Abstract
Objective
The presence of a mental health disorder with hypertension is associated with higher cardiovascular disease mortality than hypertension alone. Although earlier detection of hypertension has been demonstrated in patients with anxiety and depression, the relationship of mental health disorders to hypertension control is unknown. Our objective was to evaluate rates and predictors of incident hypertension control among patients with anxiety and/or depression compared to patients without either mental health diagnosis.
Methods
A four-year retrospective analysis included 4362 patients, ≥18 years old, who received primary care in a large academic group practice from 2008–2011. Patients met JNC 7 criteria and had a hypertension diagnosis. Kaplan-Meier analysis estimated the probability of achieving control for patients with and without anxiety and/or depression. Cox proportional hazard models were fit to identify predictors of time to control.
Results
Overall, 13% (n=573) had a baseline diagnosis of anxiety and/or depression. Those with anxiety and/or depression demonstrated more primary care and specialty visits than those without either condition. After adjustment, patients with anxiety and/or depression had faster rates of hypertension control (HR 1.22; 1.07–1.39) than patients without either diagnosis. Other associations of faster hypertension control included female gender (HR 1.32; 1.20–1.44), absence of tobacco use (HR 1.17; 1.03–1.33), Medicaid use (HR 1.27; 1.09–1.49), and a higher Adjusted Clinical Group Risk Score (HR 1.13; 1.10–1.17), a measure of healthcare utilization.
Conclusions
Greater healthcare utilization among patients with anxiety and/or depression may contribute to faster hypertension control.
Keywords: Hypertension, Anxiety, Depression, Diagnosis, Kaplan-Meier Estimate, Retrospective Studies, United States
INTRODUCTION
Hypertension is the leading risk factor for morbidity and mortality worldwide [1]. Effective management of hypertension reduces the risk of stroke, myocardial infarction, congestive heart failure, and overall mortality [2]. Patients with depression and/or anxiety represent a particularly vulnerable population as they are at higher risk for developing hypertension [3,4]. In addition, patients with co-morbid hypertension and mental health disorders are a higher-risk population for cardiovascular disease related mortality [5,6].
Depression and anxiety, which are commonly co-existing conditions [7–9], affect nearly one-fourth of the U.S. adult population [10]. There is a general association between mental health disorders and cardiovascular disease risk factors [11–13]; however, the management of hypertension in patients with common mental health disorders has received relatively little study and there is conflicting data [14]. Byrd et al. [14] found that hypertension diagnosis rates were faster in patients with depression and anxiety than in patients with neither mental health condition. This may reflect an increase in healthcare utilization among patients with anxiety and/or depression [15]. A previous study highlighted more mental health and non-mental health related visits among patients with anxiety and/or depression [9]. However, Moise et al. [16] demonstrated that patients were less likely to have intensification of hypertension treatment with a comorbid depression diagnosis. It is yet unknown how the diagnosis of either anxiety or depression affects time to incident hypertension control. Therefore, the purpose of our study was to evaluate rates of incident hypertension control among patients with anxiety and/or depression compared to patients without either diagnosis.
METHODS
Sample
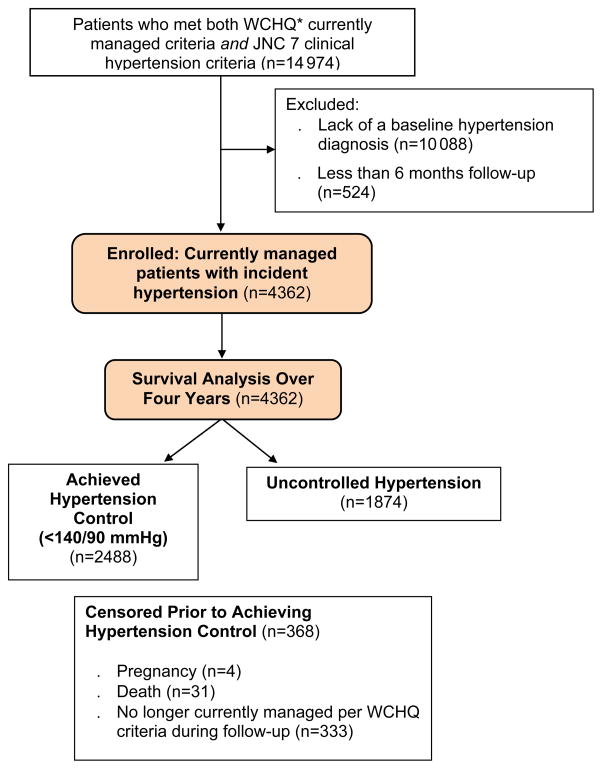
The University of Wisconsin-Madison Health Sciences Institutional Review Board approved this study with a waiver of consent. This secondary retrospective cohort analysis used electronic health record data from a large, Midwestern, multi-disciplinary academic group practice. To construct the sample (Figure 1), we identified all patients ≥18 years old who met criteria from the Wisconsin Collaborative for Healthcare Quality (WCHQ) [17,18] for being “currently managed” in the healthcare system between January 1, 2008 and December 31, 2011. WCHQ is a multi-stakeholder, voluntary consortium of Wisconsin organizations committed to publicly reporting performance measures of quality and affordability of healthcare services [19]. Per WCHQ criteria, patients had to have ≥2 billable office encounters in an outpatient, non-urgent, primary care setting, or one primary care and one office encounter in an urgent care setting, in the three years prior to study enrollment, with at least one visit in the prior two years [20]. Electronic health records were assessed for the date a patient met JNC 7 clinical blood pressure criteria for a new diagnosis of hypertension [2] (incident hypertension), meaning they had not received a previous diagnosis of or treatment for hypertension. JNC 7 criteria were used as they were the established U.S. hypertension guidelines during the reporting period. First, a patient was determined as meeting blood pressure eligibility criteria based on electronic health record data if the patient had: a) ≥3 elevated outpatient blood pressure measurements from three separate dates, ≥30 days apart, but within a two-year span (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) or b) two elevated blood pressures [21,22] (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥100 mmHg), ≥30 days apart within a two-year period. If more than one blood pressure was taken at a visit, the average was used [23]. Hospital and emergency department blood pressures were excluded. After meeting criteria for incident hypertension, patients were then excluded if they did not receive a new diagnosis of hypertension based on the Tu criteria [24] and if they had less than 6 months follow-up (Figure 1). The Tu algorithm for administrative data is used to define patients who have been diagnosed with hypertension using the following ICD-9 codes [25]: 401.x (essential hypertension), 402.x (hypertensive heart disease), 403.x (hypertensive renal disease), 404.x (hypertensive heart and renal disease), and 405.x (secondary hypertension).
Figure 1.
Study Sample: Enrollment and Analysis
*WCHQ: Wisconsin Collaborative for Healthcare Quality
Each patient meeting all eligibility criteria received an “index date” (the first date both criteria were met). A 365-day period prior to this index date was the “baseline period” to assess patients’ comorbidities and healthcare utilization. Patients continued to accrue time in the study from the index date until they achieved the study outcome (hypertension control), the study ended, or censoring occurred. Patients were censored if they died (censored day of death; n=31, 0.71%) or were no longer currently managed (censored at the end of the calendar year; n=333, 7.6%). Patients who were pregnant during the study were excluded one year before, during, and one year following pregnancy using a modified Manson approach (n=4, 0.09%) [26]. The final sample was 4362 currently managed patients with incident hypertension (Figure 1).
Primary outcome variable
The primary outcome was time (days) from the index date to achieving hypertension control, defined as three consecutive normal blood pressures (<140/90 mmHg) on three separate dates. To account for blood pressure variability, multiple clinic blood pressures were used to define hypertension control since ambulatory blood pressures were not available. Results are reported in months.
Explanatory variables
Patient and provider explanatory variables to identify barriers to achieving hypertension control were selected based on the concept of clinical inertia (delays in hypertension diagnosis and/or medication initiation/titration) [27]. Patient-related factors included: sociodemographics (age, sex, race/ethnicity, marital status, Medicaid use during the baseline or study period), behavioral risk factors (baseline tobacco use, body mass index at study entry), and comorbidities. Anxiety (ICD-9 codes: 300.0–300.02, 300.09, 300.21–300.23, 300.3, 309.24, 309.81) [28] and depression (ICD-9 codes: 300.4, 301.12, 309.0, 309.1, 311) [29] were assessed using established algorithms, requiring at least two outpatient diagnosis codes within a two-year period. Since anxiety and depression are common comorbid conditions, but often under-recognized [30], a combined variable was created (anxiety and/or depression) for analysis [14].
Patients’ morbidity burden can predict healthcare utilization which may influence hypertension control rates [31,32]. Therefore, we used the Johns Hopkins Adjusted Clinical Group (ACG) Case-Mix System (version 10.0), which assesses morbidity burden based on patient age, gender, and patterns of disease in the electronic health record to predict future healthcare resource utilization [32]. Additional measures of utilization included the number of baseline primary care, specialty, and urgent care visits. Primary care visits included those to Family Medicine/Family Practice, Internal Medicine, and a combined category of lower prevalence specialties (Obstetrics/Gynecology, Pediatrics/Adolescent Medicine) with a physician (faculty, resident, fellow), nurse practitioner, or physician assistant.
Patients were assigned to the primary care provider they saw most frequently in outpatient face-to-face Evaluation & Management visits, as reported in professional service claims [20]. Models additionally controlled for each provider’s age, specialty (Internal Medicine, Family Medicine/Family Practice, Other), and gender, which were obtained from the provider group’s human resource office and/or the American Medical Association (AMA) 2011 Masterfile data.
Statistical analysis
Analyses were conducted using SAS 9.1.3 (SAS Institute, Inc., Cary, NC) and Stata 13.1 (Stata-Corp, College Station, TX). Univariate Kaplan-Meier survival curves [33] were computed for two groups: 1) patients with anxiety and/or depression, and 2) patients with neither anxiety or depression, to evaluate the probability of achieving hypertension control as a function of time since meeting criteria for incident hypertension. Multivariate Cox proportional hazards regression analyses were conducted with robust estimates of the variance to obtain adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for achieving hypertension control. Explanatory variables included presence/absence of anxiety and/or depression, patient sociodemographic and comorbidity variables, and provider characteristics. A sensitivity analysis was performed limited to patients with Stage 2 (severe) hypertension. Tests were considered significant at p<0.05. The proportional-hazards assumption for each model was tested using a generalized linear regression of the scaled Schoenfeld residuals on functions of time [34].
RESULTS
Descriptive data
A total of 4362 patients met criteria for inclusion (Table 1). Patients with anxiety and/or depression comprised 13% (n=573) of the study population. In contrast to those without anxiety and/or depression, patients with these diagnoses were more often younger, female, not married, current or former tobacco users, and more likely to have ever received Medicaid benefits. Those with anxiety and/or depression also demonstrated higher healthcare utilization with more annual primary care and specialty visits and higher mean ACG scores than those without either condition. Study data was available for a mean (SD) of 23 (14) months. Patients with anxiety and/or depression had a shorter mean follow-up of 21 (14) months than patients without either condition, 23 (14) months (p<0.001). Among the patients identified as having anxiety and/or depression, 57% (n=327) had only anxiety coded per ICD-9 criteria and 24% (n=137) had only depression coded. The depression only group had higher healthcare utilization with 3.7 (2.3) primary care visits in the baseline year, compared to 1.8 (2.3) among the anxiety only group (p<0.001).
Table 1.
Baseline Demographics By Presence of Anxiety or Depression
| Total Population n=4362 | Diagnosis of Anxiety and/or Depression n=573 (13%) | No Diagnosis of Anxiety or Depression n=3789 (87%) | P value | |
|---|---|---|---|---|
| PATIENT CHARACTERISTICS | ||||
| Age, m (SD) | 52 (14) | 48 (14) | 52 (14) | <0.001 |
| Lowest age tertile, n (%) | 1522 (35) | 260 (45) | 1262 (33) | |
| Middle age tertile, n (%) | 1402 (32) | 181 (32) | 1221 (32) | <0.001 |
| Highest age tertile, n (%) | 1438 (33) | 132 (23) | 1306 (34) | |
| Male, n (%) | 2280 (52) | 236 (41) | 2044 (54) | <0.001 |
| Race/Ethnicity, n (%) | 0.41 | |||
| White | 3789 (87) | 504 (88) | 3285 (87) | |
| Non-White* | 573 (13) | 69 (12) | 504 (13) | |
| Marital Status, n (%) | <0.001 | |||
| Single/Divorced/Widowed | 1662 (38) | 289 (50) | 1373 (36) | |
| Married/Partnered | 2700 (62) | 284 (50) | 2416 (64) | |
| Primary Spoken Language, n (%) | 0.11 | |||
| English | 4083 (94) | 545 (95) | 3538 (93) | |
| Other | 279 (6.4) | 28 (4.9) | 251 (6.6) | |
| Tobacco Use, n (%) | <0.001 | |||
| Current Tobacco Use | 684 (16) | 107 (19) | 577 (15) | |
| Former Tobacco Use | 1051 (24) | 166 (29) | 885 (23) | |
| Never Used Tobacco | 2627 (60) | 300 (52) | 2327 (61) | |
| Body mass index, kg/m2, m (SD) | 32 (7.5) | 31 (7.7) | 32 (7.5) | 0.34 |
| BMI <25 kg/m2, n (%) | 612 (14) | 104 (18) | 508 (13) | |
| BMI 25–29 kg/m2, n (%) | 1246 (29) | 155 (27) | 1091 (29) | 0.01 |
| BMI ≥30 kg/m2, n (%) | 2504 (57) | 214 (55) | 2190 (58) | |
| On Medicaid ever†, n (%) | 399 (9.2) | 103 (18) | 296 (7.8) | <0.001 |
| JNC 7 Hypertension Stage‡, n (%) | <0.001 | |||
| Stage 1: 140–159/90–99 mmHg | 2604 (60) | 399 (70) | 2205 (58) | |
| Stage 2: 160–179/100–109 mmHg | 1758 (40) | 174 (30) | 1584 (42) | |
| Baseline Comorbid Conditions, n (%) | ||||
| Hyperlipidemia | 894 (21) | 139 (24) | 755 (20) | 0.02 |
| Diabetes mellitus | 273 (6.3) | 39 (6.8) | 234 (6.2) | 0.56 |
| ACG§ Score, young, m (SD) | 1.4 (1.3) | 1.8 (1.5) | 1.3 (1.3) | <0.001 |
| Lowest ACG tertile, n (%) | 1508 (35) | 127 (22) | 1381 (36) | |
| Middle ACG tertile, n (%) | 1400 (32) | 133 (23) | 1267 (33) | <0.001 |
| Highest ACG tertile, n (%) | 1454 (33) | 313 (55) | 1141 (30) | |
| Baseline Ambulatory Visit Counts, annual, (m, SD) | ||||
| Primary Care Visits | 2.0 (1.9) | 3.2 (2.5) | 1.8 (1.7) | <0.001 |
| Specialty Care Visits | 1.7 (2.3) | 2.0 (2.5) | 1.6 (2.3) | <0.001 |
| Urgent Care Visits | 0.39 (0.82) | 0.40 (0.82) | 0.39 (0.82) | 0.89 |
| PROVIDER CHARACTERISTICS | ||||
| Specialty Providing Majority of Ambulatory Care, n (%) | 0.64 | |||
| Primary Care|| | 3888 (89) | 514 (90) | 3374 (89) | |
| Specialty Care | 474 (11) | 59 (10) | 415 (11) | |
| Provider Age,¶ m (SD) | 46 (11) | 46 (11) | 46 (11) | 0.47 |
| Female Provider, n (%) | 1965 (45) | 289 (50) | 1676 (44) | 0.01 |
N, numerator, %, percent; m, mean; SD, standard deviation; BMI, body mass index, kg/m2, kilograms per meters squared
Non-White: Black (5.6%), Hispanic/Latino (2.1%), Asian (1.9%), Native Hawaiian/Pacific Islander (0.4%), American Indian/Alaska Native(0.3%); Unknown (2.7%)
On Medicaid at any point during the baseline or study period
JNC 7 Stage of Hypertension = severity of blood pressure elevation at study entry
ACG = Adjusted Clinical Group Case-Mix Assessment System
Primary Care = Family Medicine/Family Practice, Internal Medicine, Pediatrics/Adolescent Medicine, Obstetrics/Gynecology
AMA is the source for the raw physician data (provider ages only); statistics, tables, or tabulations were prepared by User-Customer (M.
Smith; PI: H. Johnson) using 2011 AMA Masterfile data.
Incident hypertension control rates
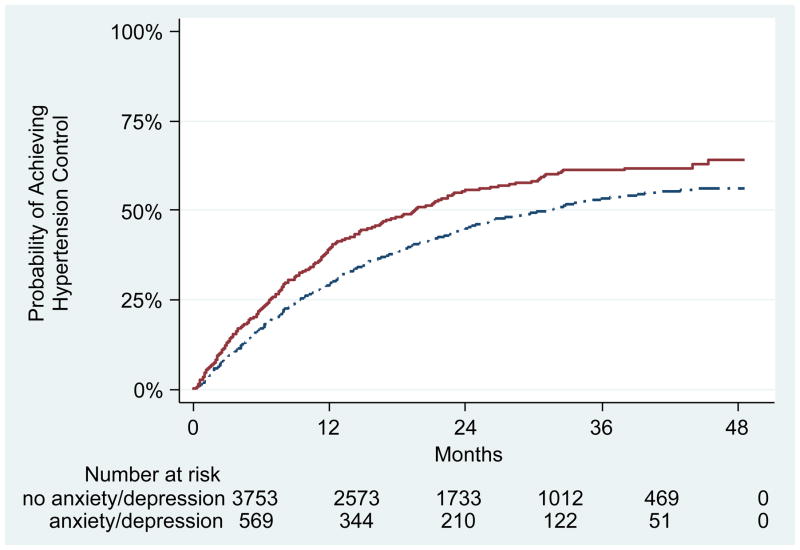
Overall, 367 (64%) of patients with anxiety and/or depression and 2121 (56%) without either diagnosis achieved hypertension control (Figure 2). Rates of hypertension control were highest during the initial 12 months after meeting incident hypertension criteria. Among those who achieved hypertension control, the mean (standard deviation) time to control was 9.6 (8.4) months for patients with anxiety and/or depression and 11.2 (9.4) months for patients without either diagnosis.
Figure 2.
Kaplan-Meier Analysis of Time to Hypertension Control
Predictors of time to hypertension control
In both unadjusted and adjusted multivariate Cox proportional hazards regression analyses (Table 2), patients with anxiety and/or depression had a faster rate of hypertension control than those without either diagnosis (HR 1.22; 1.07–1.39). Other factors associated with faster hypertension control include female gender (HR 1.32; 1.20–1.44), no history of tobacco use (HR 1.17; 1.03–1.33), ever receiving Medicaid (HR 1.27; 1.09–1.49), and a higher ACG Risk Score (HR 1.13; 1.10–1.17). No statistically significant relationship was found between time to hypertension control and provider characteristics. In additional analyses with visit frequency (not ACG score) in the model, visit frequency remained a significant predictor (HR 1.11; 1.09–1.13, p<0.001) for faster hypertension control rates (full model not shown).
Table 2.
Hazard Ratios and 95% CIs of Independent Predictors for Achieving Hypertension Control (≥18 years old; n=4362)
| Variable | Unadjusted HR (95% CI) | P value* | Adjusted HR (95% CI) | P value* |
|---|---|---|---|---|
|
Anxiety and/or Depression
|
1.32 (1.16–1.49) | <0.001 | 1.22 (1.07–1.39) | 0.003 |
| OTHER PATIENT FACTORS | ||||
| Age | ||||
| Lowest age tertile (reference) | 1.00– | – | ||
| Middle age tertile | 1.10 (0.98–1.23) | 0.09 | ||
| Highest age tertile | 1.15 (1.03–1.29) | 0.02 | ||
| Female | 1.32 (1.20–1.44) | <0.001 | ||
| Race/Ethnicity | ||||
| White | 1.00– | – | ||
| Non-White† | 0.95 (0.83–1.10) | 0.51 | ||
| Marital status | ||||
| Single (reference) | 1.00– | – | ||
| Married/Partnered | 1.05 (0.96–1.16) | 0.28 | ||
| Tobacco Use | ||||
| Current Tobacco Use (reference) | 1.00– | – | ||
| Former Tobacco Use | 1.13 (0.97–1.31) | 0.12 | ||
| Never Used Tobacco | 1.17 (1.03–1.33) | 0.02 | ||
| Body mass index, kg/m2 | ||||
| BMI <25 kg/m2 (reference) | 1.00– | – | ||
| BMI 25–29 kg/m2 | 1.00 (0.87–1.15) | 0.999 | ||
| BMI ≥30 kg/m2 | 0.92 (0.81–1.05) | 0.23 | ||
| On Medicaid Ever‡ | 1.27 (1.09–1.49) | 0.002 | ||
| ACG§ Risk Score, young index | 1.13 (1.10–1.17) | <0.001 |
HR, Hazard Ratio; CI, Confidence Interval; BMI, body mass index; kg/m2, kilograms per meters squared
Global p-value for proportional hazards assumption p=0.1687
Non-White: Black (5.6%), Hispanic/Latino (2.1%), Asian (1.9%), Native Hawaiian/Pacific Islander (0.4%), American Indian/Alaska Native (0.3%); Unknown (2.7%)
On Medicaid at any point during the baseline or study period
ACG = Adjusted Clinical Group Case-Mix Assessment System
Predictors of time to hypertension control in stage 2 hypertension
A subsequent analysis was performed limited to patients with Stage 2 hypertension (n=1758) to evaluate predictors of achieving hypertension control in patients with a greater severity of hypertension (Table 3). Patients with anxiety and/or depression continued to have a faster rate of hypertension control (HR 1.30; 1.01–1.66). Similar to our initial analysis, absence of tobacco use (HR 1.28; 1.04–1.58) and a higher ACG Risk Score (HR 1.12; 1.06–1.17) also predicted faster rates of hypertension control.
Table 3.
Hazard Ratios and 95% CIs of Independent Predictors for Achieving Hypertension Control in Stage 2 Hypertension Patients (≥18 years old; N=1758)
| Variable | Unadjusted HR (95% CI) | P value* | Adjusted HR (95% CI) | P value* |
|---|---|---|---|---|
|
Anxiety and/or Depression
|
1.35 (1.07–1.71) | 0.011 | 1.30 (1.01–1.66) | 0.04 |
| OTHER PATIENT FACTORS | ||||
| Age | ||||
| Lowest age tertile (reference) | 1.00– | – | ||
| Middle age tertile | 1.13 (0.94–1.36) | 0.19 | ||
| Highest age tertile | 1.14 (0.94–1.39) | 0.19 | ||
| Female | 1.15 (0.99–1.34) | 0.06 | ||
| Race/Ethnicity | ||||
| White (reference) | 1.00– | – | ||
| Non-White† | 0.93 (0.74–1.18) | 0.55 | ||
| Marital status | ||||
| Single (reference) | 1.00– | – | ||
| Married/Partnered | 1.16 (0.99–1.35) | 0.07 | ||
| Tobacco Use | ||||
| Current Tobacco Use (reference) | 1.00– | – | ||
| Former Tobacco Use | 1.08 (0.85–1.38) | 0.53 | ||
| Never Used Tobacco | 1.28 (1.04–1.58) | 0.02 | ||
| Body mass index, kg/m2 | ||||
| BMI <25 kg/m2 (reference) | 1.00– | – | ||
| BMI 25–29 kg/m2 | 0.91 (0.72–1.14) | 0.42 | ||
| BMI ≥30 kg/m2 | 0.87 (0.71–1.07) | 0.19 | ||
| On Medicaid Ever‡ | 1.21 (0.96–1.54) | 0.11 | ||
| ACG§ Risk Score, young index | 1.12 (1.06–1.17) | <0.001 |
HR, Hazard Ratio; CI, Confidence Interval; BMI, body mass index; kg/m2, kilograms per meters squared
Global p-value for proportional hazards assumption p= 0.1936
Non-White: Black (5.7%), Hispanic/Latino (1.7%), Asian (2.1%), Native Hawaiian/Pacific Islander (0.6%), American Indian/Alaska Native (0.4%); Unknown (3.5%)
On Medicaid at any point during the baseline or study period
ACG = Adjusted Clinical Group Case-Mix Assessment System
DISCUSSION
To our knowledge, this is the first U.S. report of rates and associations of time to incident hypertension control among patients with anxiety and/or depression. Our most significant finding is that hypertension is controlled at a faster rate in patients with anxiety and/or depression than in those without either diagnosis. Our findings are consistent with a prior U.S. study which demonstrated that hypertension is detected earlier in patients with anxiety and depression [14]. In addition, a cross-sectional study from the European PREDIMED clinical trial (Effects of the Mediterranean Diet on the Primary Prevention of Cardiovascular Diseases) likewise demonstrated that patients with depression had better hypertension control than patients without a depression diagnosis [35].
The results of our study highlight the complex relationship between hypertension management and mental health diagnoses. One reason for faster hypertension control rates is likely a higher rate of healthcare utilization among patients with mental health diagnoses. In our study, patients with anxiety and/or depression had higher mean ACG risk scores, a measure of healthcare utilization, and ACG remained an independent predictor for faster hypertension control rates. Our results suggest that greater healthcare utilization may be one mechanism by which anxiety and/or depression leads to faster hypertension control, but not the sole reason. It has been previously established that patients with mental health disorders visit healthcare providers more frequently [36] and have a higher economic burden of healthcare utilization than those without mental health disorders [37]. We showed that patients with anxiety and/or depression had significantly more primary and specialty care visits. This higher visit frequency likely results in more blood pressure measurements, supporting timely blood pressure follow-up. Additional analysis among patients with only anxiety ICD-9 codes compared to patients with only depression coded, surprisingly demonstrated greater primary care use among patients with depression. However, this analysis is limited since it has been previously documented that the two conditions commonly coexist [7–9], but not accurately reflected in administrative data. It has also been reported that patients with hypertension on antidepressant medications have lower blood pressures compared to those not on these medications [38], possibly due to decreased baroreflex sensitivity and altered neuro-endocrine pathways which may partially explain our findings [35]. It was not feasible for us to further evaluate this due to a lack of medication data (anxiety/depression medications and antihypertensives were not available).
Our multivariate analyses demonstrated that women had a significantly faster rate of hypertension control than men. This is consistent with prior studies showing that women are more likely than men to be treated for hypertension [39], and that men have a comparatively slower rate of antihypertensive medication initiation compared to women [40]. Patients without a history of tobacco use had faster hypertension control rates than current or former tobacco users. This finding is concerning because of the increased cardiovascular event risk associated with tobacco use [41,42].
One of the limitations of this study is the use of data from a single healthcare system, potentially limiting the generalizability of our findings. However, this healthcare system is one of the ten largest physician practices in the United States; in addition, our findings are in agreement with data from other healthcare systems within the U.S. and Europe [14,35]. Misclassification of hypertension or other comorbidities is a concern; however, the use of established algorithms decreases this risk. Our inclusion criteria, specifically the “currently managed” definition, may have resulted in a selection bias. Patients with more severe symptoms may have been excluded due to simultaneous use of multiple healthcare systems. The lack of ambulatory blood pressure data limits our ability to verify blood pressure control. Future studies assessing white coat and masked hypertension in this population would also be beneficial.
Conclusions
Patients with diagnoses of anxiety and/or depression demonstrated faster rates of incident hypertension control compared to patients without either mental health diagnosis. Greater healthcare utilization may contribute to timely blood pressure follow-up. Men and patients with current/former tobacco use demonstrated lower hypertension control rates, highlighting subpopulations that can be targeted to improve hypertension clinical care.
Acknowledgments
Sources of Funding: This original research was supported by the Clinical and Translational Science Award program, previously through the National Center for Research Resources (NCRR – UL1RR025011), and now by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR000427. Heather Johnson is supported by the National Heart, Lung, and Blood Institute of the NIH (K23HL112907), and also by the University of Wisconsin (UW) Centennial Scholars Program of the University of Wisconsin School of Medicine and Public Health. Christie Bartels is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (K23AR062381). Nancy Pandhi is supported by the National Institute on Aging of the NIH (K08AG029527). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Additional funding for this project was provided by the UW Health Innovation Program and the UW School of Medicine and Public Health from The Wisconsin Partnership Program.
The authors gratefully acknowledge Katie Ronk, BS and Patrick Ferguson, MPH for data preparation, and Jamie LaMantia, BS for manuscript preparation.
Footnotes
Prior Presentation: None
Conflicts of Interest: A. Ho, N. Pandhi, and H. Johnson have clinical appointments with the academic group practice that has a financial interest in delivering care to the general population from which study subjects were drawn. M. Palta is a consultant for the University of Pennsylvania and the Food and Drug Administration (FDA), and receives royalties from John Wiley. For the remaining authors, none were declared.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Ginty AT, Carroll D, Roseboom TJ, Phillips AC, de Rooij SR. Depression and anxiety are associated with a diagnosis of hypertension 5 years later in a cohort of late middle-aged men and women. J Hum Hypertens. 2013;27:187–190. doi: 10.1038/jhh.2012.18. [DOI] [PubMed] [Google Scholar]
- 4.Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30:842–851. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- 5.Axon RN, Zhao Y, Egede LE. Association of depressive symptoms with all-cause and ischemic heart disease mortality in adults with self-reported hypertension. Am J Hypertens. 2010;23:30–37. doi: 10.1038/ajh.2009.199. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Batty GD, Stamatakis E, Kivimaki M. The combined influence of hypertension and common mental disorder on all-cause and cardiovascular disease mortality. J Hypertens. 2010;28:2401–2406. doi: 10.1097/HJH.0b013e32833e9d7c. [DOI] [PubMed] [Google Scholar]
- 7.Lenze EJ, Mulsant BH, Shear MK, Schulberg HC, Dew MA, Begley AE, et al. Comorbid anxiety disorders in depressed elderly patients. Am J Psychiatry. 2000;157:722–728. doi: 10.1176/appi.ajp.157.5.722. [DOI] [PubMed] [Google Scholar]
- 8.Noyes R., Jr Comorbidity in generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:41–55. doi: 10.1016/s0193-953x(05)70205-7. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin TP, Khandker RK, Kruzikas DT, Tummala R. Overlap of anxiety and depression in a managed care population: Prevalence and association with resource utilization. J Clin Psychiatry. 2006;67:1187–1193. doi: 10.4088/jcp.v67n0803. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin RD, Davidson KW, Keyes K. Mental disorders and cardiovascular disease among adults in the United States. J Psychiatr Res. 2009;43:239–246. doi: 10.1016/j.jpsychires.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strik JJ, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. J Am Coll Cardiol. 2003;42:1801–1807. doi: 10.1016/j.jacc.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O’Connor C, Sketch MH. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2:000068. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JB, Powers JD, Magid DJ, Tavel HM, Schmittdiel JA, O’Connor PJ, et al. Detection and recognition of hypertension in anxious and depressed patients. J Hypertens. 2012;30:2293–2298. doi: 10.1097/HJH.0b013e328359b6e6. [DOI] [PubMed] [Google Scholar]
- 15.Nease DE, Jr, Volk RJ, Cass AR. Does the severity of mood and anxiety symptoms predict health care utilization? J Fam Pract. 1999;48:769–777. [PubMed] [Google Scholar]
- 16.Moise N, Davidson KW, Chaplin W, Shea S, Kronish I. Depression and clinical inertia in patients with uncontrolled hypertension. JAMA Intern Med. 2014;174:818–819. doi: 10.1001/jamainternmed.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatahet MA, Bowhan J, Clough EA. Wisconsin Collaborative for Healthcare Quality (WCHQ): lessons learned. WMJ. 2004;103:45–48. [PubMed] [Google Scholar]
- 18.Sheehy A, Pandhi N, Coursin DB, Flood GE, Kraft SA, Johnson HM, Smith MA. Minority status and diabetes screening in an ambulatory population. Diabetes Care. 2011;34:1289–1294. doi: 10.2337/dc10-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisconsin Collaborative for Health Care Quality. [Accessed January 5, 2015];WCHQ website. Available at: http://www.wchq.org/
- 20.Thorpe CT, Flood GE, Kraft SA, Everett CM, Smith MA. Effect of patient selection method on provider group performance estimates. Med Care. 2011;49:780–785. doi: 10.1097/MLR.0b013e31821b3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers MG, Tobe SW, McKay DW, Bolli P, Hemmelgarn BR, McAlister FA. New algorithm for the diagnosis of hypertension. Am J Hypertens. 2005;18:1369–1374. doi: 10.1016/j.amjhyper.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, et al. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension. 2011;57:717–722. doi: 10.1161/HYPERTENSIONAHA.110.168195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, et al. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens. 2014;32:65–74. doi: 10.1097/HJH.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu K, Chen Z, Lipscombe LL. Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. Canadian Medical Association Journal. 2008;178:1429–1435. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–26. [PMC free article] [PubMed] [Google Scholar]
- 26.Manson JM, McFarland B, Weiss S. Use of an automated database to evaluate markers for early detection of pregnancy. Am J Epidemiol. 2001;154:180–187. doi: 10.1093/aje/154.2.180. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163:2677–2678. doi: 10.1001/archinte.163.22.2677. [DOI] [PubMed] [Google Scholar]
- 28.Marciniak MD, Lage MJ, Dunayevich E, Russell JM, Bowman L, Landbloom RP, Levine LR. The cost of treating anxiety: the medical and demographic correlates that impact total medical costs. Depress Anxiety. 2005;21:178–184. doi: 10.1002/da.20074. [DOI] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Carroll D, Phillips AC, Gale CR, Batty GD. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med. 2010;72:16–19. doi: 10.1097/PSY.0b013e3181c4fca1. [DOI] [PubMed] [Google Scholar]
- 31.Campbell NR, So L, Amankwah E, Quan H, Maxwell C. Characteristics of hypertensive Canadians not receiving drug therapy. Can J Cardiol. 2008;24:485–490. doi: 10.1016/s0828-282x(08)70623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starfield B, Weiner J, Mumford L, Steinwachs D. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res. 1991;26:53–74. [PMC free article] [PubMed] [Google Scholar]
- 33.StataCorp. STATA Survival Analysis and Epidemiological Tables Reference Manual: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 34.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 35.Mejia-Lancheros C, Estruch R, Martinez-Gonzalez MA, Salas-Salvado J, Corella D, Gomez-Gracia E, et al. Blood pressure values and depression in hypertensive individuals at high cardiovascular risk. BMC Cardiovasc Disord. 2014;14:109. doi: 10.1186/1471-2261-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21:398–407. doi: 10.3122/jabfm.2008.05.070082. [DOI] [PubMed] [Google Scholar]
- 37.Thomas MR, Waxmonsky JA, Gabow PA, Flanders-McGinnis G, Socherman R, Rost K. Prevalence of psychiatric disorders and costs of care among adult enrollees in a Medicaid HMO. Psychiatr Serv. 2005;56:1394–1401. doi: 10.1176/appi.ps.56.11.1394. [DOI] [PubMed] [Google Scholar]
- 38.Dawood T, Lambert EA, Barton DA, Laude D, Elghozi JL, Esler MD, et al. Specific serotonin reuptake inhibition in major depressive disorder adversely affects novel markers of cardiac risk. Hypertens Res. 2007;30:285–293. doi: 10.1291/hypres.30.285. [DOI] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 40.Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, et al. Antihypertensive medication initiation among young adults with regular primary care use. J Gen Intern Med. 2014;29:723–731. doi: 10.1007/s11606-014-2790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodwin RD, Zvolensky MJ, Keyes KM, Hasin DS. Mental disorders and cigarette use among adults in the United States. Am J Addict. 2012;21:416–423. doi: 10.1111/j.1521-0391.2012.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rigotti NA, Clair C. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J. 2013;34:3259–3267. doi: 10.1093/eurheartj/eht352. [DOI] [PubMed] [Google Scholar]