Abstract
Adaptive responses to exercise training (ET) are crucial in maintaining physiological homeostasis and health span. Exercise-induced aerobic bioenergetic reactions in mitochondria and cytosol increase production of reactive oxygen species (ROSs), where excess of ROS can be scavenged by enzymatic as well as non-enzymatic antioxidants to protect against deleterious oxidative stress. Free radicals, however, have recently been recognized as crucial signaling agents that promote adaptive mechanisms to ET, such as mitochondrial biogenesis, antioxidant (AO) enzyme activity defense system upregulation, insulin sensitivity, and glucose uptake in skeletal muscle. Commonly used non-enzymatic AO supplements, such as vitamins C and E, a-lipoic acid, and polyphenols, in combination with ET, have been proposed as ways to prevent exercise-induced oxidative stress and hence improve adaptation responses to endurance training. Preclinical and clinical studies to date have shown inconsistent results indicating either positive or negative effects of endurance training combined with different blends of AO supplements (mostly vitamins C and E and a-lipoic acid) on redox status, mitochondrial biogenesis pathways, and insulin sensitivity. Preclinical reports on ET combined with resveratrol, however, have shown consistent positive effects on exercise performance, mitochondrial biogenesis, and insulin sensitivity, with clinical trials reporting mixed effects. Relevant clinical studies have been few and have used inconsistent results and methodology (types of compounds, combinations, and supplementation time). The future studies would investigate the effects of specific antioxidants and other popular supplements, such as a-lipoic acid and resveratrol, on training effects in humans. Of particular importance are older adults who may be at higher risk of age-related increased oxidative stress, an impaired AO enzyme defense system, and comorbidities such as hypertension, insulin resistance, and diabetes.
Keywords: Exercise Training, Dietary Antioxidants, Mitochondrial Biogenesis, Redox Status, Glucose Metabolism
1. Introduction
Exercise training (ET) is highly recommended by health professionals for its many health benefits, including improved aerobic capacity, muscle strength, maximal oxygen uptake (VO2max), and overall physical condition (15). These effects are induced by adaptive responses to regular training, including improved insulin sensitivity and glucose uptake (57), improved efficiency of enzymatic antioxidant (AO) defense system (2), and increased mitochondrial biogenesis (51). The exercise-induced reactive oxygen species (ROSs) are one of the signaling agents for inducing these biologic exercise-training adaptations (23). In preclinical models, improved efficiency of the enzymatic AO defense system by regular exercise protects cells against oxidative damage and maintains physiological homeostasis (51).
Although exercise-induced ROS production is an important signaling pathway to induce biological adaptations to training, ROS over production could also have a deleterious impact on cells and tissues, i.e., lipid and protein peroxidation (70). This concern has led some experts to suggest consuming more dietary AOs and AO-containing supplements to mitigate the ROS production that can cause excess oxidative stress during and after exercise (9, 36, 47, 65). This belief that dietary supplements are helpful, or at least safe, when used in conjunction with an exercise program, however, has recently been questioned. For example, a recent study of Ristow et al., found that supplementation with vitamin C (1000mg/day) and E (400IU/day)blunted some of the beneficial effects of exercise, such as improved insulin sensitivity, mitochondrial biogenesis level, and AO enzyme activity, in 19 untrained and 20 pre-trained healthy young individuals, and that exercise alone produced a better outcome. In contrast with the findings of Ristow et al., previous studies have shown no blunting effects of AO supplementation on changes in aerobic capacity (VO2max)(16, 74), mitochondrial biogenesis (74), and insulin sensitivity (74). At present, it is unclear whether AO supplements enhance or attenuate ET adaptive biological responses in either healthy adults or lower-functioning older adults. The purpose of this article, therefore, is to review relevant studies that have examined the effects of exercise alone and combined with AO supplementation on basic training adaptations (redox status, mitochondrial biogenesis, and glucose metabolism) in both animals and humans.
2. Literature search
Searching PubMed for manuscripts reporting effects of AO supplements on biological adaptation mechanisms to ET, we used combinations of the following keywords: exercise training, antioxidants, ROS signaling, vitamins, animal studies, clinical studies, resveratrol, redox status, superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), oxidative stress, ROS, mitochondrial biogenesis, peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), metabolism, glucose, insulin, and insulin resistance. The goal of this literature search was to find relevant publications that will improve our understanding of the beneficial, neutral, or adverse effects of AO supplements on biologic adaptation responses in combination with endurance ET. From these manuscripts, we selected studies that examined and compared exercise-training effects with and without administration of commonly used AO supplements (vitamins C and E, a-lipoic acid, coenzyme-Q10, carotene, resveratrol). Controversy around biological pathways of mitochondrial biogenesis, enzymatic AO activity, and glucose metabolism narrowed our search to only those studies that investigated an effect of ET combined with non-enzymatic AOs and training alone on adaptation response to exercise. Therefore, we included all available key markers of redox status (cytosolic superoxide dismutase (SOD1), mitochondrial superoxide dismutase (SOD2), GPx, and CAT), mitochondrial biogenesis (PGC-1α), and glucose metabolism (adenosine monophosphate (AMP)-activated protein kinase (AMPK), glucose transporter 4 (GLUT4), and insulin sensitivity). Using these criteria, we identified only a few preclinical and clinical studies. We therefore included all AO supplement compounds and combinations, endurance ET duration and types, and animal and human models. The key criterion for manuscript inclusion was a comparison between endurance-ET alone and ET combined with AO supplementation. According to these criteria 24 studies were included.
3. Normal adaptations of antioxidant enzymes, mitochondrial biogenesis, and broad metabolism to exercise training
3.1. Basic biological mechanisms during exercise
ROSs, within physiological concentration, are important signaling molecules that regulate growth, proliferation, and differentiation, and are responsible for some key adaptations to ET at the tissue and cellular levels, e.g. AO enzyme regulation (2, 31), mitochondrial biogenesis(51), and skeletal muscle hypertrophy(20).
During exercise, oxidative homeostasis is maintained by a network of AO defense mechanisms capable of producing other less-reactive species or neutralizing reactive oxygen metabolites, i.e., SOD, GPx, CAT, and thioredoxin reductase (TRX). SOD, for instance, promotes the dismutation of superoxide radicals (O2•−) and forms hydrogen peroxide and oxygen. GPx utilizes glutathione (GSH) as a reducing equivalent for hydrogen peroxide (H2O2) to form oxidized GSH and water in mitochondria and cytosol. Additionally, CAT converts H2O2 to water and oxygen (47). It has been shown in in vitro studies that ROSs (formation induced by the pro-oxidant herbicide paraquat) induced upregulation of the AO enzymes (SOD, GPx, and CAT) activity in myotubes(12). This mechanism maintains the oxidant-AO homeostasis during a skeletal muscle contraction in animal models and humans (34, 50, 62).
3.2. Adaptation of enzymatic antioxidant mechanisms to exercise training
Because prolonged exercise results in an increased production of oxidants in skeletal muscle, and hence regular activation of enzymatic AO-using mechanisms, endurance ET induces adaptations resulting in upregulation of AO enzyme activity in skeletal muscle, i.e., SOD1, SOD2, GPx, and CAT (18, 24, 26, 27, 31, 33, 48). It has been well documented that endurance ET increases total SOD activity in highly oxidative type I (the soleus) and IIa (red gastrocnemius) skeletal muscle fibers(48). Longer and more intensive endurance training promotes a greater increase in both cytosolic and mitochondrial GPx activity in oxidative skeletal muscle (type I and IIa) fibers. Endurance training also upregulates CAT activity in peroxisomes and mitochondria in highly oxidative muscles (48).
3.3. Exercise-induced oxidative stress and the mitochondrial biogenesis mechanism
Endurance training does not result in parallel increases in both oxidant and AO enzyme activity(22). The AO enzyme activity of SOD, GPx, and CAT generally increase and ROS concentration decline during normal ET(48). This mismatch seems to have an important beneficial role in ET adaptations, e.g., mitochondrial biogenesis(15)/mitohormesis(53). ROSs stimulate the mitochondrial biogenesis cascade in response to endurance ET, i.e., chronic muscle contractions(15). The newly formed mitochondria are known to be highly efficient and produce fewer ROSs for the same amount of produced adenosine triphosphate(ATP)(43). Regular ET increases expression of proteins involved in mitochondrial biogenesis, i.e., PGC-1α, nuclear respiratory factor 1 (NRF-1), and mitochondrial transcription factor A (Tfam) (Fig. 1). PGC-1α is an important transcriptional coactivator of nuclear genes encoding mitochondrial proteins, whilst Tfam regulates the expression of mitochondrial DNA (58). For example, expression of PGC-1α in skeletal muscle was significantly increased following 4 weeks of endurance ET(53), indicating a skeletal muscle contraction-stimulated mechanism of mitochondrial biogenesis. Mitochondria are also one of the main sources of ROSs, which are products of oxidative lipid and glucose metabolism during muscle contraction (53). However, the mitochondria are not the only sources of ROS during muscle contraction. For example, it has recently been shown that muscle contraction increases superoxide activity in cytosol, with a delayed increase in mitochondria. It has been proposed that nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidases are the potential sources for superoxide generation (46). Accordingly, ROS production (level of H2O2) was previously shown to increase in isolated mitochondria after acute muscle contraction in comparison with rested skeletal muscle biopsy sample (69).
Figure 1.
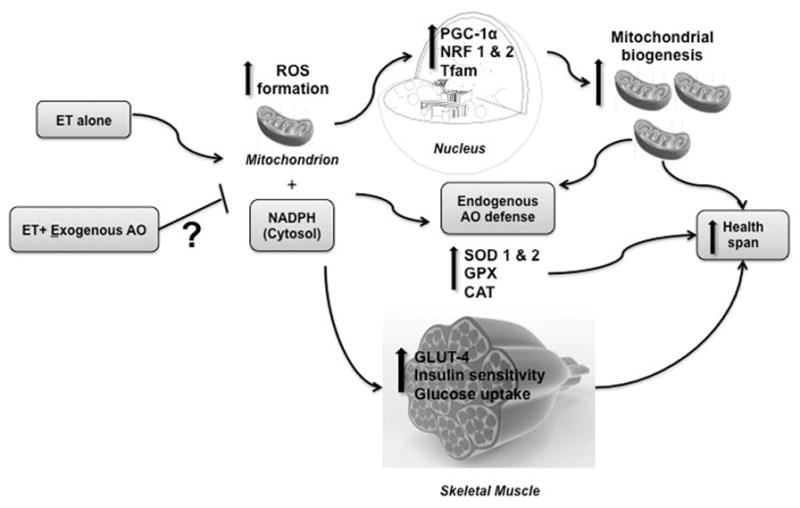
Adequate production level of reactive oxygen species (ROSs) as a signaling agent in adaptation response to exercise training (ET). This simplified scheme shows a clear potential blunting effect of antioxidant (AO) supplements on biological mechanisms when combined with ET.
Skeletal muscle contractions at high intensity increase the AMP/ATP ratio and the Ca2+ flux, thus causing upregulation of the gene expression and post-translational modification of PGC-1α by the activation of AMPK, Ca2+/calmodulin-dependent kinase(CaMK), and calcineurin A. Co-activation of PGC-1α activates nuclear respiratory factors (NRF-1 and -2), which promote the expression of Tfam that directly stimulates mitochondrial DNA replication and transcription(28, 45). This consequently enhances mitochondrial biogenesis, which results in greater oxygen consumption(28).
3.4. Exercise-induced ROS production as a signaling pathway in insulin sensitivity
Endurance ET has been shown to improve insulin sensitivity and to enhance both insulin-stimulated and non-insulin mediated glucose uptake in skeletal muscle (74). The exercise-induced enhancement in insulin-stimulated glucose disposal by skeletal muscle occurs as a result of increased protein expression of hexokinase 2(HK2) and GLUT4 (74). Muscle contraction-stimulated AMPK plays a central role in increased expression of GLUT4 and, hence, the regulation of glucose homeostasis in response to exercise. A muscle-contraction increase in AMPK activity has been correlated with GLUT4 translocation and also with non-insulin glucose transport in skeletal muscle(74). Moreover, evidence indicates that exercise-induced reactive oxygen and nitrogen species(RONS) production plays an important role in regulating signaling pathways. Nitric oxide (NO), essential for the formation of reactive nitrogen species (RNS), therefore acts as a stimulator for exercise-mediated skeletal muscle glucose uptake, showing a possible mechanism of enhanced insulin sensitivity in response to ET(74). As mentioned earlier, the muscle contraction cascade leads to aRONS-stimulated increase in expression of PGC-1α, which is an insulin-sensitivity regulator(38). The latter signaling role of RONS emphasizes its importance in insulin-sensitizing mechanisms and glycemic control (53).
This section shows the crucial role of ROS and RONS in basic adaptation mechanisms to ET. While AO supplements may optimize the training effects by protecting against exercise-induced ROS overproduction, overdosed supplementation may impair ET’s beneficial adaptation effects.
4. Dietary antioxidants: daily usage and supplementation
Dietary AO supplements, such as vitamins, a-lipoic acid, coenzyme-Q10, and resveratrol, are commonly used by more than half of the adults in the United States to maintain health and extend life span. Vitamin C and E supplements are the most commonly used and combined dietary supplements among older adults (65 to 74 years in age)(49).
The water-soluble ascorbic acid has been suggested as a very effective donor AO(10). Moreover, the fat-soluble vitamin E (mostly a-tocopherol) as an AO can scavenge lipid radicals (68, 71). Oxidized vitamin E can be transformed again to an unoxidized form by other soluble AOs such as vitamin C. That mechanism prevents vitamin E radicals from accumulating and inducing excess lipid peroxidation. The combination of vitamin C and E supplements, therefore, is most often used to potentially prevent oxidative stress and has been speculated to slow down the aging processes (68, 72).
According to dietary reference intakes (tolerable upper-intake levels) up to 2000 mg and 1000 mg of vitamin C and E, respectively, was established as the maximum level of daily nutrient intake that is likely to pose no risk of adverse effects (39). Some preclinical studies have shown that high doses of vitamins C and E may even have pro-oxidative properties and increase deleterious oxidative stress (29, 44). For example, in some cases increased levels of oxidative stress led to a lens impairment that might have contributed to developed age-related cataract (75). Supplementation of diets with smaller doses (500 mg per day of vitamin C), however, resulted in a significant increase in ascorbate levels in the plasma and, hence, reduced oxidative stress, compared with the placebo (6).
While even smaller doses of AOs may lead to reduced ROS production, it is unknown whether decreased levels of free radicals may impair ROS-driven adaptations to ET, e.g., enzymatic AO activity, mitochondrial biogenesis, and upregulated glucose metabolism. To our knowledge, there is not enough information on safe and effective dosing and interaction of vitamins and other supplements with ET in clinical studies.
Increased oxidative stress may also be deleterious in the aging process and, therefore, influence cognitive function. There is evidence that AO supplements may protect or improve cognitive function in adults aged 65 years and older (19). Supplementation with multivitamins and vitamins C, E, and B-carotene, for example, was suggested to protect the brain from oxidative damage and delay an onset of cognitive decline (11, 35). Results from a large, randomized, placebo-controlled trial, however, show that daily multivitamin supplementation had no benefit on delaying cognitive decline in adults aged 65 years and older after more than a decade of treatment intervention and follow-up (19). Lin et al, found that none of the included trials reported any benefit from supplements on cognitive function in subjects aged 69 to 95 with mild to moderate dementia (35). Impairments of cognitive function associated with intake of AO supplements are not reported in older adults. Future clinical studies will aim to investigate the potentially beneficial effects of AO supplements on cognitive function in nutrient-deficient older adults.
5. Is there evidence that antioxidant supplementation can improve or blunt beneficial exercise effects in animals and humans?
Undoubtedly, the damaging effects of excess ROS concentrations may include decreased muscular functionality, histological changes, and muscular soreness, and may attenuate exercise performance(38). This has been the rationale for consuming large quantities of non-enzymatic AO supplements, e.g., vitamins C and E and a-lipoic acid. It also has led to research into whether non-enzymatic AO supplementation could prevent ROSs’ damaging effects during exercise and thereby enhance endurance exercise performance(38)in animals and humans. In contrast to the initial protective hypothesis, preclinical studies and some recent human studies have reported controversial results on the blunting effect of non-enzymatic AO supplementation on exercise endurance training(73). Our focus will be mainly on enzymes and proteins that regulate redox biology (SOD, GPx, and CAT) and metabolism (GLUT4 and PGC-1α). We included in vivo animal and human studies on the effect of ET combined with non-enzymatic AOs only in skeletal muscle.
5.1. Exercise training and antioxidant supplements in preclinical studies
5.1.1. Redox status
It has been shown that ET alone induces upregulation of the main AO enzymes activities (SOD, GPx, and CAT) in an animal model in comparison with untrained animals. Table 1 contains preclinical studies of the effect of ET combined with AO supplementation, i.e., vitamins C and E and a-lipoic acid (1, 16, 23, 31, 37, 64). These results show either no additional(23, 31, 32, 54, 64)or a blunting effect(16, 37) on the main AO enzyme activities. It is suggested that the effect of ET combined with AOs may depend on the dosage, combination, and duration of AO supplementation, and the type of ET(1). Mostly used supplement combinations were vitamins C and E(23, 31, 54), but also vitamin C and a-lipoic acid(64) and vitamin C alone(16, 37). Interestingly, the dosages between those studies that reported no or a blunting effect did not differ. It has been shown that composition of AO supplementation may also have a different AO potential, where the induced effect can be different, e.g., between specific combinations and individual use (1). For example, a-lipoic acid increases vitamin E uptake in skeletal muscle that can amplify the overall AO effect(1). The studies that reported no additional effect of AO used a combination of vitamins E and C/a-lipoic acid(23, 31, 64). Remarkably, the results of Meier et al., and Gomez-Cabrera et al., on a blunting effect of AOs on redox status used vitamin C supplementation alone(16, 37). It also has been demonstrated that increased doses of vitamin C supplementation may increase a pro-oxidant activity and decrease tumor growth in cancer cases in mice. However, according to results of Gomez-Cabrera et al., Vitamin C supplementation combined with ET can also impair redox status in rats(16). The supplementation durations differed between the studies, but this does not seem to be a key factor in blunted expression of SOD and GPx, as studies showing no effect used longer, alike, or shorter time periods(23, 31, 54, 64).
Table 1.
Redox status in response to exercise training alone (ET) and training combined with antioxidant supplements (ET+AO) in animal and human skeletal muscle. The reverse transcription polymerase chain reaction technique was used to measure AO enzyme activities (SOD, GPx, CAT).
| Study | Species | Age | Non-enzymatic AO dose/day | Duration and type of training (tr) | Response to ET vs. sedentary: redox | Effect of ET+AO on AO enzyme activity |
|---|---|---|---|---|---|---|
| Preclinical studies | ||||||
|
| ||||||
| Leeuwenburgh et al. (34) | Wistar rats | 5 months | A-tocopherol 0.2%, ascorbic acid 0.5%, B-carotene 0.015% | 19 months; forced running on a running wheel | Increased cytosolic superoxide dismutase (SOD1) and mitochondrial superoxide dismutase (SOD2) activity; increased glutathione peroxidase (GPx) activity; no effect on CAT in skeletal muscle | No effect on SOD1, SOD2, GPx, catalase (CAT) in skeletal muscle |
| Rosa et al. (56) | Male mice | 3 months | Vitamin (Vit) E 10 mg/kg; vit C 10 mg/kg | 10 days | Increased SOD2 activity in red blood cells | No effect on SOD2 in red blood cells |
| Higashida et al. (22) | Male Wistar rats | Vit E 150 mg/kg; vit C 750 mg/kg | 9 days supplements/3 days tr; 8 weeks supplements/3 weeks tr | Increased SOD1 and SOD2 activity in skeletal muscle | No effect on SOD1 and SOD2 in skeletal muscle | |
| Strobel et al. (67) | Male Wistar | 10 weeks | Vit E 1000 IU; 1.6 g of a-lipoic acid/kg diet | 4 days/week (90 minutes/day), 14 weeks | Decreased SOD2 activity; no effect on GPx in skeletal muscle | No effect on SOD2, GPx in skeletal muscle |
| Meier et al. (40) | Mice | Vit C 12 mg/l, Q10 12 mg/l; N-acetyl-cysteine 1% | 4 weeks endurance | SOD1 expression increased (mRNA expression) in skeletal muscle | Decreased SOD1 expression (mRNA expression) in skeletal muscle | |
| Gomez-Cabrera et al. (17) | Wistar rats | 3 months | Vit C 0.24 mg/cm2 | Endurance 3 and 6 wks | Increased SOD and GPx activity (mRNA expression) in skeletal muscle | Decreased SOD2 and GPx activity (mRNA expression) in skeletal muscle |
|
| ||||||
| Clinical Studies | ||||||
|
| ||||||
| Ristow et al. (55) | 19 untrained and 20 trained healthy humans | Untrained, 26.7±4.34; trained, 25.40±2.15 | Vitamin (Vit) C 1000 mg; vit E 400 IU | Endurance 5 days/4 weeks | Increased SOD1 and 2, GPx, CAT mRNA expression in skeletal muscle | Decreased SOD1 and SOD2, GPx, CAT mRNA expression in skeletal muscle |
| Braakhuis et al. (5) | 23 trained humans | 31±8 yrs | Vit C 1000 mg | Endurance, high-intensity training, 2–3 days/3 weeks | No effect of ET on SOD, GPx, CAT activity (enzyme activity levels in erythrocytes) | Decreased SOD post-exercise; decreased CAT in erythrocytes |
| Theodorou et al. (69) | Humans; 14 vitamins and 14 placebo | Vitamins, 25.6±1.2; placebo, 26.2±1.5 | 11 weeks; Vit C 1000 mg; vit E 400 IU | Eccentric training 2 days/4 weeks | Decreased CAT activity in red blood cells | No change in CAT in red blood cells |
The reported significant reduction of AO enzyme activities does not seem to be induced by the duration or dosage of supplementation and training modalities. The only common factor of these two studies (Meier et al., and Gomez-Cabrera et al.,) out of the six listed in Table 1 (preclinical studies)is that in these experiments supplementation with vitamin C alone was used in comparison with the other reported studies. Meier et al., combined coenzyme Q10 and 1% N-acetyl-cysteine with vitamin C supplementation. However, it is unknown whether this combination had an additional blunting effect in comparison with the effect of vitamin C alone(37). This warrants future studies on impact of AO supplementation combinations with clamped dosages, duration and training modalities. This will improve our understanding of the supplement combinations and training-induced redox status changes.
5.1.2. Mitochondrial biogenesis mechanism in response to exercise training combined with antioxidant supplementation
Mitochondrial biogenesis is the essential adaptive response mechanism to endurance training and mitochondrial content in muscle is a crucial determinant of endurance capacity (1, 51). Recent preclinical studies have shown either no additional(1, 23, 64) or a blunting effect of ET+AO combination on protein expression of PGC-1α(16, 37). Expression ofPGC-1α was blunted in studies where vitamin C supplementation alone was combined with endurance ET in animals. Gomez-Cabrera et al., suggested that vitamin C supplementation during endurance training blunts one of the mitochondrial biogenesis paths, i.e., PGC-1α—NRF-1—TFAM—cytochrome C(16). Additionally, endurance training combined with vitamin C supplementation in rats blunted an improvement in running time in comparison with training alone (26.5% versus 186.7%, respectively), whereas there was no difference in VO2max(16). This is in agreement with the statement that endurance capacity depends on mitochondrial content in skeletal muscle and that VO2max is also dependent on cardiovascular training adaptations (16). While unfortunately Meier et al.,’s study reported no differences in running times, it found no differences in the peak power exercise tests performed at the end of the training period, in accordance with Gomez-Cabrera(37).
It has been shown that moderate ROS levels are crucial for signaling pathways of mitochondrial biogenesis (1, 37). Decreased expression of PGC-1α, therefore, is associated with reduced ROS-stimulated mitochondrial biogenesis. Supplementation with vitamin C decreased ROS levels, preventing enzymatic AO activity(37). Moreover, the studies of Meier et al., and Gomez-Cabrera are in agreement with the statement associating blunted expression of PGC-1α with decreased activity of AO enzymes (SOD, GPx, CAT), the primary endogenic AO defense system(16).
5.1.3. Metabolic response to exercise training combined with antioxidant supplementation in animal skeletal muscle
Increased expression of GLUT4 is one of major adaptive responses to endurance exercise in skeletal muscle (23). Additionally, studies on endurance training adaptations have shown that expression of GLUT4 is also redox-sensitive (37). Mitochondrial biogenesis and upregulated expression of GLUT4 are mediated by the PGC-1α protein, which increases in response to acute as well as endurance ET(37, 51).
Although studies on ET combined with AO supplements showed inconsistent results regarding redox status and mitochondrial biogenesis pathway, studies on the metabolic response show no difference in GLUT4 expression in response to ET with or without administered AOs.
Only two studies investigated both expressions of PGC-1α and GLUT4 in response to ET combined with AO supplements. Meier et al., found blunted PGC-1α expression and no effect on GLUT4 expression (37). On the other hand, Higashida et al., reported no effect of the ET+AO combination on expression of PGC-1α and GLUT4(23).
Taken together, animal studies have shown consistent results regarding the upregulating effect of ET alone on adaptive mitochondrial biogenesis and insulin sensitivity and glycemic regulation. Conversely, results on ET+AO were equivocal, and its potential suppressing effect on ET may depend on the dose, type of AO composition, and length of time.
5.2. Exercise training combined with administration of antioxidant supplements in healthy humans
5.2.1. Redox status
Recent studies have addressed the effects of AO supplementation on the ET adaptations in healthy humans(4, 45, 53, 66, 74). Ristow et al., and Gomez-Cabrera et al., have reported recently that AO supplementation can decrease training efficiency and prevents specific cellular adaptations of ET in healthy humans, e.g., mitochondrial biogenesis(16, 53). Recent clinical trials suggest that various AOs that directly inhibit ROS production may ameliorate the benefits of exercise that depend on ROS signaling (16, 53) or have no different effect from ET alone(66, 73).
In Table 1 (clinical studies), we present relevant double-blind placebo-controlled studies that investigated enzymatic AO defense activity during endurance ET combined with vitamin C and E supplementation in young healthy adults.
Ristow et al., have studied the effect of a 4-week endurance training regimen combined with vitamin supplementation or placebo on training adaptation in 19 untrained and 20 pre-trained healthy men. They reported a significant effect of vitamins C (1000g) and E (400 IU) supplementation in blocking ET-induced expression of AO enzyme mRNAs (SOD1 and GPx1) for the entire cohort of trained and untrained subjects in comparison with no-supplemented groups(53). These findings are in agreement with the study results of Gomez-Cabrera et al., and Braakhuis et al., where activity of SOD, GPx, and CAT (4)decreased in response to 8weeks(16) and 9weeks(4)of endurance training combined with antioxidants in untrained and trained subjects, respectively. In response to endurance and eccentric training in combination with vitamin C and E supplementation, there was no change in CAT activity in comparison with placebo (53, 66). These results seem to indicate consistently that consumption of these amounts of vitamin C and E supplements blunts SOD and GPx activities in humans(4). This may be blocking exercise-dependent production of the ROSs(4, 16)essential for hormetic stimulation of adaptive mechanisms to ET, e.g, the PGC-1α pathway of mitochondrial biogenesis. Variability in levels of significance may be training type- and duration-related.
5.2.2. Mitochondrial biogenesis
Available study results have been presented on the effects of endurance ET with vitamin C and E supplementation on PGC-1α protein expression, the mitochondrial biogenesis co-activator (Table 2). Paulsen et al., have shown that vitamin C and E supplementation blunted any rise in muscle cytosolic PGC-1α levels during an11-week endurance ET program. By contrast, PGC-1α mRNA increased only in the vitamin C and E supplementation group, and nuclear PGC-1α protein levels were unchanged in both groups. The authors explain that muscle biopsies were collected 2 to 4 days after the last training session, and they reflect no immediate activation, nuclear translocation of PGC-1α, or gene expression during exercise (45). Yfanti et al., have shown no effect of either the training or the supplementation on the basal mRNA expression of PGC-1α. Ristow et al., reported that an exercise-related induction of PGC-1α expression in skeletal muscle was strongly blunted following 4 weeks of ET in comparison with the non-AO group (53). These study results coincide with the opinion that prolonged ET (4 to 11 weeks) combined with vitamin C and E supplementation blunt ROS-stimulated cellular primary pathways of mitochondrial biogenesis that seem to block an increase in exercise performance. Yfanti et al., who observed no increase in PGC-1α expression, speculate that the result may be induced by different training modalities and the applied dose of vitamin C of 500 mg(74)versus 1000 mg in the rest of the relevant studies(16, 45, 53).
Table 2.
Mitochondrial biogenesis pathways’ stimulation in response to exercise training alone (ET) and exercise training combined with antioxidant supplements (ET+AO) in animal and human skeletal muscle, comparing effects on peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1a) signaling pathways.
| Study | Species | Age | Non-enzymatic AO dose/day | Duration and type of training (tr) | Response to ET vs. sedentary: PGC-1a | Additional effect of AO PGC-1a |
|---|---|---|---|---|---|---|
| Preclinical Studies | ||||||
|
| ||||||
| Higashida et al. (22) | Male Wistar rats | Vitamin (Vit) E 150 mg/kg; vit C 750 mg/kg | 9 days supplements (suppl)/3 days tr; 8 weeks (wks) suppl/3 wks tr | 2- to 3-fold increased expression in skeletal muscle | No effect PGC-1a expression in skeletal muscle | |
| Strobel et al. (67) | Male Wistar | 10 wks | Vit E 1000 IU; a-lipoic acid/kg diet 1.6 g | 4 days/wk (90 minutes/day), 14 wks | Increased PGC-1a mRNA and protein in skeletal muscle | No effect on PGC-1a mRNA and protein content in skeletal muscle |
| Abadi et al. (1) | Mice | Chow diet vit E 1000 IU; a-lipoic acid 0.1% | 8 wks endurance (treadmill) | Increased PGC-1a protein in skeletal muscle | No effect on PGC-1a protein content in skeletal muscle | |
| Meier et al. (40) | Mice | Vit C 12 mg/l, Q10 12mg/l; N-acetyl-cysteine 1% | 4 wks endurance | Increased expression in skeletal muscle | Blunted PGC-1a expression in skeletal muscle | |
| Gomez-Cabrera et al. (17) | Wistar rats | 3 months | Vit C 0.24 mg/cm2 | Endurance 3 and 6 wks | Increased PGC-1a expression (and Tfam) in skeletal muscle | Blunted PGC-1a expression (and Tfam) in skeletal muscle |
|
| ||||||
| Clinical Studies | ||||||
|
| ||||||
| Paulsen et al. (48) | Human trained | 29 AO, 31 Placebo group | Vitamin (Vit) C 1000 mg; vit E 235 IU | Endurance 11 weeks | Increased cytosolic PGC-1a in skeletal muscle | Blunted |
| Ristow et al. (55) | Human trained and untrained | Untrained, 26.7±4. 34; trained, 25.40±2.15 | Vit C 1000 mg; vit E 400 IU | Endurance 4 weeks | Increased PGC-1a RNA in skeletal muscle | Blunted |
| Yfanti et al. (76) | Human untrained | 29±1 | Vit C 500 mg; vit E 400 IU | Endurance 12 weeks | No difference in PGC-1a mRNA expression in skeletal muscle | No difference in PGC-1a mRNA expression in skeletal muscle |
5.2.3. Metabolic response to exercise training combined with antioxidant supplements
Exercise endurance training has been shown to improve not only exercise performance, but also other health-related functions, such as muscular glucose uptake, insulin sensitivity maintenance, and insulin-resistance improvement (53). Animal studies have shown that overexpression of skeletal muscle PGC-1α increases insulin-stimulated glucose disposal in both healthy and insulin-resistant rats. It has been shown that the insulin-sensitizing effects of endurance ET seem to be blunted by decreased ROS production induced by vitamin C and E supplementation(53). Ristow et al., have shown a significant effect of vitamin C and E supplementation on the blockage of an ET-induced improvement of insulin sensitivity, i.e., decreased expression of PGC-1α. It was determined by glucose infusion rates (p<0.001) in comparison with placebo (Table 3). While this is controversial and has not been conducted in other human studies, it is in line with some animal studies (64).
Table 3.
Metabolic response to exercise training alone (ET) and exercise training with administered antioxidant supplements (ET+AO) in animal and human skeletal muscle, comparing effects on glucose transporter 4 (GLUT4) expression.
| Study | Species | Age | Non-enzymatic AO dose/day | Duration and type of training (tr) | Response to ET vs. sedentary: GLUT4 | Additional effect of AO on GLUT4 expression |
|---|---|---|---|---|---|---|
| Preclinical Studies | ||||||
|
| ||||||
| Higashida et al. (22) | Male Wistar rats | Vitamin (Vit) E 150 mg/kg; vit C 750 mg/kg | 9 days supplements (suppl)/3 days tr; 8 weeks suppl/3 weeks tr | Increased expression of GLUT4, insulin responsiveness of glucose transport in skeletal muscle | No effect | |
| Meier et al. (40) | Mice | Vit C 12 mg/l; Q10 12 mg/l; N-acetyl-cysteine 1% | 4 weeks endurance | Increased GLUT4 expression in skeletal muscle | No effect | |
|
| ||||||
| Clinical Studies | ||||||
|
| ||||||
| Ristow et al. (55) | Human | Untrained, 26.7±4. 34; trained, 25.40±2.15 | Vitamin (Vit) C 1000 mg; vit E 400 IU | Endurance 4 weeks | Improved insulin sensitivity in skeletal muscle | Inhibition of insulin sensitivity improvement in skeletal muscle |
| Yfanti et al. (76) | Human | 29±1 | Vit C 500 mg; vit E 400 IU | Endurance 12 weeks | Increased GLUT4 expression in skeletal muscle | No additional effect on GLUT4 expression (Placebo vs. AO) in skeletal muscle |
In contrast to the latter, Yfanti et al., have shown that vitamin C and E supplementation before a 12-week training program had no effect on insulin-stimulated glucose uptake in skeletal muscle in comparison with placebo(74). The increased insulin sensitivity was associated with corresponding increases in total protein kinase B(Akt), GLUT4, and HK2 in skeletal muscle in both supplementation and placebo groups, without significant differences(74). The discrepancy between those two studies may be dependent on the dose-response of the AO supplements, as the vitamin C dose was lower (500mg)(73) where there was no effect. There is no sufficient scientific evidence, however, of impaired insulin-sensitivity improvement in response to endurance training combined with vitamin C and E supplements. This warrants more studies on the AMPK—PGC-1—GLUT4 pathway in response to endurance ET with AO supplementation in human subjects. This is important because the AOs are often combined with exercise endurance in healthy and insulin-resistant subjects. Dose-response studies of vitamin C and E supplements during ET would contribute to recommendations on effective exercise performance improvements in athletic as well as clinical settings.
In conclusion, evidence exists in both animal and human studies that exercise-induced ROSs play a crucial role in stimulating the signaling pathways for enzymatic AO activity (SOD, GPx, and CAT), mitochondrial biogenesis (expression of PGC-1α), and glucose metabolism(insulin sensitivity (GLUT4 expression) and glucose uptake). Moreover, adaptation to regular moderate ROS production during ET allows for more effective enzymatic ROS scavenging, increases mitochondrial oxidative capacity and its efficiency (new, more efficient mitochondria) and, hence, prevents deleterious overproduction of ROSs. Study results are unclear about a potential suppressive role of AO supplements in impairing these ET-induced adaptive effects.
High consumption of AOs in middle-aged and older adults may impair desired goals to improve metabolic status (obesity, lipid profile, insulin resistance, poor physical condition). Additionally, Higashida et al., (23)have suggested that the separate reports of Ristow et al., (53)and Gomez-Cabrera et al.,(16), describing a suppressive role of AO supplements on ET, have to be treated with caution because of its weak methodology. On the other hand, Gomez-Cabrera et al.(17) disagreed with Higashida et al. (23) Therefore, the unknown effects of supplemental composition, dose, and time duration on adaptive training effects necessitate further studies to establish safe AO supplement types, doses, and composition without suppressing ET’s beneficial effects.
5.3. Resveratrol as an “alternative antioxidant” and its role as an antioxidant in exercise training adaptation in preclinical and clinical studies
Resveratrol is a polyphenolic and fat-soluble compound present mainly in grapes (7). To date, there have been a number of in vitro and preclinical (25, 56, 60, 61), but also clinical studies investigating resveratrol’s AO features (14, 41, 42, 52). Resveratrol has a short biological half-life (8 to 14 minutes)(67), labile properties, and rapid metabolism and elimination. Because of its poor water solubility and instability, it converts to a less active cis form, and its beneficial AO impact is not fully understood (67).
5.3.1. Resveratrol as an antioxidant and gene regulator
Resveratrol has been studied as an AO supplement that decreases deleterious amounts of ROSs, but also as a stimulant of energetic cellular sirtuin 1 (SIRT1)- and AMPK-dependent pathways (38), both important for PGC1a activation. Study results, however, are equivocal because of resveratrol’s poor bioavailability and solubility (3). There is some evidence of in vitro results showing improved availability and uptake of resveratrol by composing it chemically with liposomal carriers. Vanaja et al., for example, found that liposomal forms of resveratrol improved its antioxidative properties and, hence, decreased oxidative damage in isolated leukocytes (67). This could potentially improve resveratrol’s bioavailability and AO effects in preclinical and clinical studies, but further studies are needed. Despite the controversies regarding the bioavailability and delivery of resveratrol, spectacular improvements on metabolism and performance induced by resveratrol supplementation have been documented (mostly in animal studies) (22, 30, 40, 52).
5.3.2. Effects of exercise training combined with resveratrol in preclinical and human studies
While a number of studies have focused on resveratrol’s effects on ET results in animal models (Table 4)(8, 22, 30, 40, 52), we are only aware of two studies that investigated a synergistic effect of combined ET with resveratrol supplementation in humans (14, 41).
Table 4.
Findings from preclinical studies of combined effects of resveratrol and physical exercise in animals, comparing exercise training alone (ET) to exercise training combined with resveratrol supplementation (REX).
| Study | Species | Age | Dosage of resveratrol | Duration and type of training | Response to ET vs. sedentary | Response to REX |
|---|---|---|---|---|---|---|
| Murase et al. (43) | Male senescence-accelerated mice | 13 weeks | 0.2% of a diet | 12 weeks | Soleus and extensor digitorum longus (EDL): increased muscle weight, tetanic contraction force; reduced glucose, triglycerides (TGs), insulin; reduced serum and muscle (EDL) thiobarbituric acid reactive substances (TBARS); increased PGC-1a and GLUT4; increased VO2 and lipid oxidation | Soleus and EDL: increased muscle weight, tetanic contraction force; reduced glucose, TGs, insulin; reduced serum and muscle (EDL) TBARS; increased PGC-1a and GLUT4; increased VO2 and lipid oxidation |
| Dolinsky et al. (9) | Male Wistar rats | 10 weeks | 4g/kg diet | 12 weeks | Increased tetanic contraction force in transverse abdominal muscle (TAM) and soleus muscle; reduced plasma TGs | No additional effect of resveratrol on tetanic muscle contraction in TAM and soleus; reduced plasma TGs; increased fat oxidation/decreased glucose oxidation (decreased respiratory exchange ratio); increased PGC-1a/tubulin ratio |
| Hart et al. (21) | Male high-capacity runner rats | 13 months | 100 mg/kg body mass (BM) | 12 weeks | Increased running distance; increased VO2max; increased Tfam, PGC-1a | Increased running distance; VO2max, Tfam, PGC-1a |
| Kim et al. (32) | Mice injected with kainate (KA) | 40 mg/kg BM | 6 weeks swimming; 60 minutes/d ay; 5 days/week | Decreased body weight and lactate threshold; decreased SOD and CAT in combination with ET+KA, but higher than with KA alone at rest | Reduced seizures and increased SOD and CAT (same as control) | |
| Ringholm et al. (54) | Mice | 3 months | 4 g/kg diet | 12 months running wheel | No effect on area under curve (AUC) for blood glucose; increased vascular endothelial growth factor (VEGF) | Increased AUC; increased VEGF |
| Ryan et al. (57) | Mice | 3–5; 26–28 months | 0.05% in diet | 3 days isometric exercise | Increased activity of SOD, GPx, CAT | SOD: no additional effect; increased GPx and CAT activity |
5.3.2.1. Preclinical studies
Hart et al., speculated that resveratrol may have induced post-training improvements in aerobic capacity by simultaneously activating different molecular pathways related to mitochondrial function (22). Indeed, some authors reported improved VO2max and running time in response to resveratrol combined with ET (22, 40). Other authors reported increased expression of PGC-1α and, hence, improved mitochondrial biogenesis (8, 22). In accordance with an improved PGC-1α signaling pathway, an enhanced metabolic adaptive response was also reported by increased GLUT4expression, insulin sensitivity, and glucose uptake (40). Additionally, two studies of the same group (Hart et al., 2014 and Hart et al., 2013) have investigated the effects of resveratrol in conjunction with ET on running distance in low capacity runner (LCR) and high capacity runner (HCR) rats, respectively. Interestingly, resveratrol supplementation combined with ET reduced running distance in LCR (21) and improved running distance in HCR rats (22). To explain this inconsistency, it was suggested that in some models resveratrol supplementation induces different results in obese prone and obese resistant rats and its effects could be influenced by metabolic status (21).
Taken together, the authors speculate that these synergistic effects may manifest as a result of providing resveratrol when cellular energy demand is high but also in rats with high levels of performance. From these basic and preclinical studies, it seems that this potential additional effect of resveratrol on training effects provides the mechanistic action through which ET combined with resveratrol supplementation (REX) may be a feasible and efficacious intervention for maintaining functional status in humans.
5.3.2.2. Human studies
Contrary to the aforementioned animal results on improved exercise performance following REX, one human study has shown a 45% larger improvement of VO2max in response to placebo after 8 weeks of high intensity training in comparison with resveratrol-supplemented subjects (Table 5)(14). The latter and other human study of Olesen et al., consistently reported that ET combined with resveratrol supplementation had no additional effect on PGC-1α, SIRT1, and AMPK-induced energetic mechanisms as compared to a placebo group (14, 41). Additionally, Scribbans et al., have also reported no additional effects of resveratrol supplementation on exercise performance and speculated that resveratrol supplementation may have a blunting effect on skeletal muscle gene expression of PGC-1α, SIRT1, and SOD2(59). Moreover, Gliemann et al., have reported that resveratrol might have blunted a beneficial effect of ET on lipid profile. They have shown that REX eliminated the training effect on low-density lipoprotein (LDL), total cholesterol/high-density lipoprotein (TC/HDL) ratio, and TGs(14).
Table 5.
Findings from clinical studies on combined effects of resveratrol and exercise training in humans, comparing exercise training alone (ET) to exercise training combined with resveratrol supplementation (REX).
| Study | Species | Age | Training | Resveratrol dosage | Response to ET vs. sedentary | Response to REX |
|---|---|---|---|---|---|---|
| Olesen et al. (44) | Men | 65±1 years | 8-week endurance | 250 mg | Increased PGC-1a mRNA; increased exercise performance; reduced TNFa mRNA | PGC-1a: no extra effect; performance (one-legged knee-extensor): no additional effect of resveratrol; no effect of resveratrol on adenosine monophosphate (AMP)-activated protein kinase (AMPK) and sirtuin 1 (SIRT1); resveratrol impaired training-induced reduction of TNFa mRNA |
| Gliemann et al. (15) | Men | 65±1 years | 8 weeks of high-intensity training | 250 mg | Greater increase in VO2max; reduced mean arterial pressure; reduced low-density lipoprotein (LDL), total cholesterol/high-density lipoprotein (TC/HDL) ratio, and triglyceride concentrations (TGs) in blood | 45% lower VO2max than in REX; no additional reduction in mean arterial pressure, only in placebo; REX abolished the training effect on LDL, TC/HDL, and TGs; no effect of REX on SIRT1 |
| Gliemann et al. (14) | Men | 65±1 years | 8 weeks of high-intensity training | 250 mg | ~20% increase in capillary to fiber ratio (C:F), an increase in the muscle protein expression of vascular endothelial growth factor (VEGF). | No increase in C:F ratio and VEGF protein expression. |
Contrary to animal study results, resveratrol did not improve exercise performance in healthy older adults. Interestingly, resveratrol has been shown to impair the observed ET-induced improvements in lipid profile. Moreover, resveratrol impaired a beneficial effect of the ET-induced improvement in mean arterial pressure (MAP)(14) and the muscle protein expression of vascular endothelial growth factor (VEGF) (13).
Given the inconsistency of existing animal data on REX and the few human studies indicating impairments in exercise performance and abolished exercise-induce lipid profile improvement following resveratrol supplementation, we suggest that this isolated human report of Gliemann et al., should not be a barrier to further investigation of resveratrol as a potential aid in improving ET effects in healthy subjects and in a clinical setting (hypertension, poor physical activity, reduced oxidative capacity). Moreover, other authors are also critical about the reported negative effects of resveratrol and have questioned Gliemann’s methodology and analyses(5, 63).
Conclusion
This review investigated basic biological adaptive mechanisms to ET in preclinical and clinical studies in response to ET combined with commonly used AO supplements. Most of the relevant animal and human studies showed neither additional nor adverse effects of ET combined with AO supplements on redox status, mitochondrial biogenesis, and glucose metabolism. Only a few animal and human studies have shown a blunting effect of ET combined with vitamin AOs on adaptive responses to ET in healthy subjects. The reason for this inconsistency could possibly lie in different AO compositions, doses, duration time of supplementation, and ET modalities. Additionally, the reported blunting effects of AO on training results could also be misleading by weak methodology of study protocols. Moreover, it is difficult to speculate about adverse or positive clinical effects of resveratrol because of a narrow body of evidence and because experiments were conducted in only healthy subjects. To date, a blunting influence of AO supplementation during ET in healthy humans remains speculative. The lack of strong evidence indicating adverse and/or positive effects and the unclear methods of AO administration in combination with ET, however, warrant future studies in healthy aging populations and individuals with chronic health conditions such as sarcopenia, hypertension, and poor physical aerobic condition, for whom training adaptations are crucial for health improvement.
Future directions
This review highlights studies on AO supplements’ adverse blunting effects on mitochondrial biogenesis pathways and suggests ROS-induced impaired signaling mechanisms. Enzymatic AO activity seems to be an efficient and effective defense against oxidative stress during ET in healthy young and middle-aged adults. Therefore, future double-blinded placebo-controlled studies should focus on investigating a potential positive or negative effect of AO supplements on key ET adaptation mechanisms in deficient older adults. Such studies may evaluate exercise responses for relevant biologic factors such as mitochondrial biogenesis, insulin, skeletal muscle glucose uptake, and redox status. Though speculative, adverse and/or positive effects of AO supplementation on ET results may depend on doses, compounds, combinations, and endurance training modalities. To address this hypothesis, dose-response clinical trials are needed to determine safe and training-efficient supplementation strategies combined with ET in both healthy and unhealthy older adults.
Acknowledgments
The manuscript does not contain clinical studies and patient data. There was no funding provided during writing this manuscript. The results of the present study do not constitute endorsement by the American College of Sports Medicine. Additionally, we recognize other reports in the area that could not be cited due to journal formatting requirements.
Footnotes
The authors declare no conflict of interest.
References
- 1.Abadi A, Crane JD, Ogborn D, et al. Supplementation with alpha-lipoic acid, CoQ10, and vitamin E augments running performance and mitochondrial function in female mice. PLoS One. 2013;8(4):e60722. doi: 10.1371/journal.pone.0060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo PM, Esposito F, Marchionni C, et al. Moderate exercise training induces ROS-related adaptations to skeletal muscles. Int J Sports Med. 2013;34(8):676–87. doi: 10.1055/s-0032-1323782. [DOI] [PubMed] [Google Scholar]
- 3.Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J Control Release. 2012;158(2):182–93. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 4.Braakhuis AJ, Hopkins WG, Lowe TE. Effects of dietary antioxidants on training and performance in female runners. Eur J Sport Sci. 2014;14(2):160–8. doi: 10.1080/17461391.2013.785597. [DOI] [PubMed] [Google Scholar]
- 5.Buford TW, Anton SD. Resveratrol as a supplement to exercise training: friend or foe? J Physiol. 2014;592(Pt 3):551–2. doi: 10.1113/jphysiol.2013.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13(9):1007–24. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 7.Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della Ragione F. Resveratrol: from basic science to the clinic. Cell Cycle. 2007;6(20):2495–510. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- 8.Dolinsky VW, Jones KE, Sidhu RS, et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590(Pt 11):2783–99. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draeger CL, Naves A, Marques N, et al. Controversies of antioxidant vitamins supplementation in exercise: ergogenic or ergolytic effects in humans? J Int Soc Sports Nutr. 2014;11(1):4. doi: 10.1186/1550-2783-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826(2):443–57. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824–34. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 12.Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27(9–10):1122–32. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 13.Gliemann L, Olesen J, Bienso RS, et al. Resveratrol Modulates the Angiogenic Response to Exercise Training in Skeletal Muscle of Aged Men. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00168.2014. [DOI] [PubMed] [Google Scholar]
- 14.Gliemann L, Schmidt JF, Olesen J, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591(Pt 20):5047–59. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. doi: 10.1155/2012/756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87(1):142–9. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Cabrera MC, Ristow M, Viña J. Antioxidant supplements in exercise: worse than useless? Am J Physiol Endocrinol Metab. 2012;302(4):E476–7. doi: 10.1152/ajpendo.00567.2011. [DOI] [PubMed] [Google Scholar]
- 18.Gore M, Fiebig R, Hollander J, Leeuwenburgh C, Ohno H, Ji LL. Endurance training alters antioxidant enzyme gene expression in rat skeletal muscle. Can J Physiol Pharmacol. 1998;76(12):1139–45. doi: 10.1139/cjpp-76-12-1139. [DOI] [PubMed] [Google Scholar]
- 19.Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159(12):806–14. doi: 10.7326/0003-4819-159-12-201312170-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handayaningsih AE, Iguchi G, Fukuoka H, et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011;152(3):912–21. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- 21.Hart N, Sarga L, Csende Z, et al. Resveratrol attenuates exercise-induced adaptive responses in rats selectively bred for low running performance. Dose Response. 2014;12(1):57–71. doi: 10.2203/dose-response.13-010.Radak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart N, Sarga L, Csende Z, et al. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem Toxicol. 2013;61:53–9. doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab. 2011;301(5):E779–84. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander J, Fiebig R, Gore M, et al. Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training. Am J Physiol. 1999;277(3 Pt 2):R856–62. doi: 10.1152/ajpregu.1999.277.3.R856. [DOI] [PubMed] [Google Scholar]
- 25.Ikizler M, Ovali C, Dernek S, et al. Protective effects of resveratrol in ischemia-reperfusion injury of skeletal muscle: A clinically relevant animal model for lower extremity ischemia. Chin J Physiol. 2006;49(4):204–9. [PubMed] [Google Scholar]
- 26.Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic Biol Med. 2008;44(2):142–52. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Ji LL, Stratman FW, Lardy HA. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys. 1988;263(1):150–60. doi: 10.1016/0003-9861(88)90623-6. [DOI] [PubMed] [Google Scholar]
- 28.Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1alpha. Exp Gerontol. 2013;48(11):1343–50. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Kang MJ, Lee SS, Koh HC. Prooxidant properties of ascorbic acid in the nigrostriatal dopaminergic system of C57BL/6 mice. Toxicology. 2012;294(1):1–8. doi: 10.1016/j.tox.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Kim IK, Song W, Lee J, Park S. The synergic effect of regular exercise and resveratrol on kainate-induced oxidative stress and seizure activity in mice. Neurochem Res. 2013;38(1):117–22. doi: 10.1007/s11064-012-0897-8. [DOI] [PubMed] [Google Scholar]
- 31.Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267(2 Pt 2):R439–45. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- 32.Leeuwenburgh C, Hansen PA, Holloszy JO, Heinecke JW. Oxidized amino acids in the urine of aging rats: potential markers for assessing oxidative stress in vivo. Am J Physiol. 1999;276(1 Pt 2):R128–35. doi: 10.1152/ajpregu.1999.276.1.R128. [DOI] [PubMed] [Google Scholar]
- 33.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272(1 Pt 2):R363–9. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 35.Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(9):601–12. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
- 36.Malaguti M, Angeloni C, Hrelia S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid Med Cell Longev. 2013;2013:825928. doi: 10.1155/2013/825928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier P, Renga M, Hoppeler H, Baum O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem Funct. 2013;31(1):51–9. doi: 10.1002/cbf.2859. [DOI] [PubMed] [Google Scholar]
- 38.Mendelsohn AR, Larrick JW. Trade-offs between anti-aging dietary supplementation and exercise. Rejuvenation Res. 2013;16(5):419–26. doi: 10.1089/rej.2013.1484. [DOI] [PubMed] [Google Scholar]
- 39.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637–40. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 40.Murase T, Haramizu S, Ota N, Hase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10(4):423–34. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- 41.Olesen J, Gliemann L, Bienso R, Schmidt J, Hellsten Y, Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J Physiol. 2014;592(Pt 8):1873–86. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olesen J, Ringholm S, Nielsen MM, et al. Role of PGC-1alpha in exercise training- and resveratrol-induced prevention of age-associated inflammation. Exp Gerontol. 2013;48(11):1274–84. doi: 10.1016/j.exger.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagliarini DJ, Calvo SE, Chang B, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–23. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paolini M, Pozzetti L, Pedulli GF, Marchesi E, Cantelli-Forti G. The nature of prooxidant activity of vitamin C. Life Sci. 1999;64(23) doi: 10.1016/s0024-3205(99)00167-8. PL 273–8. [DOI] [PubMed] [Google Scholar]
- 45.Paulsen G, Cumming KT, Holden G, et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind randomized controlled trial. J Physiol. 2014 doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson T, Kabayo T, Ng R, Chamberlain J, McArdle A, Jackson MJ. Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLoS One. 2014;9(5):e96378. doi: 10.1371/journal.pone.0096378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 2011;41(12):1043–69. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31(7):987–97. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–78. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radak Z, Asano K, Inoue M, et al. Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J Appl Physiol (1985) 1995;79(1):129–35. doi: 10.1152/jappl.1995.79.1.129. [DOI] [PubMed] [Google Scholar]
- 51.Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18(10):1208–46. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ringholm S, Olesen J, Pedersen JT, et al. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1alpha. Exp Gerontol. 2013;48(11):1311–8. doi: 10.1016/j.exger.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosa EF, Ribeiro RF, Pereira FM, Freymuller E, Aboulafia J, Nouailhetas VL. Vitamin C and E supplementation prevents mitochondrial damage of ileum myocytes caused by intense and exhaustive exercise training. J Appl Physiol (1985) 2009;107(5):1532–8. doi: 10.1152/japplphysiol.91166.2008. [DOI] [PubMed] [Google Scholar]
- 55.Ryan MJ, Jackson JR, Hao Y, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65(8):815–31. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadi G, Bozan D, Yildiz HB. Redox regulation of antioxidant enzymes: post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol Cell Biochem. 2014 doi: 10.1007/s11010-014-2051-1. [DOI] [PubMed] [Google Scholar]
- 57.Samjoo IA, Safdar A, Hamadeh MJ, Raha S, Tarnopolsky MA. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr Diabetes. 2013;3:e88. doi: 10.1038/nutd.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sano M, Fukuda K. Activation of mitochondrial biogenesis by hormesis. Circ Res. 2008;103(11):1191–3. doi: 10.1161/CIRCRESAHA.108.189092. [DOI] [PubMed] [Google Scholar]
- 59.Scribbans TD, Ma JK, Edgett BA, et al. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl Physiol Nutr Metab. 2014;39(11):1305–13. doi: 10.1139/apnm-2014-0070. [DOI] [PubMed] [Google Scholar]
- 60.Selvaraj S, Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Dose-dependent interaction of trans-resveratrol with biomembranes: effects on antioxidant property. J Med Chem. 2013;56(3):970–81. doi: 10.1021/jm3014579. [DOI] [PubMed] [Google Scholar]
- 61.Sengottuvelan M, Deeptha K, Nalini N. Resveratrol ameliorates DNA damage, prooxidant and antioxidant imbalance in 1,2-dimethylhydrazine induced rat colon carcinogenesis. Chem Biol Interact. 2009;181(2):193–201. doi: 10.1016/j.cbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S, Dewald O, Adrogue J, et al. Induction of antioxidant gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species. Free Radic Biol Med. 2006;40(12):2223–31. doi: 10.1016/j.freeradbiomed.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 63.Smoliga JM, Blanchard OL. Recent data do not provide evidence that resveratrol causes ‘mainly negative’ or ‘adverse’ effects on exercise training in humans. J Physiol. 2013;591(Pt 20):5251–2. doi: 10.1113/jphysiol.2013.262956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2011;43(6):1017–24. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 65.Tauler P, Aguilo A, Gimeno I, Fuentespina E, Tur JA, Pons A. Response of blood cell antioxidant enzyme defences to antioxidant diet supplementation and to intense exercise. Eur J Nutr. 2006;45(4):187–95. doi: 10.1007/s00394-005-0582-7. [DOI] [PubMed] [Google Scholar]
- 66.Theodorou AA, Nikolaidis MG, Paschalis V, et al. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr. 2011;93(6):1373–83. doi: 10.3945/ajcn.110.009266. [DOI] [PubMed] [Google Scholar]
- 67.Vanaja K, Wahl MA, Bukarica L, Heinle H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: Evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sci. 2013;93(24):917–23. doi: 10.1016/j.lfs.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 68.Vardi M, Levy NS, Levy AP. Vitamin E in the prevention of cardiovascular disease: the importance of proper patient selection. J Lipid Res. 2013;54(9):2307–14. doi: 10.1194/jlr.R026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasilaki A, Mansouri A, Van Remmen H, et al. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5(2):109–17. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 70.Venditti P, Di Meo S. Effect of training on antioxidant capacity, tissue damage, and endurance of adult male rats. Int J Sports Med. 1997;18(7):497–502. doi: 10.1055/s-2007-972671. [DOI] [PubMed] [Google Scholar]
- 71.Venditti P, Napolitano G, Barone D, Di Meo S. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic Res. 2014:1–32. doi: 10.3109/10715762.2014.937341. [DOI] [PubMed] [Google Scholar]
- 72.Wawrzyniak A, Gornicka M, Hamulka J, et al. alpha-Tocopherol, ascorbic acid, and beta-carotene protect against oxidative stress but reveal no direct influence on p53 expression in rats subjected to stress. Nutr Res. 2013;33(10):868–75. doi: 10.1016/j.nutres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Yfanti C, Akerstrom T, Nielsen S, et al. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc. 2010;42(7):1388–95. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 74.Yfanti C, Nielsen AR, Akerstrom T, et al. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am J Physiol Endocrinol Metab. 2011;300(5):E761–70. doi: 10.1152/ajpendo.00207.2010. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Selin J, Rautiainen S, Lindblad BE, Morgenstern R, Wolk A. High-dose supplements of vitamins C and E, low-dose multivitamins, and the risk of age-related cataract: a population-based prospective cohort study of men. Am J Epidemiol. 2013;177(6):548–55. doi: 10.1093/aje/kws279. [DOI] [PubMed] [Google Scholar]


