Abstract
Intramyocardial transplantation of cardiomyocytes (CMs), endothelial cells (ECs), and smooth muscle cells (SMCs) derived from human induced pluripotent stem cells (hiPSCs) has beneficial effects on the post-infarction heart. However, the mechanisms underlying the functional improvements remain undefined. We employed large-scale label-free quantitative proteomics to identify proteins that were differentially regulated following cellular transplantation in a swine model of myocardial infarction (MI). We identified 22 proteins that were significantly up-regulated after trilineage cell transplantation compared to both MI and Sham groups. Among them, 12 proteins, including adenylyl cyclase-associated protein 1 and tropomodulin-1, are associated with positive regulation of muscular contraction whereas 11 proteins, such as desmoplakin and zyxin, are involved in embryonic and muscular development and regeneration. Moreover, we identified 21 proteins up-regulated and another 21 down-regulated in MI, but reversed after trilineage cell transplantation. Proteins up-regulated after MI but reversed by transplantation are related to fibrosis and apoptosis. Conversely, proteins down-regulated in MI but restored after cell therapy are regulators of protein nitrosylation. Our results show that the functionally beneficial effects of trilineage cell therapy are accompanied by differential regulation of protein expression in the recipient myocardium, which may contribute to the improved cardiac function.
Keywords: Biomedicine, Cardiac regeneration, Heart disease, Label-free quantification, Large mammalian models, Stem cells
Heart failure is a devastating clinical syndrome that afflicts over 23 million people worldwide and the prevalence of heart failure is rising rapidly, imposing a significant economic burden on the health care system [1, 2]. Myocardial infarction (MI), also known as heart attack, is a common cause of heart failure due to the irreversible damage of the myocardium. Despite the rapid advancement of medical care and cardiac therapies, post-MI cardiac repair still faces significant challenges due to the limited regenerative potential of cardiomyocytes [3].
Therapies utilizing human induced pluripotent stem cells (hiPSCs), which possesses high regeneration potential, and therefore presents an unprecedented opportunity for post-MI cardiac repair [3]. Moreover, the use of hiPSCs originating from the patients’ own somatic cells avoids immunogenic complications and minimizes ethical concerns associated with embryonic stem cell therapies [4]. Preclinical studies employing hiPSC-derived cell therapies to treat large animal models of MI-induced cardiac dysfunction have yielded promising results [5, 6]. Recently, using an immunesuppressed swine model of ischemia-reperfusion (I/R) injury, we reported a significant improvement in cardiac function following the intramyocardial transplantation of hiPSCs-derived cardiomyocytes (CMs), endothelial cells (ECs), and smooth muscle cells (SMCs) combined with a 3D fibrin patch loaded with insulin-like growth factor (IGF)-encapsulated microspheres [7]. We showed that trilineage cell transplantation resulted in significant improvements in left ventricular (LV) function, as demonstrated by reduced LV wall stresses, infarct size, and apoptosis, as well as improved metabolism and contractile performance. Notably, we also achieved significant improvement in engraftment of cells from all three lineages at the site of injury [7].
Although there is evidence suggesting that paracrine signaling plays an important role in post-MI cardiac repair and regeneration following stem cell therapy [8–10], the signaling pathways activated by the therapies and the down-stream consequences in the recipient myocardium remain elusive. To further unravel the molecular mechanisms underlying the beneficial effects of trilineage cell transplantation on post-MI cardiac function, we utilized large-scale quantitative proteomics to globally assess protein expression changes in the post-MI recipient myocardium in response to trilineage cell transplantation with hiPSC-derived CMs, ECs, and SMCs.
Global proteomic analysis was performed on three groups: Sham hearts (n = 3); the periscar border zone (BZ) of hearts with post-infarction LV remodeling (MI, n = 4); and the periscar BZ of post-MI hearts with trilineage cell transplantation (CM+EC+SMC, n = 3). The experimental flow chart is shown in Supporting Information Fig. 1. We confidently identified 2997, 3154, and 2946 non-redundant proteins (combining all biological replicates) in the Sham, MI, and CM+EC+SMC groups, respectively. Complete information of all the identified proteins in each group is presented in Supporting Information Table 1. There was a high level of overlap (89.9 ± 2.3%) in the proteins identified from at least two different groups (Fig. 1A). Meanwhile, high reproducibility was exhibited across biological replicate, with similar numbers of total proteins identified in each replicate (Supporting Information Fig. 2) and 90.0 ± 5.9%, 89.9 ± 9.4%, and 88.8 ± 5.0% of the identified proteins repeatedly detected in Sham, MI, and the CM+EC+SMC group, respectively. Overall, there was no significant difference in the distribution of individual protein intensities (Supporting Information Fig. 3A) and total protein intensities (Supporting Information Fig. 3B). Additionally, more than 97% of the proteins that can be quantified in at least two replicates per group have less than a 10% coefficient of variation (CV) in their intensity values (Supporting Information Fig. 3C). This indicates high reproducibility across the replicates and showcases the high reliability of the quantification method.
Figure 1.
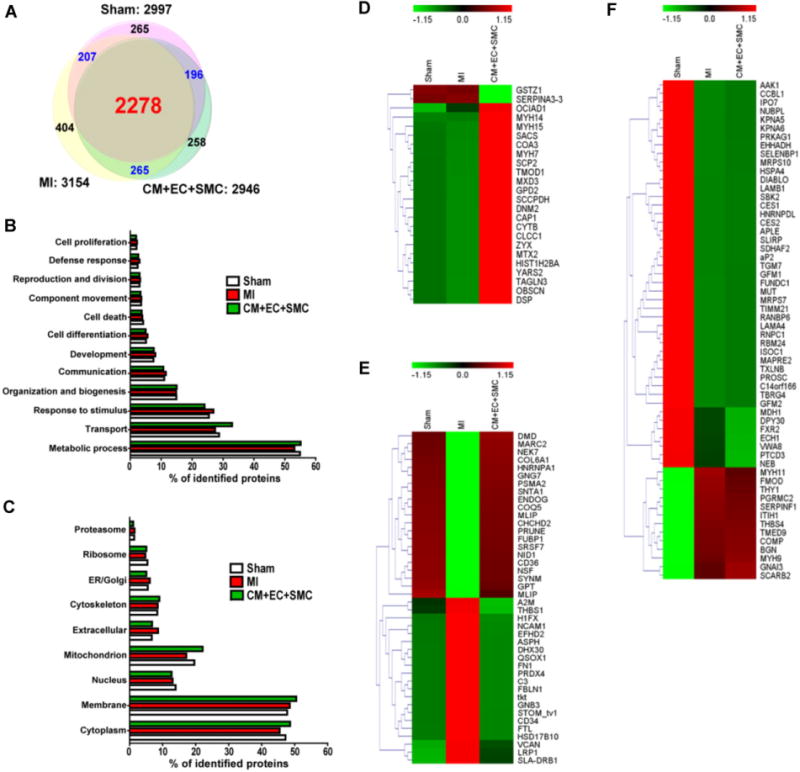
Global protein profiling of myocardium from the Sham, MI, and CM+EC+SMC groups. (A) Venn diagram showing the number of identified proteins in the Sham, MI, and CM+EC+SMC groups. GO analysis of (B) biological processes and (C) subcellular localizations of the identified proteins. (D–F) Heat maps of three distinct categories of proteins whose expression levels were altered among Sham, MI, and CM+EC+SMC groups. The heat map scale is based on Z scores (Supporting Information) ranges from −1.15 (green) to 1.15 (red) with a midpoint of 0 (black). (D) Category 1: no change in MI but altered after trilineage cell transplantation. (E) Category 2: altered in MI but reversed by trilineage cell transplantation; (F) Category 3: altered in MI but not reversed by trilineage cell transplantation.
Information regarding the biological processes and subcellular localization of the identified proteins was obtained from the Gene Ontology (GO) database (Fig. 1B and C). The majority of the identified proteins were localized to the cytoplasm and membrane, with a significant portion of proteins localized to mitochondria, which is conceivable since cardiac tissue is rich in mitochondria [11]. The majority of the proteins identified remained unchanged among Sham, MI, and CM+EC+SMC groups. For example, TGF, EIF4A, IGF, EEF1B, STAT3 and histones were detected but without significant changes among three experimental groups (Supporting Information Fig. 4A–F). Housekeeping proteins, such as β-tubulin, GAPDH, and citrate synthase also remained unchanged (Supporting Information Fig. 4G–L).
We have identified three distinct categories of proteins with altered expression: (1) no change in MI but altered after trilineage cell transplantation (Fig. 1D); (2) altered in MI but reversed by trilineage cell transplantation (Fig. 1E); (3) altered in MI but not reversed by trilineage cell transplantation (Fig. 1F). The distribution of subcellular localizations (Supporting Information Fig. 5) and biological processes (Supporting Information Fig. 6) for the proteins in these three categories were significantly different.
In the first category, we identified 24 proteins which remained unchanged between the MI and Sham groups, of which 22 of them were significantly up-regulated, and 2 were down-regulated following trilineage cell therapy (Supporting Information Table 2). Functional enrichment analysis revealed that the up-regulated proteins are involved in actin filament regulation and muscle contraction (Supporting Information Fig. S7). Among them, 12 proteins such as adenylyl cyclase-associated protein 1 (CAP1), tropomodulin-1 (TMOD1), and dynamin 2 (DNM2) (Fig. 2A–C) are associated with positive regulation of muscle contraction. Moreover, 11 of these proteins, including desmoplakin (DSP), zyxin (ZYX), and obscurin (OBSCN), are known to be involved in embryonic development and muscle regeneration (Fig. 2D–F). The Western blot results of DSP and ZYX were also consistent with the data obtained from MS analysis (Fig. 2I). Each protein was analyzed for its interactions with other proteins by STRING and NetworkAnalyst database/software. A protein–protein interactome network suggests a potential (new) cardiac regeneration pathway (Fig. 2J).
Figure 2.
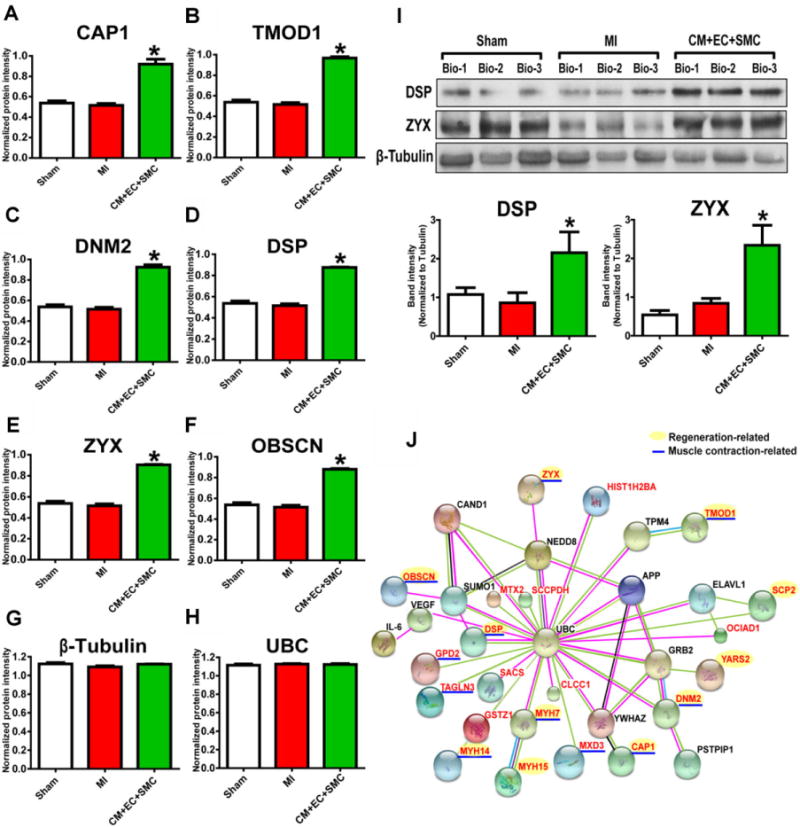
Representative proteins identified in Category 1, which showed no change in expression following MI but altered expression after trilineage cell transplantation. (A–H) Quantitative MS data of proteins significantly up-regulated by trilineage cell tranplantation. (I) Western blot confirmation of the expression change of DSP and ZYX. (J) Interactome analysis. The proteins in red were from the identified protein list. Proteins in black were obtained from the extensive interactome feature of the NetworkAnalyst software. Proteins in green are paracrine factors. * indicates p < 0.05.
The proteins in the first category constitute an extensive interacting network associated with VEGF and IL-6 signaling (Fig. 2J). Consistent with previous studies [12,13], several paracrine factors, such as VEGF and IL-6, were identified in the tissue extract from the group with trilineage cell treatment after MI [5]. A number of proteins up-regulated by trilineage cell treatment are involved in actin filament regulation and muscle contraction, indicating that restoration of contractile function may be a crucial step for cardiac repair after MI. Notably, several regulators of the contractile apparatus and cytoskeleton belong to this category, including TMOD1 and CAP1. As the primary tropomodulin isoform in CMs, TMOD1 regulates thin filament length by mediating elongation and depolymerization of actin filaments at the pointed end [14]. CAP1 is another actin binding protein that participates in the recycling of G-actin monomers and regulation of actin dynamics [15]. CAP1 is also involved in the regulation of the Ras/cAMP pathway, which is requiredfor the majority of cytoskeletal functions in vivo and in vitro [16]. Furthermore, during MI, cell death leads to complete loss of contractile function in the ischemic region [17], and therefore recovery of the injured heart following cell therapy involves regeneration of the cardiac tissues in the affected zone. 11 proteins in the first category show connection to embryonic development or cell regeneration, such as DSP and ZYX. Previous studies have shown that the level of DSP is significantly increased during liver regeneration at 24 h after partial hepatectomy in male adult mice [18]. Additionally, DSP is essential for heart growth and development during embryonic stage [19]. ZYX is enriched at focal adhesions and regulates actin cytoskeleton dynamics [20]. Moreover, another study suggested an essential role of ZYX during the epithelial-mesenchymal transition, which is an important embryonic stage for the formation of many tissues, including the development of the heart valves and the septa [21]. Specifically, ZYX is required for morphogenetic movement of the endocardial cells by mediating actin rearrangement in response to TGF-β1 during the epithelial-mesenchymal transition [21]. Thus, our findings indicate that the re-activation or up-regulation of proteins involved in the development of the embryonic heart may constitute an important mechanism for the repair and regeneration of the injured adult heart.
In the second category, 21 up-regulated and 21 down-regulated proteins in MI were reversed after trilineage cell transplantation (Supplemental Information Table 3). The proteins that were up-regulated after MI but reversed by trilineage cell transplantation include fibronectin (FN1), versican (VCAN), and thrombospondin-1 (THBS1) (Fig. 3A–E, 3I). GO analysis revealed that these proteins are primarily involved in extracellular matrix organization, the regulation of proteolysis activities, and apoptosis (Supporting Information Fig. 8). Previous study has shown that post-MI cardiac remodeling is usually accompanied by fibrosis in the infarcted and noninfarcted myocardium [22]. This is in good agreement with our finding that proteins involved in fibrosis and apoptosis, including THBS1, FTL, FN1, FBLN1, VCAN7, and VCAN8, are up-regulated following MI. Specifically, THBS1 is known to positively regulate apoptosis and can induce cell-cycle arrest [23]. Up-regulation of the proteins related to fibrosis and apoptosis was reversed after trilineage cell transplantation, in part resulting in improved heart function. This is in accordant with previous studies that demonstrated the reduction of post-MI fibrosis improves cardiac functions [24, 25]. For the proteins down-regulated post-MI but reversed by trilineage cell transplantation, including dystrophin (DMD) and alpha-1-syntrophin (SNTA1) (Fig. 3F–H), functional enrichment analysis indicated that they are involved in the regulation of protein nitrosylation and cell-substrate adhesion (Supporting Information Fig. 8). DMD and SNTA1 both of which play major roles in regulation of protein nitrosylation and muscle contraction [26], consistent with emerging evidence which showed that the S-nitrosylation of proteins plays an important role in cardioprotection [27]. SNTA1 interacts with DMD, which connects the cytoskeleton and contractile apparatus to the extracellular matrix and basement membrane. SNTA1 also plays a critical role in the regulation of voltagegated cardiac sodium channels with dysregulation, leading to ventricular arrhythmias which can occur post-MI [28]. The 42 proteins formed five protein sub-interactomes (Fig. 3J) and each of them consists of proteins with similar functions.
Figure 3.
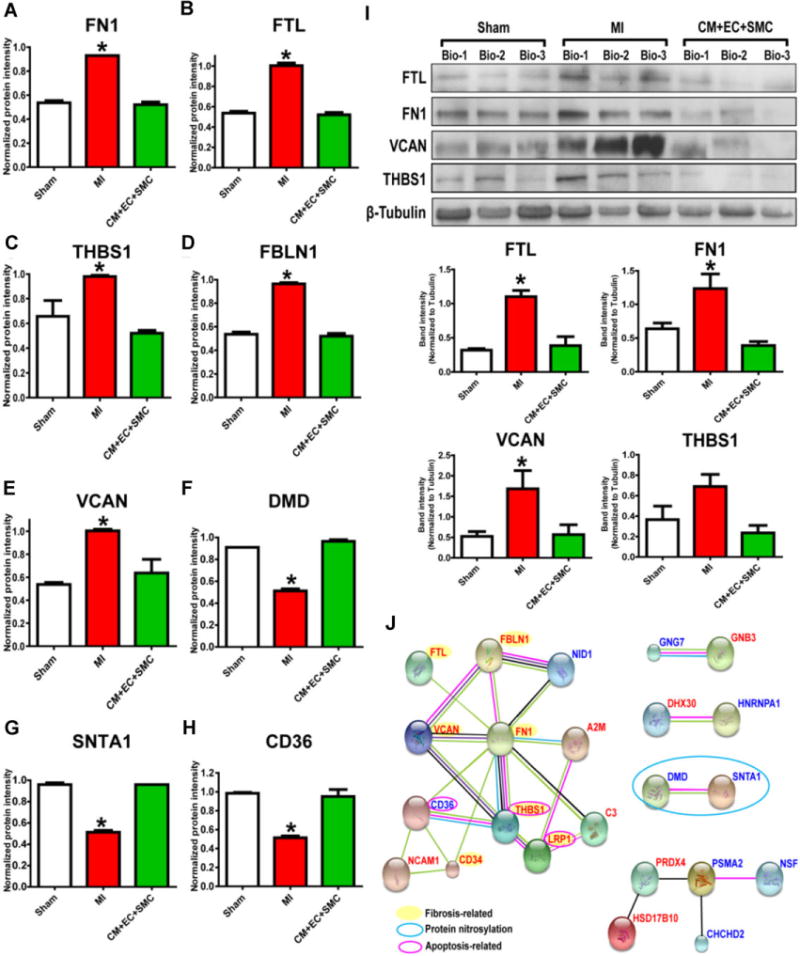
Representative proteins identified in Category 2 with altered expression after MI that was reversed by cell transplantation. (A–H) Quantitative MS data of proteins significantly altered following MI but reversed by trilineage cell transplantation. (I) Western blot confirmation of the expression change of FTL, FN1, VCAN, and THBS1. (J) Interactome analysis. The proteins up-regulated by MI but reversed by trilineage cell transplantation are indicated in red. Proteins in blue were down-regulated in MI but restored by cell therapy. *indicates p < 0.05.
Interestingly, in the third category, we identified proteins with altered expression following MI but no change in expression after trilineage cell transplantation (relative to expression in MI) (Supporting Information Table 4, Fig. 5, 6, and 9). 45 proteins were down-regulated post-MI but the protein level could not be restored after cell transplantation. Functional enrichment analysis showed that these 45 proteins were closely involved in mitochondrial protein translation and fatty acid catabolism among other functions (Supporting Information Table 3, Fig. 9A). In contrast, 13 proteins that were up-regulated by MI, but not changed relative to MI following trilineage cell transplantation, were associated with extracellular matrix organization and angiogenesis (Supporting Information Table 3, Fig. 9A). Interestingly, proteins involved in fatty acid catabolism, including delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase (ECH1), peroxisomalbifunctional enzyme (EHHADH), and methylmalonyl-CoA mutase (MUT), closely interact with proteins involved in extracellular matrix organization (Supporting Information Fig. 9B), suggesting potential mechanisms that affect post-MI mitochondrial functions.
In conclusion, our label-free quantitative proteomics approach has enabled reliable and unbiased profiling of global proteome changes and, thus, has provided insights into the mechanisms underlying improved cardiac function following trilineage cell treatment post-MI. We have detected changes in down-stream proteins that are involved in paracrine signaling in response to VEGF and IL-6, as well as other proteins that may be associated with potentially new signaling pathways. Our results provide strong evidence that the functionally beneficial effects of trilineage cell therapy are accompanied by changes in the recipient myocardial protein expression profile, including up-regulation or re-activation of proteins involved in muscle contraction, actin filament regulation, cardioprotection, regeneration, and embryonic heart development; as well as down-regulation of proteins involved in fibrosis and proteolytic activities. Collectively, these results suggest that differential regulation of protein expression following trilineage cell treatment post-MI may contribute to the observed improvement in cardiac function.
Experimental procedures
A detailed description of the experimental procedure is provided in the Supporting Information.
Swine heart model of I/R injury and cellular therapy
The detailed methods have been reported previously [5]. Briefly, female Yorkshire swine (~13 kg, Manthei Hog Farm, MN) were randomly assigned to the Sham, MI, and CM+EC+SMC experimental groups. I/R injury was surgically induced in MI and CM+EC+SMC swine by temporary occlusion of the left anterior descending coronary artery for 60 min followed by reperfusion [5]. The CM+EC+SMC group received intramyocardial transplantation of 2 million each of hiPSC-derived CMs, ECs, and SMCs directly at both the BZ and infarct zone of the injured myocardium. The MI group underwent the same treatment without the trilineage cells. At 4 weeks post-surgery, animals were sacrificed and the BZ myocardium from MI and CM+EC+SMC swine, as well as the corresponding tissue in the Sham group, was dissected for proteomic analysis. The BZ is defined as approximately 3 mm away from scar, which is a thin piece of mature fibrotic tissue that can be easily identified. Due to the relative large size of the swine heart, the border zone tissue was cut under direct vision.
Label-free proteomics for quantitative profiling of protein expression levels
All experiments on the Sham, MI, and CM+EC+SMC groups were performed with 3, 4, and 3 biological replicates, respectively. Proteins were extracted using MaSDeS [29], a mass spectrometry compatible surfactant, from 30–50 mg tissue by homogenization in HEPES buffer, followed by in-solution digestion. The protein samples were analyzed using a nanoACQUITY ultra high pressure liquid chromatography coupled to a Q Exactive (Thermo Scientific) mass spectrometer. Protein identification and quantification were performed using the SEQUEST-based Proteome Discoverer. All of the given protein intensities are presented in Log10 scale. After conversion, the intensity of each protein in the sample was divided by the median protein intensity for the entire sample for normalization. Protein changes between groups were considered significant if they: (1) passed the Kruskal-Wallis test (p < 0.05) and (2) had a greater than 1.3-fold change.
Supplementary Material
Significance of the study.
Our study is the first large-scale proteomics study of myocardial protein expression changes in the post-infarction heart following trilineage cell therapy. We have demonstrated that improved cardiac functions following trilineage cell therapy are accompanied by differential regulation of proteins in the recipient myocardium. We have also identified interesting proteins which may play an important role in post-infarction cardiac repair and regeneration following cell therapy. Furthermore, our findings have elucidated cardiac repair and regeneration pathways, which may serve as potential targets for the treatments after myocardial infarction.
Acknowledgments
We would like to thank Zachery R. Gregorich and SerifeAyaz-Guner for helpful discussions and critical reading of this manuscript. This work was supported by United States National Institutes of Health grants, R01 HL114120 (to J.Z.), and R01 HL096971, HL109810 (to Y.G.), U01 HL099773 (to T.J.K.), AHA 12GRNT12070021 (to Y.S.L.).
Abbreviations
- BZ
border zone
- CAP1
adenylyl cyclase-associated protein 1
- CD36
fatty acid translocase/CD36
- CMs
cardiomyocytes
- CV
coefficient of variation
- DMD
dystrophin
- DSP
desmoplakin
- EC
endothelial cell
- ECH1
delta(3,5)-delta(2,4)-dienoyl-CoA isomerase, mitochondrial
- EEF1B
elongation factor 1B
- EHHADH
peroxisomalbifunctional enzyme
- EIF4A
eukaryotic initiation factor-4A
- FBLN1
fibulin-1
- FN1
fibronectin
- FTL
ferritin
- hiPSC
human induced pluripotent stem cells
- I/R
ischemia reperfusion
- IGF
insulin-like growth factor
- IL-6
interleukin 6
- LV
left ventricular
- MI
myocardial infarction
- OBSCN
obscurin
- SE
standard error
- SMC
smooth muscle cells
- SNTA1
alpha-1-syntrophin
- STAT3
signal transducer and activator of transcription 3
- TGF
transforming growth factor
- THBS1
thrombospondin-1
- THBS4
thrombospondin-4
- TMOD1
tropomodulin-1
- VCAN
versican
- VEGF
vascular endothelial growth factor
- ZYX
zyxin
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site
Potential conflict of interest: T.J.K. is a co-founder and consultant for Cellular Dynamics International. All other authors have declared no conflict of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalit PA, Hei DJ, Raval AN, Kamp TJ. Induced pluripotent stem cells for post-myocardial infarction repair: remarkable opportunities and challenges. Circ Res. 2014;114:1328–1345. doi: 10.1161/CIRCRESAHA.114.300556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox IJ, Daley GQ, Goldman SA, Huard J, et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014;345:1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong Q, Ye L, Zhang P, Lepley M, Tian J, et al. Functional consequences of human induced pluripotent stem cell therapy: myocardial ATP turnover rate in the in vivo swine heart with postinfarction remodeling. Circulation. 2013;127:997–1008. doi: 10.1161/CIRCULATIONAHA.112.000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong JJ, Yang X, Don CW, Minami E, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye L, Chang YH, Xiong Q, Zhang P, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotech. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 10.Ye L, Zimmermann WH, Garry DJ, Zhang J. Patching the heart: cardiac repair from within and outside. Circ Res. 2013;113:922–32. doi: 10.1161/CIRCRESAHA.113.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Taimeh Z, Loughran J, Birks EJ, Bolli R. Vascular endothelial growth factor in heart failure. Nat Rev Cardiol. 2013;10:519–530. doi: 10.1038/nrcardio.2013.94. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Li Y, Wu Y, Wang L, et al. Interleukin-6/signal transducer and activator of transcription 3 (stat3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288:1489–1499. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almenar-Queralt A, Lee A, Conley CA, Ribas de Pouplana L, et al. Identification of a novel tropomodulin isoform, skeletal tropomodulin, that caps actin filament pointed ends in fast skeletal muscle. J Biol Chem. 1999;274:28466–28475. doi: 10.1074/jbc.274.40.28466. [DOI] [PubMed] [Google Scholar]
- 15.Bertling E, Quintero-Monzon O, Mattila PK, Goode BL, et al. Mechanism and biological role of profilin-Srv2/CAP interaction. J Cell Sci. 2007;120:1225–1234. doi: 10.1242/jcs.000158. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Ghai P, Wu H, Wang C, et al. Mammalian adenylyl cyclase-associated protein 1 (cap1) regulates cofilin function, the actin cytoskeleton, and cell adhesion. J Biol Chem. 2013;288:20966–20977. doi: 10.1074/jbc.M113.484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez AM, Guatimosim S, Dilly KW, Vassort G, et al. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 18.Cusimano A, Monga SPS. Changes in desmosomal proteins during liver regeneration after mouse partial hepatectomy. Faseb J. 2011 Meeting Abstract 791.2. [Google Scholar]
- 19.Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–941. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- 20.Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- 21.Mori M, Nakagami H, Koibuchi N, Miura K, et al. Zyxin mediates actin fiber reorganization in epithelial-mesenchymal transition and contributes to endocardial morphogenesis. Mol Biol Cell. 2009;20:3115–3124. doi: 10.1091/mbc.E09-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 23.Mirochnik Y, Kwiatek A, Volpert OV. Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets. 2008;9:851–862. doi: 10.2174/138945008785909347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Zhang L, Ni A, Zhang Z, et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:21110–21115. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Z, Sun X, Shan H, Wang N, et al. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway. Circulation. 2012;126:840–850. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 26.Wu G, Ai T, Kim JJ, Mohapatra B, et al. Alpha-1-syntrophin mutation and the long-qt syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol. 2008;1:193–201. doi: 10.1161/CIRCEP.108.769224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–6023. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang YH, Gregorich ZR, Chen AJ, Hwang L, et al. New mass-spectrometry-compatible degradable surfactant for tissue proteomics. J Proteome Res. 2015;14:1587–1599. doi: 10.1021/pr5012679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


