Abstract
A dielectrophoresis (DEP)-based method is reported to achieve highly efficient on-chip extraction of cell-laden microcapsules of any stiffness from oil into aqueous solution. The hydrogel microcapsules can be extracted into the aqueous solution by DEP and interfacial tension (IFT) forces with no trapped oil while the encapsulated cells are free from the electrical damages due to the Faraday cage effect.
Keywords: dielectrophoresis, liquid electrode, on-chip extraction, hydrogel microcapsule, interfacial tension
Microencapsulation of single cells or cell clusters in hydrogels of biocompatible polymers has wide applications in 3D cell culture, stem cell therapy, cell cryopreservation, drug delivery, and tissue engineering.[1] The polymeric hydrogel microcapsules have high permeability to the substances (both nutrients and wastes) essential for cell survival while blocking immune cells and large antibodies from entering the microcapsules to injure the encapsulated cells.[2] These cell-laden microcapsules are usually produced by emulsification and electrospray that could cause damages to cells (particularly the former).[3] More recently, microfluidics has emerged as a powerful tool for generating hydrogel microcapsules to address this issue.[4] However, one major challenge of the microfluidics approach is that after production, the hydrogel microcapsules are usually dispersed in an oil phase and difficult to be extracted for further use.[1a] This is particularly true for biomedical applications because biomedical systems are usually water rather than oil-based. For example, prolonged exposure to oil could significantly decrease the viability of mammalian cells encapsulated in the microcapsules because the oil can block the transport of nutrients and metabolic wastes.[5] As a result, timely extraction of the cell-laden microcapsules from oil into an aqueous phase is crucial for cell microencapsulation applications.
Conventionally, the hydrogel microcapsules are extracted from the oil into aqueous phase by multiple steps of centrifugation and washing, which is time-consuming with low retrieval efficiency, and could result in the formation of oily aggregates of multiple microcapsules due to the centrifugation force.[6] Recently, several methods have been proposed to achieve on-chip extraction of the hydrogel microcapsules suspended in oil into an aqueous phase. For example, the bifurcation law was utilized to transfer alginate hydrogel microcapsules from oil into an aqueous phase.[7] A mechanical filter was designed to extract microcapsules into cell culture medium.[5b] Purification (i.e., extraction) of hydrogel microcapsules was also achieved by multiple steps of oil depletion on chip.[5c] By establishing a stable interface between oil suspended with alginate hydrogel microparticles and aqueous solution, the interfacial tension was utilized to achieve on-chip extraction of the microparticles into the aqueous solution.[6] However, these methods require surface modification of the microchannel, large ratio of aqueous to oil flow rate, and/or complex geometry design. Moreover, they may not be applicable for extracting small or soft microcapsules. Therefore, better approaches for on-chip extraction of hydrogel microcapsules are in need.
To this end, we propose a dielectrophoresis (DEP)-based approach for on-chip extraction of hydrogel microcapsules. Several types of DEP devices have been developed to manipulate microcapsules on chips based on microposts,[8] metal electrodes,[9] liquid electrodes,[10] or thin-walled polydimethylsulfoxide (PDMS)[11]. In addition, the fluids surrounding the microcapsules were always aqueous (i.e., single phase) in these studies. In this communication, we developed a new type of microfluidics-DEP device with liquid electrodes to readily generate and extract cell-laden microcapsules from oil emulsion into an aqueous solution.
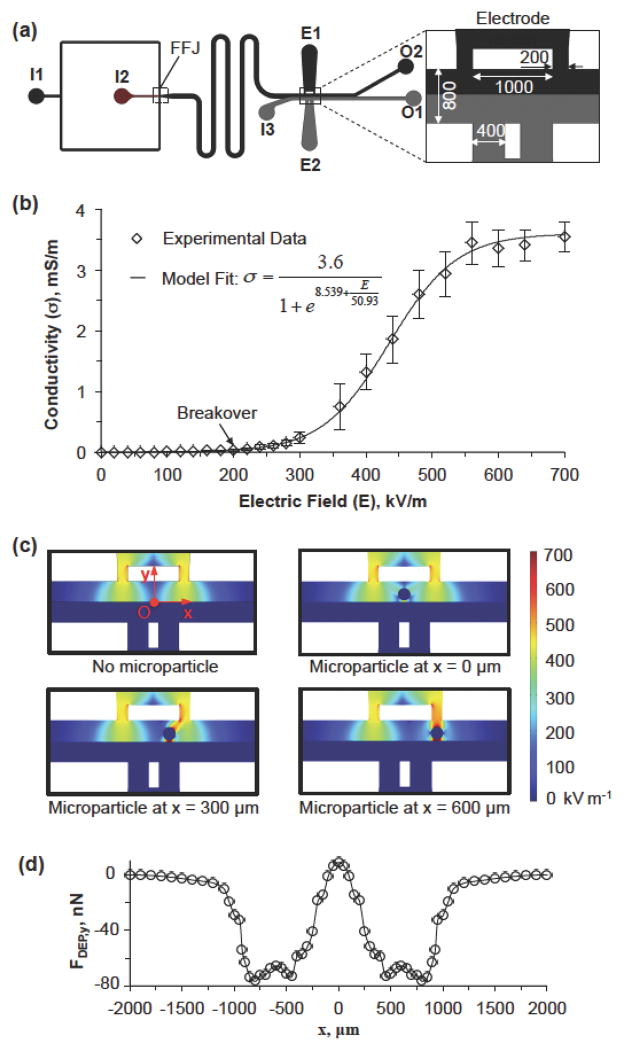
As shown in Figure 1a, the device has two inlets for introducing oil emulsion (inlet I1) and the cell-containing aqueous sodium alginate solution (inlet I2) and the two immiscible fluids meet at the nonplanar flow-focusing junction (FFJ) to generate sodium alginate microdroplets in the carrier oil phase. The nonplanar design (Figure S1) of the FFJ could prevent the resultant microdroplets from potentially sticking on the microchannel wall. The aqueous sodium alginate solution consists of 2% (w/v) sodium alginate and 0.9% (w/v) sodium chloride and is pinched into uniform microdroplets by the oil emulsion as a result of the Plateau-Rayleigh instability[12]. The microdroplets are crosslinked by Ca2+ infused in the oil emulsion into calcium alginate hydrogel microcapsules in the downstream serpentine channel.[1b] After the serpentine channel, an aqueous extraction solution consisting of 1.3% (w/v) medium-viscosity carboxymethyl cellulose (Sigma) and 0.9% (w/v) sodium chloride is introduced into the device via I3. This aqueous extraction solution has the same viscosity (~108 mPa) as the oil emulsion, which is important for the establishment of a stable interface between the oil emulsion and the aqueous extraction solution in the middle of the extraction microchannel when their flow rates are the same.[6] The flow rates of sodium alginate solution, oil emulsion, and extraction solution were 0.1, 5, and 5 ml hr−1, respectively. An electric field is applied to the liquid electrodes (E1 and E2 that are filled with oil emulsion and extraction solution, respectively) to facilitate the extraction of the microcapsules into the aqueous extraction solution. Afterward, the extracted microcapsules are collected from the aqueous outlet O1 while oil and the non-extracted microcapsules (if any) are collected from the outlet O2.
Figure 1.
The microfluidic device and modeling of electric field and DEP force in the electrode region of the device. (a) A schematic overview of the microfluidic device showing the three inlets (I1, I2, and I3), flow-focusing junction (FFJ), and two outlets (O1 and O2) together with a zoom-in view of the electrode (E1 and E2) region with dimensions (unit: μm). Compositions of fluids flowing into inlets: I1, oil emulsion; I2, 2% (w/v) sodium alginate in saline with or without cells (1.5 million/ml); I3, 1.3% (w/v) medium-viscosity carboxymethyl cellulose (Sigma) in saline; E1, the same as I1; and E2, the same as I3. (b) The conductivity of oil emulsion as a function of the DC electric field showing a breakover at ~200 kV/m. The error bars represent the standard error of mean (SEM). (c) Modeling results of the electric field distribution in the electrode region in the absence of a hydrogel microcapsule and in the presence of a hydrogel microcapsule at three different locations. (d) The y component of the DEP force that a microcapsule experiences at various locations in the electrode region. The x and y coordinates are the same as that shown in (c).
In this device, the oil emulsion used as the carrier fluid in the FFJ is also employed as half of the electrodes. Although the conductivity of pure mineral oil is nearly zero, the oil emulsion made of mineral oil and concentrated aqueous solution of calcium chloride could have a much higher conductivity above its breakover electric field, which is the turning point where the conductivity starts to increase with the further increase of the applied electric field (Figure 1b). However, the oil emulsion does not break down and can form stable interface with the aqueous extraction solution. As a result, when a direct current (DC) electric field increases above the breakover threshold (~ 200 kV m−1), the electrodes can actuate the microcapsules dispersed in the oil phase to move towards the aqueous extraction solution.
According to the theory of dielectrophoresis, a microcapsule suspended in a conductive medium could experience a net DEP force under a heterogeneous electric field. In this study, the size of the hydrogel microcapsules is comparable to that of the microchannel and their presence can significantly affect the local electrical field. Therefore, the hydrogel microcapsules should be considered in the calculation of electrical field and the DEP force on the microcapsules should be calculated based on the integration of the Maxwell stress tensor on their surfaces. Therefore, numerical simulations were performed to calculate the DEP force on a hydrogel microcapsule in the extraction channel using COMSOL-Multiphysics (version 4.3). As shown in Figure 1c, when a DC electric field is applied to the electrodes in the absence of a hydrogel microcapsule, a high and symmetric electric field is generated near the electrodes on the oil emulsion side, while it is negligible in the aqueous phase. When a microcapsule is presented in the electrode region, the distribution of electric field will be altered (Figure 1c), and the y component of the DEP force on the microcapsule is negative (Figure 1d). As a result, the microcapsules should be moved towards the aqueous phase. As shown in Supporting Information, the location where the microcapsules get extracted would be approximately 550 μm in x coordinate according to this simulation.
Because the microcapsule is hydrophilic, once they penetrate the interface between the oil emulsion and aqueous extraction solution, they will be quickly pulled into the aqueous extraction phase by interfacial tension force.[6] As the net DEP forces (~ 10–100 nN, Figure 1d) on the microcapsule during passing through the interface is much smaller than the interfacial tension force (2πrγ ~ 3000 nN, where γ ~ 5 mN m−1 is the interfacial tension), the potential pullback of hydrogel microcapsules into the oil emulsion after extraction is prohibited. In addition, it is worth noting that due to the presence of the sodium chloride as the electrolyte in the microcapsules, the electric field intensity inside the microcapsules is much lower (below 2.5 kV m−1) than that in the oil emulsion (~ 700 kV m−1) (Figure 1c) as a result of the Faraday Cage effect. This should protect the cells (if any) in the microcapsules from being damaged by the high electric field when the microcapsules are in the oil emulsion before being extracted.[13] After extraction, the electric field intensity in the aqueous extraction solution is low (Figure 1c) and not harmful to the microencapsulated cells (if any).
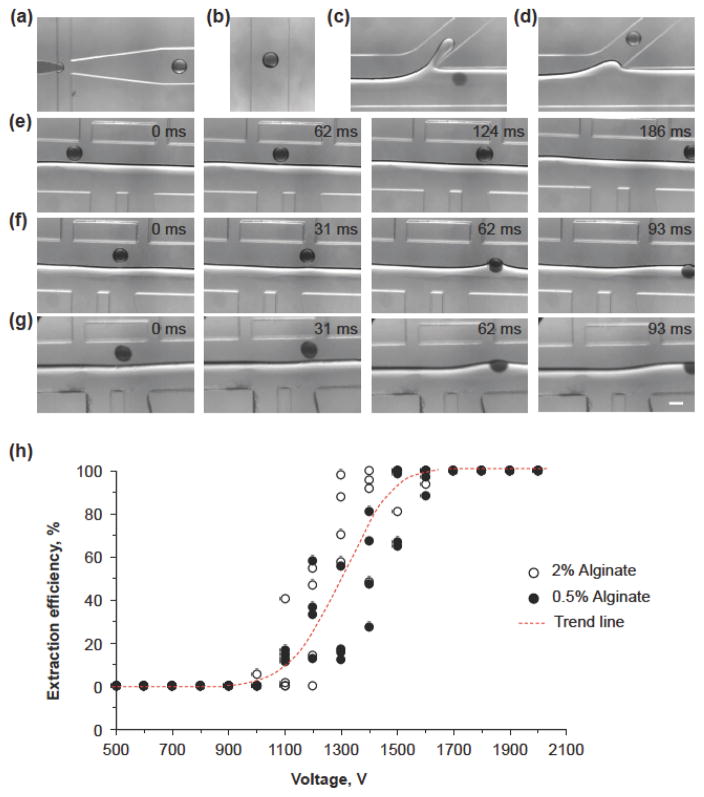
We confirmed this DEP-based extraction of hydrogel microcapsules by experimental study. To visualize the hydrogel microcapsules in the aqueous solution, a small amount (< 0.5%, w/v) of the dye amaranth (Sigma) was added in the dispersed solution. Figure 2 shows the images of the generation and transportation of the microdroplets and hydrogel microcapsules in the microchannel. Figure 2a and Movie S1 show the generation of monodisperse microdroplets at the FFJ as the flow instability falls in the dripping regimen. The sodium alginate microdroplets are crosslinked into microcapsules of calcium alginate hydrogel as they travel through the downstream serpentine channels by Ca2+ infused in the oil emulsion. Figure 2b is the microdroplets or hydrogel microcapsules travelling in the middle of the serpentine microchannel due to the dominant viscous force. Figure 2c and Movie S2 demonstrates that the extracted microcapsules exit from the outlet O1 in the aqueous phase while Figure 2d shows that the non-extracted microcapsules move out via the tilted outlet in the oil phase. Figure 2e and Movie S3 show that in the absence of an electric field, the hydrogel microcapsule moves through the electrode region in oil emulsion. When an electric field is applied, the hydrogel microcapsules could be deflected and get extracted from the oil emulsion into the aqueous phase due to DEP force (Figure 2f and Movie S4). To be noted, the hydrogel microcapsules are extracted from 450 to 650 μm in x coordinate, which is close to the aforementioned prediction from numerical simulation (550 μm).
Figure 2.
Generation and extraction of hydrogel microcapsules of calcium alginate in the microfluidic device (moving from left to right). (a) Formation of microdroplet at the flow-focusing junction (FFJ). (b) A microdroplet or hydrogel microcapsules travelling at the centerline of the serpentine microchannel. (c) A hydrogel microcapsules extracted into the aqueous phase exiting via the O1 outlet. (d) A hydrogel microcapsule in the oil emulsion exiting via the O2 outlet. (e) Movement of an alginate (2%) hydrogel microcapsule in the extraction channel (i.e., electrode region) without electrical field. (f) Extraction of 2% alginate hydrogel microcapsules with electric field 1700 V. (g) Extraction of 0.5% alginate hydrogel microcapsules with electric field 1700 V. Scale bar: 200 μm. (h) The DEP extraction efficiency of 0.5 and 2% alginate hydrogel microcapsules under various electric fields. The number (N) of microcapsules used to calculate the extraction efficiency was ~250 for each voltage. Four independent runs were performed for each alginate concentration.
Moreover, the DEP force can drive the hydrogel microcapsules towards the interface for extraction regardless of their mechanical strength. Figure 2g and Movie S5 show the migration of 0.5% (w/v) alginate hydrogel microcapsules from the oil emulsion into the aqueous phase under the DEP force even though there is apparent deformation of the hydrogel microcapsules. By contrast, although the interfacial tension-based method can extract 2% (w/v) alginate hydrogel microcapsules,[6] it is ineffective for extracting hydrogel microcapsules made of 1% (w/v) or less alginate as their mechanical strength is much weaker and they can easily deform under the shear stress in the extraction channel (Figure S2, Movies S6–8). Figure 2h summarizes the relationship between the extraction efficiency of 0.5 and 2% alginate hydrogel microcapsules and the applied electric field on the electrodes. Typically, the extraction could be initiated at approximately 1000 V. When the applied field is increased to 1700 V, all the hydrogel microcapsules in all cases can be extracted. Furthermore, the change of the alginate concentrations (and thus, mechanical strength) does not affect the extraction efficiency under various electric fields. Of note, even if the alginate droplets are not gelled (i.e., stay as liquid droplets) by replacing calcium chloride with sodium chloride in the oil emulsion, they can also be extracted with the DEP force as shown in Figure S3, Movie S9 (without DEP), and Movie S10 (with DEP) although the required voltage is decreased to 700 V for full extraction. The latter is probably due to the change of electric properties of the oil emulsion when calcium chloride is replaced by sodium chloride.[14]
To examine the effect of electric field on the viability of microencapsulated cells during DEP extraction, we encapsulated C3H10T1/2 mesenchymal stem cells in 2% alginate hydrogel and applied the maximum electric field used in this study (i.e., 2000V, Figure 2h). Fresh cells without any microencapsulation or extraction (kept at 4 °C over the ~1.5 hour duration of the experiment) and microencapsulated cells with off-chip extraction (i.e., by centrifuging and washing) were also studied in parallel as controls. For off-chip extraction, the cell-laden microcapsules, oil emulsion, and aqueous extraction solution were collected altogether in a 50 ml centrifuge tube containing 20 ml cell culture medium at 4 °C. The collected sample was then centrifuged at 300 rpm (50μg) and 4 °C for 3 min. The supernatant including most of the carrier oil emulsion was aspirated. Finally, 5 ml fresh medium was added into the centrifuge tube. After mixing the microcapsules with the medium by gentle pipetting, the sample was transferred into a clean 15 ml centrifuge tube for further study. For on-chip extraction, the cell-laden microcapsules from aqueous exit were collected in a 50 ml centrifuge tube containing 20 ml cell culture medium at 4 °C without further processing.
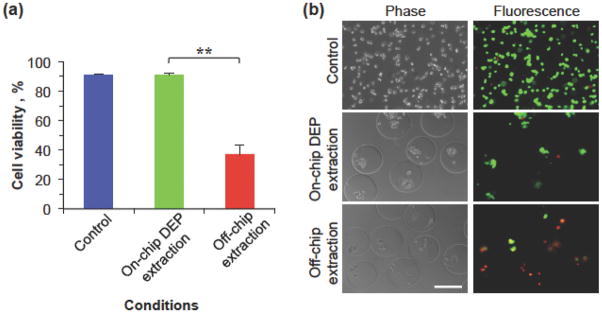
The cell viability was determined using the fluorescence-based Live/Dead cell viability assay kit (Life Technology) by incubating the cells with the assay for 10 min at 37 °C and the results are shown in Figure 3. The cell viability of the on-chip DEP extraction group is 91.1%, which is similar to that of the fresh control cells (91.3%). By contrast, the cell viability of the off-chip extraction group was significantly lower (36.7%). These cell viability data indicate that the high electric field outside microcapsules does not harm the cells in the microcapsules when they pass through the electrode regions due to the Faraday Cage effect (Figure 1c). In addition, on-chip DEP extraction can significantly reduce the time of the encapsulated cells in the oil emulsion with high concentration of calcium chloride and eliminate any oil trapped on the microcapsule surface to maintain high cell viability, compared to the conventional method with tedious procedures of centrifuging and washing. A higher cell survival for the off-chip approach might be achieved if the cells are collected for a shorter time or cells that are less sensitive to the high-salt stress are used.
Figure 3.
The effect of on-chip and off-chip extraction on the viability of microencapsulated C3H10T1/2 cells. (a) The viability of cells without microencapsulation or extraction (control), microencapsulated cells with on-chip DEP extraction (2000 V), and microencapsulated cells extracted into the aqueous phase using the conventional off-chip method (i.e., centrifuging and washing). (**): p < 0.01. The error bars represent SEM. (b) Typical phase and fluorescence images showing cell viability for the same three different groups. Scale bar: 200 μm
It is worth noting that our on-chip approach extracts the microcapsules one by one, which overcomes the issue of the formation of large oily aggregates of multiple microcapsules associated with the conventional off-chip centrifugation approach.[6] Moreover, once the DEP force drives a microcapsule to touch the interface between the oil emulsion and aqueous extraction solution, the interface between the microcapsule and surrounding oil emulsion opens up and further merges with the interface between the oil emulsion and aqueous extraction solution (Movies S3–S7). Consequently, the microcapsule is extracted into the aqueous extraction solution quickly by the interfacial tension force that is much higher than the DEP force, as discussed earlier. Therefore, the microcapsules extracted with our approach are clean with no trapped oil on their surface (Fig. 3b). Otherwise, they should be pushed back into oil emulsion by the interfacial tension force after touching the interface between the oil emulsion and the aqueous extraction solution, which we did not observe at all.
In summary, a novel DEP device with liquid electrodes was developed in this study for extracting the cell-laden microcapsules from oil emulsion into aqueous solution. When the hydrogel microcapsules pass through the electrode region, the DEP force can deflect them towards the aqueous phase regardless of their mechanical strength. Complete extraction can be achieved when the electric field is higher than 1700 V. Moreover, the DEP extraction does not compromise the viability of the microencapsulated cells. The novel DEP-based approach developed in this study is expected to greatly facilitate the wide application of the microfluidic encapsulation technology.
Modeling and experimental section
Modeling of electric field and DEP force
The AC/DC module of the COMSOL Multiphysics (version 4.3) was used for modeling the electric field. The governing equation is ∇((σ + jωε0εr)(−∇V)) = 0. The boundary condition is n·J= 0, where n is the normal vector outward from the object. J is the electrical current. The DEP force F was calculated by surface integration of the Maxwell stress tensor T, F = ∫∂Ω nT ds where ∂Ω is the surface of the microcapsule. The conductivities of the alginate solution with sodium chloride and carboxymethyl cellulose solution with sodium chloride were measured as 1.90 ± 0.05 S m−1 and 1.80 ± 0.04 S m−1, respectively, using the Agilent 85070E Dielectric Probe Kit and the Agilent E8362B PNA Network Analyzer. The relative dielectric constants of both aqueous solutions were set as 80. The conductivity of the PDMS was set as 0.83×10−12 S m−1 and its relative dielectric constants were set as 2.65.[11a] For the oil emulsion, the relationship between the conductivity and the electric field intensity was determined experimentally (Figure 1b) and its relative dielectric constant was measured as 4.0 ± 0.2 using the same approach for the aqueous solutions.
Device fabrication
The non-planar microfluidic devices were fabricated by standard soft lithography techniques. Briefly, the first layer of photosensitive epoxy (SU-8 2025, MicroChem) was spun onto a 4-inch silicon wafer. The wafer was baked on a hot plate to solidify the SU-8. Next, it was exposed to ultra violet (UV) light through the shadow mask of the first layer design and baked on the hot plate again. Similarly, the second layer of microchannels was successively imposed onto the wafer, and these two adjacent layers were aligned on an EVG620 mask aligner. Because the thicknesses of these two layers are different, the spinning speeds of SU-8, the pre- and post-exposure baking time and temperatures, and the exposure time of UV light would change according to the data sheet of SU-8 2000 from MicroChem. After patterning the features of microchannels, the wafer was developed by SU-8 developer (MicroChem) for 10 min, rinsed by isopropyl alcohol, and dried by nitrogen gas. Thereafter, polydimethylsiloxane (PDMS, Dow Corning) pre-polymer and its crosslinking agent (mass ration = 10:1) were fully mixed and poured onto the patterned wafer to make PDMS slabs (baked at 72 °C for 3 hours). After the slabs were peeled off from the wafer, they were aligned under microscope to obtain non-planar microfluidic devices. Finally, the devices were baked in oven at 72 °C for at least 72 hours to make the channel surfaces hydrophobic before use.
Measurements of mechanical stiffness of hydrogels
The measurement of the mechanical properties was performed using a TA instrument AR-1000N rheometer as described elsewhere.[1b] Briefly, alginate hydrogels of different concentrations were cut into the shape of the sample-holding plate of the rheometer and transferred onto the measuring plate. The 40 mm (diameter) parallel plates were used for the measurements. First, stress sweeps of constant frequency at 1 Hz were performed to identify the linear viscoelastic regime. The frequency sweeps were then carried out in the linear regime to obtain the storage modulus (G′) reported in this study. All rheology experiments were performed at room temperature (24 °C).
Preparation of the oil emulsion
A total of 5 ml of mineral oil (Sigma) was mixed with 93.3 μl of Span 80 (Sigma). The mixture was then emulsified with 1 ml of calcium chloride solution (1 g/ml in deionized water) using the Branson 450 Digital Sonifier at the amplitude of 20% for 1 min to obtain the oil emulsion. To prepare the oil emulsion of sodium chloride, the procedure was the same except that the aqueous solution of calcium chloride was replaced with 0.35 g/ml sodium chloride solution.
Measurements of the electric conductivity of the oil emulsion
To measure the conductivity, the oil emulsion was filled into a platinum-cured silicone tubing (Cole-Parmer). Two BD syringe needles were used as the electrodes to apply electric field and were connected to the tubing. The inner diameter of the tubing was 0.51 mm, and the length of the oil emulsion was 5 mm. A LabVIEW system was used to record the current passing through the sample and collect the data every 0.1 ms. Therefore, the conductivity can be calculated as , where I is current, L is length of the sample, S is inner area of the tubing, and V is the applied voltage.
Cell preparation
C3H10T1/2 mesenchymal stem cells (ATCC, Manassas, VA, USA) were used in this study. The cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin in 75 cm2 T-flasks at 37 °C in humidified air with 5% CO2. The medium was refreshed every two days. After detaching from the culture flask, the cells were suspended in 2% (w/v) alginate and 0.9% (w/v) sodium chloride solution and the final cell concentration was 2×106 cells/ml. The cell suspensions were kept at 4 °C before experimental use.
Supplementary Material
Acknowledgments
This work was partially supported by grants from NSF (CBET-1154965) and NIH (R01EB012108) to XH. TH was supported by the Norbert Peiker Eye Research Fellowship through the Ohio Lions Eye Research Foundation (OLERF).
Contributor Information
Haishui Huang, Department of Mechanical and Aerospace Engineering, The Ohio State University, Columbus, Ohio 43210, USA.
Mingrui Sun, Department of Biomedical Engineering, The Ohio State University, Columbus, Ohio 43210, USA.
Tyler Heisler-Taylor, Department of Biomedical Engineering, The Ohio State University, Columbus, Ohio 43210, USA.
Dr. Asimina Kiourti, Department of Electrical and Computer Engineering, The Ohio State University, Columbus, Ohio 43210, USA
Prof. John Volakis, Department of Electrical and Computer Engineering, The Ohio State University, Columbus, Ohio 43210, USA
Prof. Gregory Lafyatis, Department of Physics, The Ohio State University, Columbus, Ohio 43210, USA
Prof. Xiaoming He, Email: He.429@osu.edu, Department of Biomedical Engineering, The Ohio State University, Columbus, Ohio 43210, USA
References
- 1.a) Agarwal P, Zhao S, Bielecki P, Rao W, Choi JK, Zhao Y, Yu J, Zhang W, He X. Lab Chip. 2013;13:4525. doi: 10.1039/c3lc50678a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Choi JK, Agarwal P, Huang H, Zhao S, He X. Biomaterials. 2014;35:5122. doi: 10.1016/j.biomaterials.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang W, Yang G, Zhang A, Xu LX, He X. Biomed Microdevices. 2010;12:89. doi: 10.1007/s10544-009-9363-z. [DOI] [PubMed] [Google Scholar]; d) Lim F, Sun AM. Science. 1980;210:908. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]; e) Lee KY, Mooney DJ. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]; f) Kim C, Chung S, Kim YE, Lee KS, Lee SH, Oh KW, Kang JY. Lab Chip. 2011;11:246. doi: 10.1039/c0lc00036a. [DOI] [PubMed] [Google Scholar]; g) Zhang W, He X. J Healthcare Eng. 2011;2:427. doi: 10.1260/2040-2295.2.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang TM. Science. 1964;146:524. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 3.a) Zhang W, Zhao S, Rao W, Snyder J, Choi JK, Wang J, Khan IA, Saleh NB, Mohler PJ, Yu J, Hund TJ, Tang C, He X. J Mater Chem, B. 2013;1:1002. doi: 10.1039/C2TB00058J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Serra M, Correia C, Malpique R, Brito C, Jensen J, Bjorquist P, Carrondo MJ, Alves PM. PloS One. 2011;6:e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mumaw J, Jordan ET, Sonnet C, Olabisi RM, Olmsted-Davis EA, Davis AR, Peroni JF, West JL, West F, Lu Y, Stice SL. Int J Biomater. 2012;2012:861794. doi: 10.1155/2012/861794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu S, Nie Z, Seo M, Lewis P, Kumacheva E, Stone HA, Garstecki P, Weibel DB, Gitlin I, Whitesides GM. Angew Chem, Int Ed Engl. 2005;44:724. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- 5.a) Tan WH, Takeuchi S. Adv Mater. 2007;19:2696. [Google Scholar]; b) Hong S, Hsu HJ, Kaunas R, Kameoka J. Lab Chip. 2012;12:3277. doi: 10.1039/c2lc40558j. [DOI] [PubMed] [Google Scholar]; c) Deng Y, Zhang N, Zhao L, Yu X, Ji X, Liu W, Guo S, Liu K, Zhao XZ. Lab Chip. 2011;11:4117. doi: 10.1039/c1lc20494g. [DOI] [PubMed] [Google Scholar]; d) Agarwal P, Choi JK, Huang H, Zhao S, Dumbleton J, Li J, He X. Part Part Syst Charact. 2015;32 doi: 10.1002/ppsc.201500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, He X. Appl Phys Lett. 2014;105:143704. doi: 10.1063/1.4898040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong EH, Rondeau E, Schuetz P, Cooper-White J. Lab Chip. 2009;9:2582. doi: 10.1039/b903774h. [DOI] [PubMed] [Google Scholar]
- 8.a) Cho YK, Kim S, Lee K, Park C, Lee JG, Ko C. Electrophoresis. 2009;30:3153. doi: 10.1002/elps.200900179. [DOI] [PubMed] [Google Scholar]; b) Jen CP, Chen TW. Microsyst Technol. 2009;15:1141. [Google Scholar]
- 9.a) Li HB, Bashir R. Sens Actuators, B. 2002;86:215. [Google Scholar]; b) Rousselet J, MArkx GH, Pethig R. Colloids Surf, A. 1998;140:209. [Google Scholar]
- 10.Demierre N, Braschler T, Linderholm P, Seger U, van Lintel H, Renaud P. Lab Chip. 2007;7:355. doi: 10.1039/b612866a. [DOI] [PubMed] [Google Scholar]
- 11.a) Shafiee H, Sano MB, Henslee EA, Caldwell JL, Davalos RV. Lab Chip. 2010;10:438. doi: 10.1039/b920590j. [DOI] [PubMed] [Google Scholar]; b) Shafiee H, Caldwell JL, Sano MB, Davalos RV. Biomed Microdevices. 2009;11:997. doi: 10.1007/s10544-009-9317-5. [DOI] [PubMed] [Google Scholar]
- 12.Cubaud T, Mason TG. Phys Fluids. 2008;20:11. doi: 10.1103/PhysRevE.78.056308. [DOI] [PubMed] [Google Scholar]
- 13.Wang HY, Lu C. Anal Chem. 2006;78:5158. doi: 10.1021/ac060733n. [DOI] [PubMed] [Google Scholar]
- 14.Chanamai R, McClements DJ. Food Hydrocolloids. 2001;15:83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.