Abstract
Background
Influenza vaccination is administered throughout the influenza disease season, even as late as March. Given such timing, what is the value of vaccinating the population earlier than currently being practiced?
Methods
We used real data on when individuals were vaccinated in Allegheny County, Pennsylvania, and the following 2 models to determine the value of vaccinating individuals earlier (by the end of September, October, and November): Framework for Reconstructing Epidemiological Dynamics (FRED), an agent-based model (ABM), and FluEcon, our influenza economic model that translates cases from the ABM to outcomes and costs [health care and lost productivity costs and quality-adjusted life-years (QALYs)]. We varied the reproductive number (R0) from 1.2 to 1.6.
Results
Applying the current timing of vaccinations averted 223,761 influenza cases, $16.3 million in direct health care costs, $50.0 million in productivity losses, and 804 in QALYs, compared with no vaccination (February peak, R0 1.2). When the population does not have preexisting immunity and the influenza season peaks in February (R0 1.2–1.6), moving individuals who currently received the vaccine after September to the end of September could avert an additional 9634–17,794 influenza cases, $0.6–$1.4 million in direct costs, $2.1–$4.0 million in productivity losses, and 35–64 QALYs. Moving the vaccination of just children to September (R0 1.2–1.6) averted 11,366–1660 influenza cases, $0.6–$0.03 million in direct costs, $2.3–$0.2 million in productivity losses, and 42–8 QALYs. Moving the season peak to December increased these benefits, whereas increasing preexisting immunity reduced these benefits.
Conclusion
Even though many people are vaccinated well after September/October, they likely are still vaccinated early enough to provide substantial cost-savings.
Keywords: influenza, vaccination, economics
Influenza vaccinations are administered throughout the influenza disease season, even as late as March, the tail-end end of most seasons. This may leave individuals unprotected for a sizeable duration of the season, which can start as early as September. The longer the unprotected period, the greater the chance the individual may contract influenza, incurring potentially avoidable absenteeism, clinic visits, hospitalizations, and deaths, as our previous studies have shown for children and elderly,1,2 those who are at highest risk for poor influenza outcomes.3 Individuals who delay vaccination may not be the only ones affected. Delaying vaccination can also allow greater influenza transmission (by failing to achieve a higher level of herd protection earlier) leaving both those never get vaccinated throughout the season and even those who are vaccinated earlier at higher risk.
Although numerous efforts have been made to increase influenza vaccination coverage by convincing more people to get vaccinated, there has been comparatively less emphasis on convincing those already compliant to get immunized earlier in the season. For example, workplace-based and school-based vaccination programs and initiatives such as National Influenza Vaccination Week, which is in December.4 Late vaccinees are apparently willing to get vaccinated but may be facing obstacles to getting vaccinated earlier in the season such as busy schedules, poor access to vaccination locations, or simple oversight. Therefore, could more benefits be garnered by focusing on administering vaccines to receptive persons rather than trying to convince those who oppose vaccination to accept it?
The question remains: what is the value of vaccinating the population earlier than they are currently getting vaccinated? Historically, between 1982 and 2013, influenza activity most often peaked in February (14 seasons, or 44% of the time), followed by December (6 seasons, or 19% of the time), and January and March (5 seasons each, or 16% of the time).5 We used data on when individuals were vaccinated in Allegheny County, Pennsylvania, and 2 existing models to determine the value of getting these individuals to be vaccinated earlier: Framework for Reconstructing Epidemiological Dynamics (FRED), an agent-based model (ABM) of Allegheny County, a detailed computational simulation of the people, locations, and activities of the entire county,6,7 and FluEcon, our influenza economic model that can translate cases to influenza outcomes and costs.
METHODS
FRED ABM of Allegheny County
We used our previously described FRED ABM of Allegheny County, Pennsylvania,6–10 which comprises a total population of 1,164,880. A collaboration of investigators (P.C., S.T.B., W.D.W., and B.Y.L., currently at RTI, the Pittsburgh Supercomputing Center, and Johns Hopkins, respectively) developed the initial ABM in 2008, which was used during the 2009 H1N1 pandemic to help inform policy making at county through national levels. After which, this initial ABM was transcoded and served as the basis for the subsequently developed FRED system by investigators at the University of Pittsburgh, Carnegie Mellon University, and Pittsburgh Supercomputing Center.10 The ABM represents each individual in Allegheny County with a computational agent. Just like a person, each agent has a set of characteristics (eg, age, sex, and employment status) and is assigned to a particular household, school (if the agent is of school age), and workplace (if the agent is employed). During the simulation, the model proceeds in discrete 1-day time steps. Each simulated day, the agents move among virtual representations of the county's households, schools, workplaces, and communities, where agents interact with each other. Agents' ages and locations are based on 2010 US census data.
At any given time, an agent is in one of 4 mutually exclusive influenza states: susceptible (S), exposed (E), infectious (I), or recovered (R). An agent in the S state has a probability of contracting influenza when contacting an agent in the I state. The transmission probability depends on the agents' ages and location and the reproductive rate (R0) of the epidemic (Appendix Table A1).11–13 If the agent in the S state contracts influenza, he/she moves into the E state and remains for the duration of the latent period (distribution with a mean of 1.2 d). Once the latent period elapses, the agent transitions to the I state, in which he/she can transmit influenza to others and remain for the duration of the infectious period (distribution with a mean of 4.1 d). Once the infectious period elapses, the agent moves into the R state in which he/she is not infectious and immune to infection, and where they remained for the rest of the simulation. Vaccinating an agent in the S state had a probability (ie, the vaccine efficacy) of moving the agent to the R state 2 weeks after the vaccination occurred (to represent the time lag in the onset of immunity postvaccination). In addition, some agents could have preexisting immunity; these individuals started the simulation in the R state.
For this study, the primary output from the ABM was the number of influenza cases in each age group on each simulated day.
FluEcon: Influenza Economic Model
We developed FluEcon, our economic model, in Microsoft Excel (Microsoft, Redmond, WA) and utilized a Crystal Ball add-in (Oracle, Redwood City, CA), following our previous models,14–19 to translate influenza cases from the ABM into health outcomes and corresponding costs from the third party payer and societal perspectives. Appendix Table A2 shows the model inputs. Each influenza case from the ABM had a probability of being symptomatic. Each symptomatic case then had probabilities of seeking ambulatory care, being hospitalized, or dying from influenza, each associated with a corresponding cost. Thus, the outcome of each case determined the costs accrued. All costs and probabilities were age specific when available.
Third party payer costs included all direct costs of illness (eg, ambulatory care visit, hospitalization) derived from nationally representative data sources. Societal costs included direct and indirect (ie, productivity losses due to absenteeism from work or school and mortality) costs. The median hourly wage for all occupations estimated productivity losses and assumed an 8-hour work day. A death resulted in accruing the net present value of that person's remaining lifetime earnings, based on their life expectancy.20,21 A 3% discount rate converted all costs into 2013 $US.
Health effects were measured in quality-adjusted life-years (QALYs). Each influenza case accrued QALY values based on their age-dependent healthy QALY value attenuated by influenza's utility weight (either hospitalized or not) for the duration of their symptoms (if not hospitalized) or hospitalization (if hospitalized). For example, a child has a healthy QALY over the course of a year of 1, if he/she contracts influenza and are not hospitalized, we use the published utility weight for influenza without hospitalization (0.659) to attenuate the healthy value, resulting in 0.659 QALYs (1×0.659) for the 7-day symptom duration. By contrast, an adult has a healthy QALY value of 0.92 over a year, if he/she contracts influenza and is hospitalized, they accrue 0.473 QALYs (0.92×0.514, the published utility weight for influenza with hospitalization) for the hospitalization duration. We also considered QALYs from vaccination side effects.
Experiments
Current Coverage and Timing
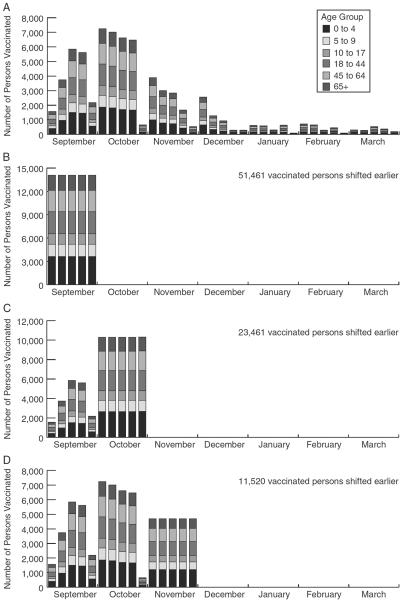
The baseline scenario had individuals vaccinated based on the days indicated by the Influenza Vaccine Effectiveness Network Data (Fig. 1) where, 66.2% of children 0–4 years old, 54.2% of children 5–9 years old, 33.7% of children 10–17 years old, 28.6% of adults 18–44 years old, 42.7% of adults 45–64 years old, and 64.9% of elderly 65 years and older were vaccinated.
FIGURE 1.
Number of persons in each age group vaccinated each week in the agent-based model. A, Current vaccination coverage and timing, B, Vaccination completed by the end of September, C, Vaccination completed by the end of October, and D, Vaccination completed by the end of November. The current vaccination coverage and timing are from Allegheny County in 2011.
Vaccinating Population Earlier
Using the same model we moved vaccinated individuals earlier; scenarios explored earlier vaccination, ensuring that vaccination was completed by the end of: (1) November, (2) October, and (3) September. In other words, anyone who typically was vaccinated after the selected month received the vaccine by the end of the specified month (eg, a person who normally received the vaccine in February was simulated to be vaccinated in November). Everyone who received the vaccine before the specified month was vaccinated at the same time as they were in the baseline simulation (Fig. 1). Additional scenarios vaccinated only children earlier, as children may be more readily vaccinated earlier (eg, enabled by target programs), are a high-risk group, and are thought to be high transmitters.
Sensitivity analyses varied R0 (1.2–1.6), the epidemic peak (December to February), and the percentage of the population with preexisting immunity (0%, 20%, and 40%), that is, those starting the simulation in the R state rather than the S state. Another scenario evaluated increases in vaccination coverage (5% and 10% increase in each age group) while maintaining the current timing.
RESULTS
Table 1 shows the epidemiologic and economic results for different vaccination scenarios for an influenza season peak of February. Figure 2 shows the corresponding epidemic curves for season peaks of December and February when there is no preexisting immunity. As influenza seasons differ year to year, our results summarize the variability in the range of costs and QALYs across varying transmission dynamics and season characteristics.
TABLE 1.
Epidemiologic and Economic Outcomes for Various Vaccination Timing Strategies for an Influenza Season With a February Peak in Alleghany County, PA (Total Population 1,164,880)
| Vaccination Timing |
|||||
|---|---|---|---|---|---|
| No Vaccination | Current Schedule | By End of September | By End of October | By End of November | |
| No preexisting immunity | |||||
| R0 1.2 | |||||
| Attack rate (%) | 32.9 | 13.7 | 12.8 | 12.8 | 12.8 |
| No. influenza cases | 382,796 | 159,035 | 149,401 | 148,943 | 149,462 |
| Direct health care costs*† | 26.1 (5.2) | 9.9 (2.0) | 9.3 (1.8) | 9.2 (1.8) | 9.3 (1.8) |
| Productivity losses*† | 84.4 (45.1) | 34.3 (18.4) | 32.2 (17.3) | 32.1 (17.2) | 32.2 (17.3) |
| QALYs lost* | 1383 (157) | 579 (65) | 544 (62) | 543 (61) | 545 (62) |
| R0 1.6 | |||||
| Attack rate (%) | 52.6 | 28.4 | 26.9 | 26.9 | 26.9 |
| No. influenza cases | 612,310 | 331,183 | 313,389 | 313,372 | 313,791 |
| Direct health care costs*† | 46.7 (9.9) | 23.7 (5.0) | 22.3 (4.7) | 22.3 (4.7) | 22.3 (4.7) |
| Productivity losses*† | 140.9 (76.5) | 72.5 (39.9) | 68.5 (37.7) | 68.5 (37.7) | 68.6 (37.7) |
| QALYs lost* | 2193 (248) | 1189 (128) | 1125 (121) | 1125 (121) | 1127 (121) |
| 20% preexisting immunity | |||||
| R0 1.2 | |||||
| Attack rate (%) | 16.8 | 2.2 | 1.8 | 1.8 | 1.8 |
| No. influenza cases | 196,040 | 25,809 | 21,379 | 21,313 | 20,915 |
| Direct health care costs*† | 12.3 (2.4) | 1.4 (0.3) | 1.2 (0.2) | 1.2 (0.2) | 1.2 (0.2) |
| Productivity losses*† | 42.0 (22.9) | 5.4 (2.9) | 4.5 (2.4) | 4.4 (2.4) | 4.4 (2.4) |
| QALYs lost* | 716 (77) | 95 (10) | 79 (8) | 79 (8) | 77 (8) |
| R0 1.6 | |||||
| Attack rate (%) | 31.2 | 14.2 | 13.3 | 13.3 | 13.3 |
| No. influenza cases | 363,181 | 164,869 | 154,792 | 155,249 | 154,961 |
| Direct health care costs*† | 25.7 (5.7) | 10.7 (2.2) | 10.0 (2.1) | 10.0 (2.1) | 10.0 (2.1) |
| Productivity losses*† | 73.5 (44.7) | 32.5 (19.5) | 30.5 (18.2) | 30.6 (18.3) | 30.5 (18.3) |
| QALYs lost* | 1305 (145) | 596 (66) | 559 (62) | 561 (62) | 560 (62) |
| 40% preexisting immunity | |||||
| R0 1.2 | |||||
| Attack rate (%) | 2.8 | 0.2 | 0.1 | 0.2 | 0.2 |
| No. influenza cases | 32,172 | 2172 | 1314 | 2305 | 1885 |
| Direct health care costs*† | 1.8 (0.3) | 0.12 (0.02) | 0.07 (0.01) | 0.12 (0.02) | 0.11 (0.02) |
| Productivity losses*† | 6.5 (3.4) | 0.4 (0.2) | 0.3 (0.1) | 0.5 (0.2) | 0.4 (0.2) |
| QALYs lost* | 118 (13) | 8 (1) | 5 (1) | 9 (1) | 7 (1) |
| R0 1.6 | |||||
| Attack rate (%) | 13.3 | 1.8 | 1.4 | 1.5 | 1.6 |
| No. influenza cases | 155,002 | 21,360 | 16,363 | 17,632 | 17,999 |
| Direct health care costs*† | 10.1 (2.0) | 1.2 (0.2) | 0.9 (0.2) | 1.0 (0.2) | 1.0 (0.2) |
| Productivity losses*† | 33.4 (18.0) | 4.5 (2.4) | 2.4 (1.8) | 3.7 (2.0) | 3.8 (2.0) |
| QALYs lost* | 563 (62) | 78 (9) | 60 (7) | 65 (7) | 66 (7) |
Does not include vaccinations costs.
Mean (SD).
Millions US$.
QALYs indicates quality-adjusted life-year
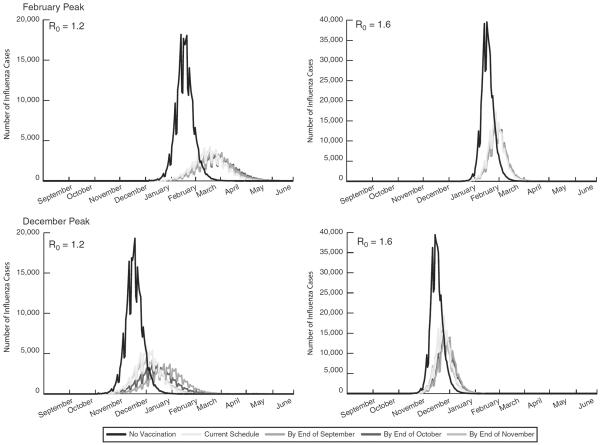
FIGURE 2.
Epidemiologic curves for an influenza season peaking in February and December with an R0 of 1.2 and 1.6.
No Vaccination
When there is no vaccination, varying R0 from 1.2 and 1.6 results in approximately 229,000 more cases generating an additional $21.0 million in direct costs, $52.0 million in productivity losses, and 807 additional QALYs (Table 1). As the amount of preexisting immunity in the population increases, the difference in the number of influenza cases and their related costs and health effects between R0 1.2 and 1.6 decrease (Table 1). This highlights the variability from season to season (as there is no one most common influenza season) and how these parameters relate to epidemiologic and economic outcomes.
Current Vaccination Schedule
Tables 1 and 2, and Figure 2 show how the current timing of vaccination reduces the epidemic curve and impacts economic outcomes. The impact of vaccination depends on the transmission dynamics of the influenza season, on R0, amount of preexisting immunity, and season peak. Vaccination can lead to a 1.9- to 14-fold decrease in attack rate, depending on these transmission dynamics. The current vaccination timing averted fewer cases when the season peaked in December (29,187–252,106 cases averted), increasing with R0 values and decreasing with higher levels of preexisting immunity.
TABLE 2.
Age Distribution of Influenza Cases and Their Related Costs for February and December Influenza Peaks With the Current Vaccination Timing (R0 1.2, No Preexisting Immunity) for the Current and Increased Coverage Rates and the Range of Cases and Costs (in Millions) When Shifting the Vaccination Timing (September to November)
| Current Vaccination Timing |
5% Increase in Coverage, Current Vaccination Timing |
10% Increase in Coverage, Current Vaccination Timing |
Shifting Vaccination Timing (September to November) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | No. Cases |
Direct Costs |
Productivity Losses |
No. Cases |
Direct Costs |
Productivity Losses |
No. Cases |
Direct Costs |
Productivity Losses |
No. Cases | Direct Costs |
Productivity Losses |
| February peak, R0 1.2, no preexisting immunity | ||||||||||||
| Preschool-aged children (0–4 y old) | 6974 | 0.6 | 1.3 | 6424 | 0.6 | 1.2 | 5537 | 0.5 | 1.0 | 6365–6382 | 0.5–0.5 | 1.2–1.2 |
| School-aged children (5–17 y old) | 71,311 | 1.5 | 12.2 | 67,553 | 1.4 | 11.2 | 62,011 | 1.3 | 10.2 | 67,539–67,680 | 1.4–1.4 | 11.6–11.6 |
| Adults (18–64 y old) | 72,029 | 7.8 | 18.5 | 67,236 | 7.3 | 16.3 | 60,461 | 6.5 | 14.7 | 67,490–67,396 | 7.3–7.3 | 17.3–17.3 |
| Elderly (65 y and older) | 8721 | 2.8 | 2.7 | 7968 | 2.5 | 2.3 | 7048 | 2.2 | 2.1 | 8008–8003 | 2.5–2.5 | 2.5–2.5 |
| Total | 159,035 | 9.9 | 34.3 | 149,182 | 9.2 | 31.0 | 135,058 | 8.3 | 28.0 | 149,401–149,462 | 9.3–9.3 | 32.2–32.2 |
| December peak, R0 1.2, no preexisting immunity | ||||||||||||
| Preschool-aged children (0–4 y old) | 9063 | 0.8 | 1.6 | 8477 | 0.7 | 1.6 | 7747 | 0.7 | 1.5 | 6768–7537 | 0.6–0.7 | 1.2–1.4 |
| School-aged children (5–17 y old) | 80,578 | 1.7 | 13.2 | 76,649 | 1.6 | 13.3 | 72,842 | 1.5 | 12.7 | 67,725–71,643 | 1.4–1.5 | 11.1–11.7 |
| Adults (18–64 y old) | 81,996 | 9.1 | 19.8 | 77,145 | 8.4 | 20.0 | 71,812 | 7.8 | 18.6 | 66,349–70,439 | 7.3–7.7 | 16.0–17.0 |
| Elderly (65 y and older) | 10,165 | 3.2 | 2.9 | 9363 | 2.9 | 2.9 | 8640 | 2.7 | 2.7 | 7821–8439 | 2.5–2.7 | 2.3–2.4 |
| Total | 181,802 | 11.4 | 38.7 | 171,635 | 10.7 | 37.9 | 161,042 | 9.9 | 35.5 | 148,663–158,059 | 9.2–9.7 | 31.5–33.5 |
Range represents shifts to September and November.
The mean total vaccination cost was $3,451,555 (SD = $325,879) for Centers for Disease Control and Prevention vaccine prices and $4,810,976 (SD = $473,959) for private sector vaccine prices. When combined with the direct costs, third party payer costs can be determined for vaccine price points. Societal costs for both vaccine price points can be calculated as the respective vaccination costs plus direct costs plus productivity losses. For example, given the current timing, with private sector vaccine costs, the third party payer costs were $16.0 million and societal costs were $52.3 million (December peak, R0 1.2).
Vaccinating All Age Groups Earlier Than the Current Schedule
When the influenza season peaks in February, shifting vaccination for all ages earlier can reduce the attack rate from 13.7% (current schedule) to 12.8% regardless of when vaccination was complete (ie, attack rate same for shifts to September, October, or November) with R0 1.2 and no preexisting immunity. Earlier vaccination could avert ~34 QALYs, $0.6 million in direct costs, and $2.1 million in productivity losses (Table 1).
Table 1 can be used to determine the costs and QALYs averted by shifting the timing of vaccination from the current schedule and to determine the cost per person countywide (divide by 1,164,880). For example, completing vaccination by the end of October can avert 10,093 cases, $0.7 million in direct costs (saving $0.6 per person countywide), $2.2 million in productivity losses (saving $2 per person countywide), and 37 QALYs (R0 1.2; no preexisting immunity). These saving thresholds represent the investment ceilings that can be made to promote earlier vaccination (ie, shift vaccination) and remain cost-neutral.
When the influenza season peaks earlier (eg, December), it is more important to vaccinate earlier. Shifting the timing of vaccination had a greater impact with a December peak. A September shift provided the greatest gains, decreasing the attack rate from 15.6% to 12.8%, averting an additional 33,139 cases, $2.2 million in direct costs, $7.2 million in productivity losses, and 120 QALYs (R0 1.2, no preexisting immunity). This decreased with the amount of preexisting immunity in the population so that at 40%, shifting vaccination timing had little impact. A September shift yielded the greatest change, averting 73 cases, $5295 in direct costs, and $17,167 in productivity losses (December peak, R0 1.2). At an R0 of 1.6, shifting vaccination could avert ≤11,687 cases and save ≤$680,627 in direct costs, and ≤$2,415,913 in productivity losses.
Figure 2 shows how earlier vaccination impacts the epidemic curves for both peaks and R0 values with no preexisting immunity. Earlier vaccination shifts the curve later in the influenza season and reduces the peak; this is most prominent with a December peak and R0 1.2, whereas earlier vaccination has little impact with a February peak and R0 1.6 as the curves are stacked on top of each other. Table 2 provides the age breakdown for these epidemic curves for R0 1.2 and no preexisting immunity, showing the month for completing vaccination had little impact with a late peak (February).
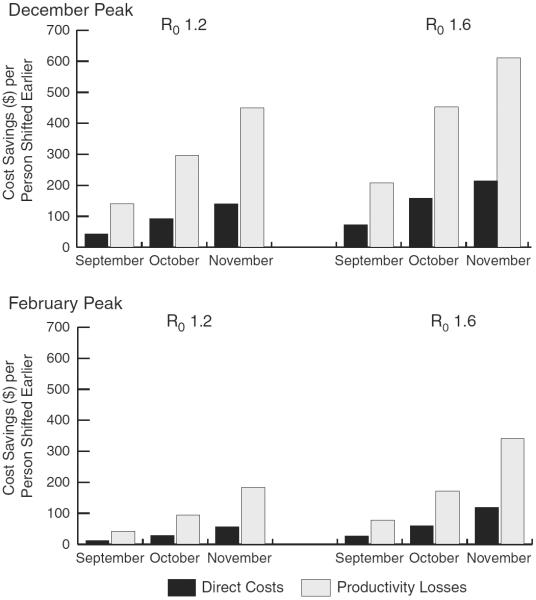
Figure 3 shows the cost-savings per vaccinated person shifted earlier for both peaks and R0 values. These savings represent the amount that could be invested per person to promote earlier vaccination and remain cost-neutral. Vaccinating everyone by the end of September (51,461 earlier vaccinations) saved $12–$72 and $41–$208 per shifted person in direct costs and productivity losses, respectively.
FIGURE 3.
Cost-savings per vaccinated person shifted earlier for December and February influenza season peaks with an R0 of 1.2 and 1.6. Direct costs and productivity losses per vaccinated person shifted if vaccinating by the end of September, October, and November.
Vaccinating Only Children Earlier Than the Current Schedule
When only vaccinating children by the end of September (everyone else remains at current schedule), there were 147,670 total cases (11,366 fewer cases than the current schedule), saving $0.6 million in direct costs, $2.3 million in productivity losses, and 42 QALYs (February peak, R0 1.2, no preexisting immunity). Compared with the other vaccination shifts, it would be better to vaccinate only children by the end of September with a February peak, but vaccinating everyone by the end of September or October would be better than only vaccinating children by the end of September with a December peak (R0 1.2). However, at R0 1.6, vaccinating everyone earlier, regardless of when, resulted in fewer cases and incurred lower costs than only vaccinating children earlier (December and February peaks).
Increasing Vaccination Coverage
Table 2 shows the epidemiologic and economic impact of increasing vaccination coverage compared with the current timing and earlier vaccination. Increasing coverage could result in attack rates of 12.8%–27.2% (5% coverage increase) and 11.6%–26.1% (10% coverage increase) for R0 1.2–1.6 (February peak). With a February peak (R0 1.2), a 5% increase in coverage among all age groups would be comparable with earlier vaccination (similar attack rates and number of cases), however, a 10% vaccination coverage increase would be better than any of the shifts (Table 2). Results were similar for R0 1.6. With a December peak, vaccinating everyone earlier would be better than a 5% or 10% increase in coverage (R0 1.2 and R0 1.6). Vaccinating children by the end of September would be better than increasing vaccination coverage by 5% at an R0 of 1.2 (regardless of the season peak) and R0 of 1.6 with a December peak. However, vaccinating children by the end of September would only be better than a 10% increase in coverage at R0 1.2 (regardless of peak).
DISCUSSION
Our study quantifies the potential benefits and cost-savings of administering influenza vaccination to the population earlier than current practice. In a majority of influenza seasons, which peak in February or later (59% over past 32 y5), the current timing of vaccination appears to be early enough. However, earlier vaccination could provide additional cost-savings in influenza seasons that peak in December or earlier (25% over past 32 y5). These cost-savings would be greater than the cost-savings garnered from increasing vaccination coverage by 5%–10% for seasons that peak in or before December (ie, earlier vaccination would be better than increasing vaccination coverage), whereas increasing coverage may be better seasons with a later peak (in or after February, regardless of R0). From season to season the comparison of increasing coverage versus earlier vaccination may have a different answer. In the long run, however, with 59% of influenza seasons peaking in February or later, increasing coverage by >5% may yield more returns than vaccinating earlier.
As influenza seasons differ from year to year, shifting vaccination earlier may have value in some seasons but not all of them and potentially not in over half. For any given season, the expected savings if completing vaccination by the end of September would range between $21,000–$1,300,000 in direct costs, $78,000–$3,800,000 in productivity losses, and 2–60 QALYs. This corresponds to up to 1.88 extra healthy days per influenza case averted or over 1 day saved/gained, 1 day of productivity, time with family or friends, etc. If vaccination were completed by the end of November, expected savings ranged from $6000–$1,100,000 in direct costs, $24,000–$3,100,000 in productivity losses, and 1–48 QALYs. Both increasing coverage and earlier vaccination would be helpful, but if having to choose, increasing coverage has value across all seasons, whereas earlier vaccination has value only were the peak is earlier than February (40% of seasons). For any given season, for coverage increases of 5%–10%, the maximum expected savings ranged from $743,000–$1,400,000 in direct costs, $1,300,000–$3,200,000 in productivity losses, and 27–56 QALYs.
Our results may provide benchmarks to help determine how much should be invested in getting people vaccinated earlier. Barriers to early vaccination include variability in timing of influenza vaccine production, variability in timing of influenza vaccine distribution, historical emphasis on distribution of vaccine in October and November, August as a vacation month and start of schools year, typically in late August. Historically, influenza vaccine production was limited by the small number of manufacturers in the US market, and time needed for egg-based production and to optimize growth for new strains. However, the market has changed with the entrance of more manufacturers, larger facilities, and cell-based and recombinant vaccines that do not require eggs. With conventional egg-based production measures it is not clear how early vaccines could be available, but newer technologies (cell-based and recombinant vaccines) could make vaccines available much earlier (eg, August).
There are several ways to increase the feasibility of earlier vaccination. First, earlier vaccination in medical settings can be achieved by a combination of automatic patient notification via e-mail, autodialing, text messaging, and express vaccination services that operate under standing orders. Second, employers and community vaccinators (eg, local pharmacies) can offer vaccination earlier. Third, vaccination could be offered in school settings early, given that many school systems start in late August. There is a tremendous amount of variability in the costs of programs to shift vaccination earlier. Rather than represent a single program (which may be difficult to cost out or vary by location), our results show the ceiling amount that can be invested in these programs and remain cost-neutral. In addition, each of these initiatives incur may have variable success in encouraging earlier vaccination. Thus, the costs of these activities must be weighed against the benefits. Systematic reviews and meta-analyses already describe ways to increase vaccination rates22; again, costs are variable and must be weighed against the benefits.
Moreover, we did not account for the possibility that influenza vaccine immunoprotection could wane over the season's course, providing disincentive to vaccination that is too early in the season. Data on such waning immunity is still equivocal. In a review, 8 of 8 studies found seroprotection lasting ≥4 months for H3N2 and 5 of 7 for H1N1 or B.23 In another study, seroprotection rates declined significantly but still met regulatory criteria (6 mo postvaccination).24 The live attenuated influenza vaccine has been shown to have protection that lasts over a year.25 The US influenza vaccine effectiveness network has found residual protection from the prior vaccine season.26
Our study endeavored to remain conservative. Vaccine efficacy varies from year to year depending on strain matching and vaccine presentation, we modeled vaccine efficacy as a range to account for these potential differences. However, actual efficacy may be higher or lower than that modeled. We did not include additional productivity losses beyond hospitalization, although a person may experience symptoms after hospitalization. Our model did not include other comorbid conditions, which may exacerbate influenza or require additional medical care and accrue additional costs. By definition, all models are simplification of real life, and as such cannot represent every possibility or outcome.27 The course of an actual epidemic may not conform to our model's data and assumptions. Our study draws data from sources of varying rigor and quality.
CONCLUSIONS
Even though many people are getting vaccinated well after the September through October timeframe, these vaccinations likely are still occurring early enough to provide substantial cost-savings. Our study quantifies the potential benefits and cost-savings of vaccinating against influenza earlier than current practice. Depending on the timing of the influenza season peak, influenza transmissibility, and preexisting immunity, vaccinating those who typically receive the vaccine later by the end of September could avert up to $3.7 million in direct costs, $10.7 million in productivity losses, and 168 QALYs. While moving vaccinations earlier from current practice yielded some benefits and cost-savings, investing in increasing coverage may yield greater benefits for later season peaks.
Acknowledgments
Supported by Cooperative Agreement FOA IP11-003, U01IP000467, funded by the Centers for Disease Control and Prevention (CDC), Agency for Healthcare Research and Quality (AHRQ) 1R01HS02331701, NICHD/NIH 5U54HD07072503, and the Bill and Melinda Gates Foundation.
APPENDIX
TABLE A1.
Agent-based Model Transmission and Contact Parameters
| Contact Group | Infectious Individual | Susceptible Individual | Transmission Probability |
|---|---|---|---|
| Household | Adult | Adult | 0.4 |
| Household | Adult | Child | 0.3 |
| Household | Child | Adult | 0.3 |
| Household | Child | Child | 0.6 |
| Elementary school | Student | Student | 0.0435 |
| Middle school | Student | Student | 0.0375 |
| High school | Student | Student | 0.0315 |
| Workplace | Adult | Adult | 0.0575 |
| Hospital | Health care worker | Health care worker | 0.0575 |
| Hospital | Health care worker | Patient | 0.01 |
| Hospital | Patient | Health care worker | 0.01 |
| Community | All | Adult | 0.00480 |
| Community | All | Child | 0.00255 |
| Social network | Location | Individual | Mean contacts per day |
| School | Classroom | Student | 13.5 |
| School | Outside classroom | Student | 15 |
| Community (weekday) | Outside of school | Student | 16.2 |
| Community (weekend) | Outside of school | Student | 24.3 |
| Community | Community | All | 32.4 |
| Workplace | Within office | Worker | 2 |
| Workplace | Outside of office | Worker | 8 |
| Health care facility | Within ward or clinic | Health care worker | 2 |
| Health care facility | Outside ward or clinic | Health care worker | 8 |
| Health care facility | With patients | Health care worker | 30 |
TABLE A2.
Select ABM Parameters and Economic Model Input Parameters
| Parameters | Mean or Median | SE or Range | Source |
|---|---|---|---|
| Other ABM parameters | |||
| Proportion receiving TIV (of those vaccinated)* | |||
| 6 mo–4 y | 93.06 | ||
| 5–9 y | 71.03 | ||
| 10–17 y | 80.11 | ||
| 18–44 y | 97.91 | ||
| 45–64 y | 100 | ||
| 65 y and older | 100 | ||
| Proportion receiving LAIV (of those vaccinated)* | |||
| 6 mo–4 y | 6.94 | ||
| 5–9 y | 28.97 | ||
| 10–17 y | 19.89 | ||
| 18–44 y | 2.09 | ||
| TIV vaccine efficacy | |||
| 6 mo–4 y | 59.0 | 51.0–65.0† | Expert Opinion28 |
| 5–9 y | 59.0 | 51.0–66.0† | Expert Opinion28 |
| 10–17 y | 59.0 | 51.0–67.0† | Expert Opinion28 |
| 18–44 y | 59.0 | 51.0–68.0† | Expert Opinion28 |
| 45–64 y | 59.0 | 51.0–69.0† | Expert Opinion28 |
| 65 y and older | 54.0 | 47.0–62.0† | Expert Opinion28 |
| LAIV vaccine efficacy | |||
| 6 mo–17 y | 83.0 | 69.0–91.0† | Expert Opinion28 |
| 18–44 y | 54.00 | 47.0–64.0† | Expert Opinion28 |
| Economic model parameters | |||
| Costs (2013 US$) | |||
| Vaccination | |||
| TIV (CDC cost per dose) | |||
| 6 mo–3 y | 10.49 | 2.49 | 29 |
| 4 y and older | 10.00 | 1.89 | 29 |
| 9 y and older | 9.70 | 1.76 | 29 |
| 18–64 y olds | 8.85 | 1.99 | 29 |
| 65 y and older | 8.50 | 1.67 | 29 |
| LAIV (CDC cost per dose) | |||
| 2–49 y olds | 17.3 | 29 | |
| TIV (private sector cost per dose) | |||
| 6 mo–3 y | 12.97 | 3.22 | 29 |
| 4 y and older | 12.96 | 1.82 | 29 |
| 9 y and older | 12.44 | 1.90 | 29 |
| 18–64 y olds | 13.04 | 2.78 | 29 |
| 65 y and older | 12.70 | 2.65 | 29 |
| LAIV (private sector cost per dose) | |||
| 2–49 y olds | 21.7 | 29 | |
| Hourly wage | 17.21 | 8.96–42.99 | 20 |
| Outpatient visit given Influenza | |||
| < 1 y old | 78.46 | 30 | |
| 1–17 y old | 83.91 | 30 | |
| 18–44 y old | 105.59 | 30 | |
| 45–64 y old | 96.26 | 31 | |
| 65–84 y old | 92.74 | 31 | |
| Hospitalization given Influenza | |||
| < 1 y old | 5650.47 | 1124.88 | 32 |
| 1–17 y old | 6824.13 | 801.07 | 32 |
| 18–44 y old | 8880.10 | 1085.17 | 32 |
| 45–64 y old | 14,703.65 | 1699.23 | 32 |
| 65–84 y old | 9810.63 | 846.85 | 32 |
| 85 y and older | 8953.97 | 901.00 | 32 |
| Probabilities | |||
| Side effects from TIV | 1.0 | Pink Book | |
| Side effects from LAIV | 7.0 | Pink Book | |
| Symptomatic influenza | 66.9 | 58.3–74.5 | 33 |
| Missing work | 72.0 | 34 | |
| Missing school | 69.0 | 35 | |
| Ambulatory care visit given influenza | |||
| 0–4 y old | 45.5 | 9.8 | 36 |
| 5–17 y old | 31.8 | 6.1 | 36 |
| 18–64 y old | 31.3 | 1.4 | 36 |
| 65 y and older | 62.0 | 2.7 | 36 |
| Hospitalization given influenza | |||
| 0–4 y old | 1.41 | 0.47 | 36 |
| 5–17 y old | 0.06 | 0.02 | 36 |
| 18–49 y old | 0.42 | 0.14 | 36 |
| 50–64 y old | 1.93 | 0.64 | 36 |
| 65 y and older | 4.21 | 1.4 | 36 |
| Morality given influenza | |||
| 0–4 y old | 0.004 | 0.001 | 36 |
| 5–17 y old | 0.001 | 0 | 36 |
| 18–49 y old | 0.009 | 0.003 | 36 |
| 50–64 y old | 0.134 | 0.045 | 36 |
| 65 y and older | 1.17 | 0.39 | 36 |
| Durations | |||
| Ambulatory care visit (h) | 4 | Assumption | |
| Work missed (d) | 1.5–4.9 | 37 | |
| School missed (d) | 2.54 | 35 | |
| Duration of symptoms (d) | 7 | 38 | |
| Hospitalization (d) | |||
| < 1 y old | 3.3 | 0.4 | 32 |
| 1–17 y old | 3.4 | 0.2 | 32 |
| 18–44 y old | 4.2 | 0.3 | 32 |
| 45–64 y old | 5.7 | 0.4 | 32 |
| 65–84 y old | 4.9 | 0.3 | 32 |
| 85 y and older | 5.5 | 0.7 | 32 |
| Utility weights | |||
| Healthy QALY | |||
| < 17 y old | 1 | 39 | |
| 18–64 y old | 0.92 | 39 | |
| 65 y and older | 0.84 | 39 | |
| Influenza, no hospitalization utility weight | 0.659 | 0.106 | 40–19 |
| Influenza, hospitalization, utility weight | 0.514 | 0.089 | 42,49,50 |
| Side effects utility weight | 0.940 | 0.041 | 18,40,42,43,47 |
Influenza Vaccine Effectiveness Network Data 2011.
95% confidence interval.
ABM indicates agent-based model; CDC, Centers for Disease Control and Prevention; LAIV, live attenuated influenza vaccine; QALY, quality-adjusted life-years; TIV, trivalent inactivated influenza vaccine.
Footnotes
R.K.Z. discloses that he has clinical research grants from Pfizer and SanofiPasteur. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Lee BY, Tai JH, Bailey RR, et al. The timing of influenza vaccination for older adults (65 years and older) Vaccine. 2009;27:7110–7115. doi: 10.1016/j.vaccine.2009.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BY, Tai JH, Bailey RR, et al. Economics of influenza vaccine administration timing for children. Am J Manag Care. 2010;16:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee BY, Shah M. Prevention of influenza in healthy children. Expert Rev Anti Infect Ther. 2012;10:1139–1152. doi: 10.1586/eri.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [Accessed April 15, 2013];Centers for Disease Control and PreventionNational Influenza Vaccination Week Atlanta, GA2013. 2013 Available at: http://cdc.gov/flu/nivw.
- 5. [Accessed November 4, 2014];Centers for Disease Control and PreventionCDC-Seasonal Influenza (Flu) CDC2013. 2014 Available at: http://www.cdc.gov/flu/about/season/flu-season.htm.
- 6.Cooley P, Lee BY, Brown ST, et al. Protecting health care workers: a pandemic simulation based on Allegheny County. Influenza Other Respir Viruses. 2010;4:61–72. doi: 10.1111/j.1750-2659.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BY, Brown ST, Cooley P, et al. Simulating school closure strategies to mitigate an influenza epidemic. J Public Health Manag Pract. 2010;16:252–261. doi: 10.1097/PHH.0b013e3181ce594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BY, Brown ST, Cooley P, et al. Vaccination deep into a pandemic wave: potential mechanisms for a “third wave” and the impact of vaccination. Am J Prevent Med. 2010;39:e21–e29. doi: 10.1016/j.amepre.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BY, Brown ST, Korch GW, et al. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine. 2010;28:4875–4879. doi: 10.1016/j.vaccine.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grefenstette JJ, Brown ST, Rosenfeld R, et al. FRED (a Framework for Reconstructing Epidemic Dynamics): an open-source software system for modeling infectious diseases and control strategies using census-based populations. BMC Public Health. 2013;13:940. doi: 10.1186/1471-2458-13-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson NM, Cummings DAT, Fraser C, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halloran ME, Ferguson NM, Eubank S, et al. Modeling targeting layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci USA. 2008;105:4639–4644. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longini IMJ, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 14.Beigi RH, Wiringa AE, Bailey RR, et al. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis. 2009;49:1784–1792. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BY, Bacon KM, Donohue JM, et al. From the patient perspective: the economic value of seasonal and H1N1 influenza vaccination. Vaccine. 2011;29:2149–2158. doi: 10.1016/j.vaccine.2010.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BY, Bailey RR, Wiringa AE, et al. Economics of employer-sponsored workplace vaccination to prevent pandemic and seasonal influenza. Vaccine. 2010;28:5952–5959. doi: 10.1016/j.vaccine.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine. 2012;30:7443–7446. doi: 10.1016/j.vaccine.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Stalter RM, Bacon KM, et al. Cost-effectiveness of adjuvanted versus nonadjuvanted influenza vaccine in adult hemodialysis patients. Am J Kidney Dis. 2011;57:724–732. doi: 10.1053/j.ajkd.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BY, Tai JHY, McGlone SM, et al. The potential economic value of a “universal” (multi-year) influenza vaccine. Influenza Other Respir Viruses. 2012;6:167–175. doi: 10.1111/j.1750-2659.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau of Labor Statistics . Occupational Employment Statistics: May 2012 National Occupational Employment and Wage Estimates. US Bureau of Labor Statistics Division of Occupational Employment Statistics; Washington, DC: [Accessed September 4, 2013]. 2012. Available at: http://www.bls.gov. [Google Scholar]
- 21.Human Mortality Database [Internet] [Accessed June 14, 2011];University of California, Berkeley (USA), and Max Planck Institute for Demographic Reseach (Germany) 2010 Available at: http://www.mortality.org.
- 22. [Accessed June 12, 2014];Guide to Community Preventive ServicesIncreasing approriate vacci-nation. 2014 Available at: http://www.thecommunityguide.org/vaccines/index.html.
- 23.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- 24.Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine. 2010;28:3929–3935. doi: 10.1016/j.vaccine.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose CS, Yi T, Walker RE, et al. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27:744–748. doi: 10.1097/INF.0b013e318174e0f8. [DOI] [PubMed] [Google Scholar]
- 26.Ohmit SE, Thompson MG, Petrie JG, et al. Infulenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58:319–327. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008;46:1139–1141. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 28.Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed April 20, 2013];Centers for Disease Control and PreventionCDC Vaccine Price List Atlanta, GA: US Department of Health and Human Services. 2013 Available at: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/2013/2013-04-16.html.
- 30.Thomson HealthcareMarketScan Research Database Ann Arbor, MI. 2008. [Google Scholar]
- 31.Centers for Medicare & Medicaid Services . Physicians Fee Schedule. US Department of Health & Human Services; Baltimore, MD: [Accessed March 16, 2011]. 2009. Available at: http://www.cms.hhs.gov/ [Google Scholar]
- 32.United States Department of Health & Human Services . HCUP Facts and Figures: Statistics on Hospital-based Care in the United States. AHRQ: Agency for Healthcare Research and Quality; Rockville, MD: [Accessed April 23, 2013]. 2010. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. [Google Scholar]
- 33.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 34.Palmer LA, Rousculp MD, Johnston SS, et al. Effect of influenza-like illness and other wintertime respiratory illnesses on worker productivity: the child and household influenza-illness and employee function (CHIEF) study. Vaccine. 2010;28:5049–5056. doi: 10.1016/j.vaccine.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Nettleman MD, While T, Lavoie S, et al. School absenteeism, parental work loss, and acceptance of childhood influenza vaccination. Am J Med Sci. 2001;321:178–180. doi: 10.1097/00000441-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 37.Kreech M, Beardsworth P. The impact of influenza on working days lost. A review of the literature. Pharmacoeconomics. 2008;26:911–924. doi: 10.2165/00019053-200826110-00004. [DOI] [PubMed] [Google Scholar]
- 38.Bridges C, Katz JM, Levandowski RA, et al. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed Elsevier; Philadelphia, PA: 2008. pp. 257–290. [Google Scholar]
- 39.Gold MR, Franks P, McCoy KI, et al. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Smith KJ, Lee BY, Nowalk MP, et al. Cost-effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine. 2010;28:7620–7625. doi: 10.1016/j.vaccine.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002;113:300–307. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 42.Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effective analysis. Am J Med. 2005;118:68–77. doi: 10.1016/j.amjmed.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Khazeni N, Hutton DW, Garber AM, et al. Effectiveness and cost-effectiveness of vaccination against pandemic influenza (H1N1) 2009. Ann Intern Med. 2009;151:829–839. doi: 10.1059/0003-4819-151-12-200912150-00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talbird SE, Brogan AJ, Winiarski AP, et al. Cost-effectiveness of treating influenzalike illness with oseltamivir in the United States. Am J Health System Pharm. 2009;66:469–480. doi: 10.2146/ajhp080296. [DOI] [PubMed] [Google Scholar]
- 45.Luce BR, Nichol KL, Belshe RB, et al. Cost-effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children ages 24–59 months in the United States. Vaccine. 2008;26:2841–2848. doi: 10.1016/j.vaccine.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 46.Perlroth DJ, Glass RJ, Davey VJ, et al. Health outcomes and costs of community mitigation strategies for an influenza pandemic in the United States. Clin Infect Dis. 2010;50:165–174. doi: 10.1086/649867. [DOI] [PubMed] [Google Scholar]
- 47.Michaelidis CI, Zimmerman RK, Nowalk MP, et al. Estimating the cost-effectiveness of a national program to eliminate disparities in influenza vaccination rates among elderly minority groups. Vaccine. 2011;29:3525–3530. doi: 10.1016/j.vaccine.2011.02.098. [DOI] [PubMed] [Google Scholar]
- 48.Mauskopf JA, Cates SC, Griffin AD, et al. Cost effectiveness of zanamivir for the treatment of influenza in a high risk population in Australia. Pharmacoeconomics. 2000;17:611–620. doi: 10.2165/00019053-200017060-00007. [DOI] [PubMed] [Google Scholar]
- 49.Lee BY, Tai JHY, Bailey RR, et al. Economics of influenza vaccine administration timing for children. Am J Manag Care. 2010;16:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 50.Jit M, Cromer D, Baguelin M, et al. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine. 2010;29:115–122. doi: 10.1016/j.vaccine.2010.08.078. [DOI] [PubMed] [Google Scholar]