Abstract
♦ Background:
The HONEYPOT study recently reported that daily exit-site application of antibacterial honey was not superior to nasal mupirocin prophylaxis for preventing overall peritoneal dialysis (PD)-related infection. This paper reports a secondary outcome analysis of the HONEYPOT study with respect to exit-site infection (ESI) and peritonitis microbiology, infectious hospitalization and technique failure.
♦ Methods:
A total of 371 PD patients were randomized to daily exit-site application of antibacterial honey plus usual exit-site care (N = 186) or intranasal mupirocin prophylaxis (in nasal Staphylococcus aureus carriers only) plus usual exit-site care (control, N = 185). Groups were compared on rates of organism-specific ESI and peritonitis, peritonitis- and infection-associated hospitalization, and technique failure (PD withdrawal).
♦ Results:
The mean peritonitis rates in the honey and control groups were 0.41 (95% confidence interval [CI] 0.32 – 0.50) and 0.41 (95% CI 0.33 – 0.49) episodes per patient-year, respectively (incidence rate ratio [IRR] 1.01, 95% CI 0.75 – 1.35). When specific causative organisms were examined, no differences were observed between the groups for gram-positive (IRR 0.99, 95% CI 0.66 – 1.49), gram-negative (IRR 0.71, 95% CI 0.39 – 1.29), culture-negative (IRR 2.01, 95% CI 0.91 – 4.42), or polymicrobial peritonitis (IRR 1.08, 95% CI 0.36 – 3.20). Exit-site infection rates were 0.37 (95% CI 0.28 – 0.45) and 0.33 (95% CI 0.26 – 0.40) episodes per patient-year for the honey and control groups, respectively (IRR 1.12, 95% CI 0.81 – 1.53). No significant differences were observed between the groups for gram-positive (IRR 1.10, 95% CI 0.70 – 1.72), gram-negative (IRR: 0.85, 95% CI 0.46 – 1.58), culture-negative (IRR 1.88, 95% CI 0.67 – 5.29), or polymicrobial ESI (IRR 1.00, 95% CI 0.40 – 2.54). Times to first peritonitis-associated and first infection-associated hospitalization were similar in the honey and control groups. The rates of technique failure (PD withdrawal) due to PD-related infection were not significantly different between the groups.
♦ Conclusion:
Compared with standard nasal mupirocin prophylaxis, daily topical exit-site application of antibacterial honey resulted in comparable rates of organism-specific peritonitis and ESI, infection-associated hospitalization, and infection-associated technique failure in PD patients.
Keywords: Honey, peritonitis, exit-site infection, mupirocin, microbiology, hospitalization, technique failure, peritoneal dialysis related infection
Peritoneal dialysis (PD)-related infections, including peritonitis and exit-site and tunnel infections, are serious complications of PD. These infections are associated with increased risks of mortality (1,2), catheter removal (3), hemodialysis transfer, and prolonged hospitalization (4).
Data from the ANZDATA Registry and from Hong Kong have shown that different spectra of microorganisms are associated with different outcomes (5–11). Compared with gram-negative organisms, gram-positive organisms account for higher proportions of both peritonitis and exit-site infections (ESI), but are also associated with better outcomes (3,8,12–15). Culture-negative peritonitis has generally better outcomes than culture-positive episodes (16) and single-organism peritonitis episodes have superior outcomes to polymicrobial peritonitis episodes (7, 8).
Topical application of mupirocin at either the exit site or intranasally has been recommended by the International Society for Peritoneal Dialysis (ISPD) in its guidelines (17) for prophylaxis against PD-related infections. Previous meta-analyses have found that mupirocin was effective in preventing Staphylococcus aureus exit-site infection and/or peritonitis (18,19). However, the agent is only active against gram-positive organisms, and has been found to be ineffective at preventing gram-negative PD-related infections (20). Furthermore, there are an increasing number of reports indicating that widespread use of mupirocin leads to the development of resistant organisms (18,21).
Over the past decade, honey has emerged as a promising therapeutic and preventive agent because of its broad-spectrum antibacterial coverage, particularly against multi-resistant organisms (22,23). Topical application of standardized antibacterial honey to hemodialysis catheter exit sites in hemodialysis patients has previously been demonstrated in a randomized controlled trial to result in infection rates similar to those with mupirocin, without the problems associated with mupirocin resistance (24).
Recently, the HONEYPOT study (25), a multi-center, multi-national, randomized controlled trial, reported that daily exit-site application of antibacterial honey was not superior to nasal mupirocin prophylaxis targeting nasal S. aureus carriers for preventing overall PD-related infections (unadjusted hazard ratio [HR] 1.12, 95% confidence interval [CI] 0.83 – 1.51; p = 0.47). In order to further evaluate the impact of exit-site application of honey on peritonitis and ESI microbiology and outcomes, this pre-specified sub-study aimed to determine whether topical antibacterial honey and mupirocin exerted differential effects on peritonitis and ESI microbiology and/or outcomes (first peritonitis-associated hospitalization, first infection-associated hospitalization, and technique failure).
Methods
Study Design and Participants
The study design and methodology (26), including the statistical analysis plan (27), have previously been described, as have the main study results (25). The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12607000537459). The study protocol was approved by ethics committees at all participating centers and all patients provided written informed consent prior to trial participation.
Adults and children of all ages with end-stage kidney disease undergoing PD were included in the trial. The exclusion criteria were ESI, tunnel infection, or peritonitis within the preceding month; current or recent (within the preceding 4 weeks) treatment with an antibiotic administered by any route; nasal carriage of mupirocin-resistant S. aureus; known hypersensitivity to, or intolerance of, honey or mupirocin; inability to provide informed consent; and history of psychological illness or disorder that interfered with the ability to understand or comply with the requirements of the study.
Participants were randomized in a 1:1 ratio to either daily exit-site application of antibacterial honey (Medihoney Antibacterial Wound Gel, Comvita, Paengaroa, New Zealand) or intranasal mupirocin prophylaxis (Bactroban, GlaxoSmithKline Limited, Melbourne, Australia) in nasal S. aureus carriers only (control; self-application twice daily to both anterior nares for 5 consecutive days each month). Usual exit-site care was performed according to local unit protocols. Randomization was stratified by study site, PD status (incident versus prevalent), and nasal carriage of S. aureus. Participants underwent a medical review and exit-site inspection in accordance with the Twardowski classification system every 2 months (28). They were followed until either completion of 24 months of follow-up, the occurrence of a study-terminating event, or the end of the study (16 June 2012), whichever came first.
The trial was open label, although microbiology staff were blinded to treatment allocation. Exit-site swabs were obtained using sterile, premoistened swabs in all suspected cases of exit-site infection (erythema, tenderness, induration, or discharge). In all cases of suspected peritonitis (e.g. abdominal pain, cloudy bags, fever, etc.), dialysate effluents were collected and inoculated in blood culture bottles. All samples were promptly sent for microscopy and culture at the local microbiology laboratory (including mupirocin-sensitivity testing of any S. aureus isolates using standard disc diffusion techniques) (26).
The primary efficacy endpoint for the trial was time to first catheter-associated infection (ESI, tunnel infection, or peritonitis, whichever came first). In this sub-study, organism-specific peritonitis and ESI rates were compared between the honey and control groups. All (first and subsequent) catheter-associated infection events were analyzed. Comparisons were also made between the 2 groups with respect to the outcomes of time to first peritonitis-associated hospitalization, time to first infection-associated hospitalization (hospitalization due primarily to peritonitis or ESI), time to PD withdrawal due to PD-related infection and causes of infection-associated technique failure (conversion from PD to hemodialysis for any duration due to peritonitis or ESI).
First peritonitis-associated hospitalization was defined as hospitalization primarily for treatment of peritonitis. First infection-associated hospitalization was defined as hospitalization primarily for treatment of any infections, including PD-related infection and non PD-related infection.
Statistical Analysis
Organism-specific peritonitis and ESI rates were analyzed by treatment group using a Poisson regression model. The incidence rate ratios (IRRs) and 95% CIs from the model were reported. Within each treatment group, infection rates were calculated as the number of infections divided by the total time at risk and expressed as episodes per patient-year at risk. In addition to analysis of individual organisms, analyses were also grouped according to larger categories, such as gram-positive, gram-negative, and polymicrobial, to increase event numbers and statistical power.
Times from randomization to first peritonitis- and first infection-associated hospitalization and time to PD withdrawal due to PD-related infection were displayed using Kaplan-Meier survival curves by treatment group. Survival curves for treatment groups were summarized using median survival times and statistically compared using the log-rank test. Unadjusted HRs were estimated from Cox proportional hazards regression models. Participants who did not have a peritonitis- or infection-associated hospitalization or PD withdrawal due to PD-related infection were censored in the survival analyses. Since the events of death, transfer to hemodialysis, renal transplant, and spontaneous recovery of renal function either prevent or alter the probability of occurrence of the PD withdrawal due to PD-related infection, competing risk survival analyses were done to test the sensitivity of results to the risk of PD withdrawal due to PD-related infection.
Results for the causes of withdrawal from PD are presented as frequencies (percentages) by intervention group. Group comparisons were performed using the chi-square test. P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
The HONEYPOT study randomized 371 participants from 26 centers to receive either honey (n = 186) or mupirocin prophylaxis (n = 185). All of these participants were included in the intention-to-treat analysis in the present sub-study. As previously reported (25), the 2 groups were well matched for all baseline characteristics. In particular, the proportion of nasal S. aureus carriers was 22% in both groups. The nasal S. aureus carriers in the control group received nasal mupirocin prophylaxis. Mupirocin-resistant S. aureus isolates were detected in 2 participants in the control group and no participants in the honey group.
Organism-Specific Peritonitis Rates
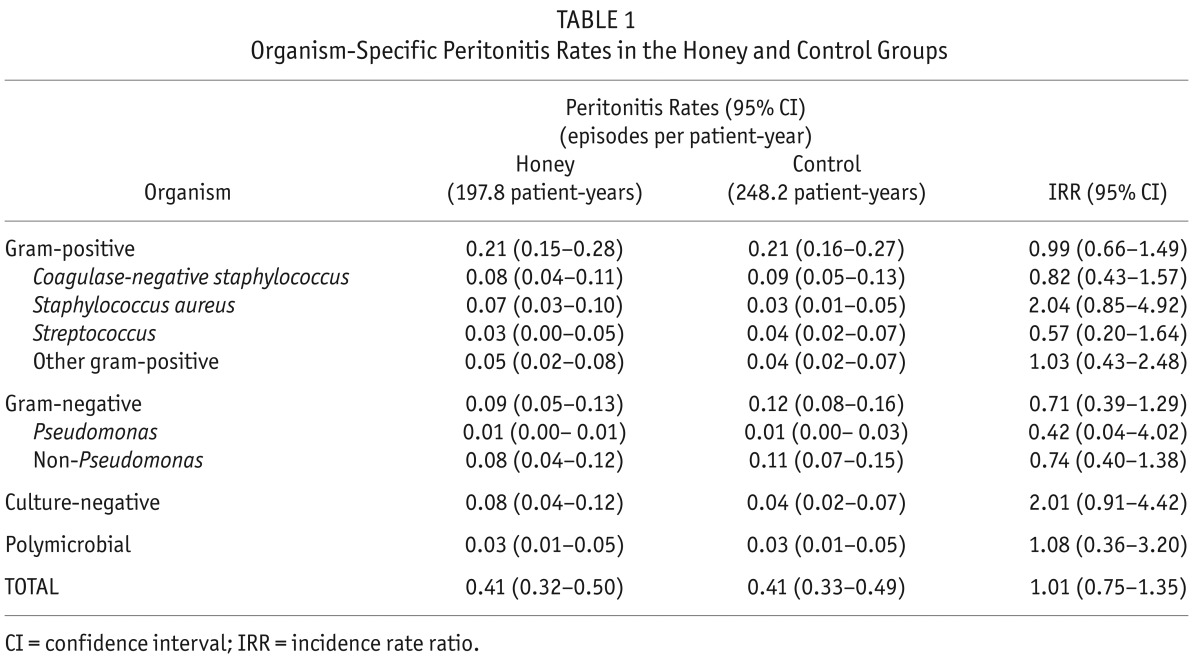
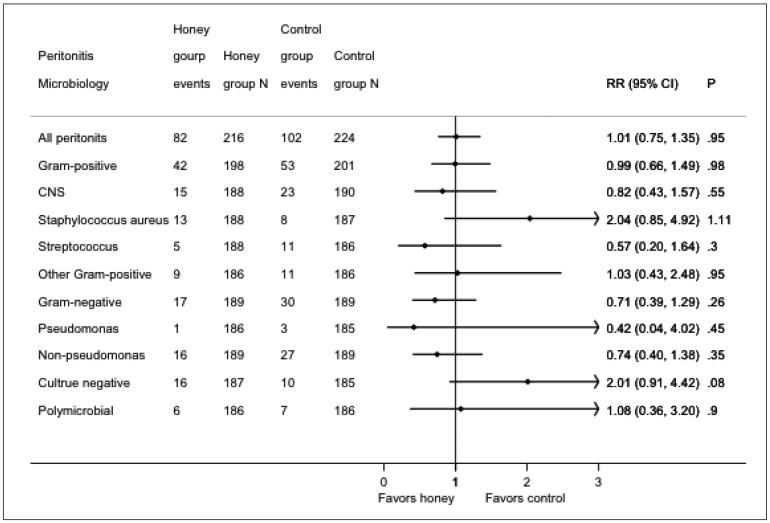
Eighty-two episodes of peritonitis occurred in 52 patients in the honey group and 102 episodes occurred in 63 patients in the control group. The mean peritonitis rates in the honey and control groups were 0.41 (95% CI: 0.32 – 0.50) and 0.41 (95% CI: 0.33 – 0.49) episodes per patient-year, respectively (IRR 1.01, 95% CI: 0.75 – 1.35; p = 0.95). No significant differences were observed between the groups for gram-positive, gram-negative, culture-negative, or polymicrobial peritonitis (Table 1, Figure 1).
TABLE 1.
Organism-Specific Peritonitis Rates in the Honey and Control Groups
Figure 1 —
Forest plot of organisms responsible for peritonitis episodes in the honey and control groups. RR = rate ratio; CI = confidence interval; CNS = coagulase-negative staphylococcus.
Organism-Specific ESI Rates
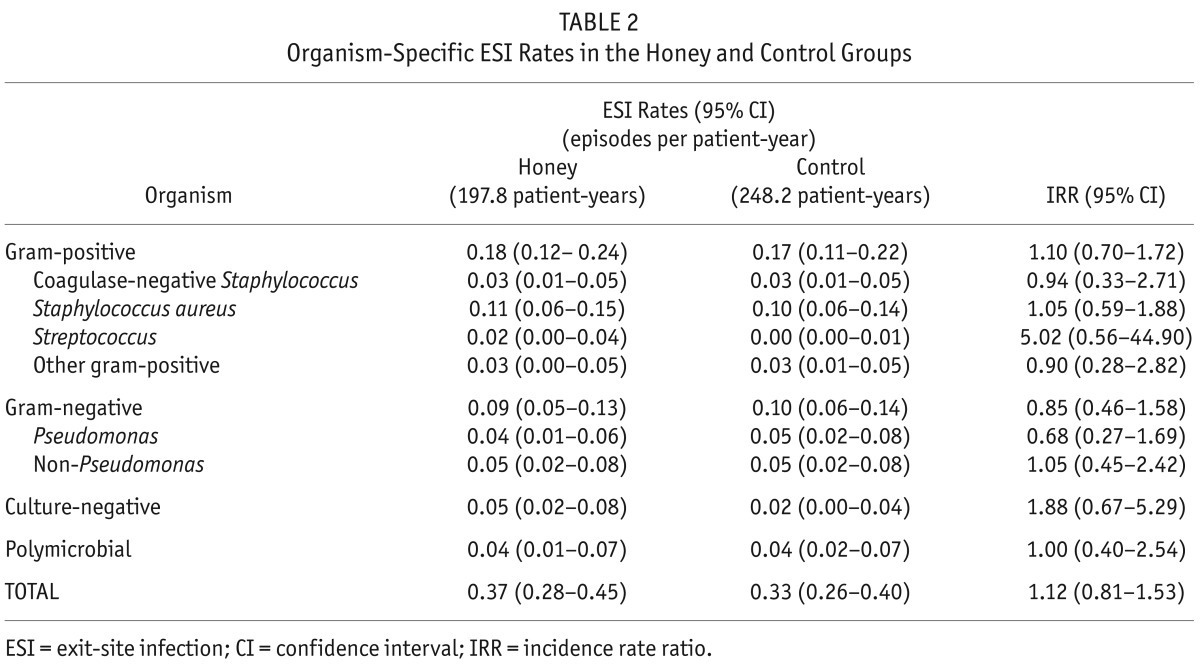
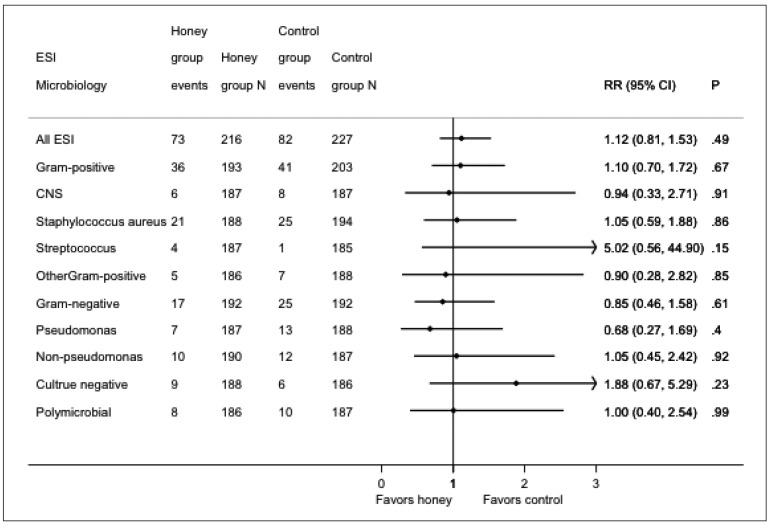
Seventy-three episodes of ESI occurred in 43 patients in the honey group and 82 episodes occurred in 40 patients in the control group. The mean ESI rates in the honey and control groups were 0.37 (95% CI: 0.28 – 0.45) and 0.33 (95% CI: 0.26 – 0.40) episodes per patient-year, respectively (IRR 1.12, 95% CI: 0.81 – 1.53; p = 0.49). No significant differences were observed between the groups for gram-positive, gram-negative, culture-negative, or polymicrobial ESI (Table 2, Figure 2).
TABLE 2.
Organism-Specific ESI Rates in the Honey and Control Groups
Figure 2 —
Forest plot of organisms responsible for ESI episodes in the honey and control groups. ESI = exit-site infection; RR = rate ratio; CI = confidence interval; CNS = coagulase-negative staphylococcus.
First Peritonitis-Associated and First Infection-Associated Hospitalization
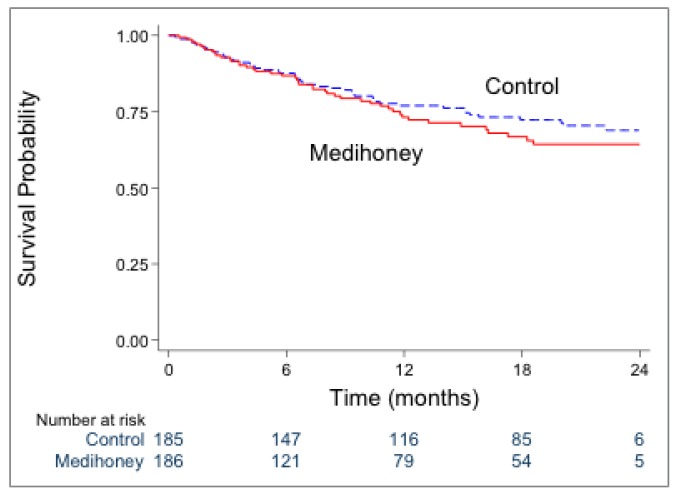
Forty-six participants (25%) in the honey group and 49 participants (26%) in the control group experienced a first peritonitis-associated hospitalization (p = 0.70). The rates of first peritonitis-associated hospitalization were similar in the honey and control groups (HR 1.17, 95% CI: 0.78 – 1.75; p = 0.45) (Figure 3).
Figure 3 —
Survival analysis of first peritonitis-associated hospitalization in the honey and control groups [HR 1.17 (95% CI 0.78–1.75); p=0.45]. HR = hazard ratio; CI = confidence interval.
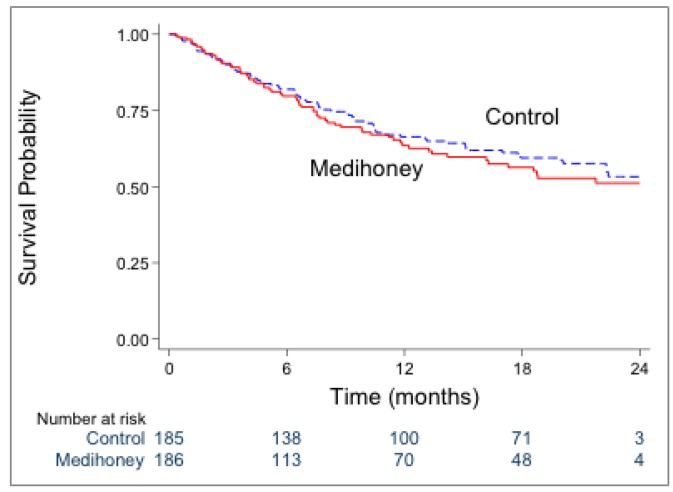
Sixty-four participants (34%) in the honey group and 71 participants (38%) in the control group experienced a first infection-associated hospitalization (p = 0.43). No significant differences in time to first infection-associated hospitalization were observed between the honey and control groups (HR 1.11, 95% CI: 0.79 – 1.56; p = 0.55) (Figure 4).
Figure 4 —
Survival analysis of first infection-associated hospitalization in the honey and control groups [HR 1.11 (95% CI 0.79–1.56); p=0.55]. HR = hazard ratio; CI = confidence interval.
Technique Survival
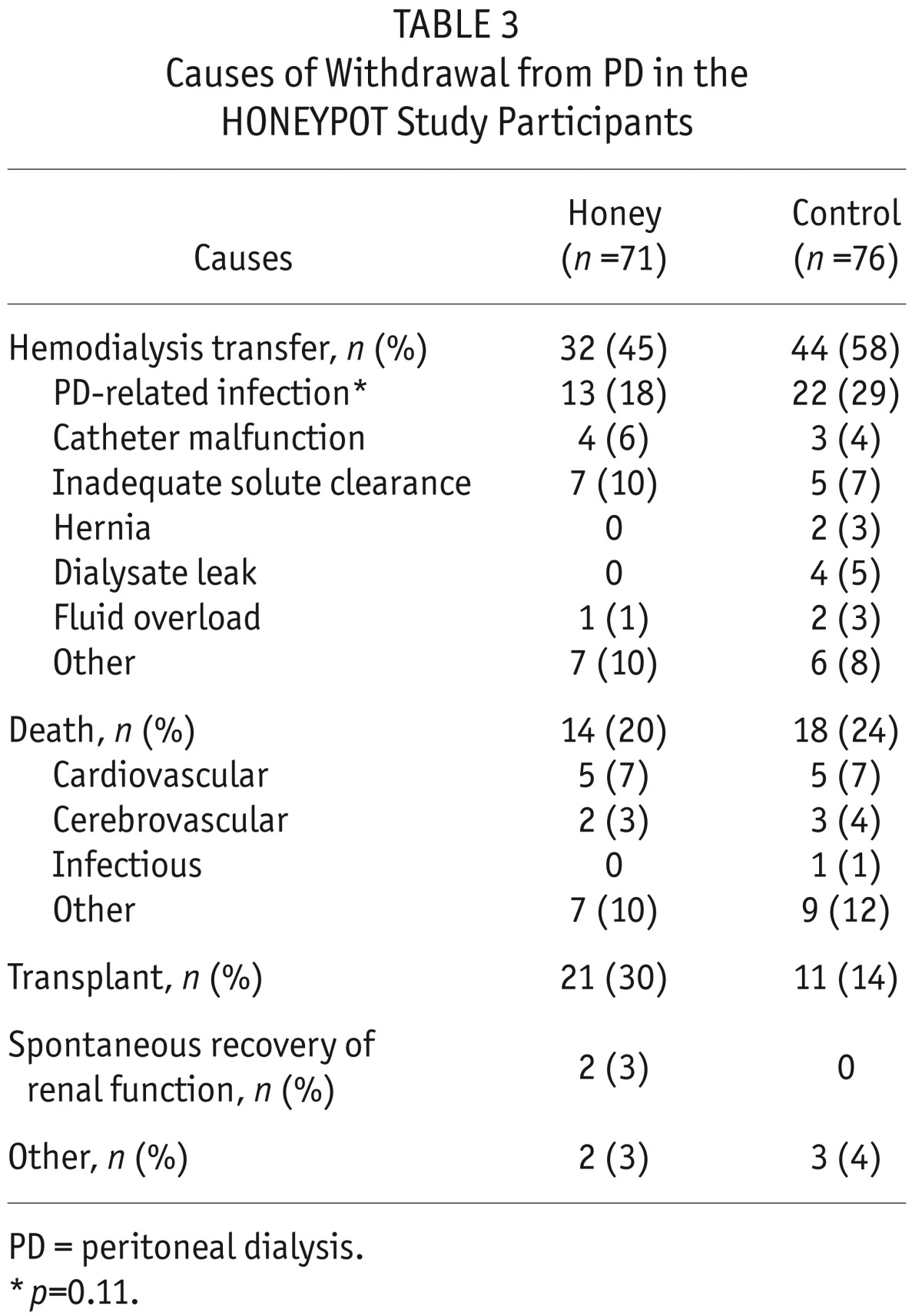
The causes of withdrawal from PD are shown in Table 3. Thirteen (18%) patients in the honey group and 22 (29%) in the control group had their catheters removed and were converted to hemodialysis due to PD-related infection, respectively (p = 0.11). One death related to infection (intra-abdominal sepsis) occurred in a patient in the control group.
TABLE 3.
Causes of Withdrawal from PD in the HONEYPOT Study Participants
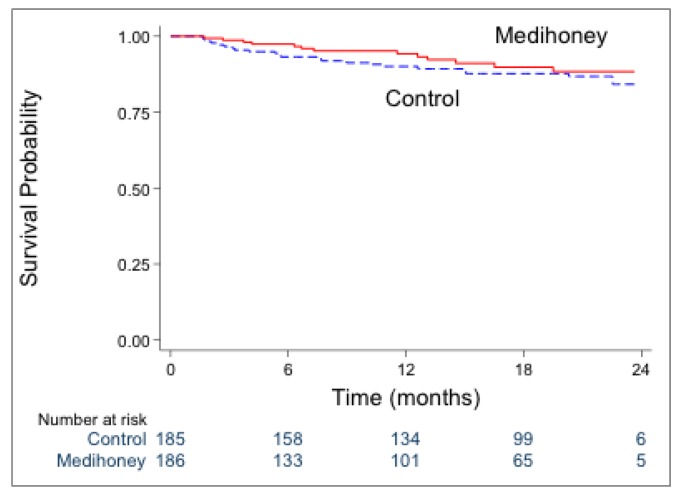
The rates of PD withdrawal due to PD-related infection were similar in the honey and control groups (HR 0.73, 95% CI: 0.37 – 1.45; p = 0.37) (Figure 5). We noted similar findings with the competing-risks survival analysis (HR 0.68, 95% CI: 0.34 – 1.35; p = 0.27).
Figure 5 —
Survival analysis of PD withdrawal due to PD-related infection in the honey and control groups [HR 0.73 (95% CI: 0.37–1.45); p=0.37]. PD = peritoneal dialysis; HR = hazard ratio; CI = confidence interval.
Discussion
This pre-specified sub-study of the HONEYPOT trial showed that, compared with standard nasal mupirocin prophylaxis targeting nasal carriage of S. aureus, daily exit-site application of antibacterial honey resulted in similar organism-specific rates of peritonitis and ESI among PD patients. The risks of first peritonitis-associated hospitalization, first infection-associated hospitalization and hemodialysis conversion due to infection were also comparable between the groups. These observations extend the main findings of the HONEYPOT trial by demonstrating that antibacterial honey does not provide any organism-specific infection control advantage over mupirocin, despite the fact that honey has a broader antimicrobial spectrum.
Honey has been reported to be effective against a large variety of microorganisms in in vitro studies, including gram-positive organisms, Staphylococcus aureus (29), methicillin-resistant Staphylococcus aureus (MRSA) (30), coagulase-negative Straphylococci (31), Streptococcus pyogenes (32,33), Escherichia coli (34), Pseudomonas aeruginosa (34–37), fungi (38), and vancomycin-resistant enterococci (39). In contrast to mupirocin, honey has a greater inhibitory effect on gram-negative bacteria than gram-positive bacteria (40) and a much more potent effect on multiple antibiotic-resistant microorganisms (22). Moreover, there are currently no known microorganisms resistant to honey (41,42). Honey has also been reported to reduce inflammation, debride necrotic tissue, reduce edema, and promote angiogenesis, granulation, and epithelialization of wounds (43). In spite of these attractive properties, very few studies have compared the effect of honey with antibiotics for either therapeutic or prophylactic purposes.
One study in Egypt (44) compared the in vitro effects of honey versus a wide variety of commonly used antibiotics (ciprofloxacin, sulbactam/ampicillin, ceftriaxone, vancomycin, imipenem, amoxicillin/clavulinic acid, ceftriaxone, and methicillin) on organisms isolated from the infected wounds of 33 burn patients. The authors reported that honey exerted greater inhibitory effects on gram-negative bacteria and MRSA than these commonly used antibiotics. Adeleke and colleagues similarly reported that honey had higher in vitro antibacterial activities against Pseudomonas aeruginosa and Escherichia coli than gentamicin in organisms isolated from infected burn wounds (45), whilst Jenkins et al. noted that honey had a superior antimicrobial effect on Staphylococcus aureus compared with vancomycin (46). However, these studies were all in vitro investigations and therefore were not necessarily generalizable to the clinical setting.
Only 1 previously published randomized controlled trial, by Johnson and colleagues, has examined the effect of topical exit-site application of honey compared with antibiotics on preventing clinical infections (24). In this study of 101 hemodialysis patients with dialysis catheters, the exit-site application of honey was not significantly associated with catheter-associated bacteremia-free survival compared with mupirocin (unadjusted HR 0.94, 95% CI 0.27 – 3.24; p = 0.92), similar to the main findings of the HONEYPOT trial in PD patients. However, organism-specific infection rates were not examined in that study due to the low number of observed infectious events (n = 11). The present sub-study of the HONEYPOT trial is therefore the first randomized controlled trial to report the clinical impact of honey on infections due to specific organisms, compared with antibiotic prophylaxis (mupirocin). Although high levels of mupirocin resistance have been reported (47,48), a low incidence of mupirocin-resistant microorganisms was found in the present study, which may have been due to the short duration of follow-up.
Apart from a neutral effect of antibacterial honey on PD-related infection rates compared with mupirocin, the current study also found that honey did not attenuate the severity of PD-related infections, as evidenced by comparable rates of first peritonitis-associated hospitalization, first infection-associated hospitalization and infection-related technique failure. These findings contrast with those of a previous meta-analysis of 7 randomized controlled trials, which demonstrated that the use of honey as a wound dressing was superior to antiseptics and/or systemic antibiotics for wound healing, maintenance of sterility, and eradication of infection (49). Some of the apparent disparity in findings with those of the present study may be explained by differences in indications for honey administration (prevention of catheter-association infection versus treatment of infected burns or post-operative wounds) and in the interventions used in the control group (mupirocin vs polyurethane film, amniotic membrane, potato peel, or silver sulphadiazine). Moreover, the systematic review examined small sample size, single-center trials of short duration and suboptimal methodological quality, 6 of which were performed by the same investigator.
Another randomized controlled trial of dressings soaked in honey vs Edinburgh University Solution of Limes (UESOL) in 32 children with 43 pyomyositis abscesses reported that honey-treated wounds demonstrated quicker healing and a shorter length of hospital stay compared with EUSOL-treated wounds (p = 0.019) (50). These results may also have differed from those of the present study as a result of the different indications for honey use and the different interventions used as a comparator.
The strengths of this study include its large sample size and involvement of many centers from 2 different countries, thereby enhancing the generalizability of the trial's findings. The pragmatic study design also closely mirrored ‘real-world’ clinical practice. Balanced against these strengths, the principal limitation of this sub-study was that analysis of PD-related infections due to some individual peritonitis organisms was limited by low event rates and therefore inadequate statistical power. For example, only 4 episodes of pseudomonas peritonitis occurred during the study (1 in the honey group and 3 in the control group). These organisms were therefore grouped into larger categories, such as gram-positive, gram-negative, and polymicrobial peritonitis or ESI, to increase event numbers and analytic power. The open-label design of the trial also potentially introduced observer and performance biases, whilst the higher withdrawal rate in the honey group (29%) potentially resulted in attrition bias. The findings of the trial may also not be generalizable to exit-site mupirocin application, which is commonly practiced in some PD centers.
In conclusion, daily topical exit-site application of antibacterial honey was not superior to nasal mupirocin prophylaxis for the prevention of catheter-associated infection, hospitalization, and technique failure in PD patients and there were no differences in the microbiological profiles of either ESI or peritonitis between the 2 treatment arms.
Disclosures
David Johnson is a consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speakers' honoraria and research grants from Fresenius Medical Care. He has previously been a consultant to Gambro Pty Ltd and was the recipient of a Gambro Research Grant which partly funded the HONEYPOT trial. Nikky Isbel, Carolyn Clark, and David Johnson received a Baxter Healthcare Renal Discoveries Extramural Program Grant which partly funded the HONEYPOT trial. David Johnson is an International Society for Peritoneal Dialysis Councillor and is a current recipient of a Queensland Government Health Research Fellowship. Alan Cass is a consultant for and has received research funds from Baxter Healthcare Pty Ltd. He has also received speakers' honoraria from Fresenius Medical Care. He is a recipient of a National Health and Medical Research Council Principal Research Fellowship. Carmel Hawley has received speakers' honoraria and research grants from Fresenius Medical Care and has been a consultant to Fresenius Medical Care. She has received research funds from Gambro Pty Ltd and was the recipient of a Queensland Health Smart Health Research Grant. All other authors have no financial conflicts of interest to declare.
Acknowledgments
Baxter Healthcare (McGaw Park, IL, USA; Renal Discoveries Extramural Grant Program), Queensland Government (Smart State Health Grant), and Gambro (Baulkham Hills, NSW, Australia) provided funding for the HONEYPOT study. Medihoney Antibacterial Wound Gel (Comvita, Paengaroa, New Zealand) was provided free of charge.
REFERENCES
- 1. Boudville N, Kemp A, Clayton P, Lim W, Badve SV, Hawley CM, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 2012; 23:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson D, Chang S, Excell L, Livingston B, Bannister K, McDonald S. Peritoneal dialysis. In: Mcdonald SP, Excell L, eds. Anzdata registry report 2006. Adelaide, South Australia: Australian and New Zealand dialysis and transplant registry; 2007:87–103. [Google Scholar]
- 3. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl 2006:S55–62. [DOI] [PubMed] [Google Scholar]
- 4. Fried L, Abidi S, Bernardini J, Johnston JR, Piraino B. Hospitalization in peritoneal dialysis patients. Am J Kidney Dis 1999; 33:927–33. [DOI] [PubMed] [Google Scholar]
- 5. Yap DY, To KK, Yip TP, Lui SL, Chan TM, Lai KN, et al. Streptococcus bovis peritonitis complicating peritoneal dialysis—a review of 10 years' experience. Perit Dial Int 2012; 32:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Shea S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Streptococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 287 cases. BMC Nephrol 2009; 10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barraclough K, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Polymicrobial peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes. Am J Kidney Dis 2010; 55:121–31. [DOI] [PubMed] [Google Scholar]
- 8. Rocha A, Rodrigues A, Teixeira L, Carvalho MJ, Mendonca D, Cabrita A. Temporal trends in peritonitis rates, microbiology and outcomes: the major clinical complication of peritoneal dialysis. Blood Purif 2012; 33:284–91. [DOI] [PubMed] [Google Scholar]
- 9. Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman J, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 2002; 22:573–81. [PubMed] [Google Scholar]
- 10. Jarvis EM, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors, treatment, and outcomes of non-pseudomonas gram-negative peritonitis. Kidney Int 2010; 78:408–14. [DOI] [PubMed] [Google Scholar]
- 11. Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Pseudomonas peritonitis in Australia: predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 2009; 4:957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 2011; 31:651–62. [DOI] [PubMed] [Google Scholar]
- 13. Prasad KN, Singh K, Rizwan A, Mishra P, Tiwari D, Prasad N, et al. Microbiology and outcomes of peritonitis in northern India. Perit Dial Int 2014; 34:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bunke C, Brier M, Golper T. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 1997; 52:524–9. [DOI] [PubMed] [Google Scholar]
- 15. Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F. Differing outcomes of gram-positive and gram-negative peritonitis. Am J Kidney Dis 1998; 32:623–8. [DOI] [PubMed] [Google Scholar]
- 16. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Culture-negative peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes in 435 cases. Am J Kidney Dis 2010; 55:690–7. [DOI] [PubMed] [Google Scholar]
- 17. Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 2011; 31:614–30. [DOI] [PubMed] [Google Scholar]
- 18. Tacconelli E, Carmeli Y, Aizer A, Ferreira G, Foreman M, D'Agata E. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin Infect Dis 2003; 37:1629–38. [DOI] [PubMed] [Google Scholar]
- 19. Strippoli G, Tong A, Johnson D, Schena F, Craig J. Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database Syst Rev 2004; 18:CD004679. [DOI] [PubMed] [Google Scholar]
- 20. Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomized, double-blind trial of antibiotic exit-site cream for prevention of exit-site infection in peritoneal dialysis patients. J Am Soc Nephrol 2005; 16:539–45. [DOI] [PubMed] [Google Scholar]
- 21. Lobbedez T, Gardam M, Dedier H, Burdzy D, Chu M, Izatt S, et al. Routine use of mupirocin at the peritoneal catheter exit site and mupirocin resistance: still low after 7 years. Nephrol Dial Transplant 2004; 19:3140–3. [DOI] [PubMed] [Google Scholar]
- 22. Blair SE, Cokcetin NN, Harry EJ, Carter DA. The unusual antibacterial activity of medical-grade leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis 2009; 28:1199–208. [DOI] [PubMed] [Google Scholar]
- 23. Miles R, Johnson D. Use of honey to prevent infections associated with medical devices. In: Cooper R, Molan P, White R. (eds). Honey: a modern wound management product. Dorset, UK: Wounds UK Books; 2008:91–105. [Google Scholar]
- 24. Johnson DW, van Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol 2005; 16:1456–62. [DOI] [PubMed] [Google Scholar]
- 25. Johnson DW, Badve SV, Pascoe EM, Beller E, Cass A, Clark C, et al. Antibacterial honey for the prevention of peritoneal-dialysis-related infections (HONEYPOT): a randomised trial. Lancet Infect Dis 2014; 14:23–30. [DOI] [PubMed] [Google Scholar]
- 26. Johnson D, Clark C, Isbel N, Hawley C, Beller E, Cass A, et al. The HONEYPOT study protocol: a randomized controlled trial of exit-site application of Medihoney antibacterial wound gel for the prevention of catheter-associated infections in peritoneal dialysis patients. Perit Dial Int 2009; 29:303–9. [PubMed] [Google Scholar]
- 27. Pascoe EM, Lo S, Scaria A, Badve SV, Beller EM, Cass A, et al. The HONEYPOT randomized controlled trial statistical analysis plan. Perit Dial Int 2013; 33:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Twardowski Z, Prowant B. Classification of normal and diseased exit sites. Perit Dial Int 1996; 16:S32–50. [PubMed] [Google Scholar]
- 29. Packer JM, Irish J, Herbert BR, Hill C, Padula M, Blair SE, et al. Specific non-peroxide antibacterial effect of manuka honey on the Staphylococcus aureus proteome. Int J Antimicrob Agents 2012; 40:43–50. [DOI] [PubMed] [Google Scholar]
- 30. Jenkins R, Burton N, Cooper R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus(MRSA) exposed to Manuka honey in vitro demonstrated down-regulation of virulence markers. J Antimicrob Chemother 2014; 69:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. French V, Cooper R, Molan P. The antibacterial activity of honey against coagulase-negative Staphylococci. J Antimicrob Chemother 2005; 56:228–31. [DOI] [PubMed] [Google Scholar]
- 32. Moussa A, Noureddine D, Mohamed HS, Abdelmelek M, Saad A. Antibacterial activity of various honey types of Algeria against Staphylococcus aureus and Streptococcus pyogenes. Asian Pac J Trop Med 2012; 5:773–6. [DOI] [PubMed] [Google Scholar]
- 33. Maddocks SE, Lopez MS, Rowlands RS, Cooper RA. Manuka honey inhibits the development of Streptococcus pyogenesbiofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 2012; 158:781–90. [DOI] [PubMed] [Google Scholar]
- 34. Mandal S, DebMandal M, Pal NK, Saha K. Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella entericaserovar Typhi. Asian Pac J Trop Med 2010; 3:961–4. [Google Scholar]
- 35. Kronda JM, Cooper RA, Maddocks SE. Manuka honey inhibits siderophore production in Pseudomonas aeruginosa. J Appl Microbiol 2013; 115:86–90. [DOI] [PubMed] [Google Scholar]
- 36. Cooper R, Halas E, Molan P. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil 2002; 23:366–70. [DOI] [PubMed] [Google Scholar]
- 37. Henriques A, Jenkins RE, Burton NF, Cooper RA. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 2011; 30:167–71. [DOI] [PubMed] [Google Scholar]
- 38. Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci 2013; 16:731–42. [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper R, Molan P, Harding K. The sensitivity to honey of gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol 2002; 93:857–63. [DOI] [PubMed] [Google Scholar]
- 40. Mohapatra DP, Thakur V, Brar SK. Antibacterial efficacy of raw and processed honey. Biotechnol Res Int 2011; 2011:917505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blair S, Cokcetin N, Harry E, Carter D. The unusual antibacterial activity of medical-grade leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis 2009; 28:1199–208. [DOI] [PubMed] [Google Scholar]
- 42. Cooper R, Jenkins L, Henriques A, Duggan R, Burton N. Absence of bacterial resistance to medical-grade manuka honey. Eur J Clin Microbiol Infect Dis 2010; 29:1237–41. [DOI] [PubMed] [Google Scholar]
- 43. Efem S, Udoh K, Iwara C. The antimicrobial spectrum of honey and its clinical significance. Infection 1992; 20:227–229. [DOI] [PubMed] [Google Scholar]
- 44. Abd-El Aal A, El-Hadidy M, El-Mashad N, El-Sebaie A. Antimicrobial effect of bee honey in comparison to antibiotics on organisms isolated from infected burns. Ann Burns Fire Disasters 2007; 20:83–8. [PMC free article] [PubMed] [Google Scholar]
- 45. Adeleke O, Olaitan J, Okpekpe E. Comparative antibacterial activity of honey and gentamicin against Escherichia coli and Pseudomonas aeruginosa. Ann Burns Fire Disasters 2006; 19:201–4. [PMC free article] [PubMed] [Google Scholar]
- 46. Jenkins R, Wootton M, Howe R, Cooper R. Susceptibility to manuka honey of Staphylococcus aureus with varying sensitivities to vancomycin. Int J Antimicrob Agents 2012; 40:88–9. [DOI] [PubMed] [Google Scholar]
- 47. Pérez-Fontán M, Rosales M, Rodríguez-Carmona A, Falcón TG, Valdés F. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 2002; 39:337–41. [DOI] [PubMed] [Google Scholar]
- 48. Conly J, Vas S. Increasing mupirocin resistance of Staphylococcus aureus in CAPD—should it continue to be used as prophylaxis? Perit Dial Int 2002; 22:649–52. [PubMed] [Google Scholar]
- 49. Moore O, Smith L, Campbell F, Seers K, McQuay H, Moore R. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med 2001; 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okeniyi J, Olubanjo O, Ogunlesi T, Oyelami O. Comparison of healing of incised abscess wounds with honey and EUSOL dressing. J Altern Complement Med 2005; 11:511–3. [DOI] [PubMed] [Google Scholar]