Abstract
♦ Background:
The impact of timing of dialysis initiation on mortality is controversial in patients with peritoneal dialysis (PD). In this study, we analyzed the impact of timing of dialysis initiation on mortality in the incident PD population.
♦ Methods:
Incident patients with PD were selected from the Clinical Research Center (CRC) registry for end-stage renal disease (ESRD), a prospective cohort study on dialysis in Korea. Patients were categorized into 3 groups according to the estimated glomerular filtration rate (eGFR) at the initiation of PD using the Modification of Diet in Renal Disease (MDRD) equation. Group A was defined as eGFR < 5 mL/min/1.73m2, group B as eGFR 5 – 10 mL/min/1.73m2, and group C as eGFR > 10 mL/min/1.73m2. Cox regression analysis was used to calculate the adjusted hazard ratio (HR) of mortality with group B as the reference. The primary outcome was all-cause mortality.
♦ Results:
A total of 495 incident PD patients were included. The number of patients in group A was 109, group B was 279, and group C was 107. The median follow-up period was 23 months. Multivariate Cox regression analysis showed that group A had a significantly higher risk of all-cause mortality compared with group B (HR 4.13, 95% confidence interval [CI], 1.55 – 11.03, p = 0.005) after adjustment for age, gender, cause of ESRD, serum albumin level, diabetes mellitus, and cardiovascular disease. There was no significant difference in mortality between group C and group B (HR 1.50, 95% CI, 0.59 – 3.80, p = 0.398) after adjustment for clinical variables.
♦ Conclusion:
An eGFR < 5 mL/min/1.73m2 at the initiation of PD was a significant risk factor for death, while an eGFR >10 mL/min/1.73m2 at the initiation of PD was not associated with improved survival compared with an eGFR of 5 – 10 mL/min/1.73m2 at the initiation of PD.
Keywords: Peritoneal dialysis, end-stage renal disease, glomerular filtration rate, mortality, survival
Initiating dialysis at the optimal time is one of the most important prognostic factors in patients with end-stage renal disease (ESRD). Current guidelines on when to initiate renal replacement therapy are based on symptoms or signs of uremia and malnutrition, as well as the glomerular filtration rate (GFR) (1–4). Most of these guidelines are primarily based on clinical evidence of uremia and malnutrition. The ideal GFR level for dialysis initiation in asymptomatic patients has not been fully clarified.
Some observational studies have demonstrated that early dialysis, defined as an estimated GFR (eGFR) > 10 mL/min/1.73m2 at the time of dialysis initiation, was associated with a decrease in mortality and complication rates (4–9), whereas other observational studies demonstrated that early dialysis had no survival benefit (10,11) or was harmful (12–17).
Recently, a randomized controlled trial, the Initiating Dialysis Early and Late (IDEAL) study, demonstrated that a planned early dialysis initiation (eGFR 10 – 14 mL/min/1.73m2) was not associated with improvement in survival or clinical outcomes compared with late dialysis initiation (eGFR 5 – 7 mL/min/1.73m2) (18,19).
Peritoneal dialysis (PD) has different potential survival factors from hemodialysis (HD), such as residual renal function, peritonitis, peritoneal protein loss, peritoneal membrane transport status and high glucose load (20–22). Furthermore, because residual renal function is an important determinant of mortality and is better preserved in patients on PD than those on HD (9), the impact of timing of dialysis initiation on survival may be different from that in HD.
The association of timing of PD initiation with survival is controversial. Some observational studies demonstrated that early PD initiation had a survival advantage (7,23), whereas other observational studies demonstrated that early PD initiation is associated with increased mortality (24) or equivalent mortality compared with late PD initiation (25). Subgroup analysis of the IDEAL study showed that early PD initiation was associated with clinical outcomes comparable to those in late PD initiation (26). This discrepancy may be due to differences in the study design or the populations of the studies.
We focused on PD patients with very late dialysis initiation, defined as an eGFR < 5 mL/min/1.73m2 at the time of PD initiation. Although the European Best Practice Guideline (EBPG) recommends that dialysis should be initiated before GFR falls before 6 mL/min/1.73 m2 (3), wide variation in the timing of dialysis initiation exists in different countries and periods. In actual clinical practice, 14 – 60% of patients with ESRD initiate dialysis at an eGFR less than 5 mL/min/1.73m2 (14,20,21).
The impact of very late dialysis initiation on mortality is not well established. Because loss of residual renal function is a risk factor for mortality in PD patients, the impact of very late dialysis initiation on mortality may be different from late dialysis initiation or early dialysis initiation. However, in previous studies, patients with very late PD initiation were not included in study populations (18,26) or were simply included in the late PD initiation group (25).
In this study, we investigated the impact of timing of PD initiation on survival, categorizing patients into 3 groups based on the eGFR at the time of PD initiation in the Clinical Research Center (CRC) registry for the ESRD cohort in Korea: group A, eGFR < 5 mL/min/1.73m2; group B, eGFR 5 – 10 mL/min/1.73m2; and group C, eGFR > 10 mL/min/1.73m2.
Methods
Study Poppulations
All patients in this study participated in the CRC registry for ESRD. This study is an ongoing observational prospective cohort study in patients with ESRD from 31 centers in Korea. The cohort started in April 2009 and included adult (> 18 years of age) dialysis patients. A total of 1,681 patients started PD during the study period. Five hundred and three patients gave informed consent and were included in this prospective cohort. Of the 503 incident PD patients, 13 patients whose eGFR could not be ascertained at enrollment were excluded for the present analysis, and the remaining 495 incident PD patients were included in the analysis.
Demographic data and clinical data were collected at enrollment. Assessment of dialysis characteristics and measurements of health were performed every 6 months until the end of follow-up. Dates and causes of mortality were immediately reported through the follow-up period. The CRC registry for ESRD was approved by the medical ethics committees of all of the participating hospitals and informed consent was obtained from all patients before inclusion.
Baseline demographic data and clinical data included age, gender, height, weight, body mass index (BMI), causes of ESRD, co-morbidities, laboratory investigations and therapeutic characteristics. Cardiovascular disease was defined as the presence of coronary artery disease, congestive heart failure, peripheral vascular disease or cerebrovascular disease. Serum hemoglobin, total cholesterol, albumin, creatinine, and urea were determined from blood samples. In all clinical laboratories of the 31 centers that participated in the study, serum creatinine levels were measured using the kinetic alkaline picrate method (Jaffe method), standardized against isotope dilution-mass spectrometry. The eGFR was calculated at the time of dialysis initiation using the Modification of Diet in Renal Disease (MDRD) 4-variable equation: eGFR = 186 × serum creatinine-1.154 × age-0.203 × 1.212 (if black) × 0.742 (if female) (27).
Incident PD patients were categorized into 3 groups according to eGFR at the time of initiation of dialysis, as follows: group A, eGFR < 5 mL/min/1.73m2; group B, eGFR 5 – 10 mL/min/1.73m2; group C, eGFR > 10 mL/min/1.73m2.
Outcomes
The clinical outcome of this study was all-cause mortality. The primary outcome was mortality. All patients were followed until death or the end of the study, with censoring of data at the time a patient underwent renal transplantation or was lost to follow-up because of patient's refusal of further participation or patient's transfer to a nonparticipating hospital. For each death, the clinical center's principal investigators completed a form that included the cause of death according to the CRC for ESRD study classification.
Statistical Analysis
Data with continuous variables and normal distributions are presented as mean ± standard deviation (SD) and those without normal distribution are presented as median with ranges as appropriate for the type of variable. Student t-test, Mann-Whitney test, one-way ANOVA test, or Kruskal-Wallis test were used, as appropriate, to determine the differences in continuous variables. Categorical variables are presented as percentages. Pearson's chi-square test or Fisher's exact test was used to determine the differences in categorical variables.
Absolute mortality rates were calculated per 100 person-years of follow-up. The survival curves for groups A, B, and C were estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazard regression model was used to calculate the hazard ratio (HR) with 95% confidence interval (CI) for all-cause mortality, using group B as the reference category. The assumption of proportional hazards over time was assessed by visual inspection of a log-minus-log survival plot. Analyses were adjusted for potential confounders using 3 models. Model 1 was adjusted for age, gender and cause of ESRD. Model 2 was adjusted for age, gender, cause of ESRD, and serum albumin level. Model 3 was adjusted for age, gender, cause of ESRD, serum albumin level, diabetes mellitus, and cardiovascular diseases. A value of p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
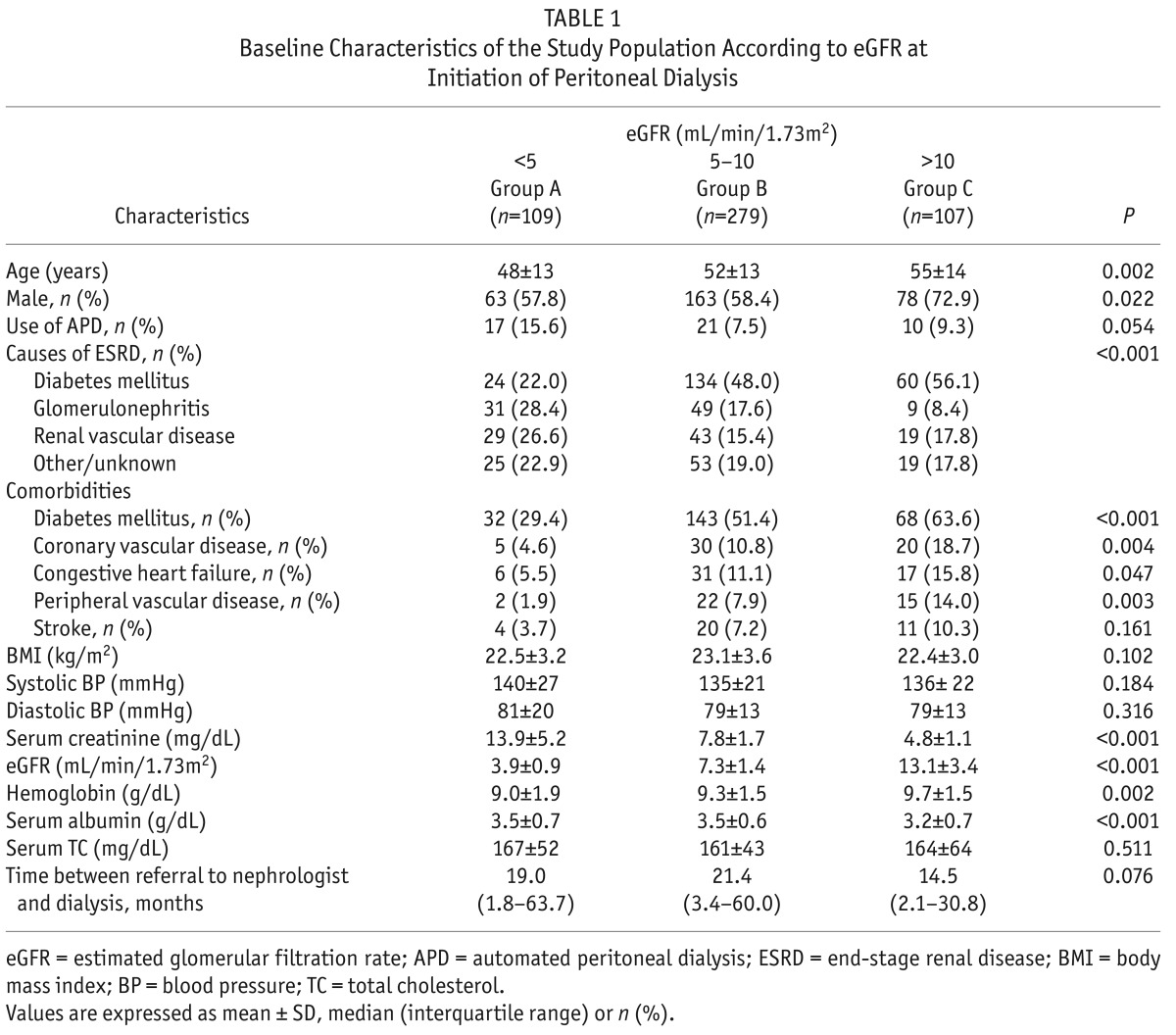
A total of 495 incident PD patients were included in the analysis. The mean age of patients was 52 ± 8 years and the mean eGFR was 7.8 ± 3.7 mL/min/1.73m2. The main causes of ESRD were diabetes (44%), renal vascular disease (18%), and glomerulonephritis (18%). Baseline characteristics of the study population according to eGFR at initiation are shown in Table 1. The number of patients in group A was 109 (22%), group B was 279 (56%) and group C was 107 (22%). The mean eGFR at the time of PD initiation ranged from 3.9 ± 0.9 mL/min/1.73m2 for patients in group A to 7.3 ± 1.4 mL/min/1.73m2 for patients in group B and 13.1 ± 3.4 mL/min/1.73m2 for patients in group C. Patients in group C were older and a higher percentage of patients were male compared to patients in group A. The use of automated PD was not significantly different among the groups. Among causes of ESRD, diabetes was more common in group C, while glomerulonephritis was more common in group A. There was a significant difference in the prevalence of the comorbidities among the groups. Diabetes, coronary vascular disease, congestive heart failure, and peripheral vascular disease were more prevalent in group C. There was no difference in body mass index, systolic blood pressure, or serum total cholesterol levels among the groups. The patients in group C had higher hemoglobin levels and lower serum albumin levels than those in groups A and B.
TABLE 1.
Baseline Characteristics of the Study Population According to eGFR at Initiation of Peritoneal Dialysis
All-Cause Mortality by eGFR at the Time of PD Initiation
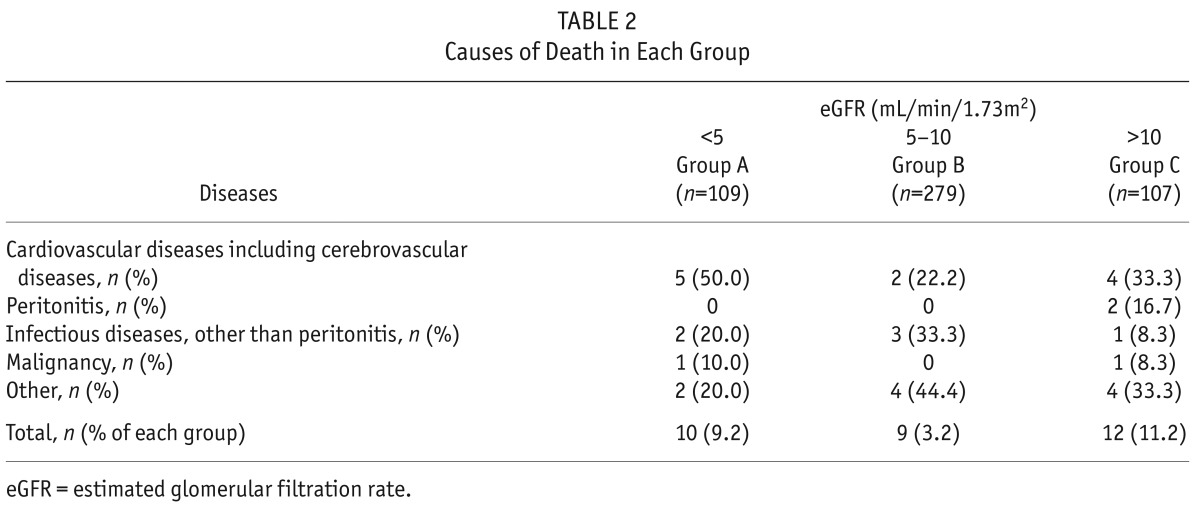
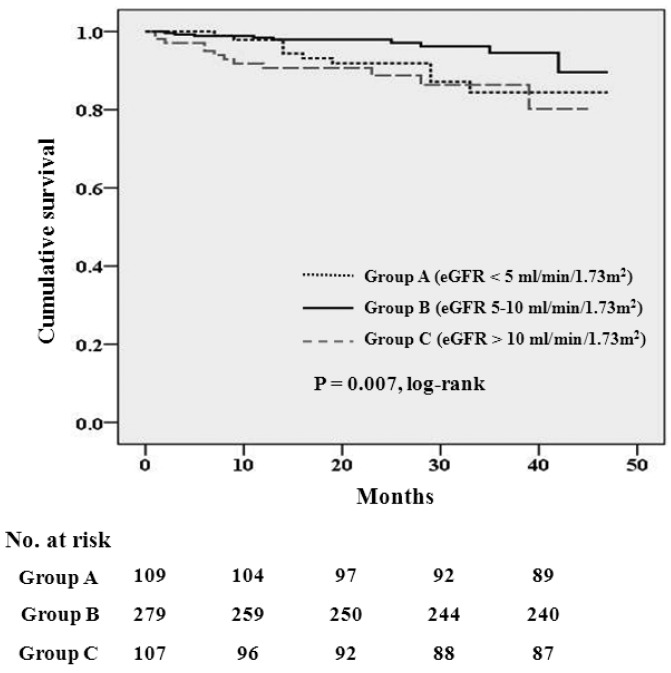
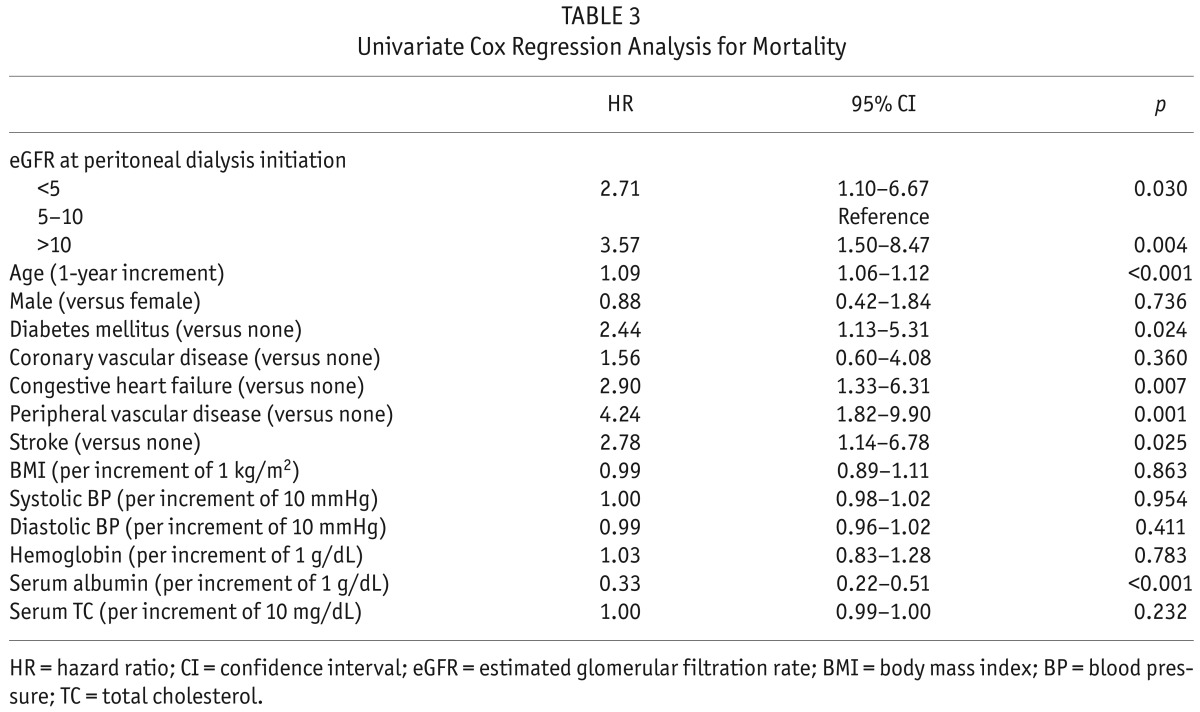
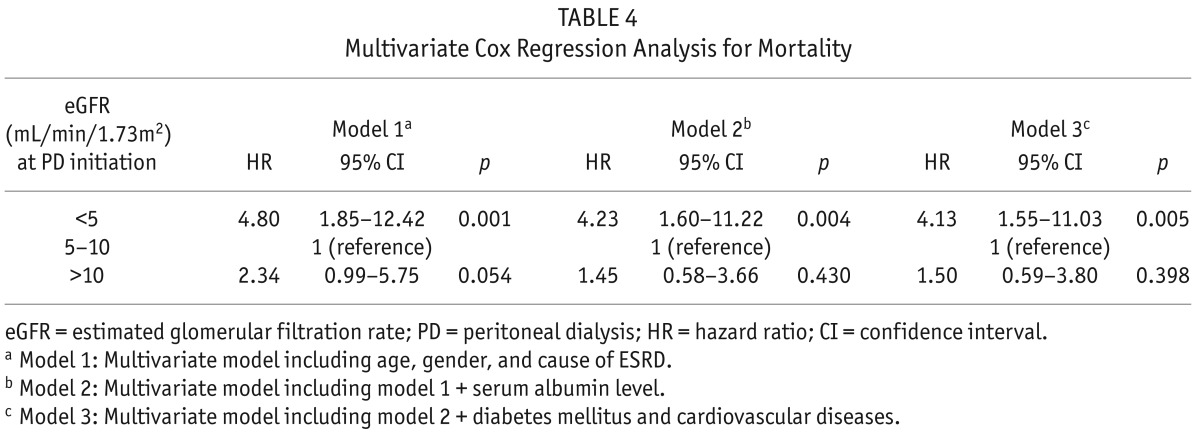
The median follow-up period was 23 months (inter-quartile range, 12 – 33 months). During the follow-up period, 51 patients left the study. The reasons for censoring included kidney transplantation (n = 24), transfer to a nonparticipating hospital (n =17), refusal of further participation (n = 3), or other (n = 7). There were 31 deaths during the follow-up period. The leading causes of death were cardiovascular diseases (36% of all deaths) and infectious diseases including peritonitis (26% of all deaths). Table 2 shows the causes of death in each group. There was no significant difference in the causes of death among the 3 groups (p = 0.585). The absolute mortality rate was 3.3 deaths per 100 person-years. Figure 1 shows the Kaplan-Meier plot of patient survival according to eGFR group at the time of PD initiation. As shown, survival was decreased in patients in both groups A and C compared to those in group B (log-rank test, p = 0.007). Table 3 shows the univariate Cox regression analysis for mortality. The hazard ratio (HR) for all-cause mortality of group C was 3.57 (95% CI, 1.50 – 8.47, p = 0.004) and the HR for mortality of group A was 2.71 (95% CI, 1.10 – 6.67, p = 0.030), using group B as the reference category. All-cause mortality was positively correlated with older age, presence of diabetes, congestive heart failure, peripheral vascular disease, and stroke, and was negatively correlated with serum albumin level. To determine whether eGFR at PD initiation is an independent predictor of all-cause mortality, multivariate Cox regression analysis was performed. As shown in Table 4, group A had significantly higher risk for all-cause mortality than group B (the reference category) in model 1 (HR 4.80, 95% CI, 1.85 – 12.42, p = 0.001), model 2 (HR 4.23, 95% CI, 1.60 – 11.22, p = 0.004), and model 3 (HR 4.13, 95% CI, 1.55 – 11.03, p = 0.005), implying that initiation of PD at eGFR < 5 mL/min/1.73m2 was an independent risk factor for death even after adjusting for demographics, laboratory data, and comorbid conditions. However, survival was not significantly different between group C and group B (the reference category) in model 1 (HR 2.34, 95% CI, 0.99 – 5.75, p = 0.054), in model 2 (HR 1.45, 95% CI, 0.58 – 3.66, p = 0.430), and model 3 (HR 1.50, 95% CI, 0.59 – 3.80, p = 0.398) implying that initiation of PD at eGFR > 10 mL/min/1.73m2 was not independently associated with mortality after adjusting for clinical variables.
TABLE 2.
Causes of Death in Each Group
Figure 1 —
Kaplan-Meier survival curve for mortality according to the estimated glomerular filtration rate at the time of peritoneal dialysis initiation (p = 0.007 by log-rank test). eGFR = estimated glomerular filtration rate; No. = number.
TABLE 3.
Univariate Cox Regression Analysis for Mortality
TABLE 4.
Multivariate Cox Regression Analysis for Mortality
Discussion
In this prospective observational study investigating the impact of timing of PD initiation on survival, we demonstrated that an eGFR < 5 mL/min/1.73m2 at PD initiation was associated with an increase in mortality, while an eGFR > 10 mL/min/1.73m2 at PD initiation was not associated with mortality when compared to an eGFR of 5 – 10 mL/min/1.73m2 at PD initiation. Our findings suggest that very late PD initiation (at eGFR < 5 mL/min/1.73m2) may be associated with increased risk of mortality.
There is a considerable number of patients with ESRD that initiate PD at eGFR < 5 mL/min/1.73m2 in clinical practice. In our study, 22.0% of patients initiated PD at eGFR < 5 mL/min/1.73 m2, and studies from other countries show a similar pattern. Twenty-three percent of patients with ESRD in the US, 13.6% in France, and 60.1% in China initiate dialysis at an eGFR < 5 mL/min/1.73 m2 (14,28,29).
The prognosis of the patients who started dialysis at an eGFR < 5 mL/min/1.73 m2 remains controversial. Lassalle et al. reported that the 2-year crude survival in patients with an eGFR < 5 mL/min/1.73 m2 at the time of dialysis initiation was higher than those with an eGFR > 20 mL/min/1.73 m2 (79% and 46%, respectively) (14). Kazmi et al. also reported that mortality in patients who initiated dialysis at an eGFR < 5 mL/min/1.73 m2 was lower than the mortality among those who initiated dialysis at an eGFR 5 – 7.5 mL/min/1.73 m2, eGFR 7.6 – 10 mL/min/1.73 m2, or eGFR > 10 mL/min/1.73 m2 (17). Yamagata et al. reported that long-term survival was not different in patients initiating dialysis at an eGFR less than 4 mL/min/1.73 m2 compared to a reference category (4 – 6 mL/min/1.73 m2) (30). However, in these studies, the modality of renal replacement therapy was predominantly HD and the proportion of PD was relatively small (3 – 11.7%), which could have made it difficult to determine the prognosis in PD patients. In subgroup analysis of the IDEAL study, mortality and clinical outcomes were not significantly different between early and late PD initiation (26). However, there was no assignment to a very late group (eGFR < 5 mL/min/1.73 m2) in the IDEAL study; therefore, the impact of PD initiation at an eGFR < 5 mL/min/1.73 m2 on survival remains unclear.
Our results demonstrating increased mortality in group A (eGFR < 5 mL/min/1.73m2 at the initiation of PD) are consistent with the Canadian-US Peritoneal Dialysis Study (CANUSA). In the reanalysis of the CANUSA study, each 5 L/week/1.73 m2 increment of GFR was associated with a 12% decrease in relative risk for 2-year mortality (8).
However, our results are inconsistent with previous studies that included mainly HD populations, which reported that late start of dialysis has a survival benefit (14,17).
The reason for increased mortality in group A (eGFR < 5 mL/min/1.73m2 at the initiation of PD) compared with group B (eGFR 5 – 10 mL/min/1.73m2 at the initiation of PD) in our study is unclear. It may be due to differences in the study design or in the populations studied. Unlike previous studies that included mainly HD populations (14,17,30), we included only PD patients. From large, registry-based studies, PD has been reported to have an early survival advantage over HD (31). The early survival advantage in PD patients compared with HD patients may be attributable, in part, to better preservation of residual renal function (32). Furthermore, the effect of the loss of GFR on mortality may be higher in patients initiating PD compared to patients initiating HD (33). Therefore, the very low residual renal function in very late PD initiation may have more of an influence on mortality than that in very late initiation of HD (20).
The results of our study are inconsistent with a previous study by Shiao et al. that reported that the initiation of PD at an eGFR < 5 mL/min/1.73 m2 had a survival benefit compared to PD initiation at an eGFR ≥ 5 mL/min/1.73 m2 (24). There are some differences in study design between our study and their study. Our study is a multicenter, prospective cohort study, while their study was a single-center, retrospective study. We categorized PD patients into 3 groups (eGFR >10 mL/min/1.73m2 vs eGFR 5 – 10 mL/min/1.73m2 vs eGFR < 5 mL/min/1.73m2), while their study categorized PD patients into 2 groups (eGFR ≥ 5 mL/min/1.73 m2 vs eGFR < 5 mL/min/1.73 m2). Randomized controlled trials are needed to further elucidate the association between very late PD initiation and mortality in ESRD patients.
Another interesting finding of our study was the absence of a significant difference in mortality between group C (eGFR > 10 mL/min/1.73m2 at the initiation of PD) and group B (eGFR 5 – 10 mL/min/1.73m2 at the initiation of PD) in the multivariate Cox regression analysis after adjustment for confounding covariates, whereas mortality in group C was increased compared to group B in the unadjusted model. These findings may be explained by the adjustment factors such as age and comorbidities. In our study, patients in group C were older and had higher comorbidities compared to patients in group B (Table 1). The statistically significantly higher HR for mortality in group C compared to group B in the unadjusted model was reduced to non-significant after adjustment for age and comorbidities. These results are similar with those of a Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) study reporting that the patients more likely to begin dialysis at a higher eGFR were older and had higher comorbidities, which may explain the inverse crude association between eGFR and survival (14). These findings are also consistent with the subgroup analysis of the IDEAL study (26), which supports the idea that the initiation of PD may be delayed until eGFR 5 – 10 mL/min/1.73m2 with careful clinical management unless patients with chronic kidney disease stage V have traditional clinical indications for the initiation of dialysis.
The strengths of our study were the relatively large number of PD patients and the multi-center design, which involved patients from 31 centers in Korea. Our study has some potential limitations. First, our study was an observational study and patients were not randomly allocated to the 3 groups. Therefore, this study may have been limited by selection bias. There were differences in the baseline characteristics such as age or comorbidities, among the study groups. Despite careful adjustment for clinical variables, unknown confounding variables may not have been adequately adjusted for in the multivariable analyses (14). Survival bias may also be considered in the interpretation of results of our study. Some patients in particularly poor condition might have died before starting dialysis. Therefore, there is a possibility that patients at lower risk for death might be over-represented in group A compared to group B or C. However, group A had significantly higher risk for all-cause mortality than group B in our study. This type of bias could not explain our results.
Given the observational nature of our study, “center effects” due to different treatment policies among the centers could also have influenced the results of our study. To evaluate the center effect, we compared mortality with and without adjusting for center. No significant center effect on mortality was observed (data not shown), which suggests that the center effect was not significant in our study.
Second, lead-time bias should be considered in the interpretation of the results of our study. As described in the NECOSAD study (11), the decreased survival in group A compared with group B in our study may be influenced by this bias because patients in group A had their observed survival timed from a later period in the natural history of their kidney disease compared with group B. Furthermore, patients were not observed from the same GFR level at the time of initiation of PD in our study. This might mean that group A had been exposed to uremic toxins for longer and this might influence their worse survival. Therefore, further randomized controlled trials looking at patients from the same GFR level is needed to confirm worse survival in group A. However, this randomized controlled trial is not likely to be feasible because many of the patients will be symptomatic and need to start dialysis at eGFR > 5 mL/min/1.73m2.
Third, late referral to a dialysis unit is independently associated with increased mortality and this may be over-represented in the patients with late start dialysis. However, there was no significant difference in time from referral to dialysis among the groups in our study (Table 1). Therefore, we cautiously assert that the referral time to dialysis did not influence the results of our study. Fourth, eGFR was calculated using the MDRD equation in our study. Although many previous studies, including the IDEAL study, used eGFR calculated by the MDRD equation to define early or late dialysis initiation (18), the NECOSAD study reported that the association between eGFR at dialysis initiation and mortality may be primarily influenced by muscle mass (34). Measurement of GFR from timed urine collections or eGFR based on serum cystatin C may be helpful to more precisely clarify the association between the timing of dialysis initiation and mortality. Fifth, in spite of the multicenter nature of our study, the cohort consisted of Korean patients and all were Asian. Thus, it is uncertain whether our results can be generalized to other ethnic groups. Sixth, the relatively low mortality rate of our study may have resulted in a lack of difference in survival between the group with eGFR > 10 mL/min/1.73m2 and the group with eGFR 5 – 10 mL/min/1.73m2 due to a type 2 statistical error.
In conclusion, very late PD initiation (eGFR < 5 mL/min/1.73 m2 at the initiation of PD) was independently associated with increased mortality, while early PD initiation (eGFR > 10 mL/min/1.73m2 at the initiation of PD) had equivalent survival with patients who initiated PD with an eGFR 5 – 10 mL/min/1.73m2.
Disclosures
The authors have no financial conflict of interest to declare.
Acknowledgments
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020). We thank the study coordinators Hye Young Lim, Nam Hee Kim, Mi Joung Moon, Hwa Young Lee, Mi Joung Kwon, Su Yeon An, Su Joung Oh and Hye Young Kwak for their contributions to this study.
REFERENCES
- 1. Hemodialysis Adequacy 2006 Work Group Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 2006; 48(Suppl 1):S2–90. [DOI] [PubMed] [Google Scholar]
- 2. Peritoneal Dialysis Adequacy 2006 Work Group Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis 2006; 48(Suppl 1):S91–7. [DOI] [PubMed] [Google Scholar]
- 3. Tattersall J, Dekker F, Heimbürger O, Jager KJ, Lameire N, Lindley E, et al. ERBP Advisory Board. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transplant 2011; 26:2082–6. [DOI] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 5. Bonomini V, Feletti C, Scolari MP, Stefoni S. Benefits of early initiation of dialysis. Kidney Int Suppl 1985; 17:S57–9. [PubMed] [Google Scholar]
- 6. Tattersall J, Greenwood R, Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol 1995; 15:283–9. [DOI] [PubMed] [Google Scholar]
- 7. Canada-USA (CANUSA) Peritoneal Dialysis Study Group Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol 1996; 7:198–207. [DOI] [PubMed] [Google Scholar]
- 8. Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–62. [DOI] [PubMed] [Google Scholar]
- 9. Churchill DN. An evidence-based approach to earlier initiation of dialysis. Am J Kidney Dis 1997; 30:899–906. [DOI] [PubMed] [Google Scholar]
- 10. Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol 2002; 13:2125–32. [DOI] [PubMed] [Google Scholar]
- 11. Korevaar JC, Jansen MA, Dekker FW, Jager KJ, Boeschoten EW, Krediet RT, et al. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet 2001; 358:1046–50. [DOI] [PubMed] [Google Scholar]
- 12. Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 2010; 5:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med 2011; 171:396–403. [DOI] [PubMed] [Google Scholar]
- 14. Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int 2010; 77:700–7. [DOI] [PubMed] [Google Scholar]
- 15. Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC, Taiwan Society of Nephrology Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant 2010; 25:2616–24. [DOI] [PubMed] [Google Scholar]
- 16. Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol 2003; 14:2305–12. [DOI] [PubMed] [Google Scholar]
- 17. Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis 2005; 46:887–96. [DOI] [PubMed] [Google Scholar]
- 18. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–19. [DOI] [PubMed] [Google Scholar]
- 19. Wong MG, Pollock CA, Cooper BA, Branley P, Collins JF, Craig JC, et al. Association between GFR estimated by multiple methods at dialysis commencement and patient survival. Clin J Am Soc Nephrol 2014; 9:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang AY. The “heart” of peritoneal dialysis. Perit Dial Int 2007; 27(Suppl 2):S228–32. [PubMed] [Google Scholar]
- 21. Krediet RT, Balafa O. Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 2010; 6:451–60. [DOI] [PubMed] [Google Scholar]
- 22. Pérez-Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274–84. [PubMed] [Google Scholar]
- 23. Tang SC, Ho YW, Tang AW, Cheng YY, Chiu FH, Lo WK, et al. Hong Kong Peritoneal Dialysis Study Group. Delaying initiation of dialysis till symptomatic uraemia—is it too late? Nephrol Dial Transplant 2007; 22:1926–32. [DOI] [PubMed] [Google Scholar]
- 24. Shiao CC, Huang JW, Chien KL, Chuang HF, Chen YM, Wu KD. Early initiation of dialysis and late implantation of catheters adversely affect outcomes of patients on chronic peritoneal dialysis. Perit Dial Int 2008; 28:73–81. [PubMed] [Google Scholar]
- 25. Oh KH, Hwang YH, Cho JH, Kim M, Ju KD, Joo KW, et al. Outcome of early initiation of peritoneal dialysis in patients with end-stage renal failure. J Korean Med Sci 2012; 27:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson DW, Wong MG, Cooper BA, Branley P, Bulfone L, Collins JF, et al. Effect of timing of dialysis commencement on clinical outcomes of patients with planned initiation of peritoneal dialysis in the IDEAL trial. Perit Dial Int 2012; 32:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–70. [DOI] [PubMed] [Google Scholar]
- 28. Obrador GT, Arora P, Kausz AT, Ruthazer R, Pereira BJ, Levey AS. Level of renal function at the initiation of dialysis in the U.S. end-stage renal disease population. Kidney Int 1999; 56:2227–35. [DOI] [PubMed] [Google Scholar]
- 29. Liu H, Peng Y, Liu F, Xiao H, Chen X, Huang A, Liu Y. Renal function and serum albumin at the start of dialysis in 514 Chinese ESRD in-patients. Ren Fail 2008; 30:685–90. [DOI] [PubMed] [Google Scholar]
- 30. Yamagata K, Nakai S, Masakane I, Hanafusa N, Iseki K, Tsubakihara Y, Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy Ideal timing and predialysis nephrology care duration for dialysis initiation: from analysis of Japanese dialysis initiation survey. Ther Apher Dial 2012; 16:54–62. [DOI] [PubMed] [Google Scholar]
- 31. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009; 53:1068–81. [DOI] [PubMed] [Google Scholar]
- 33. van der Wal WM, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT, Korevaar JC, et al. Netherlands Cooperative Study on the Adequacy of Dialysis Study Group (NECOSAD) Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant 2011; 26:2978–83. [DOI] [PubMed] [Google Scholar]
- 34. Grootendorst DC, Michels WM, Richardson JD, Jager KJ, Boeschoten EW, Dekker FW, et al. NECOSAD Study Group. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant 2011; 26:1932–37. [DOI] [PubMed] [Google Scholar]