Abstract
Purpose
Centers that care for newborns with Congenital Diaphragmatic Hernia (CDH) may impose selection criteria for offering or limiting aggressive support in those patients most severely affected. The purpose of this study was to analyze outcomes in newborns with highly severe CDH uniformly treated for survival.
Methods
We reviewed 172 consecutive inborn patients without associated lethal anomalies treated at a single institution with a dedicated CDH program. Survival, respiratory outcome, and time to discharge in the most severe 10% (or fewer) of patients based on the physiologic measures of 5-minute Apgar, CDH Study Group (CDHSG) predicted survival, need for ECMO in the first 6 hours, and need for ECMO in the first 3 hours of life were studied. We also identified patients with best PaCO2 greater than 100 and best pH less than 7.0. A multivariate model (AUC-0.92) predicting mortality was also used to define the most severe 10%.
Results
Of 172 consecutive inborn patients, 18 had a 5-minute Apgar of 3 or less, and 11 survived (61%), 10 had a 5-minute Apgar of 2 or less, and 6 survived (60%), and 6 had a 5-minute Apgar of 1 or less, and 4 survived (67%). Seventeen had a CDHSG predicted survival less than 25%, and 9 survived (53%). Thirteen of 172 required ECMO for rescue in the first 6 hours of life, and 9 survived (69%), including 7 in the first 3 hours, and 5 survived (71%). Despite focused resuscitation in the delivery room and high levels of ventilatory support, 22 patients had a best PCO2 greater than 100 and best pH less than 7.0 for 1 hour or longer. Twelve of these 22 survived to discharge (55%). Of 17 defined by multivariate predictive model as the most severe, 8 survived (47%) with zero of the 3 ECMO ineligible prematures surviving. Of the 16 (10%) most severe ECMO-eligible patients, 10 of 16 survived (63%). All survivors were discharged home on no ventilatory support greater than nasal cannula oxygen.
Conclusion
In newborn CDH patients without lethal associated anomalies, accepted measures of physiologic severity failed to predict mortality. Survival met or exceeded 50% even in the most severe 10% as defined by these measures. These data support the practice of treating each patient for survival regardless of the physiologic severity in the first hours of life, and selection criteria for not offering ECMO should be re-evaluated where practiced.
Keywords: Congenital diaphragmatic hernia, CDH, ECMO, prematurity, severity, outcomes, discharge, survival
Introduction
Congenital Diaphragmatic Hernia (CDH) is a severe and potentially life-threatening birth defect, with a wide spectrum of physiologic severity, and outcomes[1]. With widespread improvements in care based on lung preservation strategies[2–5], neonates with less severe CDH routinely survive in most centers, and newborns with more severe CDH survive at increasing rates at the best centers. Significant questions remain, however, about the viability and outcome potential of those most severely affected by the physiologic ravages of CDH[6,7].
As overall mortality in centers which measure CDH survival averages greater than 30%[8], it follows that mortality in those most severely affected will be much greater, possibly approaching 100%. Although documentation of such practice in the literature is rare, centers may apply arbitrary criteria for not offering aggressive treatment, especially escalating care to ECMO, for those infants felt to represent that most severe end of the spectrum[9].
Physiologic measures in CDH patients at birth and soon after have been shown to correlate with survival and include 5 minute Apgar score, birth weight, CDH study group predicted survival, and initial blood gas values. [8,10,11]. Inborn versus outborn status also affects measured survival at the receiving centers, as the most severe outborn patients are less likely to be transported, or to survive transport, resulting in a selection bias toward less severity at the receiving center[6]. Further, outborn patients are less likely to be prenatally diagnosed resulting in less optimal resuscitation, which raises questions about the predictive value of the physiologic data gathered in the first hours for these patients. Finally, prenatal terminations may truncate CDH severity in geographic areas where such activity is significant.
Analysis of the severe end of the CDH spectrum would therefore be best represented by studying inborn patients from a center where prenatal terminations are minimal or non-existent. To this end we studied our series of inborn patients with CDH, many of whom traveled significant distance after declining termination elsewhere, and who were treated aggressively for survival. We sought to define survival, time to discharge, and respiratory status at discharge, to address the question of whether treatment should be withheld from those with the highest severity CDH as defined by physiologic derangements at birth.
Methods
This is an IRB approved retrospective review of consecutive patients with Congenital Diaphragmatic Hernia treated at UF Health, Shands Children’s Hospital between September 1992 and December 31, 2011. A total of 268 consecutive CDH patients were identified from the cross reference of two separate medical record queries with operative records, autopsy records, a divisional database, and 2 prenatal evaluation databases. Patients with Morgagni CDH, diaphragmatic eventration, and patients in whom the diagnosis of CDH was missed and delayed more than 48 hours after delivery were not included. All patients were symptomatic in the first 6 hours of life. Of this total, 28 (10%) were judged to have lethal associated anomalies [12]. Of the remaining two hundred and forty, 172 were inborn and treated aggressively for survival, comprising the subjects of this study.
Clinical Care
The majority of patients were prenatally diagnosed and counseled at our facility. Many had been offered pregnancy termination before our evaluation, but no parents chose termination for CDH alone after our evaluation. All patients included were treated with intent to cure regardless of clinical severity, utilizing strict limitation of ventilation pressures, avoidance of hyperventilation, and use of mild sedation as previously described [3]. Medical oversight throughout the series was uniform, leading to a high degree of therapeutic consistency.
For prenatally diagnosed patients, delivery was planned to occur between 38 and 39 weeks when possible, either by attempted induction or repeat Caesarean Section. EXIT, and EXIT to ECMO procedures were not used. Preterm labor was treated with attempt to attain at least 34 weeks gestation when clinically appropriate, but not always achieved. The attending pediatric surgeon and neonatal team were present at delivery, and intubation was accomplished immediately following delivery whenever prenatal diagnosis had been made. Apgar scores were assigned by the neonatal team.
Initial ventilation was pressure limited with ambu bag or similar, utilizing peak inspiratory pressures of 20 – 25 cm of H20. Ventilator IMV rate was initially assigned at 50, 60, 80, or 100 breaths per minute based on best bedside analysis of clinical severity. Ventilation rates of 80 or higher were used for patients with severe physiologic compromise after birth defined by poor excretion of CO2 as noted by delayed colorimetric change at initial intubation, 5 minute Apgar of 3 or less, and/or preductal saturations less than 70% despite successful intubation and ventilation with 100% oxygen. High frequency oscillation was used for premature CDH patients less than 32 weeks, and occasionally for non-prematures who failed to respond to conventional ventilation.
ECMO was used only for critical instability of preductal saturations, and only after employing all available modalities to avoid ECMO (pressors, nitric oxide, steroids, and intravenous pulmonary vasodilators). Initial ECMO was veno-venous (VV) or veno-arterial (VA) but with a preference for VA ECMO in the highest severity patients as judged by anatomic severity, physiologic severity, LHR, and blood gas values. Management on ECMO was not considered different from standard and has been previously described[10].
Data collected and used for this analysis include gestational age, birth weight, Apgar scores at 1 and 5 minutes, CDH Study Group Predicted Survival[13], and post-ductal blood gas values drawn as close as possible to 1 hour of life from an umbilical artery catheter. Laboratory analysis of blood gases changed from central laboratory to point of care during the experience. Partial pressure carbon dioxide measurements (PCO2) were reported as greater than 130 mm Hg or greater than 100 mm Hg by the different systems, limiting the statistical analysis of very high values PCO2 levels. Ventilator settings were analyzed as well as data regarding use of ECMO, timing of ECMO initiation, number of ECMO days, and survival. Time to discharge was also collected, as well as respiratory status at discharge.
Analysis
Independent variables of physiologic severity including gestational age, birth weight, Apgar-1, Apgar-5, CDH Study Group predicted survival[11], need for ECMO, timing of ECMO, first pH, first PCO2, and first post-ductal PO2 were analyzed independently and in multivariate logistic regression assessing effects on the outcome variables of survival, need for ECMO, duration of ECMO, and age at discharge.
We used the R statistical software package. Fischer’s exact test was used to compare the survival groups on categorical variables and Mann-Whitney tests to compare them on continuous variables. Survival based on the worst (roughly) 10% of patients for each individual severity variable tested is reported.
To develop a best-fit multivariate model that correlated most strongly with mortality, we used logistic regression utilizing the severity variables, and employed stepwise variable elimination based on the Akaike Information Criterion. The best-fit model’s predictive ability was assessed using the area under the receiver operating characteristic curve (AUC). This multivariate model was then used to identify the most severe 10%, both including and excluding ECMO eligible infants, and using 33 weeks and 1800 grams to define eligibility.
Results
Of the total 172 consecutive inborn patients treated for survival that were without associated highly severe or lethal associated anomalies, 150 survived to discharge (87%). Eight of these were not eligible for ECMO support based on gestational age or size. Four of these eight did not survive, 3 of who met multiple measures of highest severity. Of the remaining 164 who were eligible for ECMO support, 146 survived (89 %).
Analyzing all 172 patients, highly significant relationships to survival existed for gestational age, birth weight, 1 minute Apgar, 5 minute Apgar, CDH SG predicted survival, need for ECMO, pH at 1 hours, PCO2 at 1 hour, and (post-ductal) PO2 at 1 hour (Table 1). The significance of the relationship was strongest for Apgar-1, Apgar-5; CDH Study group predicted survival, first pH and first PCO2. Time to ECMO in those patients needing ECMO support did not correlate with survival.
Table 1.
In the table below, all categorical variables are presented as “N (%)” and all continuous variables are presented as “Mean (SD); Median [25th, 75th percentile] (min, max)”
| Overall (n=172) |
Survived (n=150, 87.2%) |
Died (n=22, 12.8%) |
p | |
|---|---|---|---|---|
| CDH Side | ||||
| Left | 150 (87.2) | 132 (88.0) | 18 (12.0) | .491 |
| Right | 22 (12.8) | 18 (81.8) | 4 (18.2) | |
| Gestational age | 37.1 (2.1); 38 [37, 38] (27, 41) |
37.3 (1.8); 38 [37, 38] (31, 41) |
35.6 (3.2); 37 [35, 38] (27, 38) |
.005 |
| Birth weight | 2889 (617); 2953 [2508, 3256] (988, 4441) |
2945 (584); 2998 [2560, 3328] (1467, 4441) |
2507 (711); 2622 [2128, 3104] (988, 3535) |
.013 |
| Apgar-1 | 4.1 (2.4); 4 [2, 6] (0, 9) |
4.3 (2.3); 4.5 [2, 6] (0, 9) |
2.5 (2.1); 2 [1, 3] (0, 9) |
.0006 |
| Apgar-5 | 6.5 (2.2); 7 [5, 8] (1, 9) |
6.8 (2.0); 7 [6, 8] (1, 9) |
4.6 (2.2); 4 [3, 6] (1, 9) |
<.0001 |
| Predicted survival | 62.2 (23.3); 68.5 [46.5, 81] (2, 95) |
65.7 (21.3); 71.5 [51, 82] (4, 95) |
38.6 (23.2); 38 [21, 59] (2, 75) |
<.0001 |
| ECMO | 69 (40.1) | 51 (34.0) | 18 (81.8) | <.0001 |
| Hours to ECMO (ECMO pts only) | 46.1 (74.0); 31.5 [11.7, 42.2] (1.3, 393) |
46.4 (72.4); 32.9 [23.1, 42.6] (1.3, 393) |
45.3 (80.5); 23.9 [7.4, 38.3] (1.6, 351) |
.456 |
| pH at 1 hour | 7.2 (0.20); 7.2 [7.0, 7.3] (6.6, 7.6) |
7.2 (0.18); 7.2 [7.1, 7.3] (6.6, 7.6) |
6.9 (0.18); 6.9 [6.8, 7.1] (6.6, 7.2) |
<.0001 |
| PCO2 at 1 hour (mm Hg) | 67.5 (26.2); 65 [47, 82] (17, 145) |
63.9 (24.5); 61 [45, 76] (17, 130) |
91.9 (24.5); 98 [74, 100] (45, 145) |
<.0001 |
| PO2 at 1 hour (mm Hg) | 114 (120); 59 [45, 134] (8, 678) |
122.5 (124.2); 64.5 [46, 145] (15, 678) |
44.8 (17.4); 45.5 [34.8, 58] (8, 75) |
.001 |
To understand the predictive value for mortality of individual poor results for the physiologic variables defined above, we looked at the survival of patients who had values in the lowest 5 – 10% of the total for those variables. These are reported in Table 2.
Table 2.
Survival by Selected parameter level
| Parameter | Level | Included | Met | Survived | Percent |
|---|---|---|---|---|---|
| 5 minute apgar | <=3 | 172 | 18 | 11 | 61% |
| <=2 | 172 | 10 | 6 | 60% | |
| <=1 | 172 | 6 | 5 | 83% | |
| CDH SG Pred survival | <= 25% | 172 | 17 | 9 | 53% |
| ECMO 1st 6 hrs | 172 | 13 | 9 | 69% | |
| ECMO first 3 hrs | 172 | 7 | 5 | 71% | |
| PCO2 >100 and pH < 7.0 at 1 hr | 172 | 22 | 12 | 55% | |
| Worst 10% by model, all | 172 | 17 | 8 | 47% | |
| Worst 10% by model, ECMO eligible | 164 | 16 | 10 | 63% |
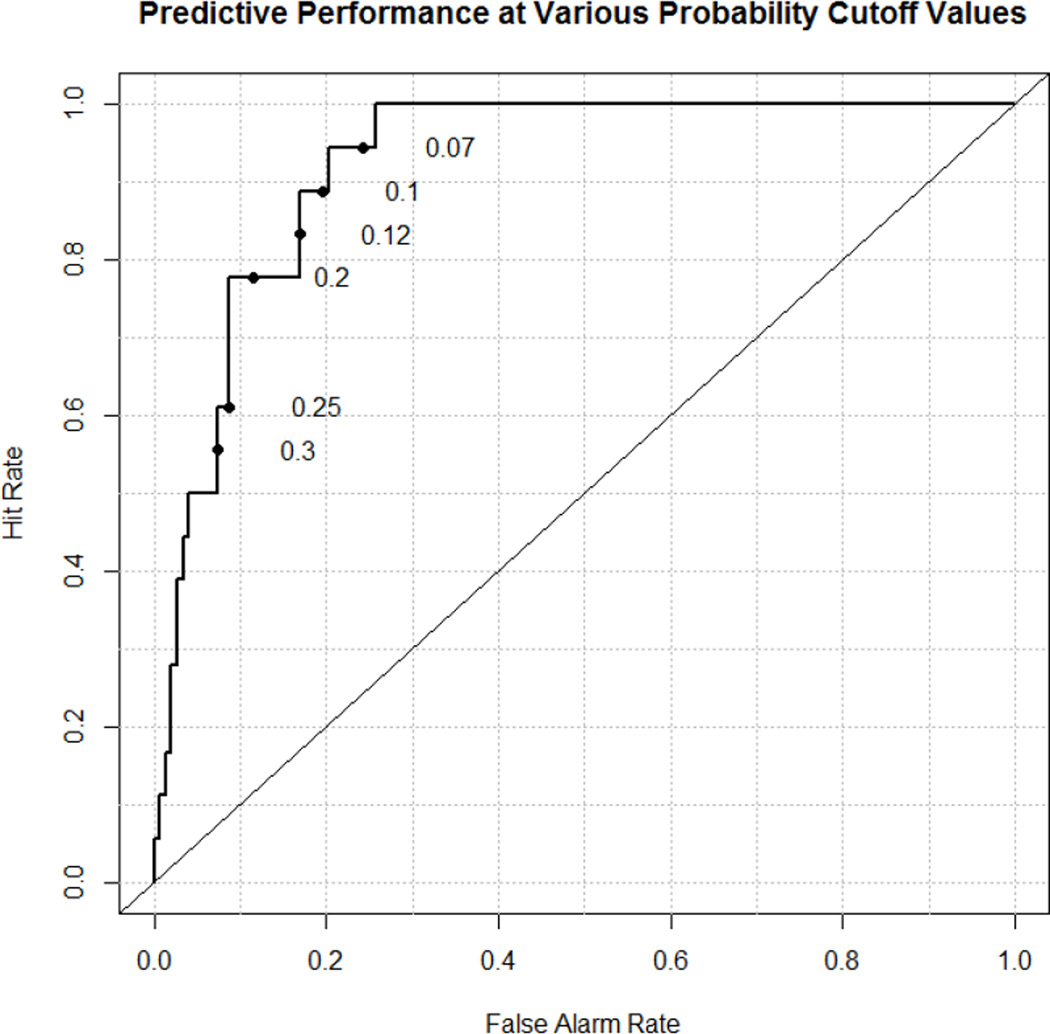
Logistic regression variable elimination algorithm was performed to develop a multivariate model of mortality based on the strongest individual predictive variables. Of these physiologic variables, the algorithm selected Apgar-1; CDH Study group predicted survival (which includes Apgar-5 and birth weight), and pH at one hour. This resulting model had an AUC of 0.922 (Figure 1). The 90th percentile of the model identified the physiologically most severe 10% of patients (n=17 of 172) which included 3 prematures not eligible for ECMO. Eight of 17 survived (47%) and all 3 non-ECMO eligible prematures died. We then developed a model removing the 8 non-ECMO eligible prematures (AUC=0.914)). Of 164 ECMO eligible CDH patients, the model defined the worst 10% (n=16) and 10 of these survived to discharge (63%). The clinical characteristics of these patients are reported in Table 3. Nineteen patients are reported. The first 17 represent the physiologically worst 10% of the total 172, and the remaining 16 after removing the prematures from the 19 listed represent the most severe 10% (n=16) of the remaining 164 ECMO eligible patients.
Figure 1.
Predictive model of Mortality based on Apgar-1, CDH SG Predicted Mortality, and pH at 1 hour (AUC-0.92)
Log(odds of death)=36.7 – 0.33*Apgar-1 – 0.030*CDSGH – 5.16*First pH
Table 3.
Severity details and outcomes of worst 20 patients predicted by model
(3 patients not eligible for ECMO due to prematurity and/or size (pts # 1, 8, and 11).
Survival worst 10% of all patients (n=17 of 172) 8/17 (47%)
Survival worst 10% of ECMO eligible patients (n=16 of 164) 10/16 (63%)
(Gestational age in weeks, Surv= Survival, D/C is time to discharge in months. Resp is oxygen at discharge.
| GA | BW(g) | Ap-1 | Ap-5 | Side | pH | PCO2 | P02 | ECMO | Surv | D/C | Resp | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 1053 | 1 | 2 | Left | 6.59 | >100 | 16 | No | No | * | * |
| 2 | 39 | 2000 | 0 | 1 | Left | 6.75 | >100 | 41 | Yes | Yes | 3.2 | 100 cc NC |
| 3 | 38 | 3200 | 1 | 2 | Right | 6.67 | >100 | 75 | Yes | No | * | * |
| 4 | 36 | 3939 | 1 | 2 | Left | 6.64 | >130 | 15 | Yes | Yes | 3.4 | 400 cc NC |
| 5 | 35 | 2645 | 0 | 4 | Left | 6.75 | 106 | 59 | Yes | No | * | * |
| 6 | 37 | 2400 | 2 | 1 | Left | 6.76 | >100 | 41 | Yes | Yes | 2.9 | 100 cc NC |
| 7 | 35 | 2040 | 1 | 4 | Left | 6.81 | 145 | 46 | Yes | No | * | * |
| 8 | 27 | 988 | 3 | 1 | Left | 6.80 | >100 | 8 | No | No | * | * |
| 9 | 37 | 2500 | 1 | 3 | Left | 6.88 | >100 | 33 | Yes | Yes | 3.7 | 300 cc NC |
| 10 | 37 | 2212 | 1 | 3 | Left | 6.95 | >100 | 62 | Yes | No | * | * |
| 11 | 33 | 1250 | 3 | 4 | Left | 6.86 | 96 | 49 | No | No | * | * |
| 12 | 39 | 2450 | 1 | 1 | Left | 7.04 | 79 | 37 | Yes | Yes | 3.4 | 100 cc NC |
| 13 | 34 | 2595 | 2 | 5 | Left | 6.85 | >130 | 37 | Yes | No | * | * |
| 14 | 35 | 1880 | 3 | 4 | Left | 6.93 | >100 | 21 | Yes | Yes | 3.6 | 100 cc NC |
| 15 | 38 | 2750 | 1 | 2 | Left | 7.07 | 67 | 44 | Yes | No | * | * |
| 16 | 37 | 3590 | 0 | 4 | Right | 6.93 | >100 | 48 | Yes | Yes | 1.6 | 400 cc NC |
| 17 | 38 | 3030 | 2 | 4 | Right | 6.88 | >100 | 33 | Yes | Yes | 4.2 | 100 cc NC |
| 18 | 33 | 1840 | 5 | 3 | Left | 6.85 | 101 | 64 | No | Yes | * | * |
| 19 | 37 | 3635 | 2 | 4 | Left | 6.80 | >130 | 37 | Yes | Yes | 3.2 | 250 cc NC |
Mean time to discharge for survivors from the most severe 10% (n= 17) was 3.25 months with a range of 1.6 to 4.2 months. All patients were discharge breathing spontaneously and without ventilation assistance or surgical airways.
Discussion
This report looks at survival, length of hospitalization, and pulmonary status at discharge in the most severe 10% of inborn CDH patients treated at a CDH referral center with high survival. As this series was entirely inborn from a center where no fetuses were terminated for lung hypoplasia and many families came from distance specifically to seek treatment for their severely affected fetus, it is likely that this series represents the full extent of CDH severity with minimal potential for selection bias.
The results show that although the individual markers of physiologic severity tested here correlated very strongly with survival across the CDH spectrum, individually they failed to predict mortality in any meaningful way even when focused on the most severely affected 10% of patients. Further, when multivariate modeling of severity was used to define the worst 10%, survival still approached 50% when including ECMO ineligible premature infants, and exceeded 60% when they were excluded.
These data demonstrate that the survival and pulmonary function potential of even the most severely affected CDH patients is significant. This fact must be considered when evaluating a CDH fetus or newborn, and when counseling parents about decisions to terminate, limit care, or treat for survival. Based on these data we urge great caution, as have others[14], when defining a CDH patient as non-survivable, as such prophecy is necessarily self-fulfilling. A foundational principal of the results presented here is a belief that even the most severely affected CDH infant can survive.
To define severity, we chose to focus on actual measures of physiologic derangement encountered at the bedside, rather than measures of predicted severity gathered prenatally, such as LHR. Lally et al from the CDH study group showed that physiologic measures at birth and soon after correlate strongly with outcome [11]. Others have expanded on this work and showed that Apgar scores, birth weight, and CDH study group predicted survival correlate with not only survival, but with need for ECMO, need for second ECMO, and duration of ECMO[8,10]. The correlation of severity indices with outcomes is especially robust in this present dataset, possibly reflecting high consistency of resuscitation and treatment in this inborn population. Additionally these data demonstrate that blood gas values in the first hour of life also correlate with survival, and add increasing granularity to predictions of mortality based on multivariate regression models[6].
In the most severe 10% as defined by the multivariate predictive equation (n=17), all fourteen ECMO-eligible patients required treatment with ECMO, whereas the 3 prematures in that most severe group, each of whom was considerably too young or small for ECMO, all died. While some have questioned whether ECMO improves survival in CDH, these data strongly support that ECMO rescue is crucial for survival in those most severely affected[15,16]. As a corollary, prematurity in combination with CDH severe enough to need ECMO behaves as a lethal associated anomaly. Any prenatal therapy for CDH that may increase premature deliveries risks decreasing potential survival by this mechanism[17].
Infants with severe CDH utilize significant resources, and survival alone is not a sufficient metric to define outcome. Focusing on the most severe 10% defined by the predictive equation, we were pleased to discover that the mean time to discharge in survivors was just 3.25 months, with a range of 1.6 – 4.2 months. Despite their severity, these patients need not languish in hospital for extended lengths of time. Further, although they were discharged home on nasal cannula oxygen, an accepted definition of respiratory “morbidity”, none required surgical airways or home ventilation. This reflects the potential for surprisingly good pulmonary function at discharge in patients most certainly affected by severe pulmonary hypoplasia.
The limitations and strengths of these data are notable. This is a retrospective review of treated patients with concerns inherent in retrospective analyses. We excluded patients with lethal associated anomalies to avoid confounding the results, which would have occurred if we included patients that had therapy withheld or results compromised because of severe associated anomalies. This could theoretically introduce a source of bias. However, only 10% of the total met the criteria of highly severe associated anomalies and are listed for review in a previous publication[12]. In addition, by limiting this study to inborns only, the bias of diminished severity inherent in outborn populations is avoided. That these data supported the development of a predictive model of mortality with an area under the curve of 0.92 correlates with a very high level of accuracy for a biologic system, and likely reflects both the strength of the associations, and the high degree of treatment consistency and outcomes obtained in this series.
We believe the central necessary components of CDH care to achieve maximal survival are strict adherence to lung protective ventilation[5,18,19] [20], repair of CDH [12,21], inclusive use of ECMO for more severe CDH as needed until lung recovery[10], and belief that the CDH infant can survive. Not all centers will achieve these results, but we hope these data will serve as a cautionary note for decisions to withhold treatments such as ECMO or CDH repair, as only by treating for survival will maximal survival be achieved. These data might also serve to support prenatal referral to a center where better results are obtained, rather than counseling families to terminate or limit support.
Conclusions
This report of the 10% most severe CDH patients defined by a variety of individual and combined severity metrics culled from 172 consecutive inborns shows that survival in excess of 60% is achievable in ECMO-eligible patients. Prematurity that precludes ECMO in these most severe patients, however, acts, as a lethal associated anomaly, and survival in these patients did not occur. Time to discharge averaged just over 3 months even in these most severe, and adherence to the treatment principles of lung protective ventilation, repair of CDH, use of ECMO as long as needed, and belief in survival potential, can result in surprisingly good survival and pulmonary outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reickert CA, Hirschl RB, Atkinson JB, Dudell G, Georgeson K, Glick P, et al. Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery. 1998;123:305–310. [PubMed] [Google Scholar]
- 2.Garcia A, Stolar CJH. Congenital diaphragmatic hernia and protective ventilation strategies in pediatric surgery. Surg Clin North Am. 2012;92:659–668. ix. doi: 10.1016/j.suc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Kays DW, Langham MR, Ledbetter DJ, Talbert JL. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg. 1999;230:340–348. doi: 10.1097/00000658-199909000-00007. discussion 348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonoff MB, Hustead VA, Groth SS, Schmeling DJ. Protocolized management of infants with congenital diaphragmatic hernia: effect on survival. J Pediatr Surg. 2011;46:39–46. doi: 10.1016/j.jpedsurg.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 5.Guidry CA, Hranjec T, Rodgers BM, Kane B, McGahren ED. Permissive hypercapnia in the management of congenital diaphragmatic hernia: our institutional experience. J Am Coll Surg. 2012;214:640–645. 647.e1. doi: 10.1016/j.jamcollsurg.2011.12.036. discussion646–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Role of admission gas exchange measurement in predicting congenital diaphragmatic hernia survival in the era of gentle ventilation. 2014;49:1197–1201. doi: 10.1016/j.jpedsurg.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Tiruvoipati R, Vinogradova Y, Faulkner G, Sosnowski AW, Firmin RK, Peek GJ. Predictors of outcome in patients with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation. J Pediatr Surg. 2007;42:1345–1350. doi: 10.1016/j.jpedsurg.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB Congenital Diaphragmatic Hernia Study Group. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 2009;44:1315–1321. doi: 10.1016/j.jpedsurg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Does a highest pre-ductal O(2) saturation. 2012;32:947–952. doi: 10.1038/jp.2012.18. [DOI] [PubMed] [Google Scholar]
- 10.Kays DW, Islam S, Richards DS, Larson SD, Perkins JM, Talbert JL. Extracorporeal life support in patients with congenital diaphragmatic hernia: how long should we treat? J Am Coll Surg. 2014;218:808–817. doi: 10.1016/j.jamcollsurg.2013.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001;36:141–145. doi: 10.1053/jpsu.2001.20032. [DOI] [PubMed] [Google Scholar]
- 12.Kays DW, Islam S, Larson SD, Perkins J, Talbert JL. Long-term maturation of congenital diaphragmatic hernia treatment results: toward development of a severity-specific treatment algorithm. Ann Surg. 2013;258:638–644. doi: 10.1097/SLA.0b013e3182a53c49. discussion644–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001;36:141–145. doi: 10.1053/jpsu.2001.20032. [DOI] [PubMed] [Google Scholar]
- 14.Yoder BA, Lally PA, Lally KP Congenital Diaphragmatic Hernia Study Group. Does a highest pre-ductal O(2) saturation <85% predict non-survival for congenital diaphragmatic hernia? J Perinatol. 2012;32:947–952. doi: 10.1038/jp.2012.18. [DOI] [PubMed] [Google Scholar]
- 15.Tam YS, Cheung HM, Tam YH, Lee KH, Lam HS, Poon TCW, et al. Clinical outcomes of congenital diaphragmatic hernia without extracorporeal membrane oxygenation. Early Hum Dev. 2012;88:739–741. doi: 10.1016/j.earlhumdev.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Does extracorporeal membrane oxygenation improve survival in neonates with congenital diaphragmatic hernia? The Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1999;34:720–724. doi: 10.1016/s0022-3468(99)90363-9. discussion724–5. [DOI] [PubMed] [Google Scholar]
- 17.Ali K, Grigoratos D, Cornelius V, Davenport M, Nicolaides K, Greenough A. Outcome of CDH infants following fetoscopic tracheal occlusion - influence of premature delivery. J Pediatr Surg. 2013;48:1831–1836. doi: 10.1016/j.jpedsurg.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Boloker J, Bateman DA, Wung JT, Stolar C. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002 doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia--a tale of two cities: the Boston experience. J Pediatr Surg. 1997;32:401–405. doi: 10.1016/s0022-3468(97)90590-x. [DOI] [PubMed] [Google Scholar]
- 20.Kays DW, Langham MR, Jr, Ledbetter DJ. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Annals of … 1999. doi: 10.1097/00000658-199909000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollinger LE, Lally PA, Tsao K, Wray CJ, Lally KP Congenital Diaphragmatic Hernia Study Group. A risk-stratified analysis of delayed congenital diaphragmatic hernia repair: does timing of operation matter? Surgery. 2014;156:475–482. doi: 10.1016/j.surg.2014.04.015. [DOI] [PubMed] [Google Scholar]