Abstract
Background
Aspartate aminotransferase-to-platelet ratio index (APRI), aspartate aminotransferase-to-alanine aminotransferase ratio (AAR), FIB-4, fibrosis index (FI), and King scores might be alternatives to the use of upper gastrointestinal endoscopy for the diagnosis of esophageal varices (EVs) in liver cirrhosis. This study aimed to evaluate their diagnostic accuracy in predicting the presence and severity of EVs in liver cirrhosis.
Material/Methods
All patients who were consecutively admitted to our hospital and underwent upper gastrointestinal endoscopy between January 2012 and June 2014 were eligible for this retrospective study. Areas under curve (AUCs) were calculated. Subgroup analyses were performed according to the history of upper gastrointestinal bleeding (UGIB) and splenectomy.
Results
A total of 650 patients with liver cirrhosis were included, and 81.4% of them had moderate-severe EVs. In the overall analysis, the AUCs of these non-invasive scores for predicting moderate-severe EVs and presence of any EVs were 0.506–0.6 and 0.539–0.612, respectively. In the subgroup analysis of patients without UGIB, their AUCs for predicting moderate-severe varices and presence of any EVs were 0.601–0.664 and 0.596–0.662, respectively. In the subgroup analysis of patients without UGIB or splenectomy, their AUCs for predicting moderate-severe varices and presence of any EVs were 0.627–0.69 and 0.607–0.692, respectively.
Conclusions
APRI, AAR, FIB-4, FI, and King scores had modest diagnostic accuracy of EVs in liver cirrhosis. They might not be able to replace the utility of upper gastrointestinal endoscopy for the diagnosis of EVs in liver cirrhosis.
MeSH Keywords: Blood Platelets; Endoscopy; Esophageal and Gastric Varices; Hypertension, Portal; Liver Cirrhosis
Background
Liver cirrhosis is one of the most common causes of death in the world [1,2]. Natural history of liver cirrhosis is primarily divided into four stages [3,4]. Stage 1, 2, 3, and 4 are characterized respectively by neither varices nor ascites, varices without ascites or bleeding, ascites with or without varices, and variceal bleeding with or without ascites, respectively. The prognosis is gradually worsened with increased stage of liver cirrhosis. Notably, the mortality is 3.4% per year in patients with varices who have never bled. By comparison, the mortality is up to 57% per year in patients with variceal bleeding. Thus, early diagnosis of varices and primary prophylaxis of variceal bleeding in high-risk patients with liver cirrhosis should be actively employed [5,6].
Upper gastrointestinal endoscopy is the golden diagnostic test of varices in liver cirrhosis. However, because of its invasiveness and discomfort, most of patients are reluctant to undergo this procedure. Recently, numerous non-invasive markers of varices have been explored in patients with liver cirrhosis [7–9]. However, they may be rarely used in clinical practices [10]. Herein, we aimed to evaluate the diagnostic accuracy of aspartate aminotransferase (AST) to platelet (PLT) ratio index (i.e., APRI), AST to alanine aminotransferase (ALT) ratio (i.e., AAR), FIB-4, fibrosis index (FI), and King scores in predicting the presence of varices and high-risk varices in liver cirrhosis. These non-invasive scores were selected, because they were readily available from regular laboratory tests and demographic data [11–15].
Material and Methods
Study design
All patients who were consecutively admitted to our hospital between January 2012 and June 2014 were considered in this retrospective study. The inclusion criteria were as follows: 1) patients were diagnosed with liver cirrhosis; 2) patients underwent both laboratory tests and endoscopic examinations. The exclusion criteria were as follows: 1) patients were diagnosed with malignant tumors; 2) patients did not undergo endoscopic examinations to evaluate the presence and degree of esophageal varices (EVs); and 3) the relevant laboratory data were missing. Notably, repeated admissions were not excluded. In other words, if one patient underwent endoscopy two or more times at different admissions during the enrollment period, all results would be included in our study. This was primarily because we just observed the association between non-invasive scores and varices. Some data had been reported in our previous papers [16–19]. This study was approved by the Ethics Committee of our hospital (number k(2015)11). Due to the retrospective nature of this study, patient written informed consents were waived.
Data collection
We collected the following data from electronic medical records: age, sex, etiology of liver diseases, ascites, hepatic encephalopathy (HE), history of upper gastrointestinal bleeding (UGIB), history of splenectomy, endoscopic findings, red blood cell (RBC), hemoglobin (Hb), white blood cell (WBC), PLT, ALT, AST, prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), albumin (ALB), total bilirubin (TBIL), alkaline phosphatase (ALP), γ-glutamine transferase (GGT) and creatinine (Cr). Additionally, we calculated the Child-Pugh [20], model for end-stage of liver disease (MELD) [21], APRI [11], AAR [12], FIB-4 [13], FI [14], and King scores [15].
Child-Pugh score = ALB score + TBIL score + INR score + ascites score + HE score
MELD score = 9.57x ln(Cr) + 3.78 × ln(TBIL) + 11.2 × ln (INR) + 6.43
APRI = [(AST/ULN) × 100]/PLT
AAR = AST/ALT
FIB-4 = (age*AST)/PLT*ALT1/2
FI = 8–0.01*PLT-ALB
King = age*AST*INR/PLT
Evaluation of EVs
Grade of EVs was classified into no, mild, moderate, and severe according to the 2008 Hangzhou consensus, which was proposed by the Chinese Society of Gastroenterology, Chinese Society of Hepatology, and Chinese Society of Digestive Endoscopy [22]. This classification is widely employed in China and is primarily based on the general rules by Japanese Society for Portal Hypertension, Baveno consensus, AASLD practice guidelines, and clinical practices in China [5,6,23]. We re-evaluated the grade of EVs by reviewing the original medical records and endoscopic results. Gastric varices were not considered in this study. Before the statistical analysis, we were blind to the correlation of EVs with non-invasive scores.
Statistical analysis
Categorical data were expressed as frequencies (percentages) and compared by using the chi-square tests. Continuous data were expressed as mean ± standard deviation and compared by using the independent sample t-tests. Receiver operating characteristic (ROC) curves were performed to evaluate and compare the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores for the prediction of EVs (moderate-severe versus no-mild EVs; with versus without EVs). The diagnostic performances were expressed as area under curve (AUC), sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predictive value, and negative predictive value. AUCs were compared by using DeLong test. Optimal cut-off values were chosen while the sum of sensitivity and specificity would be maximal. Subgroup analysis was performed in patients without any previous history of UGIB, in those with Child-Pugh class A or B+C, and in those without any previous history of splenectomy. A two-sided P<0.05 was considered statistically significant. All statistical analyses were performed by using the SPSS software version 18.0 (SPSS Inc. Chicago, IL, USA).
Results
Patients
Overall, 650 patients were eligible in our study. The characteristics of all patients are shown in Table 1. Among them, 81.4% had moderate-severe EVs, 81.8% had previous history of UGIB, and 52.6% had Child-Pugh classes B and C.
Table 1.
Overall analysis.
| Variables | Total Pts (n=650) | Moderate-large varices Pts (n=529) | No-mild varices Pts (n=121) | P value | With varices Pts (n=557) | Without varices Pts (n=93) | P value |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 425/225 | 353/176 | 72/49 | 0.132 | 373/184 | 52/41 | 0.038 |
| Age (years) | 53.54±11.75 | 53.61±11.82 | 53.27±11.48 | 0.774 | 53.38±11.85 | 54.51±11.14 | 0.393 |
| Etiology of liver diseases, n (%) | 0.396 | 0.386 | |||||
| Hepatitis B virus | 199 (30.6) | 169 (31.9) | 30 (24.8) | 176 (31.6) | 23 (24.7) | ||
| Hepatitis C virus | 46 (7.1) | 38 (7.2) | 8 (6.6) | 39 (7.0) | 7 (7.5) | ||
| Hepatitis B virus + Hepatitis C virus | 5 (0.8) | 5 (0.9) | 0 (0) | 5 (0.9) | 0 (0) | ||
| Alcohol | 154 (23.7) | 119 (22.5) | 35 (28.9) | 128 (23.0) | 26 (28.0) | ||
| Hepatitis B virus + Alcohol | 47 (7.2) | 40 (7.6) | 7 (5.8) | 41 (7.4) | 6 (6.5) | ||
| Unknown | 122 (18.8) | 91 (17.2) | 31 (25.6) | 97 (17.4) | 25 (26.9) | ||
| Others | 77 (11.8) | 67 (12.7) | 10 (8.3) | 71 (12.7) | 6 (6.5) | ||
| Ascites, n (%) | 0.029 | 0.007 | |||||
| No | 364 (56.0) | 284 (53.7) | 80 (66.1) | 298 (53.5) | 66 (71.0) | ||
| Mild | 91 (14.0) | 75 (14.2) | 16 (13.2) | 83 (14.9) | 8 (8.6) | ||
| Moderate to severe | 195 (30.0) | 170 (32.1) | 25 (20.7) | 176 (31.6) | 19 (20.4) | ||
| Hepatic encephalopathy, n (%) | 0.676 | 0.491 | |||||
| No | 637 (98.0) | 519 (98.1) | 118 (97.5) | 545 (97.8) | 92 (98.9) | ||
| Grade I–II | 13 (2.0) | 10 (1.9) | 3 (2.5) | 12 (2.2) | 1 (1.1) | ||
| Grade III–IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| History of UGIB (yes/no) | 532/118 | 467/62 | 65/56 | <0.001 | 489/68 | 43/50 | <0.001 |
| Varices, n (%) | NA | NA | |||||
| No | 93 (14.3) | 0 (0) | 93 (76.9) | 0 (0) | 93 (100) | ||
| Mild | 28 (4.3) | 0 (0) | 28 (23.1) | 28 (5.0) | 0 (0) | ||
| Moderate | 78 (12.0) | 78 (14.7) | 0 (0) | 78 (14.0) | 0 (0) | ||
| Severe | 451 (69.4) | 451 (85.3) | 0 (0) | 451 (81.0) | 0 (0) | ||
| Laboratory tests | |||||||
| RBC | 3.04±0.79 | 2.96±0.75 | 3.37±0.88 | <0.001 | 2.99±0.75 | 3.32±0.95 | <0.001 |
| Hb | 86.50±27.44 | 83.38±25.61 | 100.16±30.91 | <0.001 | 84.23±25.58 | 100.13±33.71 | <0.001 |
| WBC | 4.43±3.08 | 4.33±3.02 | 4.90±3.30 | 0.065 | 4.34±3.01 | 4.99±3.41 | 0.059 |
| PLT | 98.20±87.98 | 94.94±87.34 | 112.43±89.72 | 0.049 | 94.88±86.72 | 118.05±93.27 | 0.019 |
| TBIL | 26.25±29.22 | 25.30±26.60 | 30.40±38.54 | 0.084 | 25.84±26.74 | 28.72±41.20 | 0.38 |
| DBIL | 12.92±21.12 | 12.18±18.92 | 16.15±28.72 | 0.062 | 12.51±18.92 | 15.37±31.23 | 0.227 |
| IBIL | 13.27±10.68 | 13.08±10.32 | 14.08±12.14 | 0.353 | 13.27±10.35 | 13.24±12.52 | 0.979 |
| ALB | 33.21±6.36 | 32.80±6.38 | 34.98±6.00 | 0.001 | 32.86±6.33 | 35.30±6.20 | 0.001 |
| ALT | 34.30±57.40 | 31.07±27.93 | 48.42±118.91 | 0.003 | 31.20±27.63 | 52.87±135.00 | 0.001 |
| AST | 48.36±78.81 | 46.09±78.86 | 58.31±78.14 | 0.124 | 46.47±77.33 | 59.71±86.68 | 0.134 |
| ALP | 100.37±85.17 | 97.68±83.63 | 112.17±91.06 | 0.091 | 98.79±84.73 | 109.89±87.62 | 0.245 |
| GGT | 95.05±235.38 | 77.22±135.85 | 173.01±459.24 | <0.001 | 81.82±145.57 | 174.29±505.32 | <0.001 |
| BUN | 6.55±4.21 | 6.66±4.32 | 6.06±3.63 | 0.154 | 6.63±4.25 | 6.10±3.93 | 0.262 |
| Cr | 62.29±40.95 | 61.88±37.85 | 64.10±52.54 | 0.591 | 61.60±37.11 | 66.45±59.04 | 0.291 |
| PT | 16.02±3.45 | 16.17±3.50 | 15.36±3.13 | 0.019 | 16.17±3.46 | 15.14±3.27 | 0.008 |
| APTT | 41.93±8.82 | 41.95±9.21 | 41.85±6.90 | 0.907 | 42.05±9.12 | 41.21±6.70 | 0.396 |
| INR | 1.30±0.39 | 1.31±0.39 | 1.23±0.34 | 0.021 | 1.31±0.39 | 1.20±0.35 | 0.01 |
| Child-Pugh class, n (%) | 0.062 | 0.012 | |||||
| A | 308 (47.4) | 239 (45.2) | 69 (57.0) | 251 (45.1) | 57 (61.3) | ||
| B | 279 (42.9) | 237 (44.8) | 42 (34.7) | 248 (44.5) | 31 (33.3) | ||
| C | 63 (9.7) | 53 (10.0) | 10 (8.3) | 58 (10.4) | 5 (5.4) | ||
| Child-Pugh score | 6.60±1.76 | 7.03±1.76 | 6.64±1.72 | 0.027 | 7.04±1.78 | 6.45±1.54 | 0.003 |
| MELD score | 5.07±5.72 | 5.18±5.61 | 4.59±6.18 | 0.301 | 5.22±5.59 | 4.20±6.44 | 0.114 |
| APRI score | 2.15±3.88 | 2.15±4.11 | 2.15±2.60 | 1 | 2.16±4.03 | 2.09±2.80 | 0.864 |
| AAR score | 1.51±0.69 | 1.51±0.68 | 1.51±0.74 | 0.897 | 1.52±0.68 | 1.48±0.76 | 0.564 |
| FIB-4 score | 6.61±7.17 | 6.71±7.44 | 6.15±5.86 | 0.444 | 6.74±7.36 | 5.81±5.94 | 0.25 |
| FI score | −26.19±6.53 | −25.75±6.54 | −28.10±6.18 | <0.001 | −25.81±6.48 | −28.48±6.40 | <0.001 |
| King score | 61.17±213.86 | 63.56±235.06 | 50.74±64.08 | 0.552 | 63.21±229.33 | 48.99±67.93 | 0.553 |
AAR – AST to ALT ratio; ALB – albumin; ALP – alkaline phosphatase; ALT – alanine aminotransferase; APRI – AST to platelets ratio index; APTT – activated partial thromboplastin time; AST – aspartate aminotransferase; AUC – area under curve; BUN – blood urea nitrogen; Cr – creatinine; DBIL – direct bilirubin; FI – fibrosis index; FIB-4 – fibrosis 4 index; GGT – gamma-glutamyl transpeptidase; Hb – hemoglobin; IBIL – indirect bilirubin; INR – international normalized ratio; MELD – model for end stage liver disease; NA – not available; PLT – platelet; PT – prothrombin time; Pts – patients; RBC – red blood cell; TBIL – total bilirubin; UGIB – upper gastrointestinal bleeding; WBC – white blood cell.
Overall analysis
Moderate-severe versus no-mild EVs
Compared with the no-mild EVs group, the moderate-severe EVs group had significantly higher proportions of ascites and history of UGIB, significantly higher PT, INR, Child-Pugh score, and FI score, but significantly lower RBC, Hb, PLT, ALB, ALT, and GGT (Table 1).
FI score had the largest AUC (AUC=0.6), followed by FIB-4 (AUC=0.544), AAR (AUC=0.538), King (AUC=0.526), and APRI scores (AUC=0.506) (Figure 1A). AUC of FI score was not significantly different from that of FIB-4 (P=0.1041) or AAR score (P=0.0892), but was significantly larger than that of King (P=0.0293) and APRI scores (P=0.0093).
Figure 1.
Receiver operating characteristic curves showing the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores in predicting the presence of varices in liver cirrhosis. (A) Prediction of moderate-severe varices. (B) Prediction of varices. AUC – area under curve; PLR – positive likelihood ratio; PPV – positive predictive value; NLR – negative likelihood ratio; NPV – negative predictive value; Sen – sensitivity; Spec – specificity.
With versus without EVs
Compared with the no EVs group, the EVs group had significantly higher proportions of male, ascites, history of UGIB, and Child-Pugh class B+C, significantly higher PT, INR, Child-Pugh score, and FI score, but significantly lower RBC, Hb, PLT, ALB, ALT, and GGT (Table 1).
FI score had the largest AUC (AUC=0.612), followed by FIB-4 (AUC=0.567), AAR (AUC=0.56), King (AUC=0.55), and APRI scores (AUC=0.539) (Figure 1B). AUC of FI score was not significantly different from that of FIB-4 (P=0.2510), AAR (P=0.2167), King (P=0.1144), or APRI score (P=0.0873).
Subgroup analysis in patients without UGIB
Moderate-severe versus no-mild EVs
Compared with the no-mild EVs group, the moderate-severe EVs group had significantly higher FIB-4 and FI scores, but significantly lower PLT and ALB (Table 2).
Table 2.
Subgroup analysis of patients without UGIB.
| Variables | Total Pts (n=118) | Moderate-large varices Pts (n=62) | No-Mild varices Pts (n=56) | P value | With varices Pts (n=68) | Without varices Pts (n=50) | P value |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 69/49 | 36/26 | 33/23 | 0.924 | 38/30 | 31/19 | 0.505 |
| Age (years) | 55.09±11.02 | 55.89±10.86 | 54.21±11.24 | 0.41 | 54.90±11.59 | 55.35±10.32 | 0.828 |
| Etiology of liver diseases, n (%) | 0.041 | 0.161 | |||||
| Hepatitis B virus | 28 (23.7) | 19 (30.6) | 9 (16.1) | 19 (27.9) | 9 (18.0) | ||
| Hepatitis C virus | 8 (6.8) | 5 (8.1) | 3 (5.4) | 6 (8.8) | 2 (4.0) | ||
| Hepatitis B virus + Hepatitis C virus | 1 (0.8) | 1 (1.6) | 0 (0) | 1 (1.5) | 0 (0) | ||
| Alcohol | 30 (25.4) | 13 (21.0) | 17 (30.4) | 14 (20.6) | 16 (32.0) | ||
| Hepatitis B virus + Alcohol | 8 (6.8) | 5 (8.1) | 3 (5.4) | 5 (7.4) | 3 (6.0) | ||
| Unknown | 33 (28.0) | 11 (17.7) | 22 (39.3) | 15 (22.1) | 18 (36.0) | ||
| Others | 10 (8.4) | 8 (12.9) | 2 (3.6) | 8 (11.8) | 2 (4) | ||
| Ascites, n (%) | 0.524 | 0.172 | |||||
| No | 69 (58.5) | 34 (54.8) | 35 (62.5) | 35 (51.5) | 34 (68.0) | ||
| Mild | 18 (15.3) | 9 (14.5) | 9 (16.1) | 13 (19.1) | 5 (10.0) | ||
| Moderate to severe | 31 (26.3) | 19 (30.6) | 12 (21.4) | 20 (29.4) | 11 (22.0) | ||
| Hepatic encephalopathy, n (%) | 0.34 | 0.389 | |||||
| No | 117 (99.2) | 61 (98.4) | 56 (100) | 67 (98.5) | 50 (100) | ||
| Grade I–II | 1 (0.8) | 1 (1.6) | 0 (0) | 1 (1.5) | 0 (0) | ||
| Grade III–IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Varices, n (%) | NA | NA | |||||
| No | 50 (42.4) | 0 (0) | 50 (89.3) | 0 (0) | 50 (100) | ||
| Mild | 6 (5.1) | 0 (0) | 6 (10.7) | 6 (8.8) | 0 (0) | ||
| Moderate | 20 (16.9) | 20(32.3) | 0 (0) | 20 (29.4) | 0 (0) | ||
| Severe | 42 (35.6) | 42 (67.7) | 0 (0) | 42 (61.8) | 0 (0) | ||
| Laboratory tests | |||||||
| RBC | 3.72±0.74 | 3.68±0.69 | 3.76±0.79 | 0.571 | 3.68±0.68 | 3.78±0.82 | 0.459 |
| Hb | 116.69±25.85 | 115.45±25.58 | 118.07±26.30 | 0.585 | 115.12±25.03 | 118.84±27.03 | 0.442 |
| WBC | 4.26±2.29 | 3.94±2.39 | 4.62±2.13 | 0.106 | 3.91±2.34 | 4.74±2.14 | 0.051 |
| PLT | 90.72±59.50 | 76.92±50.82 | 106.00±64.91 | 0.007 | 76.76±50.41 | 109.70±65.88 | 0.003 |
| TBIL | 31.02±36.80 | 30.39±21.34 | 31.72±48.73 | 0.845 | 30.15±20.97 | 32.20±51.28 | 0.767 |
| DBIL | 16.79±29.45 | 15.40±17.11 | 18.33±38.93 | 0.592 | 15.33±16.68 | 18.77±41.03 | 0.533 |
| IBIL | 14.19±9.92 | 14.94±8.40 | 13.37±11.39 | 0.394 | 14.75±8.35 | 13.44±11.78 | 0.482 |
| ALB | 35.30±6.13 | 34.04±5.84 | 36.70±6.20 | 0.018 | 33.97±5.98 | 37.11±5.92 | 0.006 |
| ALT | 55.03±122.69 | 45.95±46.68 | 65.07±171.49 | 0.4 | 44.84±44.79 | 68.88±181.27 | 0.295 |
| AST | 70.42±102.52 | 74.45±106.22 | 65.95±99.02 | 0.655 | 72.15±101.98 | 68.06±104.24 | 0.832 |
| ALP | 120.34±87.20 | 124.76±99.60 | 115.46±71.59 | 0.565 | 126.68±100.69 | 111.73±64.50 | 0.36 |
| GGT | 143.04±223.66 | 138.85±240.15 | 147.68±205.94 | 0.832 | 142.13±238.07 | 144.28±204.81 | 0.959 |
| BUN | 5.61±3.35 | 5.37±2.18 | 5.89±4.29 | 0.402 | 5.37±2.11 | 5.94±4.52 | 0.365 |
| Cr | 64.85±55.02 | 58.35±27.04 | 72.05±74.35 | 0.178 | 57.34±26.15 | 75.06±78.15 | 0.084 |
| PT | 15.07±2.41 | 15.42±2.20 | 14.67±2.58 | 0.093 | 15.45±2.38 | 14.55±2.41 | 0.044 |
| APTT | 42.74±6.58 | 43.31±6.43 | 42.10±6.74 | 0.323 | 43.79±6.90 | 41.31±5.89 | 0.043 |
| INR | 1.19±0.25 | 1.23±0.24 | 1.15±0.27 | 0.094 | 1.23±0.25 | 1.14±0.25 | 0.042 |
| Child-Pugh class, n (%) | 0.633 | 0.211 | |||||
| A | 62 (52.5) | 30 (48.4) | 32 (57.1) | 31 (45.6) | 31 (62.0) | ||
| B | 47 (39.8) | 27 (43.5) | 20 (35.7) | 31 (45.6) | 16 (32.0) | ||
| C | 9 (7.6) | 5 (8.1) | 4 (7.1) | 6 (8.8) | 3 (6.0) | ||
| Child-Pugh score | 6.69±1.73 | 6.89±1.81 | 6.48±1.63 | 0.206 | 6.96±1.86 | 6.34±1.49 | 0.056 |
| MELD score | 4.72±5.83 | 5.16±4.71 | 4.24±6.88 | 0.392 | 4.97±4.77 | 4.39±7.07 | 0.591 |
| APRI score | 3.13±5.13 | 3.69±6.44 | 2.50±3.01 | 0.209 | 3.58±6.18 | 2.51±3.13 | 0.261 |
| AAR score | 1.58±0.84 | 1.68±0.89 | 1.48±0.79 | 0.206 | 1.66±0.87 | 1.48±0.80 | 0.266 |
| FIB-4 score | 8.24±8.27 | 9.87±9.66 | 6.45±5.98 | 0.024 | 9.58±9.38 | 6.42±6.10 | 0.04 |
| FI score | −28.21±6.23 | −26.81±5.83 | −29.76±6.25 | 0.01 | −26.74±5.98 | −30.21±6.07 | 0.002 |
| King score | 81.27±176.82 | 101.92±231.32 | 58.40±78.43 | 0.183 | 97.82±221.78 | 58.76±80.67 | 0.237 |
AAR – AST to ALT ratio; ALB – albumin; ALP – alkaline phosphatase; ALT – alanine aminotransferase; APRI – AST to platelets ratio index; APTT – activated partial thromboplastin time; AST – aspartate aminotransferase; AUC – area under curve; BUN – blood urea nitrogen; Cr – creatinine; DBIL – direct bilirubin; FI – fibrosis index; FIB-4 – fibrosis 4 index; GGT – gamma-glutamyl transpeptidase; Hb – hemoglobin; IBIL – indirect bilirubin; INR – international normalized ratio; MELD – model for end-stage liver disease; NA – not available; PLT – platelet; PT – prothrombin time; Pts – patients; RBC – red blood cell; TBIL – total bilirubin; UGIB – upper gastrointestinal bleeding; WBC – white blood cell.
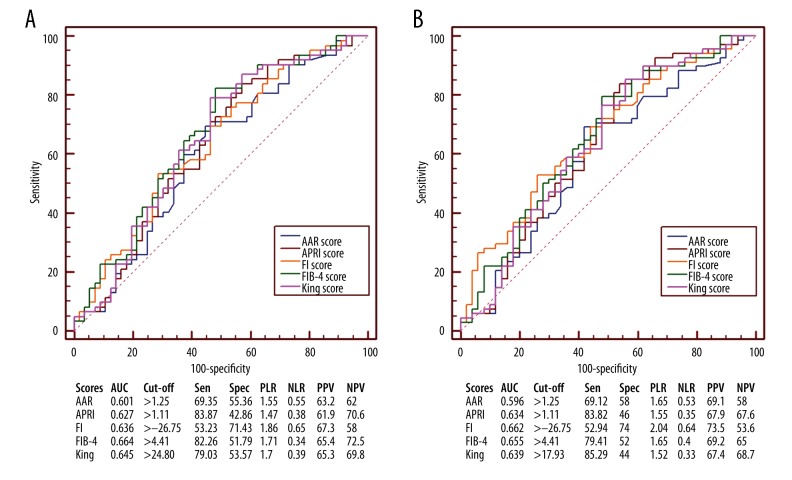
FIB-4 score had the largest AUC (AUC=0.664), followed by King (AUC=0.645), FI (AUC=0.636), APRI (AUC=0.627), and AAR scores (AUC=0.601) (Figure 2A). AUC of FIB-4 score was not significantly different from that of FI (P=0.6317), King (P=0.3537), AAR (P=0.3037), or APRI score (P=0.1571).
Figure 2.
Receiver operating characteristic curves showing the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores in predicting the presence of varices in liver cirrhosis without UGIB. (A) Prediction of moderate-severe varices. (B) Prediction of varices. AUC – area under curve; PLR – positive likelihood ratio; PPV – positive predictive value; NLR – negative likelihood ratio; NPV – negative predictive value; Sen – sensitivity; Spec – specificity.
With versus without EVs
Compared with the no EVs group, the EVs group had significantly higher PT, APTT, INR, FIB-4 score, and FI score, but significantly lower PLT and ALB (Table 2).
FI score had the largest AUC (AUC=0.662), followed by FIB-4 (AUC=0.655), King (AUC=0.639), APRI (AUC=0.634), and AAR scores (AUC=0.596) (Figure 2B). The AUC of FI score was not significantly different from that of FIB-4 (P=0.9120), King (P=0.6968), APRI (P=0.6530), or AAR score (P=0.3083).
Subgroup analysis in patients without UGIB at Child-Pugh class A
Moderate-severe versus no-mild EVs
Compared with the no-mild EVs group, the moderate-severe EVs group had significantly higher PT and INR, but a significantly lower WBC (Table 3).
Table 3.
Subgroup analysis of patients without UGIB at Child-Pugh class A.
| Variables | Total Pts (n=62) | Moderate-large varices Pts (n=30) | No-mild varices Pts (n=32) | P value | With warices Pts (n=31) | Without varices Pts (n=31) | P value |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 33/29 | 17/13 | 16/16 | 0.599 | 17/14 | 16/15 | 0.799 |
| Age (years) | 54.61±11.50 | 55.19±11.42 | 54.06±11.74 | 0.702 | 55.09±11.24 | 54.12±11.93 | 0.741 |
| Etiology of liver diseases, n (%) | 0.159 | 0.244 | |||||
| Hepatitis B virus | 22 (35.5) | 14 (46.7) | 8 (25.0) | 14 (45.2) | 8 (25.8) | ||
| Hepatitis C virus | 3 (4.8) | 2 (6.7) | 1 (3.1) | 2 (6.5) | 1 (3.2) | ||
| Hepatitis B virus + Hepatitis C virus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Alcohol | 11 (17.7) | 4 (13.3) | 7 (21.9) | 4 (12.9) | 7 (22.6) | ||
| Hepatitis B virus + Alcohol | 3 (4.8) | 2 (6.7) | 1 (3.1) | 2 (6.5) | 1 (3.2) | ||
| Unknown | 20 (32.3) | 6 (20.0) | 14 (43.8) | 7 (22.6) | 13 (41.9) | ||
| Others | 3 (4.8) | 2 (6.7) | 1 (3.1) | 2 (6.5) | 1 (3.2) | ||
| Ascites, n (%) | 0.947 | 1 | |||||
| No | 58 (93.5) | 28 (93.3) | 30 (93.8) | 29 (93.5) | 29 (93.5) | ||
| Mild | 4 (6.5) | 2 (6.7) | 2 (6.3) | 2 (6.5) | 2 (6.5) | ||
| Moderate to severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Hepatic encephalopathy, n (%) | NA | NA | |||||
| No | 62 (100) | 30 (100) | 32 (100) | 31 (100) | 31 (100) | ||
| Grade I–II | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Grade III–IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Varices, n (%) | NA | NA | |||||
| No | 31 (50.0) | 0 (0) | 31 (96.9) | 0 (0) | 31 (100) | ||
| Mild | 1 (1.6) | 0 (0) | 1 (3.1) | 3 (3.2) | 0 (0) | ||
| Moderate | 8 (12.9) | 8 (26.7) | 0 (0) | 8 (25.8) | 0 (0) | ||
| Severe | 22 (35.5) | 22 (73.3) | 0 (0) | 22 (71.0) | 0 (0) | ||
| Laboratory tests | |||||||
| RBC | 3.95±0.71 | 3.40±0.57 | 3.90±0.83 | 0.588 | 3.99±0.56 | 3.91±0.84 | 0.637 |
| Hb | 122.03±25.39 | 123.23±23.38 | 120.91±27.47 | 0.722 | 123.00±23.02 | 121.06±27.91 | 0.767 |
| WBC | 3.82±1.52 | 3.37±1.10 | 4.24±1.75 | 0.023 | 3.38±1.08 | 4.26±1.78 | 0.022 |
| PLT | 87.34±50.03 | 76.10±44.14 | 97.88±53.52 | 0.087 | 76.48±43.45 | 98.19±54.38 | 0.088 |
| TBIL | 19.43±9.53 | 20.66±7.61 | 18.27±11.04 | 0.327 | 20.57±7.50 | 18.29±11.22 | 0.35 |
| DBIL | 7.66±3.93 | 7.93±3.25 | 7.34±4.52 | 0.56 | 7.88±3.20 | 7.37±4.59 | 0.614 |
| IBIL | 11.72±5.90 | 12.63±4.78 | 10.87±6.75 | 0.242 | 12.53±4.74 | 10.92±6.85 | 0.287 |
| ALB | 38.68±4.62 | 38.09±4.44 | 39.23±4.79 | 0.338 | 38.12±4.37 | 39.23±4.87 | 0.347 |
| ALT | 45.19±50.60 | 46.07±48.51 | 44.38±53.25 | 0.897 | 46.00±47.70 | 44.39±54.13 | 0.901 |
| AST | 51.81±51.21 | 56.17±61.74 | 47.72±39.50 | 0.521 | 55.61±60.78 | 48.00±40.12 | 0.563 |
| ALP | 100.61±70.72 | 109.89±94.03 | 91.92±37.51 | 0.321 | 113.83±95.02 | 87.40±27.91 | 0.142 |
| GGT | 104.42±177.76 | 89.47±144.00 | 118.44±205.82 | 0.526 | 105.42±167.13 | 103.42±190.57 | 0.965 |
| BUN | 5.10±2.34 | 5.22±1.30 | 4.99±3.03 | 0.704 | 5.26±1.30 | 4.94±3.07 | 0.594 |
| Cr | 60.24±49.09 | 54.74±10.69 | 65.39±67.66 | 0.398 | 54.95±10.57 | 65.53±68.78 | 0.4 |
| PT | 14.11±1.55 | 14.51±1.62 | 13.74±1.42 | 0.05 | 14.42±1.67 | 13.81±1.39 | 0.119 |
| APTT | 40.95±5.49 | 41.21±5.67 | 40.70±5.39 | 0.718 | 41.32±5.61 | 40.58±5.43 | 0.6 |
| INR | 1.09±0.15 | 1.14±0.16 | 1.05±0.14 | 0.036 | 1.13±0.17 | 1.06±0.14 | 0.089 |
| Child-Pugh score | 5.35±0.48 | 5.33±0.48 | 5.38±0.49 | 0.737 | 5.32±0.48 | 5.39±0.50 | 0.603 |
| MELD score | 2.42±3.99 | 3.13±3.17 | 1.76±4.58 | 0.179 | 3.06±3.14 | 1.78±4.66 | 0.21 |
| APRI score | 2.29±2.75 | 2.44±2.73 | 2.15±2.80 | 0.68 | 2.40±2.69 | 2.18±2.84 | 0.75 |
| AAR score | 1.29±0.44 | 1.29±0.36 | 1.29±0.50 | 0.979 | 1.28±0.36 | 1.30±0.50 | 0.835 |
| FIB-4 score | 6.26±5.03 | 6.84±4.46 | 5.71±5.53 | 0.382 | 6.73±4.42 | 5.79±5.61 | 0.462 |
| FI score | −31.55±4.68 | −30.85±4.44 | −32.20±4.87 | 0.258 | −30.88±4.37 | −32.21±4.95 | 0.266 |
| King score | 51.28±70.67 | 57.61±75.38 | 45.34±66.61 | 0.499 | 56.39±74.42 | 44.17±67.55 | 0.573 |
AAR – AST to ALT ratio; ALB – albumin; ALP – alkaline phosphatase; ALT – alanine aminotransferase; APRI – AST to platelets ratio index; APTT – activated partial thromboplastin time; AST – aspartate aminotransferase; AUC – area under curve; BUN – blood urea nitrogen; Cr – creatinine; DBIL – direct bilirubin; FI – fibrosis index; FIB-4 – fibrosis 4 index; GGT – gamma-glutamyl transpeptidase; Hb – hemoglobin; IBIL – indirect bilirubin; INR – international normalized ratio; MELD – model for end-stage liver disease; NA – not available; PLT – platelet; PT – prothrombin time; Pts – patients; RBC – red blood cell; TBIL – total bilirubin; UGIB – upper gastrointestinal bleeding; WBC – white blood cell.
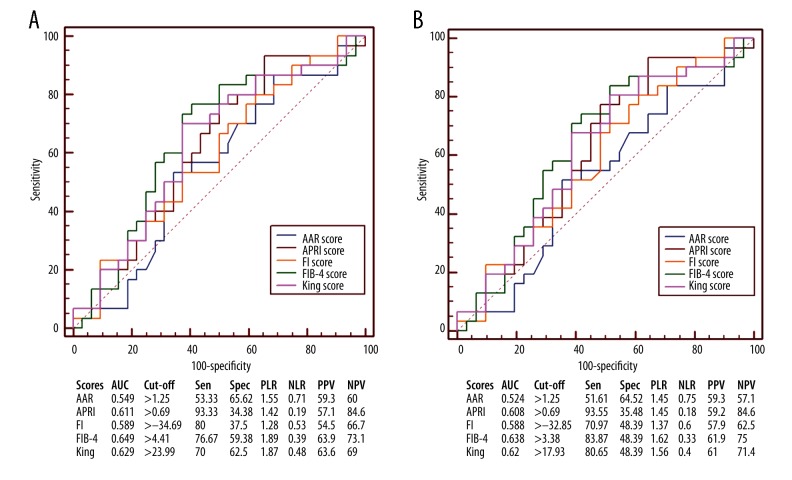
FIB-4 score had the largest AUC (AUC=0.649), followed by King (AUC=0.629), APRI (AUC=0.611), FI (AUC=0.589), and AAR scores (AUC=0.549) (Figure 3A). AUC of FIB-4 score was not significantly different from that of King (P=0.5172), FI (P=0.4906), APRI (P=0.3419), or AAR score (P=0.3025).
Figure 3.
Receiver operating characteristic curves showing the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores in predicting the presence of varices in liver cirrhosis without UGIB at Child-Pugh class A. (A) Prediction of moderate-severe varices. (B) Prediction of varices. AUC – area under curve; PLR – positive likelihood ratio; PPV – positive predictive value; NLR – negative likelihood ratio; NPV – negative predictive value; Sen – sensitivity; Spec – specificity.
With versus without EVs
Compared with the no EVs group, the EVs group had a significantly lower WBC (Table 3).
FIB-4 score had the largest AUC (AUC=0.638), followed by King (AUC=0.62), APRI (AUC=0.608), FI (AUC=0.588), and AAR scores (AUC=0.524) (Figure 3B). The AUC of FIB-4 score was not significantly different from that of FI (P=0.5732), King (P=0.5542), APRI (P=0.4411), or AAR score (P=0.2463).
Subgroup analysis in patients without UGIB at Child-Pugh class B and C
Moderate-severe versus no-mild EVs
Compared with the no-mild EVs group, the moderate-severe EVs group had a significantly higher FI score, but significantly lower PLT and ALB (Table 4).
Table 4.
Subgroup analysis patients without UGIB at Child-Pugh class B and C.
| Variables | Total Pts (n=56) | Moderate-large varices Pts (n=32) | No-mild varices Pts (n=24) | P value | With varices Pts (n=37) | Without varices Pts (n=19) | P value |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 36/20 | 19/13 | 17/7 | 0.376 | 21/16 | 15/4 | 0.101 |
| Age (years) | 55.63±10.55 | 56.55±10.45 | 54.41±10.78 | 0.457 | 54.74±12.02 | 57.36±6.79 | 0.383 |
| Etiology of liver diseases, n (%) | 0.355 | 0.617 | |||||
| Hepatitis B virus | 6 (10.7) | 5 (15.6) | 1 (4.2) | 5 (13.5) | 1 (5.3) | ||
| Hepatitis C virus | 5 (8.9) | 3 (9.4) | 2 (8.3) | 4 (10.8) | 1 (5.3) | ||
| Hepatitis B virus + Hepatitis C virus | 1 (1.8) | 1 (3.1) | 0 (0) | 1 (2.7) | 0 (0) | ||
| Alcohol | 19 (33.9) | 9 (28.1) | 10 (41.7) | 10 (27.0) | 9 (47.4) | ||
| Hepatitis B virus + Alcohol | 5 (8.9) | 3 (9.4) | 2 (8.3) | 3 (8.1) | 2 (10.5) | ||
| Unknown | 13 (23.2) | 5 (15.6) | 8 (33.3) | 8 (21.6) | 5 (26.3) | ||
| Others | 7 (12.5) | 6 (18.8) | 1 (4.2) | 6 (16.2) | 1 (5.3) | ||
| Ascites, n (%) | 0.763 | 0.436 | |||||
| No | 11 (19.6) | 6 (18.8) | 5 (20.8) | 6 (16.2) | 5 (26.3) | ||
| Mild | 14 (25.0) | 7 (21.9) | 7 (29.2) | 11 (29.7) | 3 (15.8) | ||
| Moderate to severe | 31 (55.4) | 19 (59.4) | 12 (50.0) | 20 (54.1) | 11 (57.9) | ||
| Hepatic encephalopathy, n (%) | 0.382 | 0.47 | |||||
| No | 55 (98.2) | 31 (96.9) | 24 (100) | 36 (97.3) | 19 (100) | ||
| Grade I–II | 1 (1.8) | 1 (3.1) | 0 (0) | 1 (2.7) | 0 (0) | ||
| Grade III–IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Varices, n (%) | NA | NA | |||||
| No | 19 (33.9) | 0 (0) | 19 (79.2) | 0 (0) | 19 (100) | ||
| Mild | 5 (8.9) | 0 (0) | 5 (20.8) | 5 (13.5) | 0 (0) | ||
| Moderate | 12 (21.4) | 12 (37.5) | 0 (0) | 12 (32.4) | 0 (0) | ||
| Severe | 20 (35.7) | 20 (62.5) | 0 (0) | 20 (54.1) | 0 (0) | ||
| Laboratory tests | |||||||
| RBC | 3.47±0.70 | 3.38±0.68 | 3.57±0.72 | 0.321 | 3.41±0.66 | 3.57±0.76 | 0.418 |
| Hb | 110.79±25.26 | 108.16±25.74 | 114.29±24.72 | 0.373 | 108.51±25.02 | 115.21±25.83 | 0.352 |
| WBC | 4.76±2.84 | 4.48±3.08 | 5.13±2.50 | 0.398 | 4.36±2.97 | 5.53±2.48 | 0.147 |
| PLT | 94.46±68.76 | 77.69±57.08 | 116.83±77.46 | 0.034 | 77.00±56.17 | 128.47±79.29 | 0.007 |
| TBIL | 43.86±49.60 | 39.50±25.78 | 49.66±70.19 | 0.453 | 38.18±25.02 | 54.90±77.92 | 0.236 |
| DBIL | 26.93±40.35 | 22.41±21.48 | 32.97±56.61 | 0.337 | 21.58±20.53 | 37.37±62.91 | 0.168 |
| IBIL | 16.93±14.50 | 17.10±10.37 | 16.71±15.11 | 0.911 | 16.61±10.16 | 17.56±16.44 | 0.789 |
| ALB | 31.57±5.42 | 30.24±4.23 | 33.34±6.35 | 0.033 | 30.50±4.86 | 33.65±5.96 | 0.038 |
| ALT | 65.91±170.15 | 45.84±45.67 | 92.67±255.18 | 0.313 | 43.86±42.84 | 108.84±286.09 | 0.178 |
| AST | 91.02±136.48 | 91.59±134.20 | 90.25±142.36 | 0.971 | 86.00±125.87 | 100.79±158.35 | 0.705 |
| ALP | 142.19±98.51 | 138.69±104.10 | 146.84±92.52 | 0.762 | 137.44±105.29 | 151.43±85.69 | 0.619 |
| GGT | 185.80±260.43 | 185.16±299.19 | 186.67±203.82 | 0.983 | 172.89±282.97 | 210.95±214.69 | 0.609 |
| BUN | 6.18±4.14 | 5.50±2.78 | 7.08±5.39 | 0.161 | 5.46±2.62 | 7.57±5.96 | 0.072 |
| Cr | 69.96±60.95 | 61.72±36.15 | 80.93±83.08 | 0.247 | 59.35±34.22 | 90.62±91.26 | 0.069 |
| PT | 16.12±2.74 | 16.27±2.36 | 15.92±3.22 | 0.638 | 16.31±2.51 | 15.75±3.18 | 0.479 |
| APTT | 44.72±7.15 | 45.28±6.57 | 43.98±7.94 | 0.506 | 45.85±7.26 | 42.51±6.55 | 0.097 |
| INR | 1.30±0.29 | 1.32±0.27 | 1.28±0.33 | 0.651 | 1.32±0.28 | 1.27±0.33 | 0.489 |
| Child-Pugh score | 8.18±1.36 | 8.34±1.31 | 7.96±1.43 | 0.299 | 8.32±1.42 | 7.89±1.24 | 0.268 |
| MELD score | 7.27±6.49 | 7.07±5.15 | 7.54±8.06 | 0.79 | 6.57±5.32 | 8.63±8.32 | 0.265 |
| APRI score | 4.05±6.77 | 4.86±8.48 | 2.97±3.28 | 0.305 | 4.57±7.93 | 3.04±3.57 | 0.429 |
| AAR score | 1.90±1.05 | 2.03±1.08 | 1.73±1.01 | 0.286 | 1.97±1.04 | 1.77±1.09 | 0.502 |
| FIB-4 score | 10.44±10.40 | 12.70±12.16 | 7.42±6.53 | 0.06 | 11.97±11.60 | 7.46±6.87 | 0.126 |
| FI score | −24.51±5.65 | −23.02±4.23 | −26.51±6.71 | 0.021 | −23.27±4.84 | −26.94±6.43 | 0.02 |
| King score | 114.47±242.56 | 143.47±310.32 | 75.81±90.42 | 0.306 | 132.53±290.18 | 79.31±96.92 | 0.442 |
AAR – AST to ALT ratio; ALB – albumin; ALP – alkaline phosphatase; ALT – alanine aminotransferase; APRI – AST to platelets ratio index; APTT – activated partial thromboplastin time; AST – aspartate aminotransferase; AUC – area under curve; BUN – blood urea nitrogen; Cr – creatinine; DBIL – direct bilirubin; FI – fibrosis index; FIB-4 – fibrosis 4 index; GGT – gamma-glutamyl transpeptidase; Hb – hemoglobin; IBIL – indirect bilirubin; INR – international normalized ratio; MELD – model for end-stage liver disease; NA – not available; PLT – platelet; PT – prothrombin time; Pts – patients; RBC – red blood cell; TBIL – total bilirubin; UGIB – upper gastrointestinal bleeding; WBC – white blood cell.
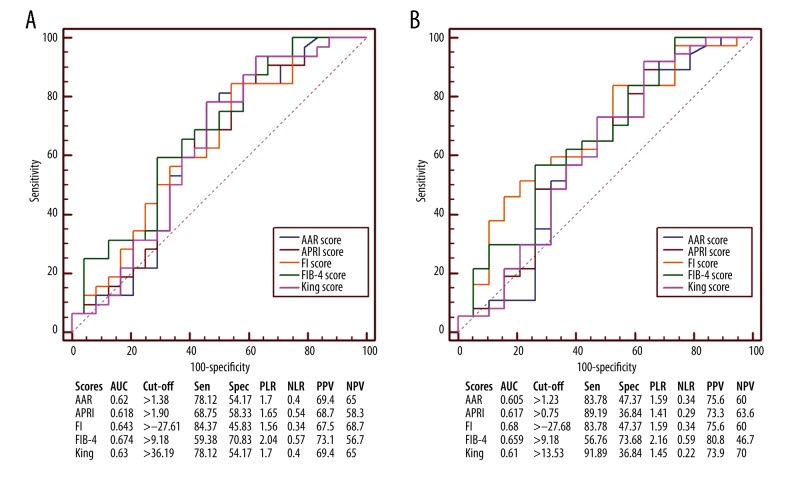
FIB-4 score had the largest AUC (AUC=0.674), followed by FI (AUC=0.643), King (AUC=0.63), AAR (AUC=0.62), and APRI scores (AUC=0.618) (Figure 4A). The AUC of FIB-4 score was not significantly different from that of FI (P=0.7411), AAR (P=0.5294), King (P=0.2340), or APRI score (P=0.1717).
Figure 4.
Receiver operating characteristic curves showing the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores in predicting the presence of varices in liver cirrhosis without UGIB at Child-Pugh classes B and C. (A) Prediction of moderate-severe varices. (B) Prediction of varices. AUC – area under curve; PLR – positive likelihood ratio; PPV – positive predictive value; NLR – negative likelihood ratio; NPV – negative predictive value; Sen – sensitivity; Spec – specificity.
With versus without EVs
Compared with the no EVs group, the EVs group had a significantly higher FI score, but significantly lower PLT and ALB (Table 4).
FI score had the largest AUC (AUC=0.68), followed by FIB-4 (AUC=0.659), APRI (AUC=0.617), King (AUC=0.61), and AAR scores (AUC=0.605) (Figure 4B). The AUC of FI score was not significantly different from that of FIB-4 (P=0.8261), APRI (P=0.5687), King (P=0.5217), or AAR score (P=0.5058).
Subgroup analysis in patients without UGIB or splenectomy
Moderate-severe versus no-mild EVs
Compared with the no-mild EVs group, moderate-severe EVs group had significantly higher FIB-4 and FI scores, but significantly lower PLT and ALB (Table 5).
Table 5.
Subgroup analysis of patients without UGIB or splenectomy.
| Variables | Total Pts (n=112) | Moderate-large varices Pts (n=57) | No-mild varices Pts (n=55) | P value | With varices Pts (n=62) | Without varices Pts (n=50) | P value |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 66/46 | 33/24 | 33/22 | 0.821 | 35/27 | 31/19 | 0.553 |
| Age (years) | 55.19±10.50 | 55.49±10.91 | 54.88±10.15 | 0.757 | 55.06±10.73 | 55.35±10.32 | 0.885 |
| Etiology of liver diseases, n (%) | 0.047 | 0.149 | |||||
| Hepatitis B virus | 28 (25.0) | 19 (33.3) | 9 (16.4) | 19 (30.6) | 9 (18.0) | ||
| Hepatitis C virus | 6 (5.4) | 3 (5.3) | 3 (5.5) | 4 (6.5) | 2 (4.0) | ||
| Hepatitis B virus + Hepatitis C virus | 1 (0.9) | 1 (1.8) | 0 (0) | 1 (1.6) | 0 (0) | ||
| Alcohol | 28 (25.0) | 11 (19.3) | 17 (30.9) | 12 (19.4) | 16 (32.0) | ||
| Hepatitis B virus + Alcohol | 7 (6.3) | 4 (7.0) | 3 (5.5) | 4 (6.5) | 3 (6.0) | ||
| Unknown | 32 (28.6) | 11 (19.3) | 21 (38.2) | 14 (22.6) | 18 (36.0) | ||
| Others | 10 (9.0) | 8 (14.0) | 2 (3.6) | 8 (12.9) | 2 (4.0) | ||
| Ascites, n (%) | 0.495 | 0.202 | |||||
| No | 66 (58.9) | 31 (54.4) | 35 (63.6) | 32 (51.6) | 34 (68.0) | ||
| Mild | 16 (14.3) | 8 (14.0) | 8 (14.5) | 11 (17.7) | 5 (10.0) | ||
| Moderate to severe | 30 (26.8) | 18 (31.6) | 12 (21.8) | 19 (30.6) | 11 (22.0) | ||
| Hepatic encephalopathy, n (%) | 0.324 | 0.367 | |||||
| No | 111 (99.1) | 56 (98.2) | 55 (100) | 61 (98.4) | 50 (100) | ||
| Grade I–II | 1 (0.9) | 1 (1.8) | 0 (0) | 1 (1.6) | 0 (0) | ||
| Grade III–IV | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Varices, n (%) | NA | NA | |||||
| No | 50 (44.6) | 0 (0) | 50 (90.9) | 0 (0) | 50 (100) | ||
| Mild | 5 (4.5) | 0 (0) | 5 (9.1) | 5 (8.1) | 0 (0) | ||
| Moderate | 17 (15.2) | 17 (29.8) | 0 (0) | 17 (27.4) | 0 (0) | ||
| Severe | 40 (35.7) | 40 (70.2) | 0 (0) | 40 (64.5) | 0 (0) | ||
| Laboratory tests | |||||||
| RBC | 3,72±0.75 | 3.67±0.70 | 3.77±0.80 | 0.511 | 3.67±0.69 | 3.78±0.82 | 0.452 |
| Hb | 116.52±25.46 | 114.95±24.51 | 118.15±26.54 | 0.509 | 114.65±24.19 | 118.84±27.03 | 0.388 |
| WBC | 4.22±2.32 | 3.88±2.47 | 4.58±2.12 | 0.111 | 3.80±2.39 | 4.74±2.14 | 0.032 |
| PLT | 86.51±56.75 | 68.74±40.66 | 104.93±65.01 | 0.001 | 67.81±39.71 | 109.70±65.88 | ﹤0.01 |
| TBIL | 31.39±37.63 | 30.93±21.79 | 31.87±49.17 | 0.895 | 30.74±21.52 | 32.20±51.28 | 0.839 |
| DBIL | 17.21±30.15 | 15.98±17.66 | 18.49±39.27 | 0.662 | 15.95±17.29 | 18.77±41.03 | 0.625 |
| IBIL | 14.14±10.01 | 14.89±8.38 | 13.36±11.49 | 0.42 | 14.70±8.39 | 13.44±11.78 | 0.509 |
| ALB | 35.33±6.03 | 33.82±5.63 | 36.89±6.09 | 0.007 | 33.89±5.78 | 37.11±5.92 | 0.005 |
| ALT | 54.96±125.56 | 44.88±46.45 | 65.42±173.05 | 0.389 | 43.74±44.76 | 68.88±181.27 | 0.294 |
| AST | 70.61±104.86 | 75.81±110.16 | 62.22±99.78 | 0.595 | 72.66±106.15 | 68.06±104.24 | 0.819 |
| ALP | 120.29±85.90 | 129.11±102.22 | 111.15±64.53 | 0.271 | 127.19±99.89 | 111.73±64.50 | 0.346 |
| GGT | 145.40±228.06 | 146.47±249.14 | 144.29±206.25 | 0.96 | 146.31±246.88 | 144.28±204.81 | 0.963 |
| BUN | 5.62±3.42 | 5.40±2.24 | 5.86±4.33 | 0.476 | 5.37±2.16 | 5.94±4.52 | 0.379 |
| Cr | 65.27±56.43 | 58.48±28.12 | 72.31±75.01 | 0.196 | 57.37±27.32 | 75.06±78.15 | 0.099 |
| PT | 15.03±2.43 | 15.37±2.23 | 14.68±2.60 | 0.136 | 15.42±2.39 | 14.55±2.41 | 0.057 |
| APTT | 42.46±6.48 | 42.97±6.26 | 41.97±6.72 | 0.416 | 43.41±6.82 | 41.31±5.89 | 0.088 |
| INR | 1.19±0.25 | 1.22±0.23 | 1.15±0.27 | 0.153 | 1.23±0.25 | 1.14±0.25 | 0.06 |
| Child-Pugh class, n (%) | 0.478 | 0.206 | |||||
| A | 59 (52.7) | 27 (47.4) | 32 (58.2) | 28 (45.2) | 31 (62.0) | ||
| B | 45 (40.2) | 26 (45.6) | 19 (34.5) | 29 (46.8) | 16 (32.0) | ||
| C | 8 (7.1) | 4 (7.0) | 4 (7.3) | 5 (8.1) | 3 (6.0) | ||
| Child-Pugh score | 6.69±1.72 | 6.91±1.79 | 6.45±1.63 | 0.16 | 6.97±1.85 | 6.34±1.49 | 0.054 |
| MELD score | 4.69±5.98 | 5.13±4.90 | 4.24±6.94 | 0.432 | 4.94±4.97 | 4.39±7.07 | 0.627 |
| APRI score | 3.22±5.24 | 3.90±6.68 | 2.51±3.04 | 0.163 | 3.79±6.43 | 2.51±3.13 | 0.198 |
| AAR score | 1.59±0.85 | 1.72±0.91 | 1.46±0.78 | 0.119 | 1.68±0.89 | 1.48±0.80 | 0.219 |
| FIB-4 score | 8.51±8.38 | 10.42±9.85 | 6.53±6.00 | 0.014 | 10.19±9.57 | 6.42±6.10 | 0.017 |
| FI score | −28.20±6.15 | −26.51±5.59 | −29.94±6.26 | 0.003 | −26.57±5.76 | −30.21±6.07 | 0.002 |
| King score | 83.77±180.99 | 107.44±240.35 | 59.24±78.90 | 0.16 | 103.95±231.20 | 58.76±80.67 | 0.19 |
AAR – AST to ALT ratio; ALB – albumin; ALP – alkaline phosphatase; ALT – alanine aminotransferase; APRI – AST to platelets ratio index; APTT – activated partial thromboplastin time; AST – aspartate aminotransferase; AUC – area under curve; BUN – blood urea nitrogen; Cr – creatinine; DBIL – direct bilirubin; FI – fibrosis index; FIB-4 – fibrosis 4 index; GGT – gamma-glutamyl transpeptidase; Hb – hemoglobin; IBIL – indirect bilirubin; INR – international normalized ratio; MELD – model for end-stage liver disease; NA – not available; PLT – platelet; PT – prothrombin time; Pts – patients; RBC – red blood cell; TBIL – total bilirubin; UGIB – upper gastrointestinal bleeding; WBC – white blood cell.
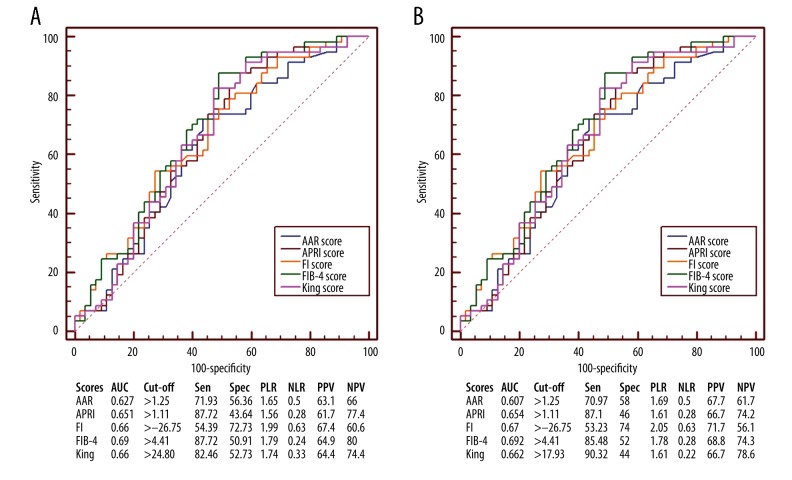
FIB-4 score had the largest AUC (AUC=0.69), followed by FI and King (AUC=0.66 for both of them), APRI (AUC=0.651), and AAR scores (AUC=0.627) (Figure 5A). The AUC of FIB-4 score was not significantly different from that of FI (P=0.6041), AAR (P=0.2949), APRI (P=0.1353), or King score (P=0.1330).
Figure 5.
Receiver operating characteristic curves showing the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores in predicting the presence of varices in liver cirrhosis without UGIB or splenectomy. (A) Prediction of moderate-severe varices. (B) Prediction of varices. AUC – area under curve; PLR – positive likelihood ratio; PPV – positive predictive value; NLR – negative likelihood ratio; NPV – negative predictive value; Sen – sensitivity; Spec – specificity.
With versus without EVs
Compared with the no EVs group, the EVs group had significantly higher FIB-4 and FI scores, but significantly lower WBC, PLT, and ALB (Table 5).
FIB-4 score had the largest AUC (AUC=0.692), followed by FI (AUC=0.67), King (AUC=0.662), APRI (AUC=0.654), and AAR scores (AUC=0.607) (Figure 5B). The AUC of FIB-4 score was not significantly different from that of FI (P=0.7167), AAR (P=0.1783), APRI (P=0.1578), or King score (P=0.1423).
Discussion
Non-invasive markers of varices are primarily derived from non-invasive assessment of liver fibrosis. For example, APRI was first developed by Wai and colleagues to identify the presence of significant fibrosis and liver cirrhosis in patients with chronic hepatitis C [11]. Similarly, AAR, FIB-4, FI, and King scores were originally used for the assessment of liver fibrosis and its severity in patients with hepatitis C [12–15]. More importantly, they were calculated based on some regular laboratory data (i.e., AST, ALT, ALB, INR, and PLT). By comparison, several other non-invasive markers might not be easily accessible, such as Forns’ index (composed of age, GGT, cholesterol, and PLT [24]), Fibrometer (composed of PLT, prothrombin index, AST, alpha-2 macroglobulin, hyaluronate, urea, and age [25]), and Hepascore (composed of bilirubin, GGT, hyaluronic acid, alpha-2 macroglobulin, age, and sex) [26]. Indeed, cholesterol, hyaluronic acid or hyaluronate, and alpha-2 macroglobulin are not detected in our everyday clinical practices, although our recent study has explored the predictive role of four major serum liver fibrosis markers, including hyaluronic acid, laminin, amino-terminal propeptide of type III procollagen, and collagen IV, for predicting the presence of gastroesophageal varices in 118 patients with liver cirrhosis [16]. Thus, only APRI, AAR, FIB-4, FI, and King scores, rather than Forns’ index, Fibrometer, or Hepascore, were evaluated in the present study.
The characteristics of our study population should be noted, as follows.
First, considering that a valid score can be generalized for any clinical conditions, all cirrhotic patients undergoing endoscopic examinations should be eligible for our study.
Second, the history of UGIB was not restricted in the overall analysis. Because not all episodes of acute UGIB were attributed to the varices in patients with liver cirrhosis [27], we should also identify whether the source of acute UGIB was from varices, peptic ulcer, or others. Indeed, this was important and helpful in choosing the appropriate drugs.
Third, moderate and severe EVs were ascribed to one group, because the treatment strategy was similar in both of them [5].
Fourth, in our study, only a very low proportion of patients presented with grade I–II hepatic encephalopathy at their admissions, and none of them presented with grade III–IV hepatic encephalopathy. This could be because patients must be clearly conscious during upper gastrointestinal endoscopic examinations.
Our study demonstrated that the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores was modest. These findings were largely consistent with the results of our recent meta-analysis (PROSPERO registration number: CRD42015017519) [28]. Additionally, it appeared that FIB-4 and FI scores had better diagnostic accuracy than other non-invasive scores. However, their diagnostic accuracy was not significantly different among most comparative analyses.
Our study also showed that the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores might be gradually improved as the study population was further refined (Figure 6). These findings suggested that candidates undergoing non-invasive assessment of varices should be appropriately selected. Indeed, if there was a history of splenectomy in a patient with liver cirrhosis, the PLT would remarkably increase and then return back to a normal level [29]. In this setting, the association of PLT with portal hypertension would be also masked, thereby weakening the diagnostic accuracy of non-invasive scores which include PLT.
Figure 6.
Areas under curves showing the diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores in different study populations. (A) Prediction of moderate-severe varices. (B) Prediction of varices.
Except for the retrospective nature, it should be acknowledged that a majority of patients undergoing endoscopic examinations had positive EVs in our study. This phenomenon might be primarily because most of our patients were at a more advanced stage or had decompensated cirrhosis and our physicians preferred to prescribe the endoscopy to patients with more severe liver cirrhosis. Given the potential bias of patient selection, the eligibility criteria should be refined in further prospective studies.
Conclusions
APRI, AAR, FIB-4, FI, and King scores had modest diagnostic accuracy for varices in liver cirrhosis. It would be difficult to replace the use of upper gastrointestinal endoscopy for the diagnosis of varices by these non-invasive scores. In future, an optimal non-invasive score should be established and validated in prospective multicenter studies.
Abbreviations
- AST
aspartate aminotransferase
- PLT
platelets
- APRI
aspartate aminotransferase-to-platelet ratio
- ALT
alanine aminotransferase
- AAR
aspartate aminotransferase-to-alanine aminotransferase ratio
- FI
fibrosis index
- RBC
red blood cell
- Hb
hemoglobin
- WBC
white blood cell
- PT
prothrombin time
- APTT
activated partial thromboplastin time
- INR
international normalized ratio
- ALB
albumin
- TBIL
total bilirubin
- ALP
alkaline phosphatase
- GGT
γ-glutamine transferase
- Cr
creatinine
- MELD
model for end-stage liver disease
- ROC
receiver operating characteristic curve
- AUC
area under curve
- EV
esophageal varices
- UGIB
upper gastrointestinal bleeding
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–61. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PM. Management of patients with decompensated cirrhosis. Clin Med. 2015;15(2):201–3. doi: 10.7861/clinmedicine.15-2-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006;44:217–31. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Sanyal AJ, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 6.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–68. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Kim MY, Jeong WK, Baik SK. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J Gastroenterol. 2014;20(15):4300–15. doi: 10.3748/wjg.v20.i15.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Franchis R, Dell’Era A. Invasive and noninvasive methods to diagnose portal hypertension and esophageal varices. Clin Liver Dis. 2014;18(2):293–302. doi: 10.1016/j.cld.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20(45):16811–19. doi: 10.3748/wjg.v20.i45.16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi X, Guo X, Li H, Liu X, Deng H. Knowledge about non-invasive diagnostic tests for varices in liver cirrhosis: A questionnaire survey to the Gastroenterology Branch of the Liaoning Medical Association, China Gastroenterol Rep (Oxf), 2015 [Epub ahead of print] doi: 10.1093/gastro/gov031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 12.Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163(2):218–24. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 13.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 14.Ohta T, Sakaguchi K, Fujiwara A, et al. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006;60(2):77–84. doi: 10.18926/AMO/30729. [DOI] [PubMed] [Google Scholar]
- 15.Cross TJ, Rizzi P, Berry PA, et al. King’s Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21(7):730–38. doi: 10.1097/MEG.0b013e32830dfcb3. [DOI] [PubMed] [Google Scholar]
- 16.Qi X, Li H, Chen J, et al. Serum liver fibrosis markers for predicting the presence of gastroesophageal varices in liver cirrhosis: a retrospective cross-sectional study Gastroenterol Res Pract, 2015 [In press] doi: 10.1155/2015/274534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X, Peng Y, Li H, et al. Diabetes is associated with an increased risk of in-hospital mortality in liver cirrhosis with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2015;27(4):476–77. doi: 10.1097/MEG.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Qi X, Dai J, et al. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8(1):751–57. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C, Qi X, Li H, et al. Correlation of serum liver fibrosis markers with severity of liver dysfunction in liver cirrhosis: a retrospective cross-sectional study. Int J Clin Exp Med. 2015;8(4):5989–98. [PMC free article] [PubMed] [Google Scholar]
- 20.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–49. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 21.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Society of Gastroenterology, Chinese Society of Hepatology, Chinese Society of Endoscopy, Chinese Medical Association. [Consensus on prevention and treatment for gastroesophageal varices and variceal hemorrhage in liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2008;16(8):564–70. ]in Chinese] [Google Scholar]
- 23.The general rules for recording endoscopic findings on esophageal varices. Jpn J Surg. 1980;10(1):84–87. doi: 10.1007/BF02468653. [DOI] [PubMed] [Google Scholar]
- 24.Forns X, Ampurdanes S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36(4 Pt 1):986–92. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 25.Patel K, Muir AJ, McHutchison JG. Validation of a simple predictive model for the identification of mild hepatic fibrosis in chronic hepatitis C patients. Hepatology. 2003;37(5):1222. doi: 10.1053/jhep.2003.50159. author reply 1223. [DOI] [PubMed] [Google Scholar]
- 26.Adams LA, Bulsara M, Rossi E, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51(10):1867–73. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 27.Cremers I, Ribeiro S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Therap Adv Gastroenterol. 2014;7(5):206–16. doi: 10.1177/1756283X14538688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng H, Qi X, Guo X. Diagnostic accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex scores in predicting the presence of esophageal varices in liver cirrhosis: a systematic review and meta-analysis. Medicine. 2015 doi: 10.1097/MD.0000000000001795. [Accepted] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick PA, Murphy KM. Splenomegaly, hypersplenism and coagulation abnormalities in liver disease. Baillieres Best Pract Res Clin Gastroenterol. 2000;14(6):1009–31. doi: 10.1053/bega.2000.0144. [DOI] [PubMed] [Google Scholar]