Peritonitis is the major complication of peritoneal dialysis (PD), and severe or repeated episodes of peritonitis cause long-term complications that potentially lead to peritoneal membrane failure (1).
Mesothelial cells play an important role in regulating the inflammatory response in the peritoneal cavity, with production of pro-inflammatory cytokines and chemokines. These cytokines promote the return of the mesothelial cells to normal phenotype but, at the same time, contribute to peritoneal fibrosis (2). Cell-free DNA (cfDNA) is composed of circulating extracellular DNA fragments that originate from necrotic and apoptotic cells, reflecting inflammation (3). Cell-free DNA is present in blood and urine in various healthy and pathological conditions (4–5). High levels of cfDNA have also been reported in many clinical nephrological conditions, such as chronic kidney disease, hemodialysis, and PD (6–7). However, there are no data on cfDNA in PD patients with peritonitis.
The aim of this study was to evaluate the variation in plasma levels of cfDNA subsequent to peritonitis as an indication of inflammation, apoptosis, and tissue damage in a population of PD patients.
Materials and Methods
We enrolled 54 PD patients: 25 without any history of peritonitis (group A), 21 whose last episode of peritonitis was more than 3 months prior to inclusion (group B), and 8 who had had an episode of peritonitis within 3 months prior to enrolment (group C). Peritoneal dialysis patients with medical histories of comorbidities, such as active infection (exception from peritonitis), were excluded from the study to avoid confounding factors. Blood samples were collected from all 54 patients and centrifuged twice. Cell-free DNA was extracted from plasma using a DNA isolation kit and quantified by real-time quantitative polymerase chain reaction (PCR) for the β-actin gene. For each run, 1 aliquot of stock DNA was serially diluted to generate a 6-point standard calibration curve. Results are expressed as genome equivalents (GE)/mL; 1 GE/mL equals 6.6 pg DNA. Plasma caspase-3, interleukin (IL)-1β and IL-6 concentration were measured by enzyme-linked immuno-sorbent assay (ELISA). All the numerical variables are presented as mean ± standard deviation. Significance at the p < 0.05 level was assessed by non-parametric Mann-Whitney tests for 2 groups of data and Kruskal-Wallis for 3 or more groups by means of the SPSS software package (SPSS, Chicago, IL, USA). Spearman's rho correlations were calculated to verify the correlation between variables.
Results and Discussion
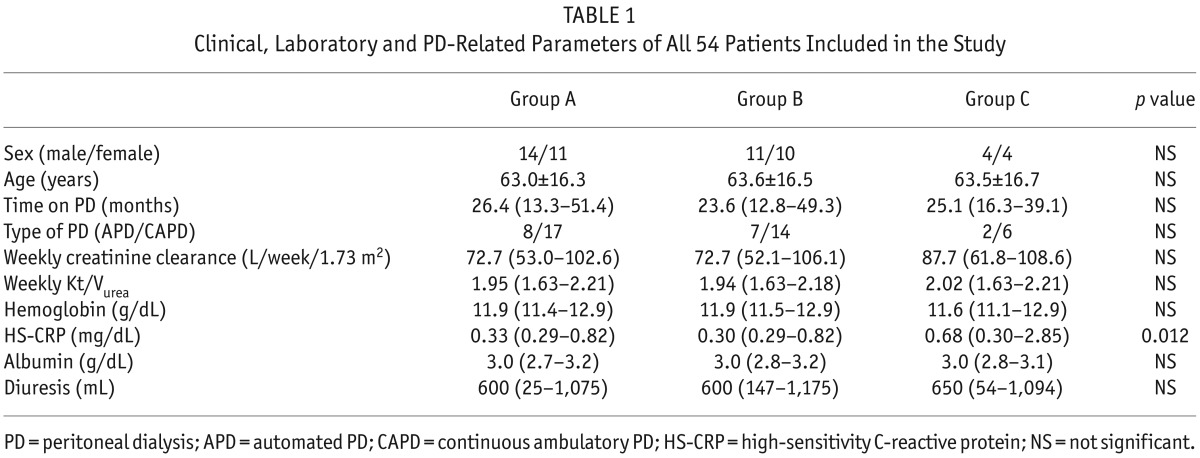
A total of 54 chronic PD patients (29 male; mean age 63.1 ± 16.3, median time on PD 25.5 months interquartile range [IQR]: 13.2 – 49.4) were enrolled (Table 1).
TABLE 1.
Clinical, Laboratory and PD-Related Parameters of All 54 Patients Included in the Study
Twenty-four patients had a first episode of peritonitis and responded to first-line antibiotics (75% gram-positive, 25% gram-negative), whereas 5 subjects had a relapsing episode of peritonitis (50% gram-positive, 50% gram-negative) but subsequently responded to intraperitoneal antibiotics. At day 10 from the peritonitis treatment, white blood cell (WBC) count in the peritoneal effluent was normal in all patients. No patient required catheter removal and no patients died during the study period. Twelve patients had multiple episodes (maximum = 5) of peritonitis, and 17 patients had a single episode of peritonitis in their clinical history. The median number of days from the start of the last peritonitis was 583 days (IQR 265 – 925) in group B, and 12 days (IQR 2 – 27) in group C.
Group A and B patients showed a lower high-sensitivity C-reactive protein (HS-CRP), and this parameter was significantly elevated in group C. In fact, its levels rise dramatically during inflammatory processes, such as peritonitis, and return to normal with resolution of the acute episode.
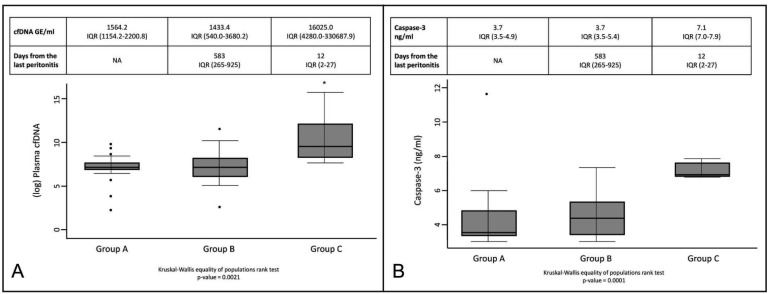
Median plasma cfDNA levels were 1,564.2 GE/mL (IQR 1,154.2 – 2,200.8) in group A, 1,433.4 GE/mL (IQR 540.0 – 3,680.2) in group B and 16,025.0 GE/mL (IQR 4,280.0 – 33,0687.9) in group C. The level of cfDNA in group C was significantly higher when compared with group A and group B (p < 0.001) (Figure 1A). However, plasma cfDNA levels in group A and group B did not differ significantly. No significant difference was found between the levels of plasma cfDNA in PD subjects with new (3,355.2 GE/mL; IQR 774.9 – 17,145.4) and relapsing (2,330.0 GE/mL; IQR 574.1 – 11,691.3) episodes of peritonitis and no significant difference was found between the levels of plasma cfDNA in PD subjects with 1 (2,330.0 GE/mL; IQR 696.6 – 8,019.57) or multiple (3,448.0 GE/mL; IQR 1,235.9 – 28,432.5) episodes of peritonitis.
Figure 1 —
cfDNA and caspase-3 levels in PD patients. A: Comparison of plasma cfDNA levels between group A, group B and group C; B: Comparison of plasma caspase-3 levels between group A, group B, and group C. cfDNA = cell-free DNA; PD = peritoneal dialysis; IQR = interquartile range; NA = not applicable; GE = genome equivalents.
No statistically significant relationship was observed between HS-CRP and cfDNA levels (p = 0.3); similarly, no statistically significant relationship was observed between HS-CRP and days from the start of the last peritonitis. Our results showed that HS-CRP returned to normal levels more quickly than cfDNA. On the contrary, cfDNA decreased slowly after an episode of peritonitis. We speculated that cfDNA may be related to the gradual recovery of the peritoneal tissue after an episode of peritonitis. We hypothesized that cfDNA may be derived from apoptotic events in peritoneal and/or inflammatory cells induced by peritonitis. Although these findings are provocative, the design of the study does not allow us to make these conclusions. Furthermore, we theorized that the cfDNA decline may be inversely connected with the peritoneal membrane repair process from this damage. In general, the elimination process of cfDNA from the blood remains largely unknown; it is speculated that liver, spleen, and kidney may be involved in cfDNA disappearance (4).
Our data suggested an increased intraperitoneal production of cfDNA during the course of the peritonitis episode and, consequently, the release of cfDNA into the blood circulation.
Furthermore, we analyzed the 29 patients with peritonitis according to their clinical history. A significant negative correlation was observed between plasma cfDNA concentration and days from the start of the last peritonitis (Spearman's rho = -0.74, p < 0.01). We performed a univariate linear regression of log(cfDNA) on days from the start of the last peritonitis, and we observed that each 1-unit increase in days from the start of peritonitis was associated with a 0.402% decrease in cfDNA levels. Lower levels of cfDNA were detected in patients with a longer peritonitis-free period; moreover, cfDNA levels tend to progressively decrease in correlation with peritonitis-free time. Based on these results, we hypothesized the presence of a threshold beyond which no difference can be observed between subjects with previous peritonitis and subjects without peritonitis. Further studies are necessary to define this threshold. After this threshold period, we may consider these patients similarly to the way we consider patients without peritonitis in their history.
The level of plasma caspase-3 in group C was significantly increased when compared with groups A and B (p < 0.001) (Figure 1B). A significant positive correlation was observed between caspase-3 and cfDNA levels (Spearman's rho = 0.5, p < 0.01). We speculated that the abundance of cfDNA present in PD patients who had had peritonitis in the previous 3 months was potentially due to apoptotic events. Indeed, Moreira et al. hypothesized the induction of cfDNA by apoptotic events in febrile patients and considered cfDNA as a direct marker of cell apoptosis (8). Based on our findings, we hypothesized that cfDNA induced by peritonitis may strongly activate the innate immune system to promote pro-inflammatory events and cytokine networks up-regulation.
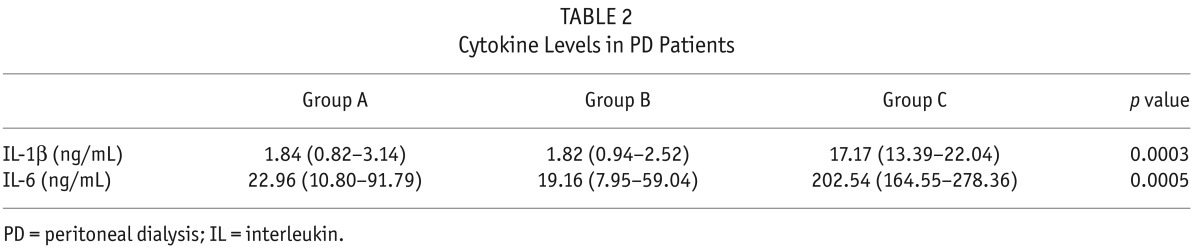
In addition, we examined the inflammatory state of these patients. Plasma pro-inflammatory cytokine levels (IL-1β and IL-6) were measured in all 54 PD patients (Table 2). Plasma IL-1β and IL-6 levels were significantly elevated in group C compared with groups A and B (p < 0.001). However, no significant difference was found in these cytokine levels between the 2 latter groups. A significant positive correlation was observed between IL-6 levels and caspase-3 and cfDNA levels (Spearman's rho = 0.7 and 0.4, both p < 0.05). A positive correlation was found between IL-6 and IL-1β levels (Spearman's rho = 0.4, p < 0.05) and between IL-1β and caspase-3 levels (p < 0.05, Spearman's rho = 0.28). However, no significant relationship was found between IL-1β and cfDNA levels. These data suggest that cfDNA production subsequent to peritonitis may be an indicator of inflammation, apoptosis induced by peritoneal damage in this acute event. Furthermore, based on IL-1β results, we hypothesized that cfDNA production may not be mediated by the inflammasome complex. However, we are unable to directly address this hypothesis in this study.
TABLE 2.
Cytokine Levels in PD Patients
Conclusion
Our study is the first report to address the association between dynamic changes of cfDNA and the peritoneal membrane repair process in PD-related peritonitis. Cell-free DNA could potentially serve as an index for noninvasive monitoring of tissue damage and apoptosis after peritonitis and the reverse peritoneal membrane repair from this damage.
REFERENCES
- 1. Brown EA. Peritonitis: limiting the damage. Nephrol Dial Transplant 2005; 20:1539–41. [DOI] [PubMed] [Google Scholar]
- 2. Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, Jimenez-Heffernan JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 2007; 18:2004–13. [DOI] [PubMed] [Google Scholar]
- 3. Lichtenstein AV, Melkonyan HS, Tomei LD, Umansky SR. Circulating nucleic acids and apoptosis. Ann N Y Acad Sci 2001; 945:239–49. [DOI] [PubMed] [Google Scholar]
- 4. Jung K, Fleischhacker M, Rabien A. Cell-free DNA in the blood as a solid tumor biomarker—a critical appraisal of the literature. Clin Chim Acta 2010; 411:1611–24. [DOI] [PubMed] [Google Scholar]
- 5. Dwivedi DJ, Toltl LJ, Swystun LL, Pogue J, Liaw KL, Weitz JI, et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care 2012; 16:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pajek J, Kveder R, Gucek A, Skoberne A, Bren A, Bucar M, et al. Cell-free DNA in the peritoneal effluent of peritoneal dialysis solutions. Ther Apher Dial 2010; 14:20–6. [DOI] [PubMed] [Google Scholar]
- 7. Korabecna M, Opatrna S, Wirth J, Rulcova K, Eiselt J, Sefrna F, et al. Cell-free plasma DNA during peritoneal dialysis and hemodialysis and in patients with chronic kidney disease. Ann N Y Acad Sci 2008; 1137:296–301. [DOI] [PubMed] [Google Scholar]
- 8. Moreira VG, Prieto B, Rodriguez JS, Alvarez FV. Usefulness of cell-free plasma DNA, procalcitonin and C-reactive protein as markers of infection in febrile patients. Ann Clin Biochem 2010; 47:253–8. [DOI] [PubMed] [Google Scholar]