Int. 2015 doi: 10.1111/bju.13132. [DOI] [PubMed] [Google Scholar]
- 29.Zona C, Avoli M. Lamotrigine reduces voltage-gated sodium currents in rat central neurons in culture. Epilepsia. 1997;38:522–525. doi: 10.1111/j.1528-1157.1997.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 30.Clouse AK, Jugus MJ, Eisennagel SH, Laping NJ, Westfall TD, et al. Voltage-gated Na+ channel blockers reduce functional bladder capacity in the conscious spontaneously hypertensive rat. Urology. 2012;79:1410. doi: 10.1016/j.urology.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Koh SD, Kim TW, Jun JY, Glasgow NJ, Ward SM, et al. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol. 2000;527 Pt 1:149–162. doi: 10.1111/j.1469-7793.2000.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jun JY, Choi S, Yeum CH, Chang IY, Park CK, et al. Noradrenaline inhibits pacemaker currents through stimulation of beta 1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine. Br J Pharmacol. 2004;141:670–677. doi: 10.1038/sj.bjp.0705665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolan JA, Muenkel HA, Burns MG, Pellegrino SM, Fraser CM, et al. Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J Pharmacol Exp Ther. 1994;269:1000–1006. [PubMed] [Google Scholar]
- 34.Aizawa N, Igawa Y, Nishizawa O, Wyndaele J. Effects of CL316,243, a beta 3-adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol Urodyn. 2010;29:771–776. doi: 10.1002/nau.20826. [DOI] [PubMed] [Google Scholar]
- 35.Igawa Y, Aizawa N, Homma Y. Beta3-adrenoceptor agonists: possible role in the treatment of overactive bladder. Korean J Urol. 2010;51:811–818. doi: 10.4111/kju.2010.51.12.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loutochin O, Al Afraa T, Campeau L, Mahfouz W, Elzayat E, et al. Effect of the anticonvulsant medications pregabalin and lamotrigine on urodynamic parameters in an animal model of neurogenic detrusor overactivity. Neurourol Urodyn. 2012;31:1197–1202. doi: 10.1002/nau.21214. [DOI] [PubMed] [Google Scholar]
- 37.Kuwabara Y, Kuwahara K, Takano M, Kinoshita H, Arai Y, et al. Increased expression of HCN channels in the ventricular myocardium contributes to enhanced arrhythmicity in mouse failing hearts. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000150. [DOI] [PMC free article] [PubMed] [Google Scholar]


